Abstract
1. Experiments have been performed to test the hypothesis that the group II fibres from the secondary endings of the muscle spindle provide an excitatory contribution to the tonic stretch reflex of the decerebrate cat. They have consisted of studying the effect of fusimotor paralysis by procaine, applied to the muscle nerve, on the reflex response to the combined stimuli of stretch (5-9 mm at 5 mm/sec) and of high-frequency vibration (100-150 Hz, 150 μm).
2. The reflex response to the combined stimuli was found to be paralysed in two distinct stages which paralleled those of the ordinary stretch reflex described earlier. The two phases of paralysis may be attributed to an early paralysis of the γ efferents followed by a later paralysis of the Ia afferents and α motor fibres. However, the Ia discharges elicited by the combined stimuli, unlike those elicited by simple stretch, should have remained unchanged on γ efferent paralysis since the Ia firing frequency may be presumed to have been clamped at the vibration frequency by the occurrence of one-to-one `driving'. The early reduction of the response to the combined stimuli may thus be attributed to the removal of a stretchevoked autogenetic excitatory input other than that long known to be provided by the Ia pathway. This supports the view that the spindle group II fibres have such an action, since their firing will be appropriately reduced on γ efferent paralysis by removal of their pre-existing fusimotor bias; there is no evidence for the existence of any other group of fibres with the right properties.
3. Recording of compound action potentials and of single units confirmed the great sensitivity of the γ efferents to procaine but showed that the group II fibres were nearly as resistant as the Ia fibres and α motor fibres.
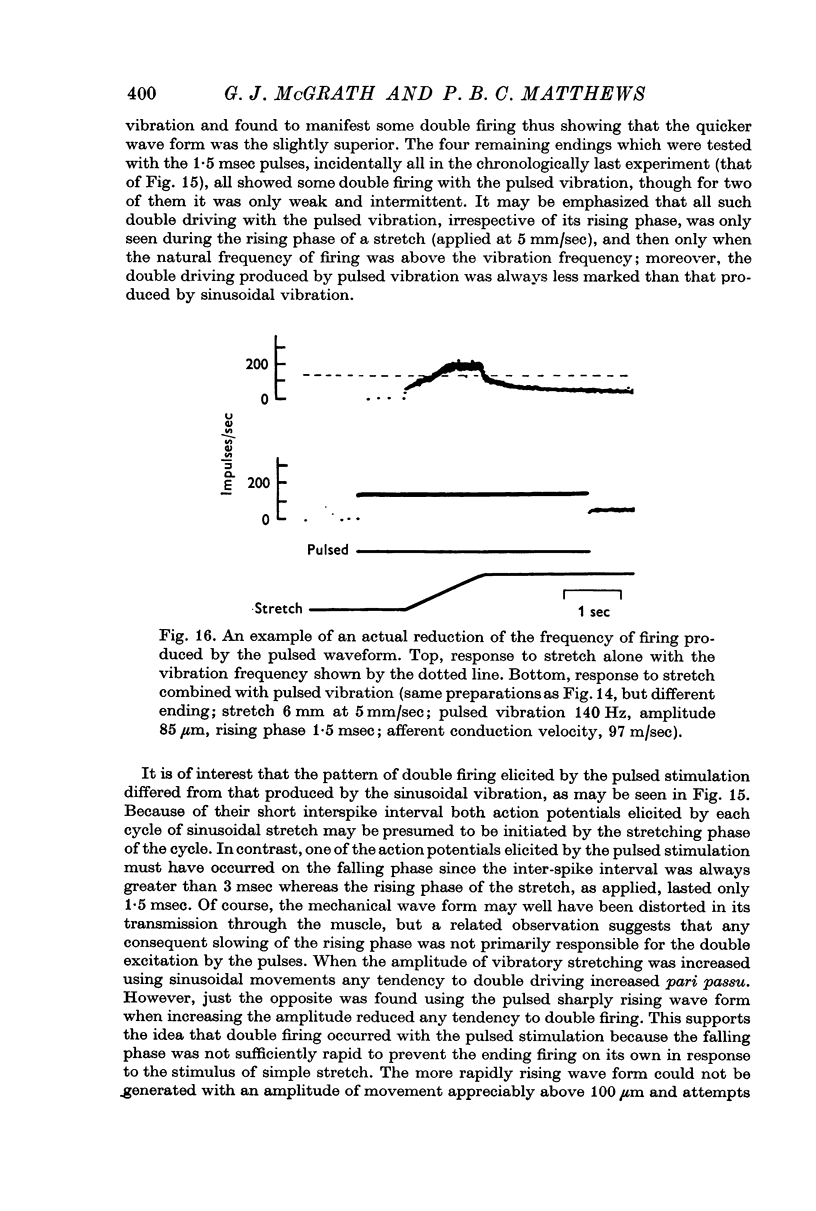
4. The reliability of one-to-one driving of the Ia discharges by the vibration was tested in control experiments in which the reflex was elicited by an asymmetrical vibratory waveform with a rapid rising phase (1·5 or 1·9 msec at 140 Hz) and a slower falling phase. Recordings from single units showed that the use of this wave form greatly diminished any tendency to double driving (2 spikes/cycle of vibration) during the dynamic phase of stretch and never elicited it during the static phase of stretch when the reflex measurements were made. These `pulsed' vibrations elicited reflex contractions which were of the same general size and which were paralysed in the same two phases by procaine as those elicited by sinusoidal vibrations. This eliminates the possibility that the early phase of paralysis might have been due to conversion of the pattern of Ia firing from double to single driving on γ efferent paralysis.
5. Wedensky inhibition of the afferent fibres could not be held responsible for the early phase of paralysis.
6. The results are taken to strengthen the hypothesis that the spindle group II fibres contribute excitation rather than inhibition to the stretch reflex. The particular support derived from the present experiments is that all measurements of the size of the reflex at various times were made with the muscle at the same length so that the findings cannot be attributed to the tension-length properties of muscle. The detailed mechanism of the excitation, however, remains to be established and certain of the present findings suggest that it may not be a direct one.
Full text
PDF




































Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown M. C., Engberg I., Matthews P. B. The relative sensitivity to vibration of muscle receptors of the cat. J Physiol. 1967 Oct;192(3):773–800. doi: 10.1113/jphysiol.1967.sp008330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cangiano A., Lutzemberger L. The action of selectively activated group II muscle afferent fibers on extensor motoneurons. Brain Res. 1972 Jun 22;41(2):475–478. doi: 10.1016/0006-8993(72)90519-7. [DOI] [PubMed] [Google Scholar]
- Cook W. A., Duncan C. C. Contribution of group I afferents to the tonic stretch reflex of the decerebrate cat. Brain Res. 1971 Oct 29;33(2):509–513. doi: 10.1016/0006-8993(71)90127-2. [DOI] [PubMed] [Google Scholar]
- Emonet-Dénand F., Jami L., Joffroy M., Laporte Y. Absence de réflexe myotatique après blocage de la conduction dans les fibres du groupe. C R Acad Sci Hebd Seances Acad Sci D. 1972 Mar 6;274(10):1542–1545. [PubMed] [Google Scholar]
- Goodwin G. M., McGrath G. J., Matthews P. B. The tonic vibration reflex seen in the acute spinal cat after treatment with DOPA. Brain Res. 1973 Jan 30;49(2):463–466. doi: 10.1016/0006-8993(73)90443-5. [DOI] [PubMed] [Google Scholar]
- Grillner S. Is the tonic stretch reflex dependent upon group II excitation? Acta Physiol Scand. 1970 Mar;78(3):431–432. doi: 10.1111/j.1748-1716.1970.tb04679.x. [DOI] [PubMed] [Google Scholar]
- Grillner S. The role of muscle stiffness in meeting the changing postural and locomotor requirements for force development by the ankle extensors. Acta Physiol Scand. 1972 Sep;86(1):92–108. doi: 10.1111/j.1748-1716.1972.tb00227.x. [DOI] [PubMed] [Google Scholar]
- JANSEN J. K., MATTHEWS P. B. The effects of fusimotor activity on the static responsiveness of primary and secondary endings of muscle spindles in the decerebrate cat. Acta Physiol Scand. 1962 Aug;55:376–386. doi: 10.1111/j.1748-1716.1962.tb02451.x. [DOI] [PubMed] [Google Scholar]
- Joyce G. C., Rack P. M., Westbury D. R. The mechanical properties of cat soleus muscle during controlled lengthening and shortening movements. J Physiol. 1969 Oct;204(2):461–474. doi: 10.1113/jphysiol.1969.sp008924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATTHEWS P. B., RUSHWORTH G. The discharge from muscle spindles as an indicator of gamma efferent paralysis by procaine. J Physiol. 1958 Mar 11;140(3):421–426. [PMC free article] [PubMed] [Google Scholar]
- MATTHEWS P. B., RUSHWORTH G. The selective effect of proCaine on the stretch reflex and tendon jerk of soleus muscle when applied to its nerve. J Physiol. 1957 Feb 15;135(2):245–262. doi: 10.1113/jphysiol.1957.sp005707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATTHEWS P. B. The effect of the local application of procaine on the stretch reflex of the soleus muscle of the cat decerebrated by anaemia. J Physiol. 1958 Mar 11;140(3):408–420. [PMC free article] [PubMed] [Google Scholar]
- Marsden C. D., Merton P. A., Morton H. B. Servo action in human voluntary movement. Nature. 1972 Jul 21;238(5360):140–143. doi: 10.1038/238140a0. [DOI] [PubMed] [Google Scholar]
- Matthews P. B. Evidence that the secondary as well as the primary endings of the muscle spindles may be responsible for the tonic stretch reflex of the decerebrate cat. J Physiol. 1969 Oct;204(2):365–393. doi: 10.1113/jphysiol.1969.sp008918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. B., Stein R. B. The regularity of primary and secondary muscle spindle afferent discharges. J Physiol. 1969 May;202(1):59–82. doi: 10.1113/jphysiol.1969.sp008795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. B. The reflex excitation of the soleus muscle of the decerebrate cat caused by vibbration applied to its tendon. J Physiol. 1966 May;184(2):450–472. doi: 10.1113/jphysiol.1966.sp007926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath G. H., Matthews P. B. Support for an autogenetic excitatory reflex action of the spindle secondaries from the effect of gamma blockade by procaine. J Physiol. 1970 Sep;210(2):176P–177P. [PubMed] [Google Scholar]
- Morelli M., Nicotra L., Barnes C. D., Cangiano A., Cook W. A., Jr, Pompeiano O. An apparatus for producing small-amplitude high-frequency sinusoidal stretching of the muscle. Arch Ital Biol. 1970 Apr;108(2):222–232. [PubMed] [Google Scholar]
- Paintal A. S. Block of conduction in mammalian myelinated nerve fibres by low temperatures. J Physiol. 1965 Sep;180(1):1–19. [PMC free article] [PubMed] [Google Scholar]
- Thoden U., Magherini P. C., Pompeiano O. Evidence that presynaptic inhibition may decrease the autogenetic excitation caused by vibration of extensor muscles. Arch Ital Biol. 1972 May;110(1):90–116. [PubMed] [Google Scholar]
- Westbury D. R. A study of stretch and vibration reflexes of the cat by intracellular recording from motoneurones. J Physiol. 1972 Oct;226(1):37–56. doi: 10.1113/jphysiol.1972.sp009972. [DOI] [PMC free article] [PubMed] [Google Scholar]



