Abstract
1. Voltage clamp experiments were carried out on squid axons perfused with an isotonic solution of 25 mM-CsF + sucrose and placed in a Na-free solution of 100 mM-CaCl2 + sucrose.
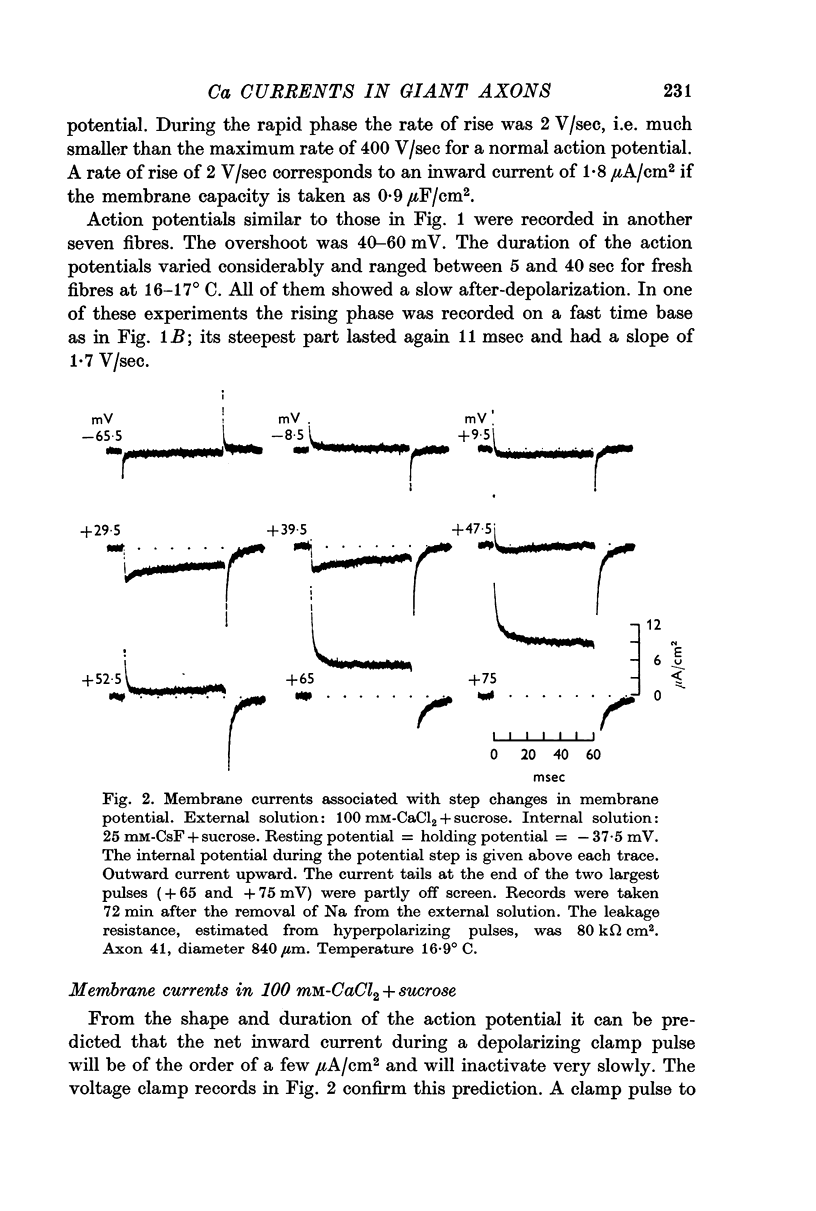
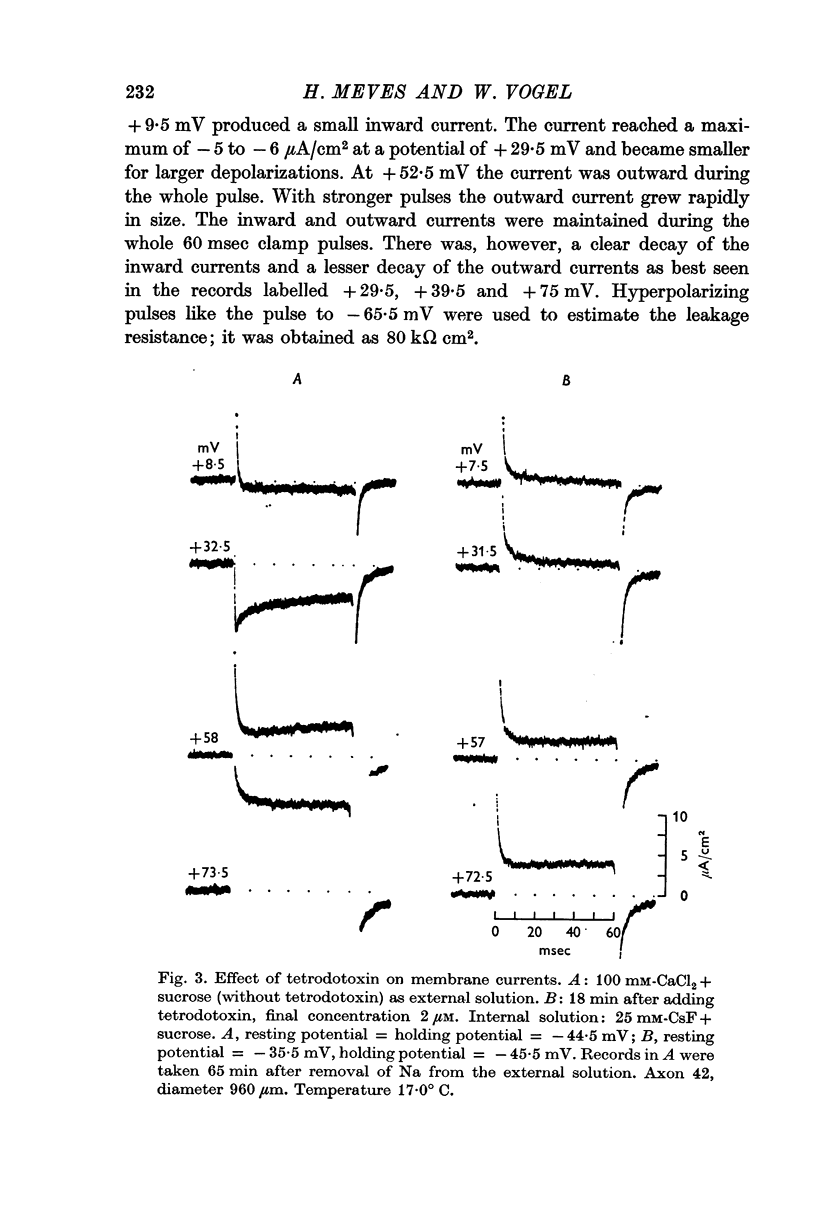
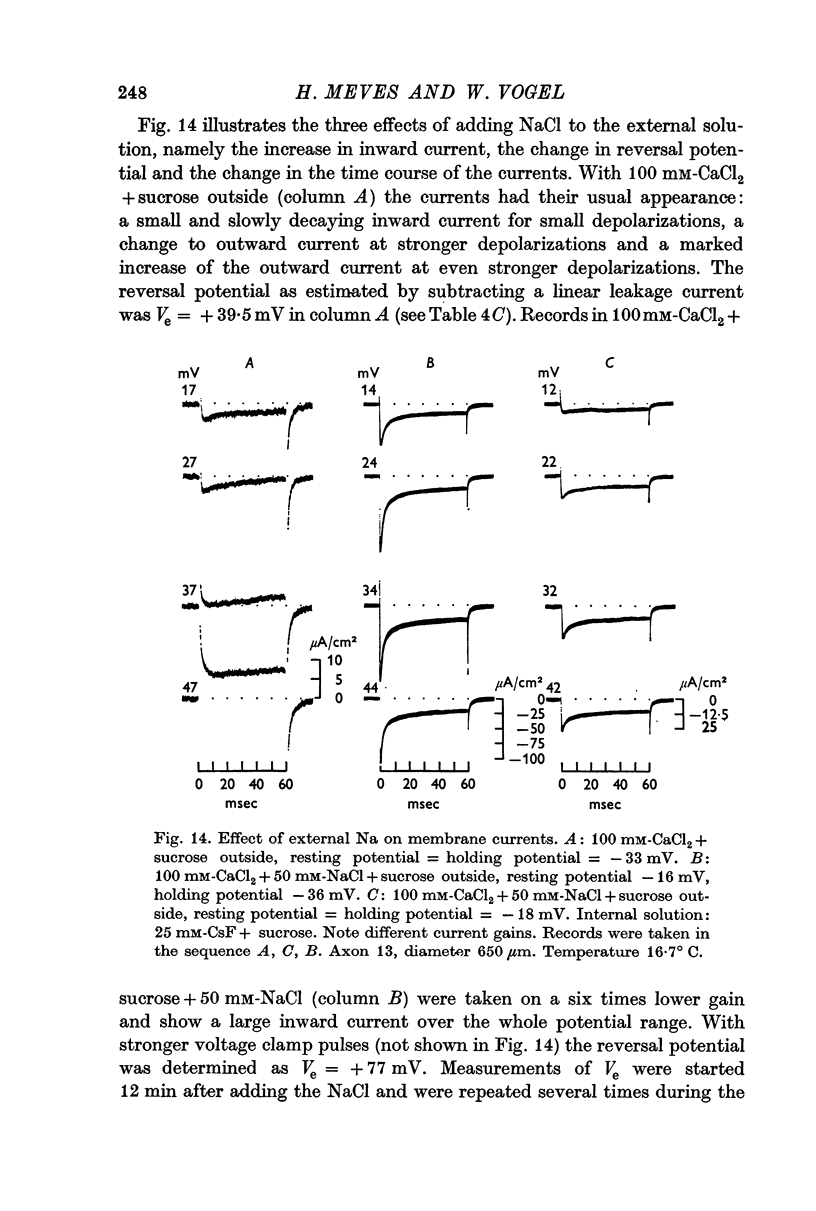
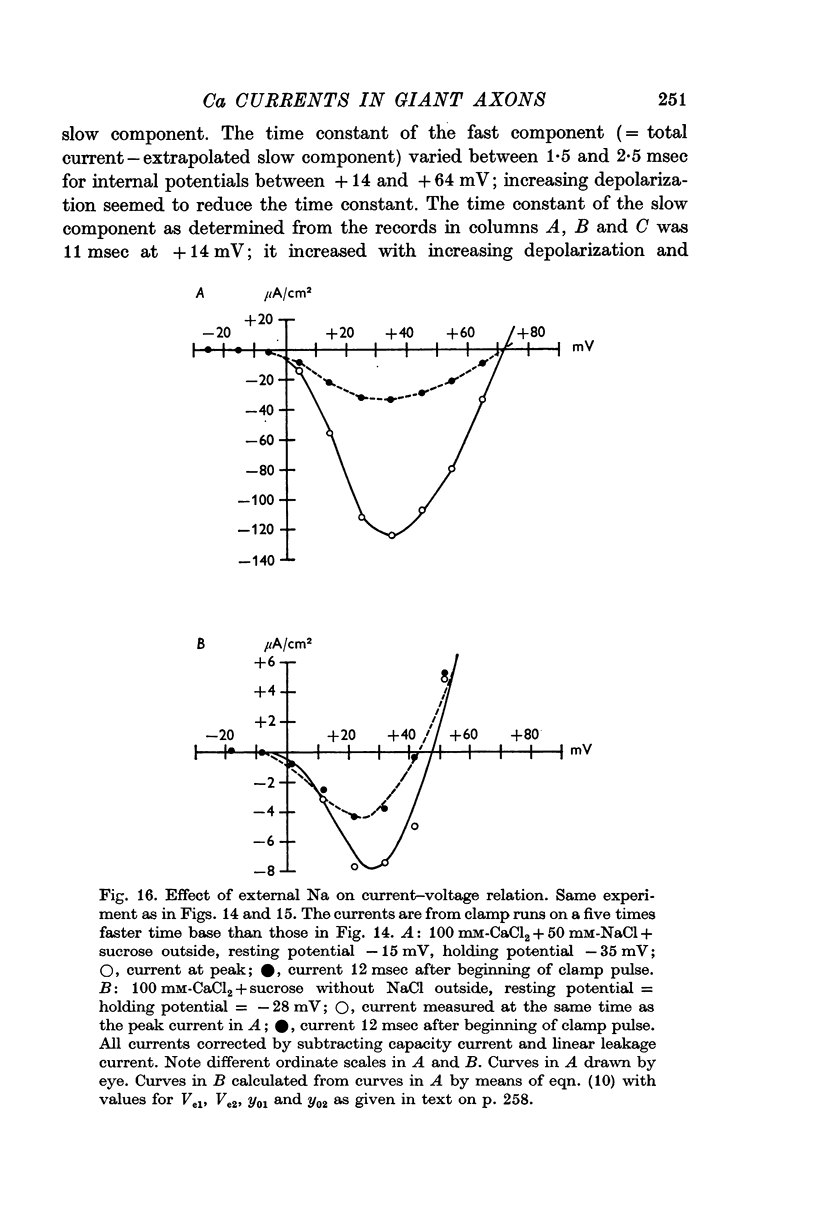
2. Depolarizing voltage steps produced inward currents of 4-6 μA/cm2 peak amplitude which decayed slightly during a 60 msec pulse; the inward current disappeared when the internal potential reached +50 to +60 mV and became outward for larger depolarizations.
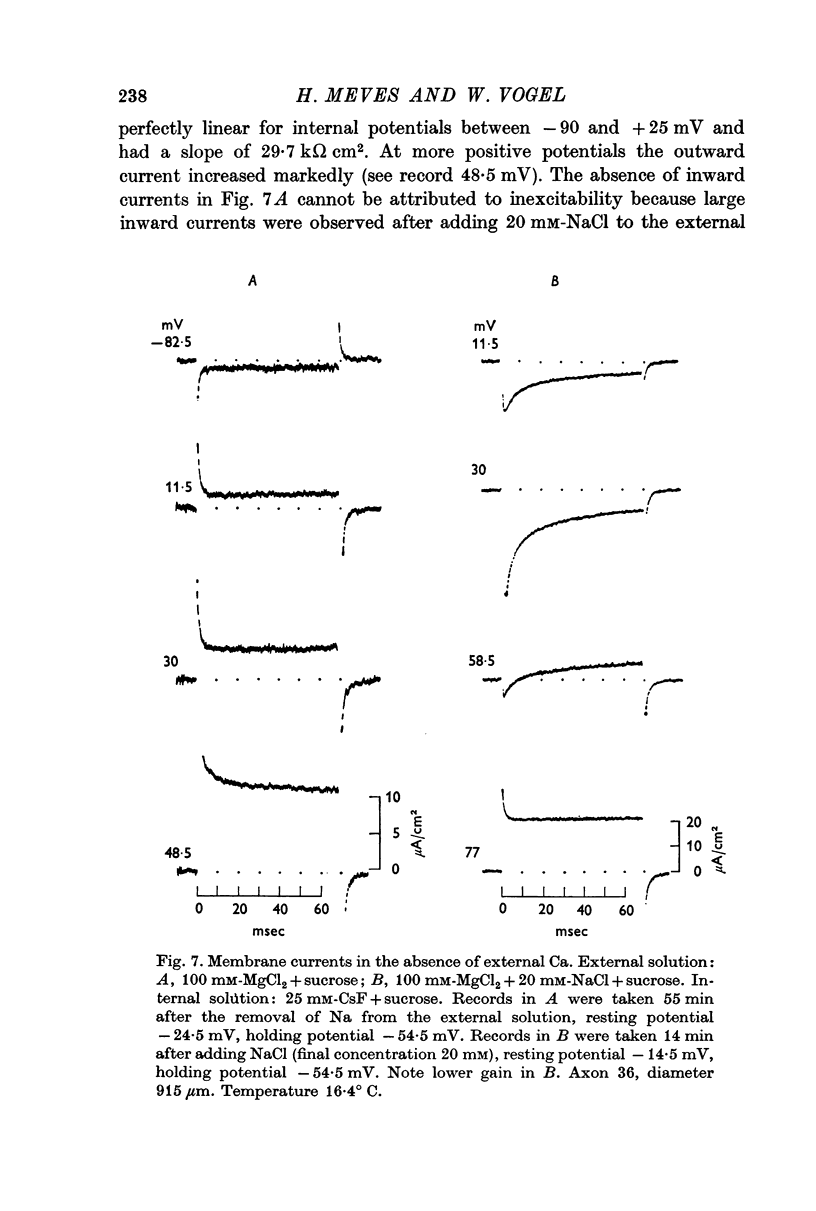
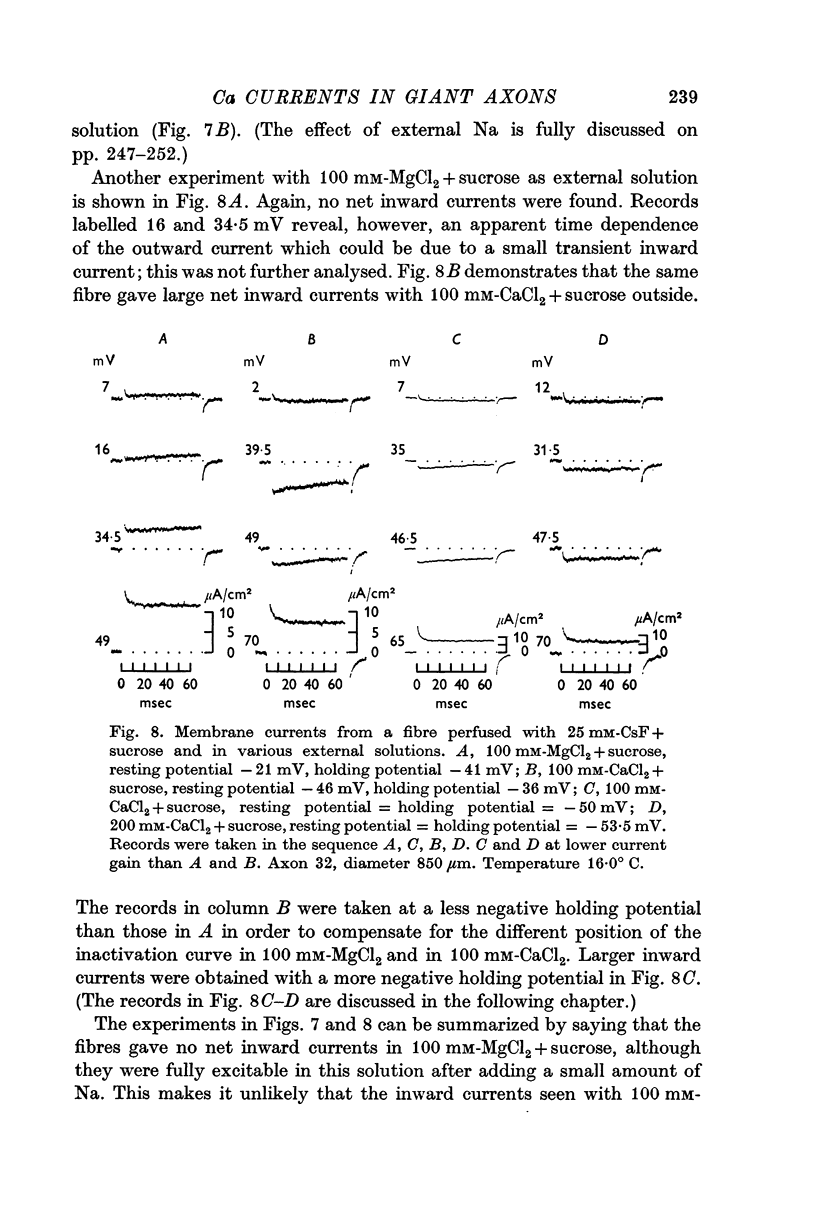
3. Tetrodotoxin completely blocked the inward current and part of the outward current. No inward currents were seen with 100 mM-MgCl2 + sucrose as the external solution. Substituting acetate for external Cl- did not abolish the tetrodotoxin-sensitive outward currents.
4. It is concluded that the inward current is carried by Ca and the tetrodotoxin-sensitive outward current by Cs ions, both moving through the Na channel.
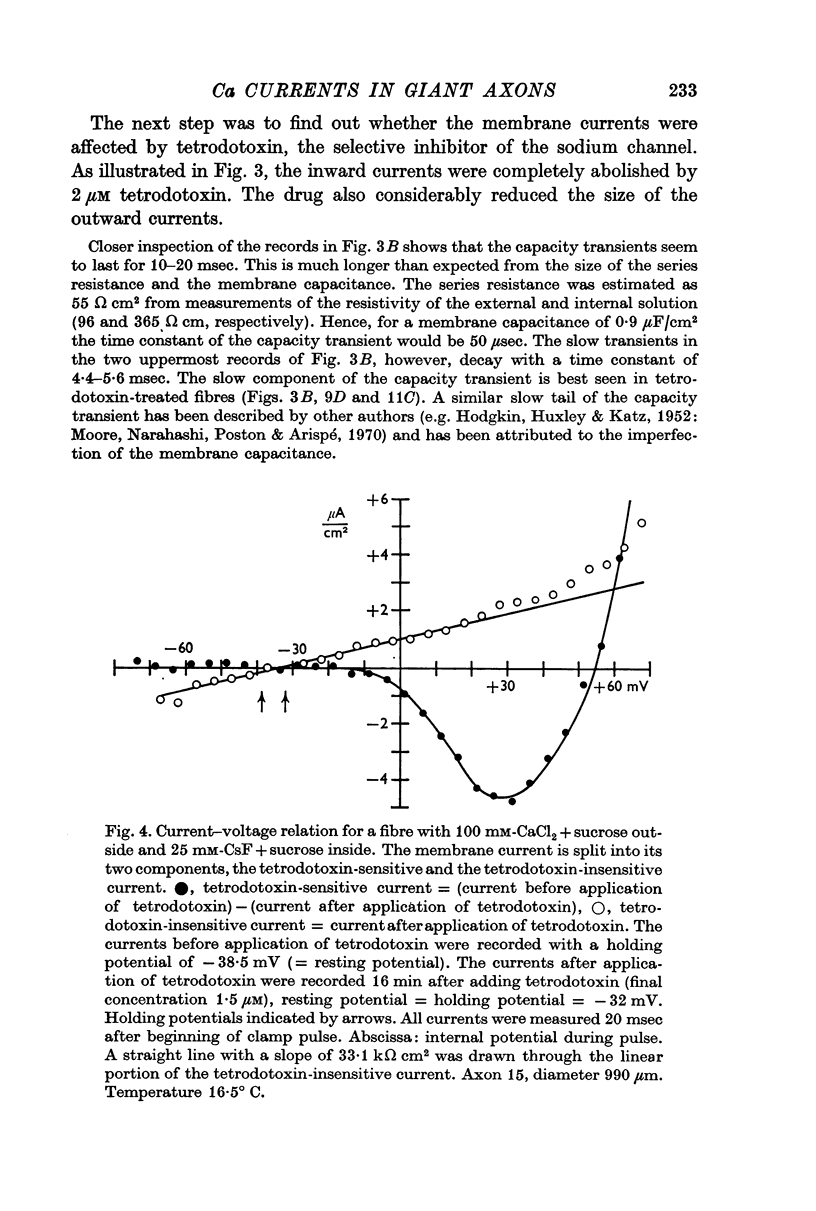
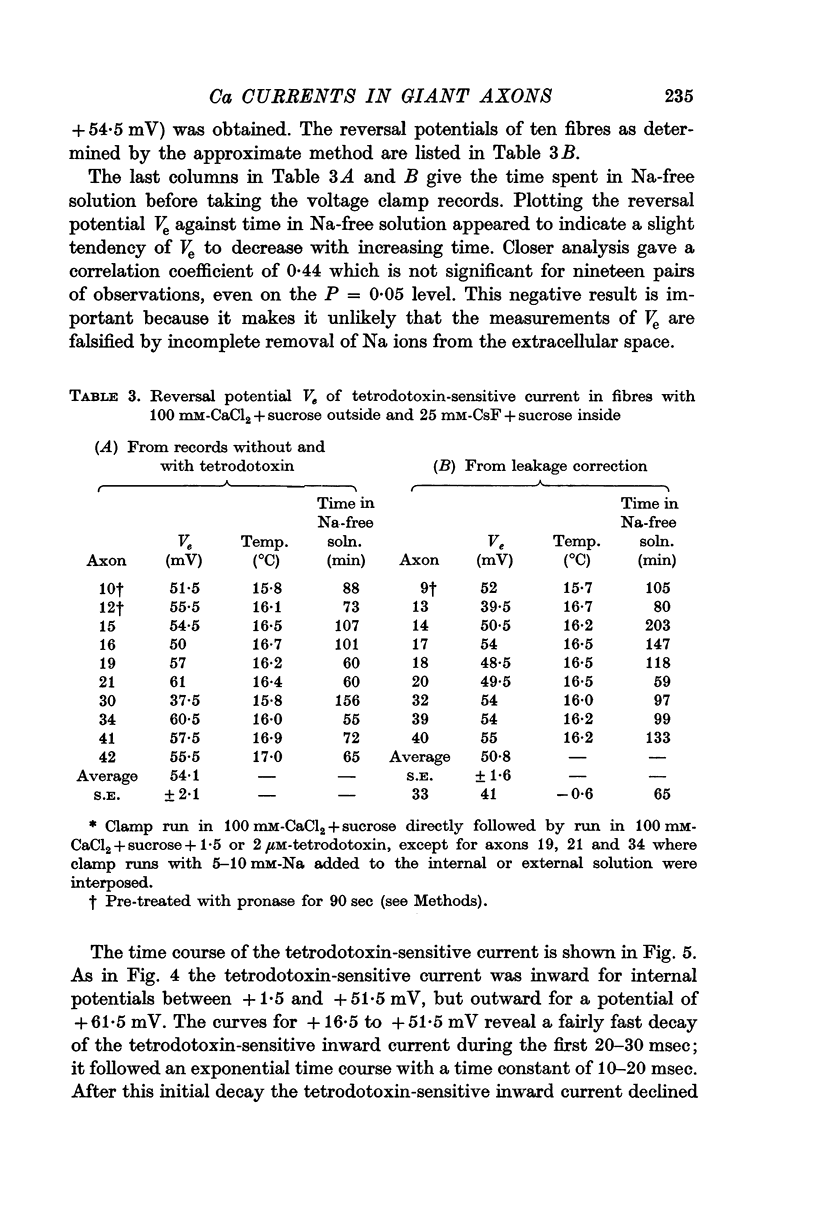
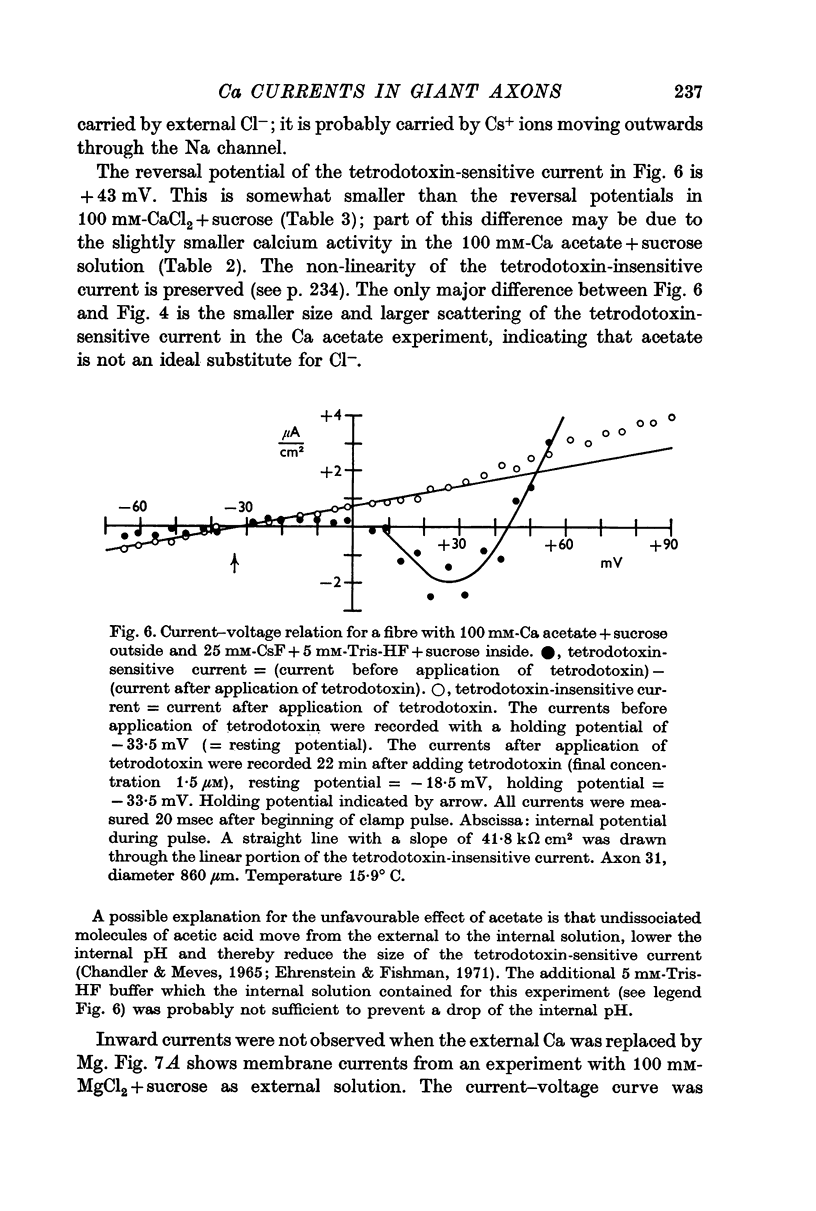
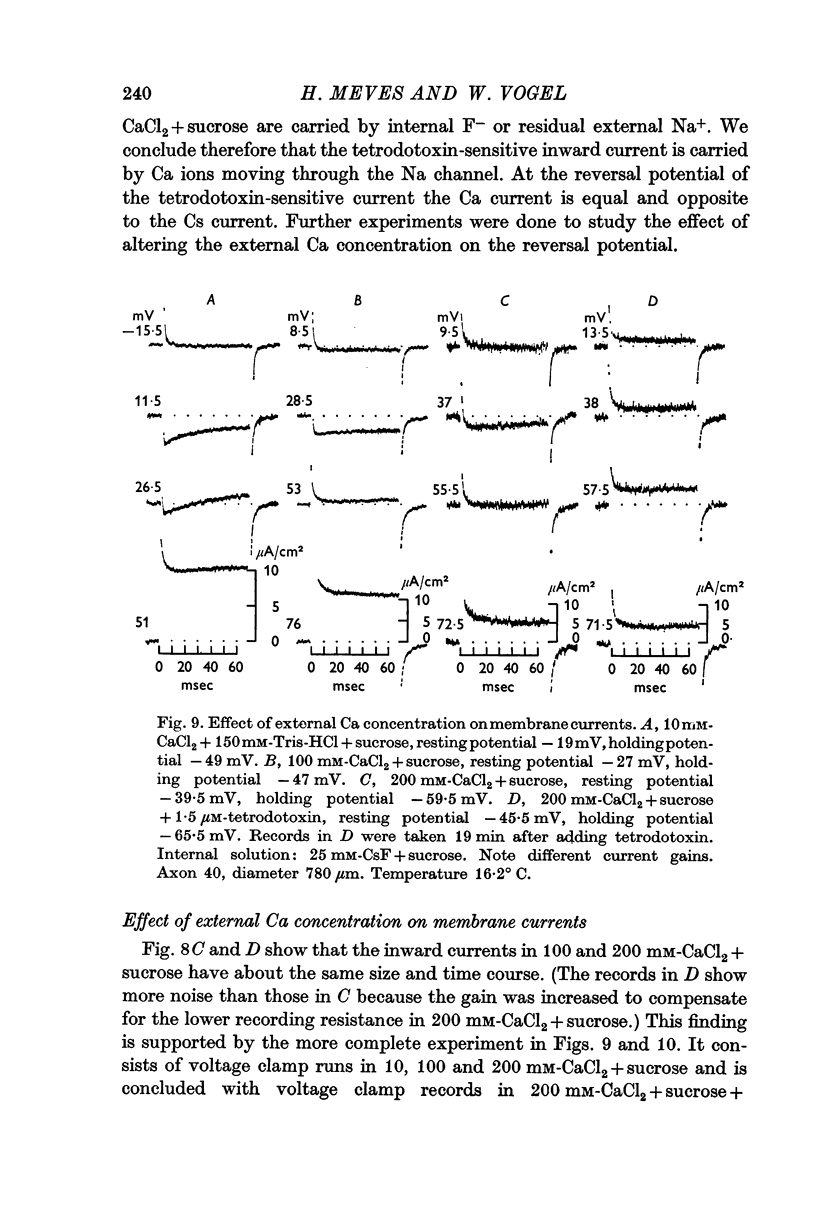
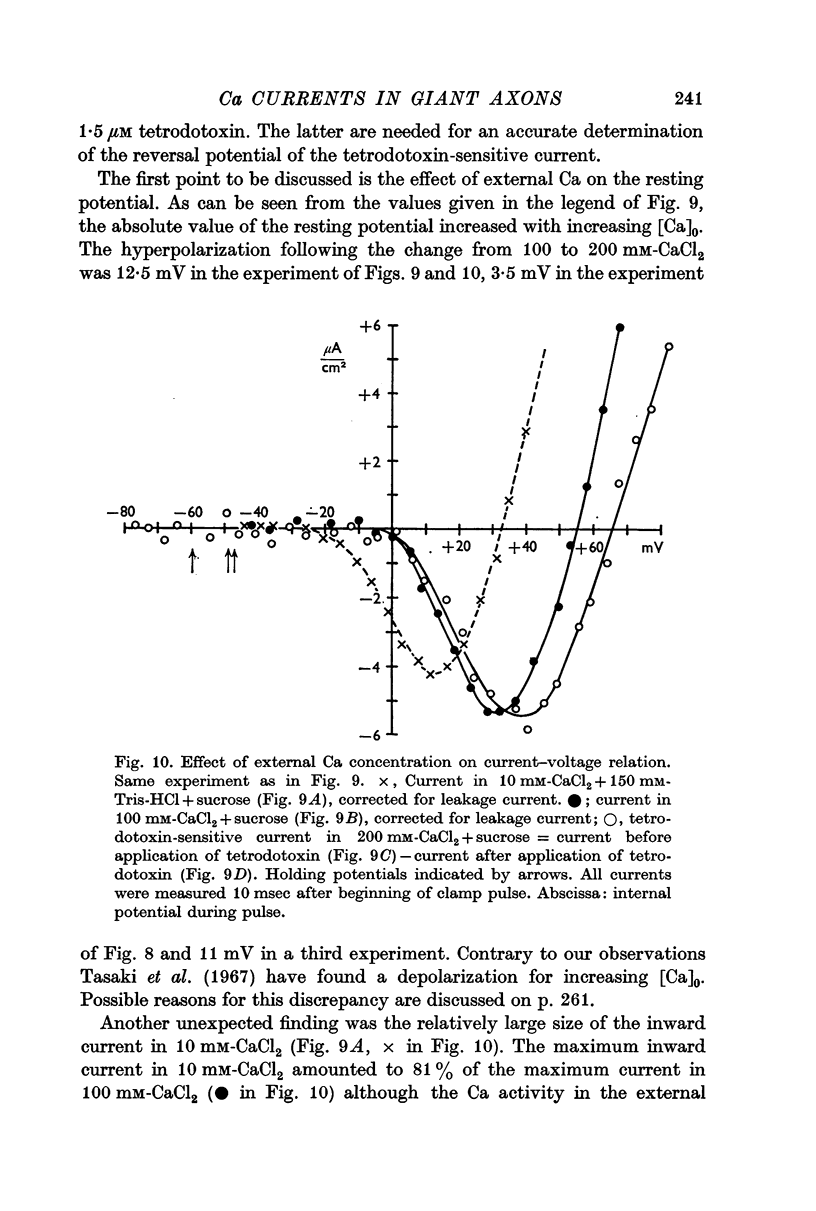
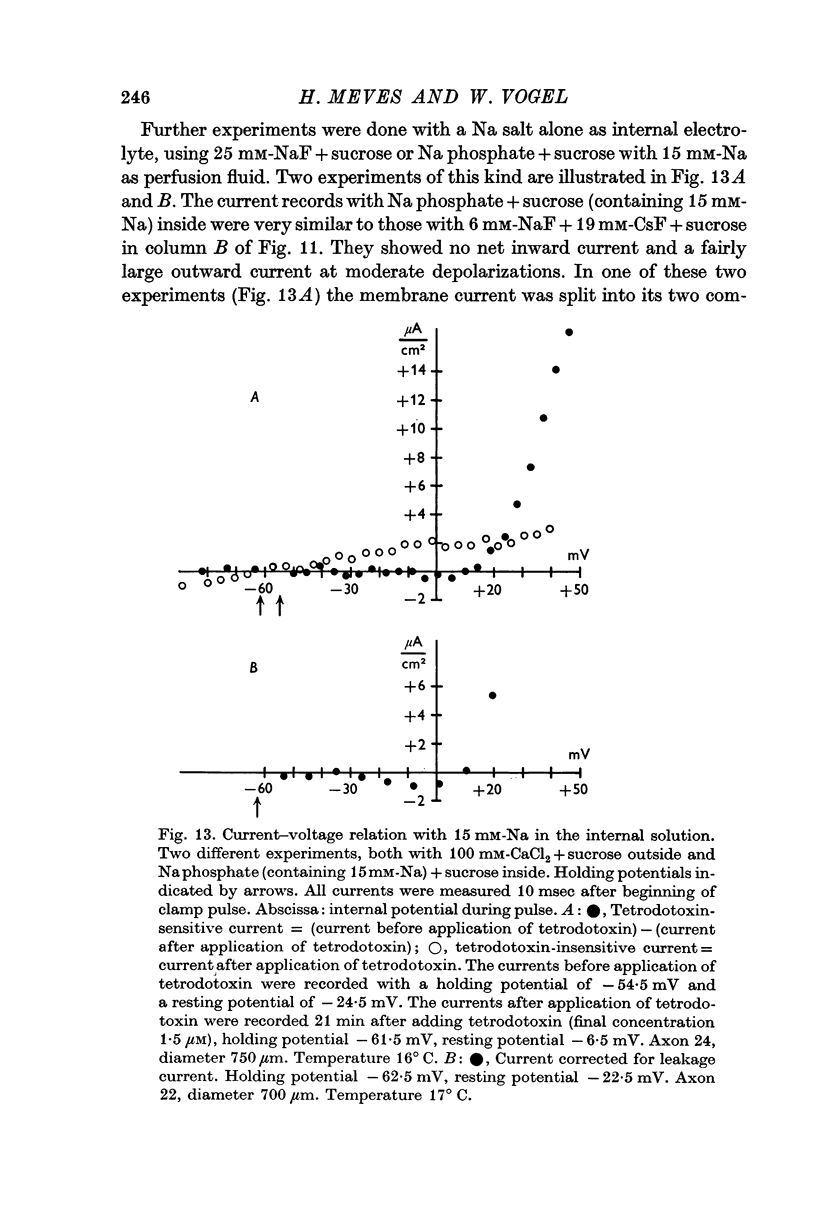
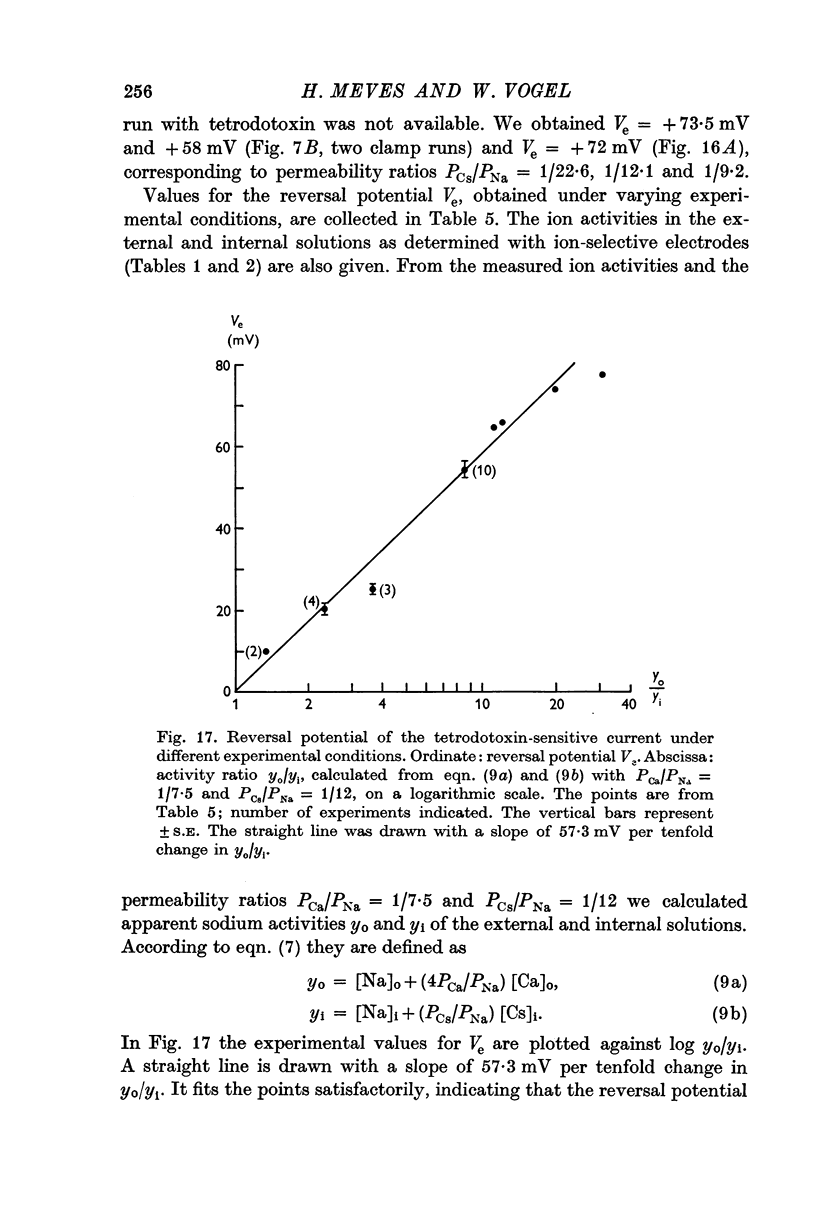
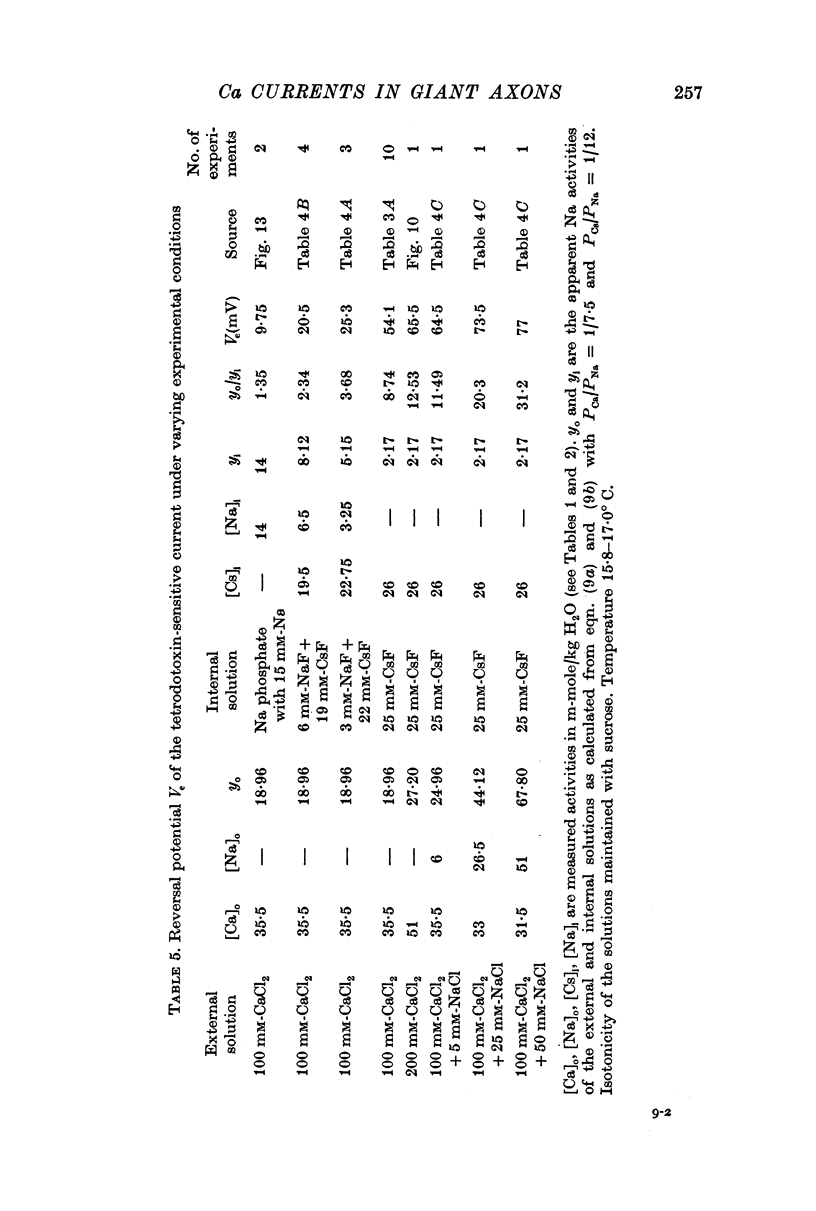
5. The reversal potential of the tetrodotoxin-sensitive current was in the average +54 mV. Raising the external Ca concentration or adding NaCl to the external solution increased the reversal potential; lowering the external Ca concentration or replacing the internal CsF by a Na salt decreased the reversal potential.
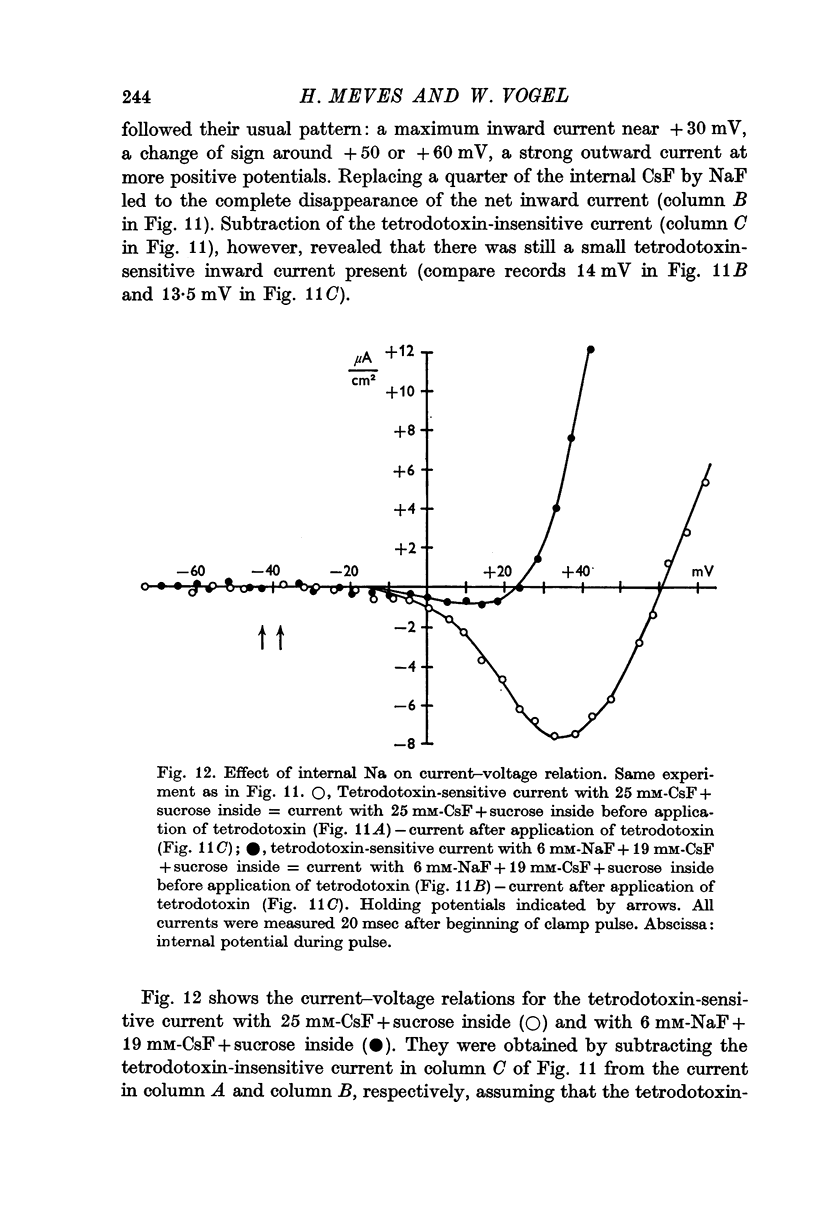
6. From the reversal potentials of the tetrodotoxin-sensitive current measured with varying external and internal solutions the relative permeabilities of the Na channel were calculated as PCa/PCs = 1/0·6, PCa/PNa = 1/10 to 1/7 and PCs/PNa = 1/22 to 1/9 by means of the constant field equation. The permeability ratios suggest that under these experimental conditions the Na channel is still primarily permeable to Na ions, although its selectivity is relatively small.
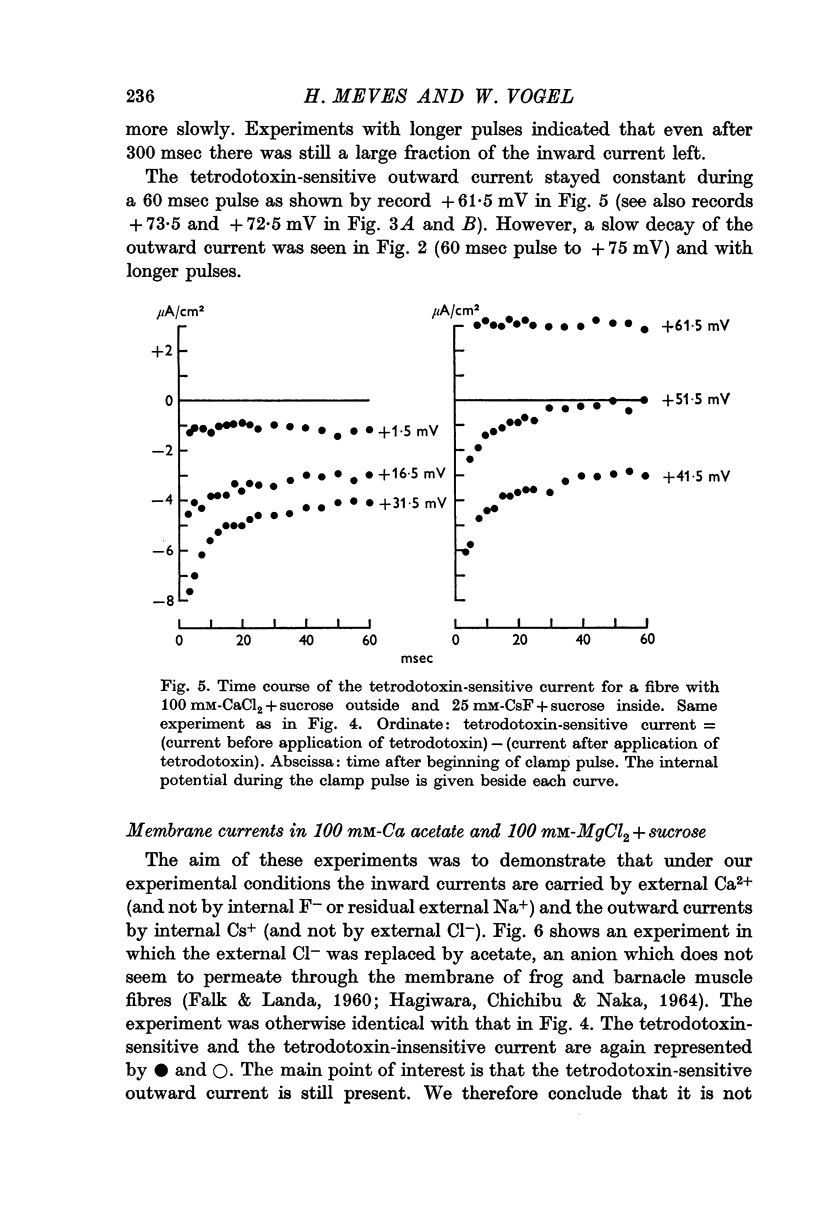
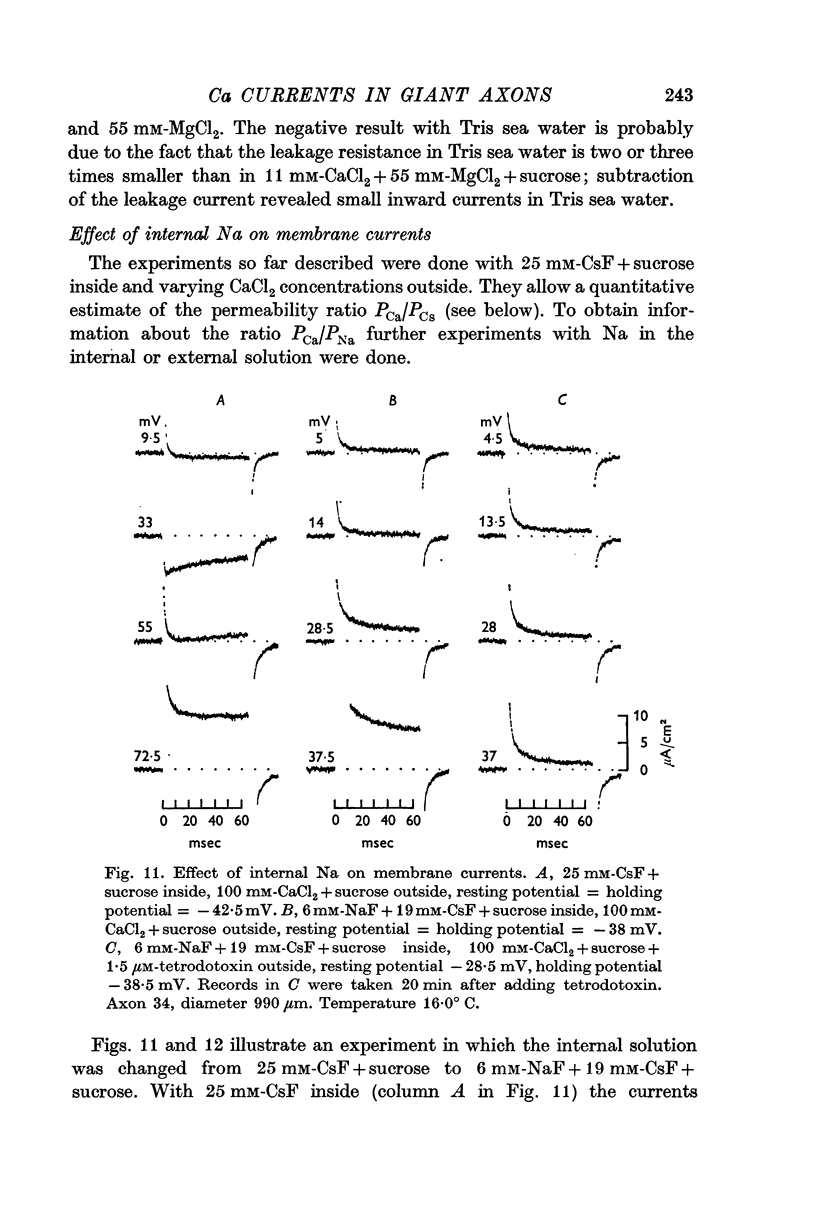
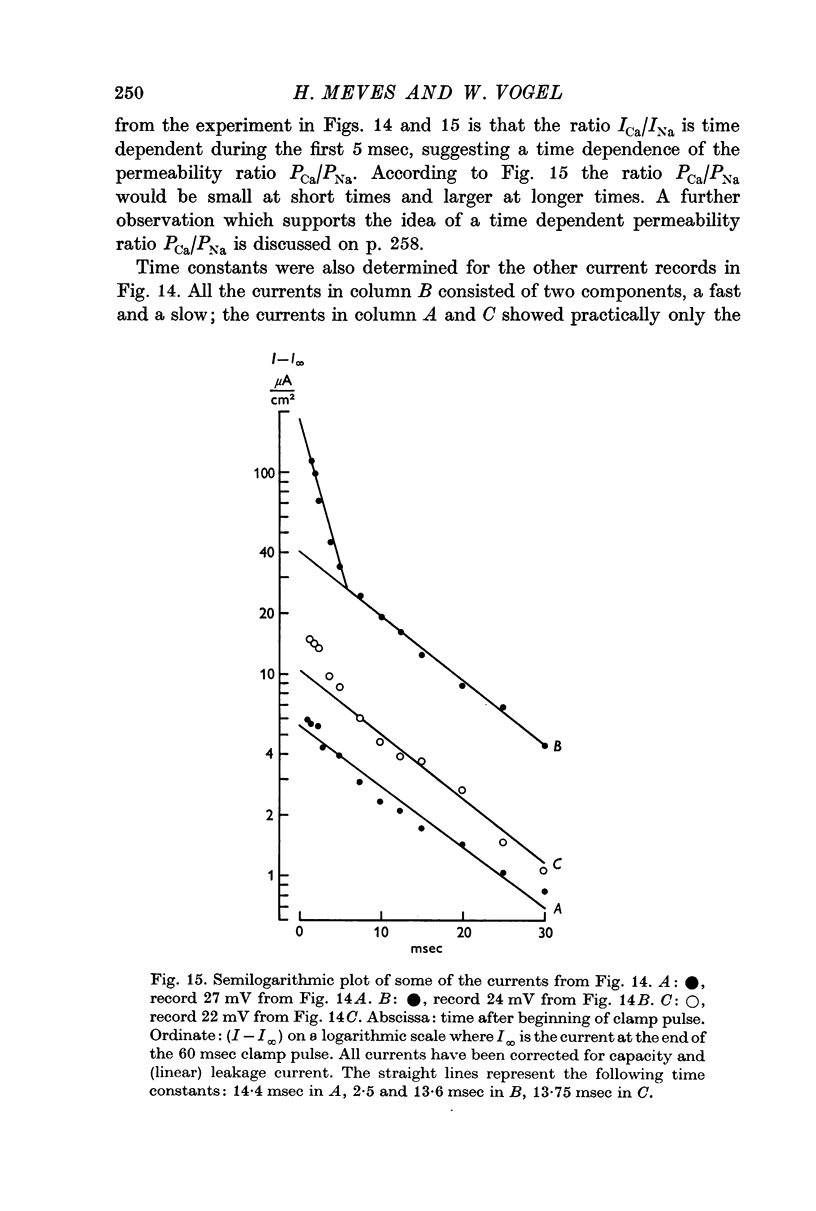
7. The time course of the tetrodotoxin-sensitive Ca inward current was different from the time course of the Na inward current. The Na current consisted of an initial peak followed by a more slowly decaying component, the Ca current showed only the slow component.
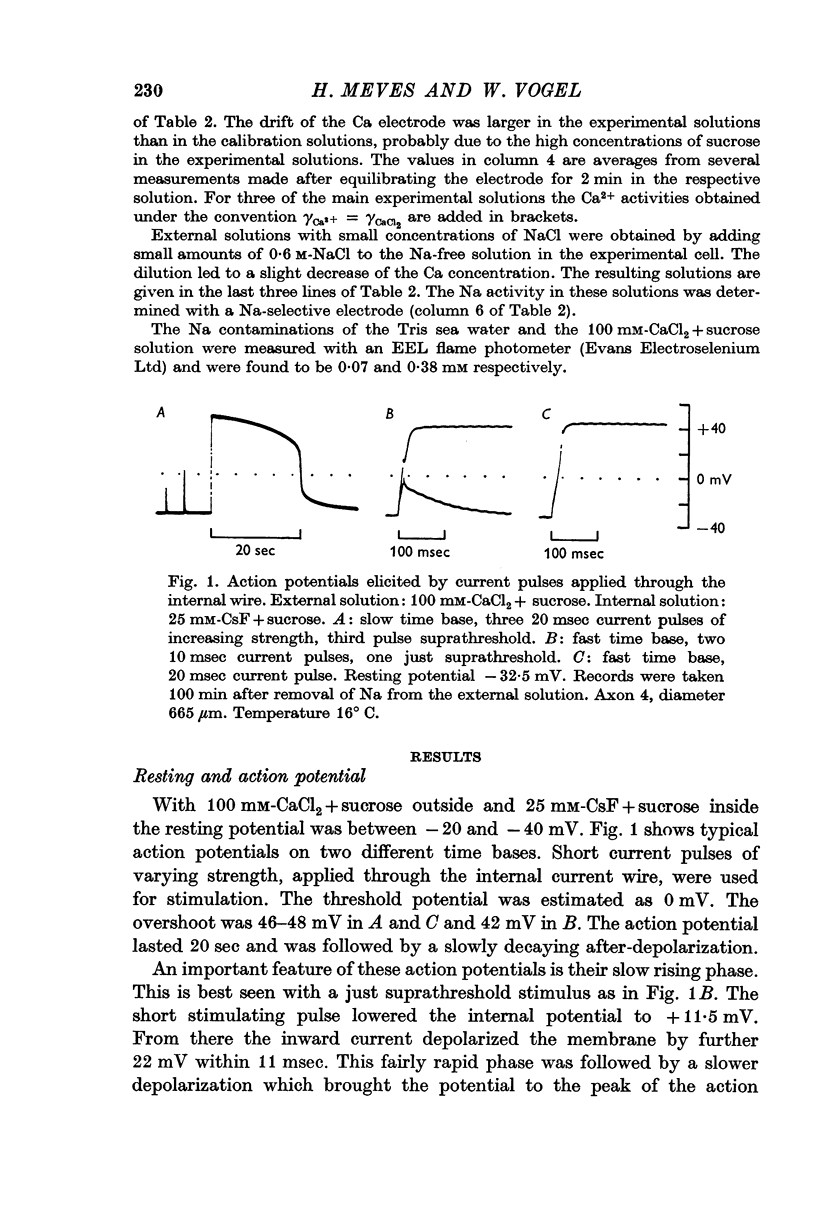
8. The slowly inactivating tetrodotoxin-sensitive Ca inward currents give rise to the long lasting action potentials which have first been observed by Tasaki and coworkers under similar conditions.
Full text
PDF



















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADELMAN W. J., TAYLOR R. E. Leakage current rectification in the squid giant axon. Nature. 1961 Jun 3;190:883–885. doi: 10.1038/190883a0. [DOI] [PubMed] [Google Scholar]
- Adelman W. J., Jr, Senft J. P. Voltage clamp studies on the effect of internal cesium ion on sodium and potassium currents in the squid giant axon. J Gen Physiol. 1966 Nov;50(2):279–293. doi: 10.1085/jgp.50.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAKER P. F., HODGKIN A. L., MEVES H. THE EFFECT OF DILUTING THE INTERNAL SOLUTION ON THE ELECTRICAL PROPERTIES OF A PERFUSED GIANT AXON. J Physiol. 1964 Apr;170:541–560. doi: 10.1113/jphysiol.1964.sp007348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAKER P. F., HODGKIN A. L., SHAW T. I. Replacement of the axoplasm of giant nerve fibres with artificial solutions. J Physiol. 1962 Nov;164:330–354. doi: 10.1113/jphysiol.1962.sp007025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Hodgkin A. L., Ridgway E. B. Depolarization and calcium entry in squid giant axons. J Physiol. 1971 Nov;218(3):709–755. doi: 10.1113/jphysiol.1971.sp009641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Rojas E., Taylor R. E. Sodium and potassium conductance changes during a membrane action potential. J Physiol. 1970 Dec;211(3):729–751. doi: 10.1113/jphysiol.1970.sp009301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Goldman D. E. The action of certain polyvalent cations on the voltage-clamped lobster axon. J Gen Physiol. 1968 Mar;51(3):279–291. doi: 10.1085/jgp.51.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Hodgkin A. L., Meves H. The effect of changing the internal solution on sodium inactivation and related phenomena in giant axons. J Physiol. 1965 Oct;180(4):821–836. doi: 10.1113/jphysiol.1965.sp007733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Rate constants associated with changes in sodium conductance in axons perfused with sodium fluoride. J Physiol. 1970 Dec;211(3):679–705. doi: 10.1113/jphysiol.1970.sp009299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Slow changes in membrane permeability and long-lasting action potentials in axons perfused with fluoride solutions. J Physiol. 1970 Dec;211(3):707–728. doi: 10.1113/jphysiol.1970.sp009300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Sodium and potassium currents in squid axons perfused with fluoride solutions. J Physiol. 1970 Dec;211(3):623–652. doi: 10.1113/jphysiol.1970.sp009297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Voltage clamp experiments on internally perfused giant axons. J Physiol. 1965 Oct;180(4):788–820. doi: 10.1113/jphysiol.1965.sp007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenstein G., Fishman H. M. Evidence against hydrogen-calcium competition model for activation of electrically excitable membranes. Nat New Biol. 1971 Sep 1;233(35):16–17. doi: 10.1038/newbio233016a0. [DOI] [PubMed] [Google Scholar]
- FALK G., LANDA J. F. Prolonged response of skeletal muscle in the absence of penetrating anions. Am J Physiol. 1960 Feb;198:289–299. doi: 10.1152/ajplegacy.1960.198.2.289. [DOI] [PubMed] [Google Scholar]
- FATT P., GINSBORG B. L. The ionic requirements for the production of action potentials in crustacean muscle fibres. J Physiol. 1958 Aug 6;142(3):516–543. doi: 10.1113/jphysiol.1958.sp006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B. Sodium permeability in toad nerve and in squid nerve. J Physiol. 1960 Jun;152:159–166. doi: 10.1113/jphysiol.1960.sp006477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA S., CHICHIBU S., NAKA K. I. THE EFFECTS OF VARIOUS IONS ON RESTING AND SPIKE POTENTIALS OF BARNACLE MUSCLE FIBERS. J Gen Physiol. 1964 Sep;48:163–179. doi: 10.1085/jgp.48.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F., KATZ B. Measurement of current-voltage relations in the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):424–448. doi: 10.1113/jphysiol.1952.sp004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Movements of labelled calcium in squid giant axons. J Physiol. 1957 Sep 30;138(2):253–281. doi: 10.1113/jphysiol.1957.sp005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Charges and potentials at the nerve surface. Divalent ions and pH. J Gen Physiol. 1968 Feb;51(2):221–236. doi: 10.1085/jgp.51.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meves H., Vogel W. Calcium inward currents in internally perfused giant axons of Loligo forbesi. J Physiol. 1972 Oct;226(2):89P–90P. [PubMed] [Google Scholar]
- Shatkay A. Individual activity of calcium ions in pure solutions of CaCl2 and in mixtures. Biophys J. 1968 Aug;8(8):912–919. doi: 10.1016/S0006-3495(68)86528-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaki I., Lerman L., Watanabe A. Analysis of excitation process in squid giant axons under bi-ionic conditions. Am J Physiol. 1969 Jan;216(1):130–138. doi: 10.1152/ajplegacy.1969.216.1.130. [DOI] [PubMed] [Google Scholar]
- Tasaki I., Watanabe A., Lerman L. Role of divalent cations in excitation of squid giant axons. Am J Physiol. 1967 Dec;213(6):1465–1474. doi: 10.1152/ajplegacy.1967.213.6.1465. [DOI] [PubMed] [Google Scholar]
- Tasaki I., Watanabe A., Singer I. Excitability of squid giant axons in the absence of univalent cations in the external medium. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1116–1122. doi: 10.1073/pnas.56.4.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A., Tasaki I., Lerman L. Bi-ionic action potentials in squid giant axons internally perfused with sodium saltssalts. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2246–2252. doi: 10.1073/pnas.58.6.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A., Tasaki I., Singer I., Lerman L. Effects of tetrodotoxin on excitability of squid giant axons in sodium-free media. Science. 1967 Jan 6;155(3758):95–97. doi: 10.1126/science.155.3758.95. [DOI] [PubMed] [Google Scholar]


