Abstract
Catalytic and regulatory domains of the Rel/Spo homolog of Streptococcus equisimilis affecting (p)ppGpp synthesis and degradation activities have been defined, and opposing activities of the purified protein and its fragments have been compared. Two major domains of the 739-residue RelSeq protein are defined by limited proteolytic digestion. In vitro assays of the purified N-terminal half-protein reveal synthesis of (p)ppGpp by an ATP-GTP 3′-pyrophosphotransferase as well as an ability to degrade (p)ppGpp by a Mn2+-dependent 3′-pyrophosphohydrolase. Removal of the C-terminal half-protein has reciprocal regulatory effects on the activities of the N-terminal half-protein. Compared to the full-length protein, deletion activates (p)ppGpp synthesis specific activity about 12-fold and simultaneously inhibits (p)ppGpp degradation specific activity about 150-fold to shift the balance of the two activities in favor of synthesis. Cellular (p)ppGpp accumulation behavior is consistent with these changes. The bifunctional N-terminal half-protein can be further dissected into overlapping monofunctional subdomains, since purified peptides display either degradation activity (residues 1 to 224) or synthetic activity (residues 79 to 385) in vitro. These assignments can also apply to RelA and SpoT. The ability of RelSeq to mediate (p)ppGpp accumulation during amino acid starvation in S. equisimilis is absent when the protein is expressed ectopically in Escherichia coli. Fusing the N-terminal half of RelSeq with the C-terminal domain of RelA creates a chimeric protein that restores the stringent response in E. coli by inhibiting unregulated degradation and restoring regulated synthetic activity. Reciprocal intramolecular regulation of the dual activities may be a general intrinsic feature of Rel/Spo homolog proteins.
Probably all cells respond to nutrient exhaustion in a manner that enables them to survive or adapt to the stress. These responses are perhaps most extensively studied in enterobacteria, where they are found accompanied by global effects on gene expression as well as complex metabolic and physiological changes. Among the many regulators mediating these adjustments are GDP and GTP derivatives with a pyrophosphate group on the 3′-hydroxyl position of ribose, i.e., GDP 3′-diphosphate (ppGpp) and GTP 3′-diphosphate (pppGpp), collectively abbreviated as (p)ppGpp (for reviews, see references 7 and 9). There is a growing awareness that eubacteria have exploited (p)ppGpp regulatory circuits in different ways. These include an unusual instance where they provide an essential function in Staphylococcus aureus (14). Among its dispensable functions, (p)ppGpp is thought to signal nutritional stress to initiate fruiting body development in Myxococcus xanthus (13, 20) and to activate quorum sensing in Pseudomonas aeruginosa (11). Involvement of (p)ppGpp in the regulation of antibiotic production in Streptomyces coelicolor (43) is thought to occur, along with regulation of colicin synthesis in Escherichia coli (25). These signal nucleotides probably contribute to virulence in Legionella pneumophila (18) and in Mycobacterium tuberculosis (37). Genes encoding proteins for (p)ppGpp synthesis seem common in the genomes of eubacteria, and one example has been reported in plants (47).
In E. coli and its close relatives, the metabolism of (p)ppGpp is governed by two homologous proteins, RelA and SpoT. The RelA protein catalyzes (p)ppGpp synthesis in a reaction requiring its binding to ribosomes bearing codon-specified uncharged tRNA (21). The major role of the SpoT protein in (p)ppGpp metabolism is the breakdown of (p)ppGpp by a manganese-dependent (p)ppGpp pyrophosphohydrolase activity (1, 22, 45). The spoT gene is also deduced to encode a weak (p)ppGpp synthesis activity in vivo that is normally obscured by its more abundant degradation activity (23, 35, 50). However, the deduced SpoT (p)ppGpp-synthetic activity is not detectable in vitro. There is no consensus as to whether the synthetic activity of SpoT is regulated (15, 35), and there is no knowledge as to how the degradation activity of SpoT is regulated. Deletion mapping in vivo concluded that the SpoT catalytic domains responsible for (p)ppGpp degradation and synthesis are localized within the N-terminal half of the protein on distinct but overlapping regions (15). Although the SpoT protein is deduced to possess a weak residual activity reminiscent of that of its RelA homolog, the converse is not true: RelA has not been observed to possess (p)ppGppase activity. The basis of this defect may be the absence of an HD domain, implicated as a key feature of a metal-dependent phosphohydrolase superfamily of proteins (2).
Eubacteria distantly related to the family Enterobacteraciae (such as species of Bacillus, Streptomyces, and Myxococcus) also have long been known to undergo a stringent response to amino acid starvation as they accumulate (p)ppGpp; rel mutants that abolish both responses also exist. In some instances, a protein with RelA-like ribosome-dependent (p)ppGpp-synthetic activity can be demonstrated; recent examples are from Bacillus subtilis (48, 49), S. coelicolor (8, 28), (29) and M. xanthus (20, 41). Sequencing revealed these genes to encode proteins related to the RelA/SpoT family. Although there are exceptional instances of multiple rsh (for rel spo homolog) genes present in a single organism (43, 47), a single bifunctional rsh gene could well represent an ancestral state (34). Genome sequences have revealed so far that species with a single rsh gene are much more common than those with separate (and probably specialized) Rel and Spo functions (34).
We wished to understand how opposing catalytic activities are mutually regulated when they coexist in the same Rel/Spo protein to prevent a futile cycle of catalysis. A recent well-studied example is provided by M. tuberculosis. The functional uniqueness of the product of a single rsh gene in the M. tuberculosis genome, called RelMtb, has been established by gene disruption (37). The RelMtb protein displays a ribosome-dependent (p)ppGpp-synthetic reaction activated by codon-specific uncharged tRNA binding in addition to a (p)ppGpp pyrophosphohydrolase activity (4). It is notable that the hydrolase activity of RelMtb is weakened simultaneously with activation of the synthetic activity on ribosomes (3).
The Rel/Spo homolog from Streptococcus equisimilis, called RelSeq, presents a special case to study for two reasons. First, each of the dual activities of RelSeq is evident in reactions containing only the purified protein and its nucleotide substrates (30). This allows us to ask whether the coordination of antagonistic activities is an intrinsic property of the protein. The second reason we are interested in RelSeq is that its dual activities are displayed in a host-specific manner. The (p)ppGpp-synthetic function of RelSeq is like that of RelA in its native host, while its high-level degradation activity (like that of SpoT) is the dominant activity when the RelSeq protein is expressed in E. coli (30, 31). This unusual feature provides a striking example of host-specific effects on regulation of the dual activities that is still poorly understood.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Bacterial strains and plasmids are listed in Table 1. The pURS1 plasmid is a pUC19 derivative, carrying relSeq from S. equisimilis H46A, used as a template for PCR amplification of relSeq fragments (30). The fragments were cloned, and their expression was regulated using pBAD vectors (17). Cells were grown with aeration at 32°C in Luria broth (LB) or morpholinepropanesulfonic acid (MOPS) minimal medium (36) containing 2 mM phosphate (0.2 mM phosphate for uniform 32P labeling), 0.4% glycerol, 40 μg (each) of the 20 amino acids/ml, and 20 μg (each) of adenosine, guanosine, cytidine, uridine, and thymidine per ml. Arabinose or glucose was present as indicated, and ampicillin (100 μg/ml) was added for plasmid maintenance. Aminotriazole (AT) plates contained 15 mM 3-amino-1,2,4-triazole from Sigma-Aldrich (38). Minimal medium was supplemented with 1 mM (each) serine, methionine, and glycine for SMG plates (46).
TABLE 1.
Bacterial strains, plasmids, and PCR primers
| Strain or plasmid | Genotype or constructiona | Reference or source |
|---|---|---|
| E. coli | ||
| CF1648 | Wild-type MG1655 | 50 |
| CF1652 | As CF1648 but ΔrelA251 | 50 |
| CF1693 | As CF1652 but ΔspoT207 | 50 |
| CF4941 | As 1648 but galK2 zib-563::Tn10 ΔrelA251 | 30 |
| CF4943 | As 4941 but spoT203 | 30 |
| BL21(λDE3) | BL21 (λDE3 lysogen; T7 expression vector) | Novagen |
| Plasmid | ||
| pUC19 | Vector; lacZpo; ColE1 replicon; Apr | |
| pURS1 | As pUC19 but carrying relSeq from S. equisimilis H46A in lacZpo antisense orientation | 30 |
| pET21 | Expression Vector; T7lacpo; pBR replicon; Apr | Novagen |
| pUM99 | As pET21; RelSeq (aa 1-385); C-terminal His tag | This study |
| pBAD18 | Vector; BADpo; pBR replicon; Apr | 17 |
| pBAD22A | Vector; BADpo; pBR replicon; Apr | 17 |
| pUM66 | As pBAD18; RelSeq (aa 1-385); C-terminal His tag | This study |
| pUM108 | As pBAD18; RelSeq (aa 1-224); C-terminal His tag | This study |
| pUM9 | As pBAD18; RelSeq (aa 79-385); C-terminal His tag | This study |
| pUM109 | As pBAD18; RelSeq (aa 1-739); C-terminal His tag | This study |
| pUM110 | As pBAD18; RelSeq (aa 1-385); C-terminal His tag and RelSeq (aa 386-739) coded by separate ORFs | This study |
| pUM111 | As pBAD18; RelSeq (aa 1-385) and RelSeq (aa 386-739) coded by separate ORFs | This study |
| pUM112 | As pBAD18; RelSeq (aa 1-347) and RelSeq (aa 348-739) coded by separate ORFs | This study |
| pUM113 | As pBAD18; RelSeq (aa 386-739 fused to aa 1-385) | This study |
| pUM116 | As pBAD18; SpoT (aa 1-702); C-terminal His tag | This study |
| pUM117 | As pBAD18; SpoT (aa 1-378); C-terminal His tag | This study |
| pLB15 | As pBAD22A; RelA | This study |
| pLB16 | As pBAD22A; RelSeq | This study |
| pLB18 | As pBAD22A; RelA (aa 1-392) fused to RelSeq (aa 383-739) | This study |
| pLB19 | As pBAD22A; RelSeq (aa 1-382) fused to RelA (aa 394-744) | This study |
ORF, open reading frame; aa, amino acids.
(p)ppGpp measurements.
(p)ppGpp accumulation was measured in cells grown and uniformly labeled with 32Pi in MOPS medium (supplemented as described above) by published procedures (6) adapted to accommodate arabinose induction. Overnight cultures contained 0.8 mM phosphate and 0.02% glucose to insure repression of BADpo transcription. The cultures were diluted 1:100 in fresh medium containing 0.2 mM phosphate and 0.02% arabinose for BADpo induction. At an A600 of ≅0.05, carrier-free 32Pi (150 μCi/ml) was added, and the cells were further incubated until they reached an optical density at 600 nm of ≅0.3 and then sampled. In some experiments (see Fig. 6), 1 mg of dl-serine hydroxamate (Sigma-Aldrich)/ml was added to similarly arabinose-induced, uniformly 32Pi-labeled cultures at an A600 of 0.3. Nucleotide extraction and quantitation was done as described previously (31). Amounts of pppGpp and ppGpp were expressed as fractions of the sum of blank corrected GTP-plus-(p)ppGpp pools after normalizing their differing molar phosphate contents.
FIG. 6.
The RelA C-terminal domain restores (p)ppGpp accumulation response to amino acid starvation in the RelSeq/RelA chimera. Cultures were grown and uniformly 32P labeled in MOPS containing 0.002% arabinose for low-level protein induction. Serine hydroxamate was added at time zero. The strains tested were CF4941 derivatives containing pLB15 (RelA), pLB18 (RelA/RelSeq), pLB16 (RelSeq), and pLB19 (RelSeq/RelA).
Purification of RelSeq and SpoT protein fragments.
Purification of the native RelSeq protein is described elsewhere (30). We shall designate the amino-terminal and carboxy-terminal protein domains of RelSeq N and C, with addition of a six-His tag indicated by H, immediately followed by the amino acid residues present excluding the six histidines. Thus, NCH 1-739 denotes the full-length RelSeq protein with a C-terminal six-His tag. The RelSeq fragments NH 1-224 and NH 1-385 as well as NCH 1-739 were purified from 1-liter LB cultures of the CF1652 relA deletion strain bearing appropriate plasmids (Table 1), grown to an A600 between 0.7 and 0.9, and then induced for 2.5 h with 0.2% arabinose. Cells were harvested by centrifugation, frozen, and then resuspended in 25 ml of binding buffer (300 mM NaCl, 50 mM sodium phosphate [pH 8.0], 5 mM imidazole) containing 0.1 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride hydrochloride serine protease inhibitor and lysed by incubation for 30 min on ice with 130 μg of lysozyme/ml followed by sonication. After removal of cell debris by centrifugation at 10,000 rpm in a Sorval SS-34 rotor for 20 min, the protein was batch purified with Ni-nitrilotriacetic acid resin (Qiagen) according to the instructions of the supplier but using a wash buffer containing 300 mM NaCl, 50 mM sodium phosphate (pH 8.0), 10% glycerol, and 40 mM imidazole. Proteins were eluted from Ni-nitrilotriacetic acid columns with a step gradient of 0.1 to 0.5 mM imidazole in five increments of 0.1 mM in elution buffer (1 M NaCl, 50 mM sodium phosphate [pH 6.0], 20% glycerol). The desired fraction, consisting of a major protein band with trace amounts of other proteins, was localized by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), dialyzed against 50 mM bis-Tris-propane (pH 9.0) containing 300 mM NaCl, and stored at −20°C. The bis-Tris-propane buffer {1,3-bis[tris(hydroxymethyl)methylamino]-propane} was obtained from Sigma Chemical Co.
Preparations of the SpoT NH 1-379 and SpoT NCH proteins were similar but scaled down 10-fold with purification on Ni-silica spin columns (Qiagen) and with 50 mM HEPES buffer substituted for phosphate in the binding buffer, which also contained 1 mM β-mercaptoethanol; washing with 40 mM imidazole, and elution with 150-μl washes of 0.5 M imidazole instead of a step gradient. The eluate was dialyzed against 50 mM HEPES (pH 8.0), 5% glycerol, 300 mM NaCl, and 1 mM β-mercaptoethanol.
Purification of His-tagged RelSeq NH 79-385 required a still more modified procedure to enhance solubility. Culture growth was at a lower temperature (26°C), and induction was done by adding 0.02% arabinose. The frozen cells were resuspended in 25 ml of a binding buffer that contained 50 mM HEPES (pH 8.0), 0.1 M LiCl, 0.1% Triton X-100, and 10 mM imidazole, as well as 0.1 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride hydrochloride. Cell lysis and removal of the debris was done as described above. His-Bind-Quick columns (NOVAGEN) with an Ni2+ cellulose matrix were washed according to the manufacturer's instructions with 30 ml of binding buffer, washed with 40 ml of buffer containing 40 mM imidazole, and finally eluted with five increments of imidazole step gradient but using an elution buffer containing 50 mM HEPES (pH 8.0), 0.1 M LiCl, 0.1% Triton X-100, and 10% glycerol.
Assay for pppGpp synthetase.
For detection of pppGpp-synthetic activity, 20-μl reaction mixtures generally containing 8 mM ATP, 6 mM GTP, 25 mM bis-Tris-propane (pH 9.0), 150 mM NaCl, and 15 mM MgCl2 were incubated at 37°C, and reactions were initiated by enzyme addition. At intervals, 4-μl reaction samples were mixed with 2 μl of 3 M formic acid and resolved by polyethyleneimine (PEI) thin-layer chromatography (6). Synthesized (p)ppGpp was visualized by UV absorbance and/or by labeling using either [γ-32P]ATP with GTP as a substrate or [α-32P]GTP with ATP as a substrate. Specific activities were calculated by defining 1 U as 1 μmol/min/mg of protein and correcting for blanks lacking enzyme. The ImageQuant program was used for quantitation, with data acquired with a Storm scanning densitometer. Synthetase assays with SpoT proteins included a 20-min preincubation with sufficient EDTA to insure chelation of manganese ions copurifying with the enzyme.
Assay for (p)ppGppase.
Pyrophosphohydrolase activity was measured in 20-μl reaction mixtures containing 2 mM ppGpp, 25 mM bis-Tris-propane (pH 9.0), 10 mM MgCl2, and 1 mM MnCl2 at 37°C with the reaction initiated by enzyme addition. Samples were processed as described above. Labeled substrate 3′-[β-32P](p)ppGpp was purified (30), and activity could be monitored by the disappearance of the substrate and the stoichiometric appearance of labeled pyrophosphate, which comigrates with GTP on PEI-cellulose chromatograms. In some instances, labeled pppGpp was allowed to accumulate in synthetase reactions containing MgCl2 at concentrations equal to the total nucleotides, and hydrolysis was initiated by the addition of 2 mM MnCl2. Preincubation reactions contained either [α-32P]GTP and ATP (6 mM each) to form 5′-[α-32P]pppGpp (see Fig. 4) or 6 mM [γ-32P]ATP and 4 mM GTP to form 3′-[β-32P]pppGpp (see Fig. 5).
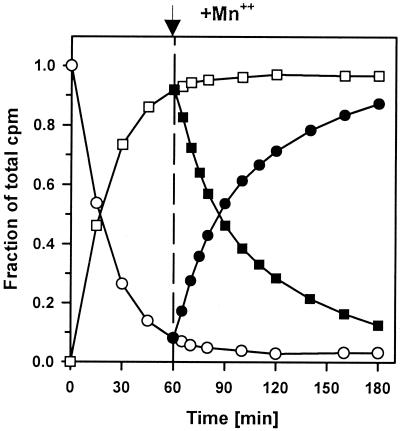
FIG. 4.
Complete degradation of pppGpp under conditions of substrate-limited synthesis. The 150-μl reaction mixtures contained 6 mM [α-32P]GTP, 6 mM ATP, 13 mM MgCl2, 150 mM NaCl, 25 mM bis-Tris-propane, and RelSeq NH 1-385. MnCl2 (2 mM) was added 60 min after the start of the synthesis reaction. Symbols: circles, GTP; squares, pppGpp; open symbols, Mg2+; solid symbols, Mg2+ and Mn2+.
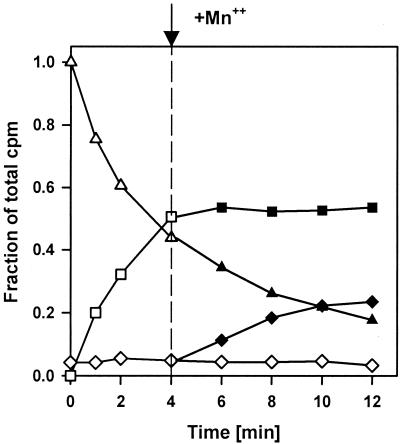
FIG. 5.
Simultaneous pppGpp synthesis and degradation in vitro. The 40-μl reaction mixtures contained [γ-32P]ATP (6 mM), 4 mM GTP, 10 mM MgCl2, 1 mM dithiothreitol, 150 mM NaCl, 25 mM bis-Tris-propane, and RelSeq NH 1-385. MnCl2 (2.5 mM) was added 4 min after the reaction was started. Symbols: triangles, ATP; squares, pppGpp; diamonds, PPi; open symbols, Mg2+; solid symbols, Mg2+ and Mn2+. The reactions are represented as follows: synthesis (Mg2+), ATP + GTP → pppGpp + AMP + H2O; degradation (Mn2+), ppGpp + H2O → GTP + PPi; net reaction, ATP → AMP + PPi.
RESULTS
RelSeq consists of two domains that are separated by a proteolytically sensitive region.
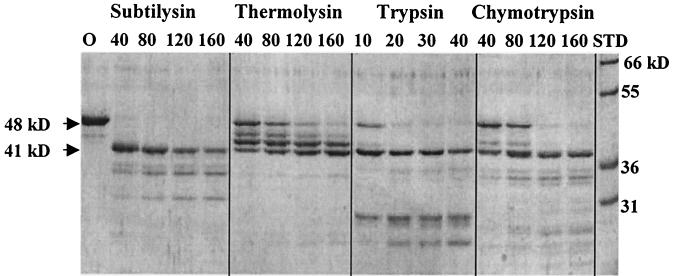
Studies of RelA and SpoT from E. coli suggest a two-domain structure: the N-terminal half-protein has the catalytic activity of RelA or SpoT, and the C-terminal half-protein is implicated in regulation of RelA (15, 16, 40). The deduced protein sequences of rsh genes from a variety of sources also show central regions of unusual sequence heterogeneity (28). The SpoT protein has also been noted to possess a central protease-sensitive region (K. Ikehara, personal communication). We were therefore led to ask whether the functional domains of the rsh homolog from S. equisimilis could be mapped as regions of relative protease resistance. We found that limited trypsin digestion of the 739-residue RelSeq protein initially gave two (41- and 50-kDa) peptides on SDS-PAGE (data not shown). We also found that the full-length RelSeq protein was difficult to work with because of solubility problems, whether or not a six-His tag was present on either end. A 385-amino-acid peptide containing the entire N-terminal domain, all 16 amino acids in the region of sequence heterogeneity (the putative trypsin-sensitive region), and several residues beyond was found more soluble. More detailed proteolytic digestion studies with the 48-kDa RelSeq NH 1-385 protein are presented in Fig. 1, where cleavage with subtilysin, thermolysin, trypsin, or chymotrypsin can be seen to give an initially relatively stable product with an apparent molecular mass of about 41 kDa. Analysis of the trypsin-cleaved 41-kDa peptide by mass spectrometry indicated that its C-terminal residue was lysine-347 (data not shown). This position coincides with the upstream boundary of the central region of sequence heterogeneity for RelSeq. We take this to mean that the RelSeq NH 1-385 protein contains the proteolytically insensitive N-terminal region present in the original full-length protein together with most of the small proteolytically sensitive central region. We next turned to asking whether deletion of residues 386 to 739 affects the catalytic activities of the RelSeq protein. Changes in (p)ppGpp-synthetic activity and its regulation have been deduced to occur when a large C-terminal portion of RelA is deleted (40).
FIG. 1.
Protease cleavage of RelSeq NH 1-385. Reaction mixtures (20 μl) containing 4 μg of purified protein, 4 mM MgCl2, 5% glycerol, 50 mM NaCl, 20 mM Tris (pH 7.9), and 1 mM EDTA were incubated at room temperature for 30 min. Subtilysin, thermolysin, trypsin, or chymotrypsin was present at the concentrations (in nanograms) indicated above the lanes. The reaction was stopped by the addition of an equal volume of 2× SDS loading buffer and boiling for 2 min, and the products were run on a 12% gel. Lane O, uncleaved protein; lane STP, protein size standards.
RelSeq NH 1-385 shows altered (p)ppGpp synthesis and degrading activity in vivo.
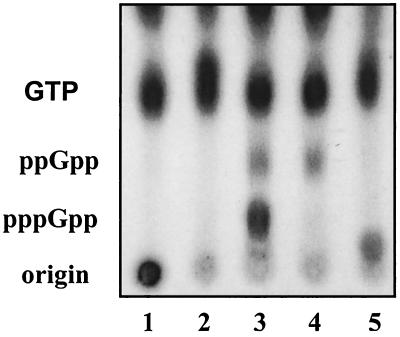
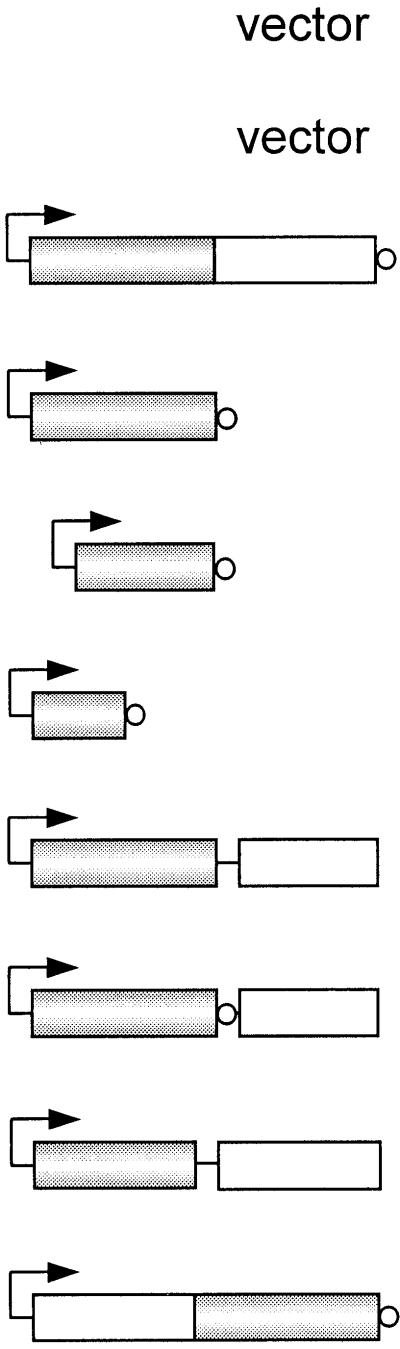
The RelSeq NH 1-385 protein was conditionally expressed by varying arabinose concentrations after being cloned in a pBAD18 vector (pUM66) present in a host with relA deleted, strain CF1652 (Table 1). A very-small-colony phenotype for CF1652 (pUM66) compared to the vector control, CF1652 (pBAD18), was observed for this construct on LB-agar plates containing 0.2% arabinose. Titrations revealed that reducing the arabinose concentration 10-fold (to 0.02%) only partly minimized the growth defects and still resulted in the accumulation of significant amounts of (p)ppGpp. Steady-state growth in 0.02% arabinose yielded ppGpp at 10% and pppGpp at 20% of the sum of GTP-pppGpp-ppGpp pools (Fig. 2). This accumulation of 30% (p)ppGpp reversed the sensitivity of the ΔrelA host to growth on AT plates as well as on SMG plates (Table 2). Both plate tests indicated that at least wild-type levels of (p)ppGpp were induced during the stringent response (38, 46). This behavior differs from that of full-length RelSeq induced to similar levels, which does not elevate (p)ppGpp (30).
FIG. 2.
Accumulation of ppGpp and pppGpp in E. coli CF1652 (ΔrelA) containing pBAD18 (vector control) (lane 1), BADpo NCH 1-739 (full-length RelSeq) (lane 2), pBAD NH 1-385 (lane 3), BADpo NH 79-385 (lane 4), and BADpo NH 1-224 (lane 5). Resolved nucleotides were extracted from uniformly 32P-labeled cultures grown under steady-state conditions in 0.02% arabinose. The radioactive spot near the origin in lane 5 is not reproducible and not identified.
TABLE 2.
Growth on LB-agar plates or M9 minimal medium plates containing AT or SMG
| Strain | Constructa | Growth on LB + 0.2% arabinoseb | Growth on M9 containing 0.02% arabinosec
|
|
|---|---|---|---|---|
| AT | SMG | |||
| relA+(pBAD18) | 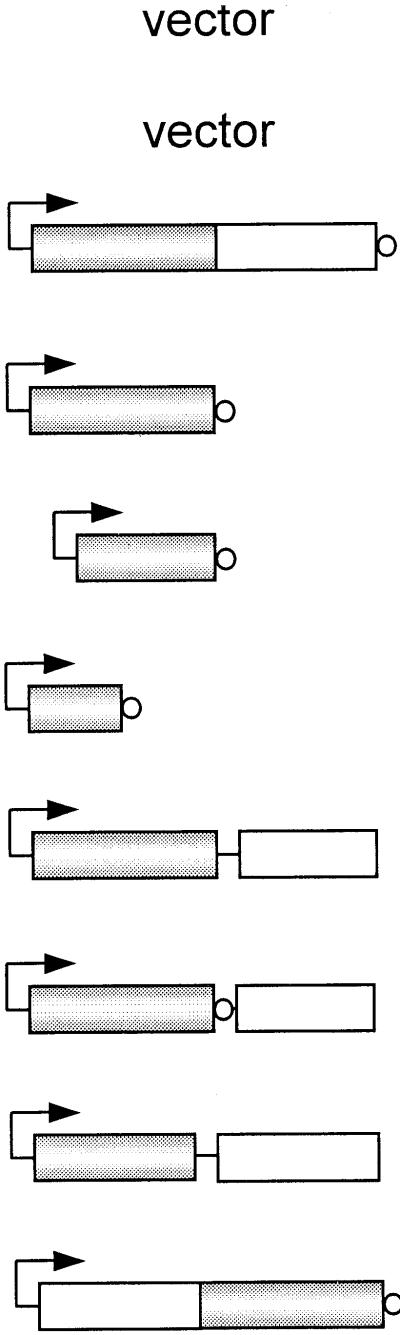 |
+++ | + | + |
| ΔrelA(pBAD18) | +++ | − | − | |
| ΔrelA(pUM109) | +++ | − | − | |
| ΔrelA(pUM66) | ++ | + | + | |
| ΔrelA(pUM9) | + | + | + | |
| ΔrelA(pUM108) | +++ | − | − | |
| ΔrelA(pUM111) | ++ | + | + | |
| ΔrelA(pUM110) | ++ | + | + | |
| ΔrelA(pUM112) | ++ | + | + | |
| ΔrelA(pUM113) | ++ | + | + | |
Arrows indicate BADpo promoter; shaded bars represent RelSeq amino acids 1 to 385 or scaled fractions thereof; open bars represent RelSeq amino acids 347 to 739 or fractions thereof; small circles represent six-His tag.
Colony sizes: +++, wild type; ++, smaller; +, very small.
+, growth; −, no growth.
The spoT203 allele gives an elevated steady-state (p)ppGpp level and thereby confers a slow-growth phenotype on relA mutant hosts (39). Expression of RelSeq NH 1-385 under the control of the BADpo promoter in the spoT203 host with relA deleted confers an even slower growth phenotype than the corresponding vector control due to excessive (p)ppGpp-synthetic activity (Table 3; see below). We are unable to detect net degrading activity for NH 1-385 by complementing a spoT mutation (Table 3), whereas full-length RelSeq shows strong degrading activity by this in vivo test (30).
TABLE 3.
Tests for reversal of growth inhibition of an E. coli spoT203 mutant by expression of selected RelSeq fragments
Specific activities of RelSeq NH 1-385 for both synthesis and degradation differ from those of full-length RelSeq.
The inability to complement a spoT mutation in vivo does not necessarily mean the RelSeq NH 1-385 protein is completely devoid of (p)ppGppase activity. It reflects instead a net difference in the balance of synthesis and degradation. Table 4 shows that the purified protein fragment both synthesizes and degrades pppGpp in vitro under conditions where each reaction is assayed separately. The specific activities in Table 4 are calculated from initial reaction velocities measured under conditions of substrate excess with observed rates that are linear with both time and enzyme concentration. Comparing the observed specific activities of NH 1-385 with those of the full-length (NC 1-739) protein reveals that synthetic activity is activated about 12-fold (2.15 U/0.17 U) after correcting for the fact that the NH 1-385 peptide is about half the molecular weight of full-length RelSeq. Compared to NC 1-739, degradation activity of NH 1-385 is inhibited by about 150-fold (52 U/0.35 U), again correcting for the molecular weights of the compared proteins (Table 4). The in vivo differences in (p)ppGpp levels shown in Fig. 2 can be expected to reflect changes in the balance between degrading and synthetic activities. The net specific activities apparent from these in vitro assays change from about 50-fold (52 U − 0.17 U) in favor of (p)ppGpp degradation for native RelSeq to about 3.6-fold (4.3 U − 0.7 U) in favor of (p)ppGpp synthesis for NH 1-385. The low values are somewhat imprecise due to our inability to measure weak specific activities with the same accuracy as strong activities.
TABLE 4.
Specific activities of purified RelSeq fragments
| Enzyme | pppGpp synthetase activity units (μmol/min/mg) | pppGpp hydrolase activity units (μmol/min/mg) |
|---|---|---|
| RelSeq NC 1-739 | 0.17 | 52.0 |
| RelSeq NCH | 0.54 | 0.35 |
| RelSeq NH 1-385 | 4.3 | 0.7 |
| RelSeq NH 79-385 | 0.22 | 0.0 |
| RelSeq NH 1-224 | 0.0 | 0.46 |
It is noteworthy that the simple addition of the six-His affinity tag to the C terminus of full-length RelSeq (Table 4, NCH) has regulatory effects nearly equivalent to those of the C-terminal deletion. Affinity tag addition inhibits degradation activity (52 U − 0.35 U = ∼50-fold) and activates synthetic activity (3-fold; 0.54 U/0.17 U). This results in very low activities for both synthesis and degradation for NCH, with only a net 1.5-fold excess of synthesis over degradation. Given our estimate of a modest net activity favoring synthesis, we were surprised to see that overexpression of NCH neither inhibits growth nor complements a relA mutant by AT or SMG growth tests (Table 2) but that NCH does complement a spoT203 mutation (Table 4). This is the only example where in vivo behavior is inconsistent with in vitro activity measurements (see Discussion).
Donor and acceptor specificities for the RelSeq NH 1-385-catalyzed synthesis reaction.
Using [γ-32P]ATP as a pyrophosphate donor, we tested GTP, GDP, GMP, dGDP, ITP, IDP, and XTP as possible acceptors of the reaction and found a GTP > ITP > GDP preference for RelSeq NH 1-385 (Table 5), whereas the preference of RelA is GTP = GDP > ITP (10). RelMtb has a similar acceptor specificity, but preferences among GTP, GDP, and ITP are quantitatively uncertain (4). The specificities of pyrophosphate donors were tested using [α-32P]GTP as an acceptor; ATP and dATP showed activity, but not ITP, XTP, UTP, or CTP (Table 5). This donor specificity is similar to that of RelA but not to that of RelMtb.
TABLE 5.
Substrate specificity of RelSeq NH 1-385
| Donorac | Acceptorbc
|
||
|---|---|---|---|
| Substrate | Activity (U) | Substrate | Activity (U) |
| ATP | 4.3 | GTP | 4.3 |
| dATP | 3.7 | GDP | 0.6 |
| ITP | NDc | GMP | |
| XTP | NDc | dGDP | |
| UTP | NDc | ITP | 1.9 |
| CTP | NDc | IDP | |
| XTP | |||
Measured with donors at 8 mM using 6 mM GTP as acceptor substrate.
Measured with acceptors at 6 mM using 8 mM ATP as donor substrate.
ND, none detectable; minimum detectable activity, 0.01 U.
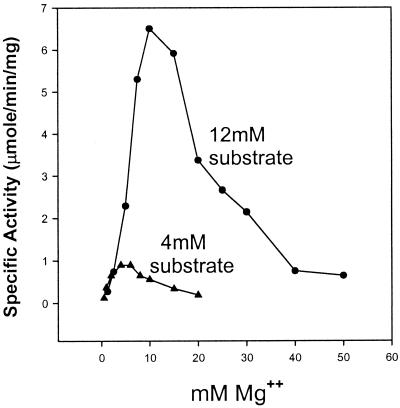
The Mg2+ optimum parallels the total nucleotide substrate concentration in the pppGpp synthesis reaction. Figure 3 shows the effects on specific activity of varying the Mg2+ ion concentration at fixed concentrations of total nucleotide substrates at either 4 or 12 mM while keeping the ATP/GTP ratio constant at 1:3. This ratio was determined to be optimal in preliminary experiments. In Fig. 3, when the total substrate concentration is 4 mM, a broad Mg2+ optimum is found extending from about 3 to 7 mM. At a total substrate concentration of 12 mM, the Mg2+ optimum shifts to 10 to 15 mM, peaking at about 12 mM. The observed optima for MgCl2 in the reaction approximate the total nucleotide substrate concentrations present, as if ATP and GTP are presented to the enzyme as complexes with Mg2+ ions. A similar conclusion was reached previously using RelMtb and is discussed in detail elsewhere (3).
FIG. 3.
The magnesium optimum is determined by the total nucleotide substrate concentration. The MgCl2 concentration (abscissa) was varied at total nucleotide substrate concentrations of either 12 or 4 mM and a fixed ATP/GTP ratio of 1:3.
Approximating Km values for ATP and GTP.
We estimate the Km for GTP to be approximately 2 mM (data not shown). It was measured at 8 mM ATP by covarying the concentrations of GTP and MgCl2. The Km for ATP is at least 5 mM, measured at 6 mM GTP and varying the concentrations of ATP and MgCl2. This estimate is qualified because double reciprocal plots of initial reaction rates obtained versus ATP gave concave upward kinetics, suggesting cooperativity with ATP.
RelSeq NH 1-224 peptide degrades (p)ppGpp without synthesis.
We constructed pUM108 (Table 1) to allow arabinose induction of RelSeq NH 1-224, the portion of RelSeq homologous to the SpoT peptide fragment deduced from in vivo behavior to have (p)ppGpp degradation, but not synthesis, activity (15). Expression of NH 1-224 complements the E. coli spoT203 slow-growth phenotype in vivo, as expected (Table 3). This behavior is similar to that of the native full-length RelSeq protein, with a net activity strongly favoring degradation (30). Moreover, no detectable (p)ppGpp accumulates during steady-state growth in 0.2% arabinose of an E. coli ΔrelA host overexpressing NH 1-224 (Fig. 2, lane 5). The purified NH 1-224 protein fragment displayed (p)ppGpp degradation activity in vitro, but unlike full-length RelSeq (30), we could not detect (p)ppGpp synthesis activity (Table 4).
RelSeq NH 79-385 can synthesize but not degrade (p)ppGpp.
We also constructed the plasmid pUM9 to express the RelSeq NH 79-385 homolog of the SpoT peptide that shows (p)ppGpp synthesis and not degradation in vivo (15). Steady-state induction of RelSeq NH 79-385 conferred (p)ppGpp accumulation (12% of the total guanine nucleotide pool defined as before) on the CF1652 strain with relA deleted (Fig. 2, lane 4), along with growth inhibition (Table 3). As for strains overproducing the NH 1-385 protein, this accumulation of ppGpp allows complementation of a relA mutation, as judged by AT and SMG plate tests (Table 2).
The purified NH 79-385 peptide catalyzes pppGpp synthesis but not detectable (p)ppGpp hydrolysis in vitro (Table 4). The specific synthetic activity of NH 79-385 is nearly the same as that of native RelSeq (0.22 versus 0.17 U) and much lower than that of NH 1-385 (4.3 versus 0.22 U) (Table 4). The NH 79-385 peptide showed at least as strong a preference for GTP over GDP as a pyrophosphate acceptor as NH 1-385, since we were unable to detect ppGpp synthesis using GDP and ATP as substrates (data not shown).
A futile cycle: simultaneous formation and degradation of pppGpp in vitro.
By simply manipulating nucleotide substrates and Mn2+ ion availability in reactions containing RelSeq, it is possible to separate or combine pppGpp synthesis with simultaneous degradation. The latter reflects a futile metabolic cycle. The net reaction is hydrolysis of ATP to pyrophosphate and AMP with (p)ppGpp as an intermediate (see the legend to Fig. 5). RelSeq NH 1-385 was chosen for this demonstration because both synthesis and degradation are active (Table 4).
Figure 4 presents a control reaction in which pppGpp is first preformed and then degraded by the addition of Mn2+ under conditions where pppGpp synthesis is limited by ATP exhaustion. The presence of only Mg2+ and [α-32P]GTP equimolar with ATP results in accumulation of 5′-[α-32P]pppGpp stoichiometric with the disappearance of labeled GTP (Fig. 4). The subsequent addition of Mn2+ after pppGpp synthesis is complete (Fig. 4) leads to degradation of pppGpp and an apparently stoichiometric rejuvenation of [α-32P]GTP. Under these conditions, AMP and inorganic pyrophosphate (PPi) are not labeled and are therefore not monitored.
For simultaneous (p)ppGpp synthesis and degradation, ATP is present in excess over GTP to insure its presence when GTP is completely consumed in the synthetic reaction so that regeneration of GTP by pppGpp pyrophosphorolysis will allow renewed synthesis (Fig. 5). The labeled substrate is changed to [γ-32P]ATP to allow monitoring of both 3′-[β-32P]pppGpp and the labeled PPi product of pppGpp pyrophosphorolysis. Accumulation of labeled pppGpp occurs as before in the presence of only Mg2+; the time scale in Fig. 5 is more condensed than that in Fig. 4 because more NH 1-385 activity is present for trivial reasons. Accumulation of pppGpp stops after 4 min; thereafter, the addition of Mn2+ initiates pppGpp degradation, as indicated by the appearance of labeled PPi (Fig. 5). The continued decrease of [γ-32P]ATP after the addition Mn2+ together with constant levels of pppGpp attest to persistent pppGpp synthesis during pppGpp hydrolysis to GTP. After incubations long enough to exhaust ATP, pppGpp disappears, with virtually all label appearing in PPi (data not shown). Side reactions, such as complete hydrolysis of nucleotides or PPi to orthophosphate, are apparently minimal under these conditions, since the total label in ATP, pppGpp, and PPi after 12 min of incubation is nearly equivalent to the initial label in ATP at time zero (Fig. 5).
A RelSeq/RelA chimera is responsive to amino acid starvation in E. coli.
The C-terminal domain of RelA is needed for its activation by ribosomes and amino acid starvation (40). Since full-length RelSeq, as well as NH 1-385, is unresponsive to activation by amino acid starvation in E. coli, we asked whether the C terminus of RelA might restore regulation of the catalytic activities of the RelSeq N-terminal domain. The pLB19 plasmid was constructed to encode a RelSeq/RelA chimera containing amino acids 1 to 382 of RelSeq followed by amino acids 394 to 744 of RelA. A RelA/RelSeq inverse chimera, plasmid pLB18, was made to express amino acids 1 to 393 of RelA followed by amino acids 383 to 739 of RelSeq. All chimeras were constructed without affinity tags to avoid the sort of regulatory effects seen with RelSeq NCH described above. Figure 6 shows the (p)ppGpp accumulation responses of each chimera expressed in a host with relA deleted when serine hydroxamate was added to uniformly labeled cultures. Cells were grown in medium containing 0.002% arabinose for low-level steady-state protein induction, and equivalent induction of protein was verified by SDS-PAGE (data not shown). It can be seen in Fig. 6 that the RelSeq/RelA chimera has a (p)ppGpp accumulation response very similar to that of wild-type RelA. This suggests that the RelA C terminus, itself lacking any reported catalytic activities, mediates three regulatory functions. The first is the strong inhibition of otherwise constitutive pppGpp degradation (compared to full-length RelSeq). The second is activation of pppGpp synthesis during starvation, compared to full-length RelSeq or to the RelA/RelSeq chimera. The third is the reduction of high steady-state basal pppGpp levels with RelSeq NH 1-385 (Fig. 2 and Table 2). For the RelA/RelSeq chimera, the modest steady-state elevation of (p)ppGpp that is not stimulated by amino acid starvation is reminiscent of a RelA C-terminal deletion (32, 44).
Attempts to obtain trans-complementation with the RelSeq C-terminal domain.
Overexpression of various E. coli RelA peptide fragments is known to restore (p)ppGpp-synthetic activity by trans complementation as well as to give altered regulation (16, 32). We attempted to reconstitute a functional full-length RelSeq with the phenotype of pUM109 by overexpression of RelSeq N- and C-terminal domains as separate open reading frames on the same plasmid, as for pUM110-pUM113 (Table 3). None of the four constructs behaved like full-length NC or NCH with respect to (p)ppGpp accumulation phenotypes in vivo.
Demonstration of in vitro SpoT (p)ppGpp-synthetic activity.
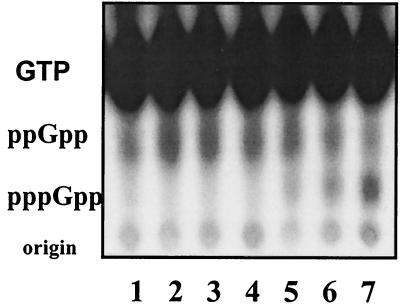
The E. coli SpoT protein has been deduced from in vivo behavior to possess (p)ppGpp-synthetic activity (15, 50), but this could not be verified in vitro (30). We found that the SpoT NH 1-378 protein has (p)ppGpp-synthetic activity evident when assayed as for RelSeq but with longer incubations (Fig. 7, lanes 5 to 7). A specific synthetic activity of only about 1 nmol/min/mg of SpoT protein fragment is found, rather than 4 μmol/min/mg for RelSeq NH 1-385. Although the observed synthetic activity of this SpoT fragment is very weak, it is not demonstrable at all with full-length SpoT NCH (Fig. 7, lanes 2 to 4) or with native SpoT. This behavior is consistent with the notion that deletion of the C terminus of SpoT, as with RelSeq, activates its latent synthetic activity. It is worth pointing out that (p)ppGpp degradation is absent under these conditions because Mg2+, rather than Mn2+, ions are present.
FIG. 7.
The SpoT N-terminal half-protein synthesizes (p)ppGpp in vitro. Shown is a PEI-cellulose thin-layer chromatogram of reaction aliquots incubated for 2 h that contained 8 mM ATP, 6 mM [α-32P]GTP, 15 mM MgCl2, 1 mM β-mercaptoethanol, 150 mM NaCl, and 50 mM HEPES (pH 8.0). Lane 1, no enzyme control; lanes 2 to 4, SpoT NCH at 2, 4, and 8 μg/ml, respectively; lanes 5 to 7, SpoT NH 1-378 at 3.5, 7, and 14 μg/ml, respectively.
DISCUSSION
The domain structure of RelSeq, the protein homolog in the Rel/Spo family from S. equisimilis, has been explored. This purified protein catalyzes opposing reactions in (p)ppGpp metabolism. Similar basal activities in the presence of substrate are reported for another Rel/Spo homolog (RelMtb), with the exception that RelSeq shows much less synthetase activity. Unlike RelSeq, RelMtb can also be activated by heterologous E. coli ribosomes (4), and its altered kinetic properties have been characterized in some detail (3). The authors concluded that RelMtb probably possesses two catalytic sites, each simultaneously showing maximal rates of opposing reactions with appropriate substrates. In the additional presence of ribosomes, mRNA, and codon-specified uncharged tRNA, RelMtb increased its transferase activity and almost completely lost its hydrolysis activity; this is thought to be due to an allosteric effect communicated to the hydrolysis active site (3). We find with RelSeq that protease sensitivity defines two domains by cleaving near the center of the protein. The N-terminal domain retains both catalytic activities, while removal of the C-terminal half of the protein alters opposing catalytic activities in a manner that simulates allosteric effects of biological activation of RelMtb binding to ribosomes (3). The central finding of this work is that the C-terminal domain of RelSeq is involved in reciprocal regulation of the two opposing activities present in the N-terminal domain. This sort of regulation seems to ensure that both synthesis and degradation activities are not coinduced when one activity is altered, thereby minimizing futile cycling.
The existence of a large separate regulatory domain differentially affecting dual catalytic activities, documented here for RelSeq, is very likely to apply to RelA, SpoT (discussed below), and possibly other Rel/Spo homologs, considering the sequence relatedness of this gene family (34). This feature is not found among five other examples in the superfamily of single-gene-encoded enzymes with opposing activities: EnvZ kinase/phosphatase (12), isocitrate dehydrogenase kinase/phosphatase (33), glutathionylspermidine synthetase/amidase (27), adenyl transferase adenylylation/deadenylylation (24), and 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (51).
As noted above, RelSeq expressed in E. coli has a net hydrolysis activity uninfluenced by regulatory signals that affect RelA. Whether (p)ppGpp accumulates or vanishes under the control of RelSeq depends on the balance of opposing activities. Full-length RelSeq has a net specific activity so strongly in favor of degradation that it can reverse the stringent response when relA is maximally induced with IPTG (isopropyl-β-d-thiogalactopyranoside) in E. coli (30). Deletion of the RelSeq C terminus, exemplified by NH 1-385, enhances synthesis 12-fold and inhibits degradation 150-fold compared to full-length RelSeq (Table 4) such that the net strong degrading activity of the full-length RelSeq is reversed to clearly favor synthesis. Growth tests (Tables 2 and 3) reinforce the notion that our in vitro estimates of net activities of altered RelSeq proteins are physiologically significant. A SpoT NH 1-378 protein, the homolog of RelSeq NH 1-385, shows in vitro synthetic activity for the first time for this protein (Fig. 7), suggestive of a parallel behavior of residual synthetic activity of SpoT with that of RelSeq.
It is notable that RelSeq NCH does not behave in vivo as we would predict from its activities in vitro. Instead, it behaves as if degradation is the dominant activity in vivo because cells in which it is induced are AT sensitive and SMG sensitive (Table 2) and also do not show growth inhibition (Table 3). One explanation could be that the error inherent in assaying very low and nearly equal in vitro specific activities of RelSeq NCH (Table 4) could affect our estimates of net activity. Alternatively, in vivo factors could contribute to the difference.
Possible functions of the C-terminal domain.
There is no doubt that RelSeq NCH shows a strong inhibition of degradation activity compared to full-length RelSeq lacking the C-terminal six-His tag (Table 4). Therefore, regulation of degradation activity could involve an interaction between the extreme C terminus and some portion of the N-terminal residues (1 to 224) that catalyze degradation. There is evidence of a potentially analogous situation for RelA; addition of several amino acids to the C terminus of RelA by suppression of an amber codon inactivates synthetic activity (5). Nevertheless, our attempts to obtain differential regulation by providing various C-terminal fragments in trans have been unsuccessful. Recent results suggest that oligomerization of RelA through its C terminus is involved in activation of (p)ppGpp synthesis (16). We do not know if the monomer or dimer is the species able to bind to ribosomes and undergo the conformational change to activate synthesis. This might be important, because the phosphatase activity of 6-phospho-2-kinase/fructose 2,6-bisphosphatase is activated by homodimerization (51). Dimerization of RelSeq does not occur under the conditions that are effective for RelA (U. Mechold and G. Glaser, unpublished data).
As a possibly separate issue, the C-terminal domain of RelSeq is missing some function needed for ribosomal activation in E. coli because joining the RelA C-terminal domain to the RelSeq N-terminal domain largely restores the potential to receive the signal for activation upon amino acid starvation (Fig. 6). This could occur as an indirect effect operating through the ribosome or a direct intramolecular regulatory effect. In the latter case, the RelA C-terminal domain is viewed as interacting with the heterologous RelSeq N-terminal catalytic domain to regulate opposing activities. This would again suggest that these domains are functionally modular. Since RelMtb, RelSco, and RelBsu can be activated under conditions of amino acid starvation in E. coli (4, 29, 49), the apparent incompatibility of the C terminus of RelSeq with ribosomal activation is clearly not a general characteristic of bifunctional Rel/Spo homologs. We do not know the individual activities for the RelSeq/RelA chimeric protein or how they change as a function of ribosomal association or possibly dimerization. The RelSeq/RelA chimera does retain an ability to degrade ppGpp in vivo with a 1-min half-life after the addition of chloramphenicol to cells undergoing the stringent response (data not shown); this strong degradation activity is not apparent during the stringent response to serine hydroxamate addition (Fig. 6).
Catalytic subdomains.
We have not mapped the minimal domains necessary for the catalytic activities of RelSeq. We have verified the expected singular biochemical activities for two purified peptide subdomain fragments of RelSeq predicted from the SpoT deletion map deduced from in vivo activities (15). The activities of these subfragments are verifiable by growth tests in vivo (Tables 2 and 3), while absence of the second activity can be shown only in vitro. Degradation activity is found for residues 1 to 224, and synthesis activity is found for residues 79 to 385 (Table 4). Thus, there are 145 shared, central residues (residues 79 to 224), as well as unique flanking regions necessary but not sufficient for each function: 1 to 78 for degradation and 225 to 385 for synthesis. The role of the central core region (79 to 224) could be that it harbors a common acceptor for pyrophosphate transfer from either pppGpp or ATP and thereby functions for both synthesis and degradation. A similar role, but for a phosphate acceptor-donor, has been proposed for the His-243 residue of the bifunctional EnvZ kinase/phosphatase (12). Genetic testing of this model for RelSeq is in progress, since mutants defective in one or the other activity can now be isolated. Mutants isolated in monofunctional subdomains and reassembled into NH 1-385 or the full-length protein might allow more precise mapping of individual catalytic domains, as well as defining sites affecting regulation within the catalytic domain. It will be of interest to see how these mutants affect the structures of the two domains derived from crystallization studies.
Our assignments of the catalytic subdomains for RelSeq and SpoT are not in complete agreement with the boundaries for the catalytic activities of the cloned rshA RelA/SpoT homolog from S. coelicolor (29). Although differences may well exist among Rel/Spo homologs, several of the RelSco fragments were found by the authors to be metabolically unstable in vivo, and therefore different activity assignments are uncertain.
Patterns of pppGpp accumulation.
The synthetically active RelSeq NH 1-385 and RelSeq NH 79-385 show remarkable differences in patterns of (p)ppGpp accumulation (Fig. 2). Hosts expressing RelSeq NH 1-385, but not NH 79-385, show excessive levels of pppGpp reminiscent of a gppA mutation (6). We do not know why. The net specific synthetic activity of RelSeq NH 1-385 is at least 10-fold higher than for NH 79-385, and each protein has little or no degradation activity (Table 4). Excessive pppGpp synthesis might titrate enzymes responsible for the conversion of pppGpp to ppGpp. Two such enzymes are known to display this activity in vitro, Gpp (19) and Ppx (26). However, only gpp mutants have this phenotype (6, 42). Titration of Gpp activity was proposed previously as a possible explanation for the preferential accumulation of pppGpp when RelA is activated in E. coli cells also expressing full-length RelSeq (30). RelSeq NH 1-385 does not show a preferential ability to degrade ppGpp compared to pppGpp (data not shown). Preferential use of GTP over GDP seems to be ruled out, since Table 5 indicates that the NH 79-385 peptide shows, if anything, a stronger preference for GTP over GDP than NH 1-385, which accumulates excessive pppGpp.
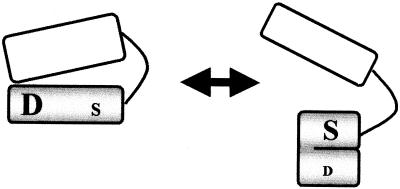
A model for a switch.
Figure 8 is a cartoon model of a switch that seems to achieve reciprocal regulation of the dual activities of RelSeq. The RelSeq bifunctional catalytic domain consists of residues 1 to 347, and its regulatory domain (residues 363 to 739) is thought to be linked by a solvent-exposed, flexible hinge of 16 amino acids, as judged from sequence homologies and protease sensitivities. The two domains can be aligned with the extreme C terminus in apposition with the N terminus to activate the degradation site while simultaneously inhibiting the synthesis site, giving an activity state for the native enzyme of degradation on, synthesis off. When contact between the C and N domains is altered, the N domain has an intrinsic tendency to fold, thereby activating synthesis and inhibiting degradation: degradation off, synthesis on. The N-C contacts can be altered by deletion, by sequestration through ribosomal binding, and by modification of the extreme C terminus with a six-His tag, as well as by C-C dimerization.
FIG. 8.
Cartoon depicting reciprocal regulation of the dual activities of RelSeq. The shaded N-terminal domain fragment contains catalytic sites for degradation (D) and synthesis (S), with the font sizes indicating relative activities. It is linked to the C-terminal regulatory domain (open) by a hinge region accessible to proteases.
Acknowledgments
We thank Kenji Ikehara (Nara Women's University, Nara, Japan) for sharing information on the protease sensitivity of the SpoT protein prior to publication. Jeffrey Kowalak in the Section on Metabolic Analysis and Mass Spectrometry (NICHD, NIH) identified K347 as the C-terminal residue remaining after the first trypsin cleavage of RelSeq NH 1-385 by matrix-assisted laser desorption ionization-time of flight analysis. Mark Thompson and Craig Hyde of the Laboratory of Structural Biology Research (NIAMS, NIH) contributed to the purification protocols for the RelSeq protein fragments and to defining optimal solubility conditions. We are grateful to Chris Reed for his preliminary work with RelSeq fragments.
REFERENCES
- 1.An, G., J. Justesen, R. J. Watson, and J. D. Friesen. 1979. Cloning the spoT gene of Escherichia coli. J. Bacteriol. 137:1100-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aravind, L., and E. V. Koonin. 1998. The HD domain defines a new superfamily of metal-dependent phosphohydrolases. Trends Biochem. Sci. 23:469-472. [DOI] [PubMed] [Google Scholar]
- 3.Avarbock, D., A. Avarbock, and H. Rubin. 2000. Differential regulation of opposing RelMtb activities by the aminoacylation state of a tRNA.ribosome.mRNA.RelMtb complex. Biochemistry 39:11640-11648. [DOI] [PubMed] [Google Scholar]
- 4.Avarbock, D., J. Salem, L. S. Li, Z. M. Wang, and H. Rubin. 1999. Cloning and characterization of a bifunctional RelA/SpoT homologue from Mycobacterium tuberculosis. Gene 233:261-269. [DOI] [PubMed] [Google Scholar]
- 5.Breeden, L., and M. Yarus. 1982. Amber suppression relaxes stringent control by elongating stringent factor. Mol. Gen. Genet. 187:254-264. [Google Scholar]
- 6.Cashel, M. 1994. Detection of (p)ppGpp accumulation patterns in Escherichia coli mutants, p. 341-356. In K. W. Adolph (ed.), Methods in molecular genetics, vol. 3, part A. Academic Press, New York, N.Y. [Google Scholar]
- 7.Cashel, M., D. R. Gentry, V. J. Hernandez, and D. Vinella. 1996. The stringent response, p. 1458-1496. In F. C. Neidhardt (ed.), Escherichia coli and Salmonella: cellular and molecular biology, vol. 1. ASM Press, Washington, D.C. [Google Scholar]
- 8.Chakraburtty, R., and M. Bibb. 1997. The ppGpp synthetase gene (relA) of Streptomyces coelicolor A3(2) plays a conditional role in antibiotic production and morphological differentiation. J. Bacteriol. 179:5854-5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatterji, D., and A. K. Ojha.2001. Revisiting the stringent response: ppGpp and starvation signaling. Curr. Opin. Microbiol. 4:160-165. [DOI] [PubMed] [Google Scholar]
- 10.Cochran, J. W., and R. W. Byrne. 1974. Isolation and properties of a ribosome-bound factor required for ppGpp and pppGpp synthesis in Escherichia coli. J. Biol. Chem. 249:353-360. [PubMed] [Google Scholar]
- 11.Delden, C. V., R. Compte, and M. Bally. 2001. Stringent response activates quorum sensing and modulates cell density-dependent gene expression in Pseudomonas aeruginosa. J. Bacteriol. 183:5376-5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dutta, R., and M. Inouye. 1996. Reverse phosphotransfer from OmpR to EnvZ in a kinase−/phosphatase+ mutant of EnvZ (EnvZ.N347D), a bifunctional transducer of Escherichia coli. J. Biol. Chem. 271:1424-1429. [DOI] [PubMed] [Google Scholar]
- 13.Garza, A. G., B. Z. Harris, B. M. Greenberg, and M. Singer. 2000. Control of asgE expression during growth and development of Myxococcus xanthus. J. Bacteriol. 182:6622-6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gentry, D., T. Li, M. Rosenberg, and D. McDevitt. 2000. The rel gene is essential for in vitro growth of Staphylococcus aureus. J. Bacteriol. 182:4995-4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gentry, D. R., and M. Cashel. 1996. Mutational analysis of the Escherichia coli spoT gene identifies distinct but overlapping regions involved in ppGpp synthesis and degradation. Mol. Microbiol. 19:1373-1384. [DOI] [PubMed] [Google Scholar]
- 16.Gropp, M., Y. Strausz, M. Gross, and G. Glaser. 2001. Regulation of Escherichia coli RelA requires oligomerization of the C-terminal domain. J. Bacteriol. 183:570-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guzman, L. M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammer, B. K., and M. S. Swanson. 1999. Co-ordination of Legionella pneumophila virulence with entry into stationary phase by ppGpp. Mol. Microbiol 33:721-731. [DOI] [PubMed] [Google Scholar]
- 19.Hara, A., and J. Sy. 1983. Guanosine 5′-triphosphate, 3′-diphosphate 5′-phosphohydrolase. Purification and substrate specificity. J. Biol. Chem. 258:1678-1693. [PubMed] [Google Scholar]
- 20.Harris, B. Z., D. Kaiser, and M. Singer. 1998. The guanosine nucleotide (p)ppGpp initiates development and A-factor production in Myxococcus xanthus. Genes Dev. 12:1022-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haseltine, W. A., and R. Block. 1973. Synthesis of guanosine tetra- and pentaphosphate requires the presence of a codon-specific, uncharged transfer ribonucleic acid in the acceptor site of ribosomes. Proc. Natl. Acad. Sci. USA 70:1564-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heinemeyer, E. A., and D. Richter. 1977. In vitro degradation of guanosine tetraphosphate (ppGpp) by an enzyme associated with the ribosomal fraction in Escherichia coli. FEBS Lett. 84:357-361. [DOI] [PubMed] [Google Scholar]
- 23.Hernandez, V. J., and H. Bremer. 1991. Escherichia coli ppGpp synthetase II activity requires spoT. J. Biol. Chem. 266:5991-5999. [PubMed] [Google Scholar]
- 24.Jaggi, R., W. C. van Heeswijk, H. V. Westerhoff, D. L. Ollis, and S. G. Vasudevan. 1997. The two opposing activities of adenylyl transferase reside in distinct homologous domains, with intramolecular signal transduction. EMBO J. 16:5562-5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuhar, I., J. P. van Putten, D. Zgur-Bertok, W. Gaastra, and, B. J. Jordi. 2001. Codon-usage based regulation of colicin K synthesis by the stress alarmone ppGpp. Mol. Microbiol. 41:207-216. [DOI] [PubMed] [Google Scholar]
- 26.Kuroda, A., H. Murphy, M. Cashel, and A. Kornberg. 1997. Guanosine tetra- and pentaphosphate promote accumulation of inorganic polyphosphate in Escherichia coli. J. Biol. Chem. 272:21240-21243. [DOI] [PubMed] [Google Scholar]
- 27.Kwan, D. S., C. H. Lin, S. Chen, J. K. Coward, C. T. Walsh, and J. M. Bollinger, Jr. 1997. Dissection of glutathionylspermidine synthetase/amidase from Escherichia coli into autonomously folding and functional synthetase and amidase domains. J. Biol. Chem. 272:2429-2436. [DOI] [PubMed] [Google Scholar]
- 28.Martinez-Costa, O. H., P. Arias, N. M. Romero, V. Parro, R. P. Mellado, and F. Malpartida. 1996. A relA/spoT homologous gene from Streptomyces coelicolor A3(2) controls antibiotic biosynthetic genes. J. Biol. Chem. 271:10627-10634. [DOI] [PubMed] [Google Scholar]
- 29.Martinez-Costa, O. H., M. A. Fernandez-Moreno, and F. Malpartida. 1998. The relA/spoT-homologous gene in Streptomyces coelicolor encodes both ribosome-dependent (p)ppGpp-synthesizing and -degrading activities. J. Bacteriol. 180:4123-4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mechold, U., M. Cashel, K. Steiner, D. Gentry, and H. Malke. 1996. Functional analysis of a relA/spoT gene homolog from Streptococcus equisimilis. J. Bacteriol. 178:1401-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mechold, U., and H. Malke. 1997. Characterization of the stringent and relaxed responses of Streptococcus equisimilis. J. Bacteriol. 179:2658-2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Metzger, S., G. Schreiber, E. Aizenman, M. Cashel, and G. Glaser. 1989. Characterization of the relA1 mutation and a comparison of relA1 with new relA null alleles in Escherichia coli. J. Biol. Chem. 264:21146-21152. [PubMed] [Google Scholar]
- 33.Miller, S. P., E. J. Karschnia, T. P. Ikeda, and D. C. LaPorte. 1996. Isocitrate dehydrogenase kinase/phosphatase. Kinetic character of the wild-type and two mutant proteins. J. Biol. Chem. 271:19124-19128. [DOI] [PubMed] [Google Scholar]
- 34.Mittenhuber, G. 2001. Comparative genomics and evolution of genes encoding bacterial (p)ppGpp synthetases/hydrolases (the Rel, RelA and SpoT proteins). J. Mol. Microbiol. Biotechnol. 3:585-600. [PubMed] [Google Scholar]
- 35.Murray, K. D., and H. Bremer. 1996. Control of spoT-dependent ppGpp synthesis and degradation in Escherichia coli. J. Mol. Biol. 259:41-57. [DOI] [PubMed] [Google Scholar]
- 36.Neidhardt, F. C., P. L. Bloch, and D. F. Smith. 1974. Culture medium for enterobacteria. J. Bacteriol. 119:736-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Primm, T. P., S. J. Andersen, V. Mizrahi, D. Avarbock, H. Rubin, and C. E. Barry III. 2000. The stringent response of Mycobacterium tuberculosis is required for long-term survival. J. Bacteriol. 182:4889-4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rudd, K. E., B. R. Bochner, M. Cashel, and J. R. Roth. 1985. Mutations in the spoT gene of Salmonella typhimurium: effects on his operon expression. J. Bacteriol. 163:534-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarubbi, E., K. E. Rudd, and M. Cashel. 1988. Basal ppGpp level adjustment shown by new spoT mutants affect steady state growth rates and rrnA ribosomal promoter regulation in Escherichia coli. Mol. Gen. Genet. 213:214-222. [DOI] [PubMed] [Google Scholar]
- 40.Schreiber, G., S. Metzger, E. Aizenman, S. Roza, M. Cashel, and G. Glaser. 1991. Overexpression of the relA gene in Escherichia coli. J. Biol. Chem. 266:3760-3767. [PubMed] [Google Scholar]
- 41.Singer, M., and D. Kaiser. 1995. Ectopic production of guanosine penta- and tetraphosphate can initiate early developmental gene expression in Myxococcus xanthus. Genes Dev. 9:1633-1644. [DOI] [PubMed] [Google Scholar]
- 42.Somerville, C. R., and A. Ahmed. 1979. Mutants of Escherichia coli defective in the degradation of guanosine 5′-triphosphate, 3′-diphosphate (pppGpp). Mol. Gen. Genet. 169:315-323. [DOI] [PubMed] [Google Scholar]
- 43.Sun, J., A. Hesketh, and M. Bibb. 2001. Functional analysis of relA and rshA, two relA/spoT homologues of Streptomyces coelicolor A3(2). J. Bacteriol. 183:3488-3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Svitil, A. L., M. Cashel, and J. W. Zyskind. 1993. Guanosine tetraphosphate inhibits protein synthesis in vivo. A possible protective mechanism for starvation stress in Escherichia coli. J. Biol. Chem. 268:2307-2311. [PubMed] [Google Scholar]
- 45.Sy, J. 1977. In vitro degradation of guanosine 5′-diphosphate, 3′-diphosphate. Proc. Natl. Acad. Sci. USA 74:5529-5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uzan, M., and A. Danchin. 1976. A rapid test for the relA mutation in E. coli. Biochem. Biophys. Res. Commun. 69:751-758. [DOI] [PubMed] [Google Scholar]
- 47.van der Biezen, E. A., J. Sun, M. J. Coleman, M. J. Bibb, and, J. D. Jones. 2000. Arabidopsis RelA/SpoT homologs implicate (p)ppGpp in plant signaling. Proc. Natl. Acad. Sci. USA 97:3747-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wendrich, T. M., C. L. Beckering, and, M. A. Marahiel. 2000. Characterization of the relA/spoT gene from Bacillus stearothermophilus. FEMS Microbiol. Lett. 190:195-201. [DOI] [PubMed] [Google Scholar]
- 49.Wendrich, T. M., and M. A. Marahiel. 1997. Cloning and characterization of a relA/spoT homologue from Bacillus subtilis. Mol. Microbiol. 26:65-79. [DOI] [PubMed] [Google Scholar]
- 50.Xiao, H., M. Kalman, K. Ikehara, S. Zemel, G. Glaser, and M. Cashel. 1991. Residual guanosine 3′,5′-bispyrophosphate synthetic activity of relA null mutants can be eliminated by spoT null mutations. J. Biol. Chem. 266:5980-5990. [PubMed] [Google Scholar]
- 51.Yuen, M. H., X. L. Wang, H. Mizuguchi, K. Uyeda, and C. A. Hasemann. 1999. A switch in the kinase domain of rat testis 6-phosphofructo-2-kinase/fructose-2-6-bisphosphatase. Biochemistry 1999:12333-12342. [DOI] [PubMed] [Google Scholar]