Abstract
The major heat shock genes of Helicobacter pylori are regulated by the HspR repressor. In the present study we characterize the transcriptional response of the three known HspR-dependent promoters Pcbp, Pgro, and Phrc to different environmental stresses. A temperature shift from 37 to 42°C causes a typical heat shock response at all three promoters characterized by an immediate and strong induction phase of transcription and a subsequent adaptation phase, which is specific for each promoter and whose onset is determined partially by the half-lives of the respective mRNAs. Exposure to high osmolarity induces a similar response on the Pgro and Pcbp promoters while no such response is detectable at the Phrc promoter. Puromycin treatment induces transcription from all three HspR-dependent promoters, indicating that different environmental stresses are intracellularly sensed by the regulatory machinery through the accumulation of nonnative proteins. The implications of these data for the regulatory network controlling the heat shock response in H. pylori are discussed.
The heat shock proteins of the gastric pathogen Helicobacter pylori have been studied in some detail both because of their general role in the protection of the bacteria from the hostile environment represented by the human stomach and because of their involvement in specific pathogenic processes. The H. pylori GroEL (Hsp60) homologue (12, 20) has been proposed to play a role in regulating the activity of the nickel-dependent urease enzyme, which generates ammonia ions from the hydrolysis of urea and therefore protects the bacteria from the low pH of the stomach lumen (4, 6). Its cochaperone GroES (Hsp10) is thought to contribute to this regulation by controlling the availability of nickel ions by means of its intrinsic metal-binding activity (10, 20). GroEL as well as another major heat shock protein, DnaK (Hsp70), can furthermore be found in association with the outer membrane, and this surface localization has been suggested to modify the glycolipid binding specificity of H. pylori cells at a low pH (5, 9, 15).
Because of their multiple functions in both the general stress response and in specific disease mechanisms, the H. pylori heat shock proteins are expected to be tightly regulated in their expression levels. It was recently demonstrated that transcription of the groESL, hrcA-grpE-dnaK, and cbpA-hspR-orf operons encoding the major chaperones of H. pylori is negatively regulated by HspR (17). HspR is a homologue of the repressor of the dnaK operon of Streptomyces coelicolor (2, 7). By RNA analyses and footprint experiments with purified protein preparations, we have shown that HspR represses transcription by binding to regions of 75 bp on the three chaperone gene-transcribing promoters Pgro, Phrc, and Pcbp. In an attempt to identify the environmental stresses, which induce transcription from these promoters, we subjected H. pylori cultures to both osmotic shock and thermal shock at 45°C. While the Pgro and Pcbp promoters were found to be strongly inducible by treatment with 300 mM NaCl, no induction could be observed on either promoter by incubation of cultures at 45°C, indicating that heat shock does not induce transcription from HspR-dependent promoters. Recently, however, Homuth and coworkers (8) have shown that the Pgro and Phrc promoters are strongly inducible by a mild heat shock at 42°C, suggesting that HspR can indeed mediate the transcriptional response to a sudden temperature increase. In the present work we determine in detail the transcriptional response of the three HspR-dependent promoters to different environmental stresses. We show that a temperature shift to 42°C causes a typical heat shock response at all three promoters, characterized by a fast and strong induction of transcription and a subsequent shutoff phase, whose onset is determined largely by the stability of the respective mRNAs. Treatment with 300 mM NaCl causes a similar response on both Pgro and Pcbp while transcription from Phrc is essentially unaffected under these conditions. Puromycin treatment also induced transcription from the HspR-regulated promoters, indicating that misfolded proteins represent the intracellular signal that is sensed by the transcriptional machinery.
MATERIALS AND METHODS
Media and growth conditions.
H. pylori G27 (24) cells were recovered from frozen stocks on Columbia agar plates containing 5% horse blood, 0.2% cyclodextrin, and Dent's or Skirrow's antibiotic supplement under microaerophilic conditions (Oxoid) for 2 to 3 days. After passage on fresh plates, bacteria were cultured in a 5% CO2-95% air atmosphere. Liquid cultures of H. pylori were grown in modified brucella broth containing Dent's or Skirrow's antibiotic supplement and 5% fetal calf serum in a 5% CO2-95% air atmosphere. When required, rifampin or puromycin was added to a final concentration of 80 or 20 μg/ml, respectively. Osmotic shock of H. pylori cultures was performed by adding NaCl to a final concentration of 300 mM NaCl. Heat shock was achieved by incubation of H. pylori cultures in a water bath at 42°C. It should be noted that previous heat shock experiments in our laboratory were performed by incubation of liquid cultures in microaerophilic jars (17). Differences in the induction profiles of heat shock promoters with respect to previously published experiments might therefore be attributable to these different growth conditions.
RNA preparation.
H. pylori G27 was grown in 260 ml of modified brucella broth at 37°C to mid-log phase and then exposed to various stresses. At different time intervals after stress exposure, 25-ml samples were harvested and stored at −20°C. Cells were lysed in 3.7 ml of 100 mM Tris-HCl (pH 7.5), 2 mM Na2-EDTA, and 1% sodium dodecyl sulfate for 5 min at 95°C. After 10 min of incubation on ice in the presence of 80 mM KCl, cellular debris was removed by centrifugation at 8,000 rpm for 10 min in a JA20 rotor (Beckman). To 3.5 ml of supernatant, 4.56 g of CsCl was added, and the RNA was sedimented by centrifugation in an SW65 rotor (Beckman) for 15 to 20 h at 35,000 rpm. The RNA pellet was then resuspended in 500 μl of TE (10 mM Tris-HCl [pH 8], 1 mM Na2-EDTA), extracted once with an equal volume of phenol-chloroform (1:1), ethanol precipitated, resuspended in 200 μl of TE, reprecipitated, and stored at −20°C.
Quantification of transcripts.
Pgro- and Phrc-specific transcripts were detected by reverse transcription with oligonucleotides groS (5′-GACCCTTTCTCCTAATGGCTG) and hrcA (5′-CAAACGCATCTAACAAACTCTC) as described by Spohn and Scarlato (17). Pcbp-specific transcripts were detected by S1 nuclease mapping with a 674-bp-specific DNA probe (17). This DNA fragment was cloned into the pGem3 vector (Promega) as a PCR product with oligonucleotide pair cbp1-cbp2 (5′-attattggatccACCCCAAGACGCGCTAAAGCCC and 5′-attattgAATTCTTGGGTTAGGGGGATTTTAAGGG, capital letters indicate H. pylori sequences and underlined sequences represent the BamHI and EcoRI recognition sites, respectively) and comprises the 5′ regions of the cbpA gene and the upstream open reading frame HP1023. Briefly, the DNA fragment was 5′ end labeled at its EcoRI site in the presence of [γ-32P]ATP (5,000 Ci/mmol; Amersham) and T4 polynucleotide kinase (New England Biolabs). Approximately 20 fmol of the labeled fragment was coprecipitated with 15 μg of total H. pylori RNA and resuspended in 20 μl of hybridization buffer (80% formamide, 60 mM Tris-Cl [pH 7.5], 400 mM NaCl, 0.4 mM EDTA). The mixture was denatured at 100°C for 3 min and then incubated at the annealing temperature of 43°C. After 16 h of incubation, 180 μl of ice-cold S1 buffer (33 mM Na-acetate [pH 5.2], 5 mM ZnSO4, 250 mM NaCl) and 1 μl of S1 nuclease (400 U/μl) were added and S1 digestion was carried out for 30 min at 37°C. Samples were then extracted once with phenol-chloroform, ethanol precipitated, resuspended in 5 μl of sequencing loading buffer, heat denatured, subjected to 6% urea polyacrylamide gel electrophoresis, autoradiographed, and exposed to a PhosphorImager (Molecular Dynamics) for radioactivity quantification. As a control experiment, the flaB transcription was assayed by primer extension with oligonucleotide flaB as described in Spohn and Scarlato (18).
RESULTS
Three HspR-regulated promoters exhibit a typical heat shock response at 42°C.
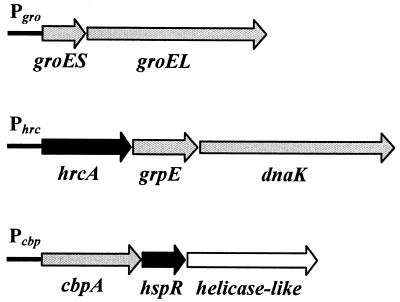
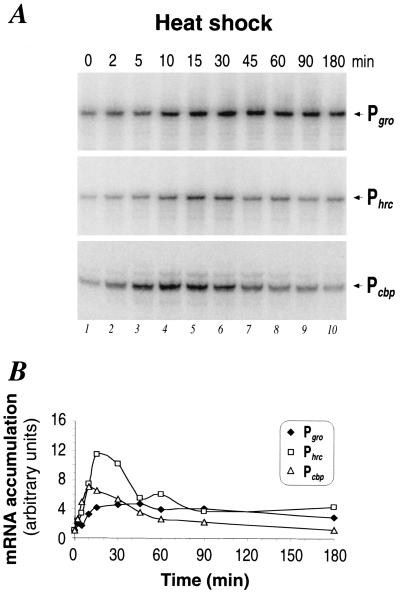
The structural organization of the three HspR-regulated operons and the respective promoters are schematically depicted in Fig. 1. Pgro transcribes one bicistronic mRNA encoding the chaperonin machinery GroESL; Phrc transcribes a tricistronic mRNA encoding the putative repressor HrcA, the chaperone DnaK, and its cochaperone GrpE (8, 17); and Pcbp is likely to transcribe a tricistronic mRNA encoding the DnaJ homologue CbpA, the HspR repressor, and a putative DNA helicase (17). To investigate the transcriptional response of these promoters to a sudden temperature increase, total RNA was isolated from H. pylori strain G27 grown at 37°C and at different time points after upshift of the culture to 42°C. Promoter-specific mRNA was then detected by reverse transcription with specific oligonucleotides or by S1 nuclease mapping with specific DNA probes, respectively, and quantified by exposure of urea-acrylamide gels to a PhosphorImager. The results of this analysis are shown in Fig. 2. Figure 2A shows that the amount of each transcript increases rapidly after temperature upshift, starting at 2 min and reaching a maximum at 10 to 15 min. As evident from Fig. 2B, the ratio of this increase of transcription is different for the three promoters, ranging from 5-fold for Pgro to 7-fold for Pcbp and 11-fold for Phrc. The phase of maximal induction is followed by a decline phase during which transcript amounts gradually decrease. In the case of Pcbp, this shutoff phase starts immediately after the peak of induction at about 10 min and brings back transcript amounts to about twice the pre-heat shock levels within 1 h and to a level comparable to the unstressed situation after 3 h. Phrc transcript amounts accumulate at the maximal level for about 10 min and then begin to decline, reaching a new steady-state level of transcription within 90 min, which is about fourfold higher than the uninduced level. Conversely, Pgro transcript levels do not decrease significantly after the initial induction phase and remain at a high level until the end of the time course at 3 h after temperature upshift.
FIG. 1.
Structural organization of H. pylori chaperone genes (17, 21). Grey arrow bars indicate chaperone genes, black arrow bars indicate regulatory genes (hspR and hrcA), and the white arrow bar indicates a putative helicase-like gene. Chaperone genes groES and groEL code for the HspA (Hsp10) and HspB (Hsp60) proteins (20); cbpA encodes a protein with 30% amino acid identity (in a 288-amino-acid overlap) to the cochaperone curved DNA binding protein CbpA from E. coli, a homologue of DnaJ (22); hspR encodes a regulatory protein with 46% identity (in a 91-amino-acid overlap) to HspR, the negative regulator of heat shock genes in S. coelicolor (2); the helicase-like open reading frame encodes a protein with 30% identity within 421 amino acids to a hypothetical helicase-like protein from Haemophilus influenzae; hrcA encodes a protein with 28% identity (in a 71-amino-acid overlap) to the heat-inducible transcriptional repressor HrcA from B. subtilis (23); and grpE and dnaK encode the GrpE and DnaK (Hsp70) chaperones, respectively.
FIG. 2.
Heat shock response of chaperone genes. (A) Primer extension (Pgro, Phrc) and S1 nuclease (Pcbp) analyses of H. pylori RNA extracted from cells grown at 37°C (0 min) (lane 1) or upon temperature shift to 42°C (lanes 2 to 10). The time interval at which RNA was extracted is indicated, in minutes, above each lane. The products of RNA elongation or protection by S1 nuclease are marked on the rightmost side of the autoradiography by the names of the corresponding promoters: Pgro, Phrc, and Pcbp. (B) Pattern of RNA accumulation at the indicated promoters as obtained by PhosphorImager analysis quantifications of the radioactive bands shown in panel A. It is worth mentioning that primer extensions conducted on the cbpA mRNA gave unsatisfactory results. By contrast, S1 nuclease mapping turned out to function very well in mapping both the transcription start site and the regulation of transcription upon stress conditions.
We conclude that the three HspR-regulated promoters of H. pylori exhibit a typical heat shock response at 42°C. All three promoters are strongly and rapidly induced after temperature upshift. The adaptation phase following this initial induction phase follows different kinetics for the different promoters.
Differences in the adaptation kinetics are largely due to differences in mRNA stabilities.
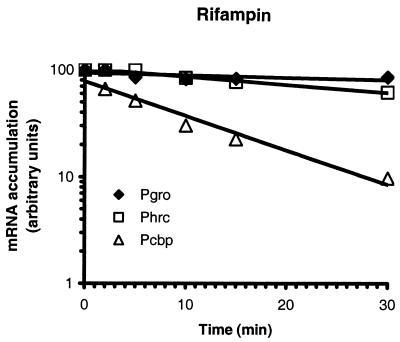
To establish whether the kinetic differences observed in the shutoff phase of transcription are consequences of a possible differential regulation of the three heat shock promoters by the HspR repressor or of regulatory events at the posttranscriptional level, we decided to estimate the half-lives of the respective mRNAs. For that purpose, H. pylori G27 cells were first exposed to a 15-min heat shock at 42°C to induce transcription and then treated with rifampin to stop transcriptional initiation. RNA was prepared before and at different time intervals after the addition of rifampin, and the amount of each specific transcript was monitored by reverse transcription and S1 nuclease mapping. Figure 3 shows that the amount of Pcbp-specific transcript decreases rapidly after rifampin treatment, with a half-life which calculates to ∼6 min. Phrc and Pgro transcripts on the other hand appear to be particularly stable; after 30 min of rifampin treatment, 65% of the initial amount of Phrc-specific transcript and 85% of Pgro-specific transcript can still be detected. By extrapolation, the half-lives of the Phrc and Pgro transcripts can therefore be estimated to 40 to 45 min and more than 90 min, respectively. These are unusually long half-lives. In fact, in Bacillus subtilis the groE transcript shows an estimated half-life of 5 min under heat shock and non-heat shock conditions (26).
FIG. 3.
Estimation of mRNA half-lives at 42°C. Data points represent PhosphorImager analysis quantifications of radioactive bands obtained by primer extension or protection by S1 nuclease of RNA extracted before (0 min) and after the addition of rifampin to growing cells at the indicated time intervals.
The differences in the adaptation kinetics between the three heat shock promoters can therefore probably be attributed to differences in the stabilities of the respective mRNAs. Transcriptional shutoff becomes evident immediately at the Pcbp promoter because of the short half-life of this mRNA, whereas the long half-lives of the Phrc and Pgro mRNAs allow detection of shutoff only with a delay or not at all, respectively.
In order to investigate whether the initial induction of transcription detected after heat shock at 42°C is in part mediated by temperature-dependent changes in mRNA stability, we also measured the half-lives of the mRNAs transcribed from Pgro and Phrc at 37°C. No significant differences could be observed in the stabilities of these mRNAs between heat shock and non-heat shock conditions (data not shown), indicating that the initial induction of transcription is solely the result of regulatory events at the level of transcription initiation.
The Pcbp and Pgro promoters are also induced by osmotic shock.
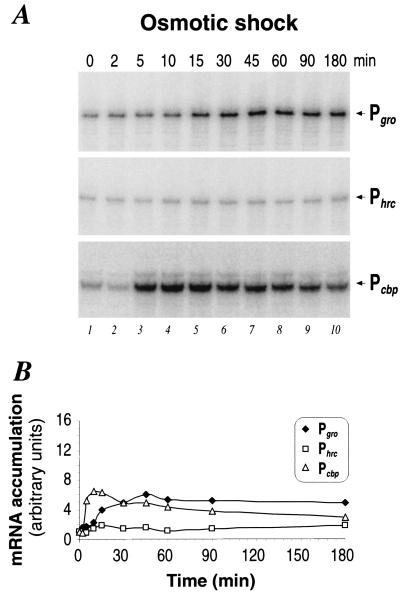
Previous work had shown that salt treatment of H. pylori cells can induce transcription from the Pcbp and Pgro promoters but not from the Phrc promoter (17). To study in more detail the transcriptional response of the three HspR-regulated promoters to a sudden increase in osmolarity, we isolated total RNA from H. pylori G27 immediately before and at different time intervals after the addition of 300 mM NaCl and assayed transcript levels as before by primer extension and S1 nuclease mapping and subsequent PhosphorImager analysis. Figure 4A shows that the Pcbp and Pgro promoters are induced rapidly after salt treatment, reaching a maximum after 10 and ∼30 min, respectively. As shown in Fig. 4B, the induction ratios for both promoters are similar to those found for heat shock treatment, i.e., ∼6- to 7-fold for Pcbp and 5- to 6-fold for Pgro. Also, the induction kinetics resemble those observed after thermal upshift, with Pcbp starting shutoff of transcription immediately after the peak of induction and Pgro transcript levels remaining at the maximal induced level for up to 3 h after temperature upshift. In agreement with previously published results (17), no significant induction could be observed at the Phrc promoter. Transcription of this promoter remains at the basal level during the whole time course of osmotic shock treatment. Similar results have been obtained by adding 440 mM sucrose to growing cells (data not shown).
FIG. 4.
Osmotic shock response of chaperone genes. (A) Primer extension (Pgro, Phrc) and S1 nuclease (Pcbp) analyses of H. pylori RNA extracted from cells grown at 37°C (0 min) (lane 1) in normal medium or upon addition of 300 mM NaCl (lanes 2 to 10). Symbols are as described in the legend to Fig. 2. (B) Pattern of RNA accumulation at the indicated promoters as obtained by PhosphorImager analysis quantifications of the radioactive bands shown in panel A.
Nonnative proteins induce an HspR-dependent stress response.
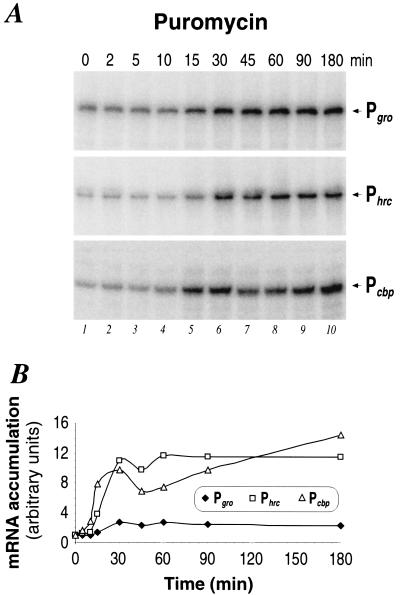
The fact that environmental stresses as diverse as osmolarity changes and temperature increases can induce the same transcriptional response at two of the HspR-regulated promoters supports the hypothesis that these stresses are not sensed directly by the regulatory apparatus but converge into a common intracellular signal which is then perceived and transduced into a transcriptional response. In Escherichia coli and B. subtilis this intracellular signal is believed to be represented by unfolded or aberrant proteins that accumulate under various conditions of environmental stress, triggering in this way the onset of the heat shock response. To investigate if nonnative proteins can induce a heat shock response of the HspR-dependent promoters, we isolated total RNA from H. pylori cells before and at different time intervals after the addition of puromycin, a tRNA analog that incorporates into nascent polypeptide chains and leads to premature translation termination. Transcription from the three HspR-dependent promoters was monitored as before by primer extension and S1 mapping and subsequent PhosphorImager analysis of urea-acrylamide gels. Figure 5A shows that all three promoters are induced rapidly after the addition of puromycin, with transcript levels reaching a maximum after about 30 min. As shown in Fig. 5B, Phrc and Pcbp transcript amounts increase about 10- to 12-fold, whereas Pgro-specific transcripts increase only about 3-fold. In contrast to the heat and osmotic shock responses shown in Fig. 2 and 4, respectively, transcript levels fail to decline after this initial induction phase and remain at the maximal level for the whole time course of the experiment. As a control, the P112 promoter transcribing the flaB mRNA (18, 19) was also included in this analysis, showing that transcription from this promoter was essentially unaffected by puromycin treatment (data not shown).
FIG. 5.
Transcriptional response of chaperone genes to accumulation of misfolded polypeptides. (A) Primer extension (Pgro, Phrc) and S1 nuclease (Pcbp) analyses of H. pylori RNA extracted from cells grown at 37°C (0 min) (lane 1) or upon the addition of puromycin (lanes 2 to 10). Symbols are as described in the legend to Fig. 2. (B) Pattern of RNA accumulation at the indicated promoters as obtained by PhosphorImager analysis quantifications of the radioactive bands shown in panel A.
We conclude that aberrant proteins induced by the addition of puromycin can elicit a heat shock response of the HspR-regulated promoters of H. pylori.
DISCUSSION
Bacteria respond to conditions of stress by synthesizing chaperones that protect the cell from damage by preventing protein denaturation, aggregation, or misfolding (see reference 11 and references therein). The chaperones of the human gastric pathogen H. pylori have been studied in some detail because of their possible involvement in specific virulence mechanisms (9, 12, 20, 25). It has been shown that the groESL, hrcA-grpE-dnaK, and cbpA-hspR-orf operons, encoding the major chaperones of H. pylori, are transcribed by an RNA polymerase containing the vegetative sigma factor σ80 and regulated negatively by the transcriptional repressor HspR (17). Very recently, by using an in vitro selection and amplification approach, two new chromosomal binding sites of the HspR protein were identified (3). Surprisingly, these HspR binding sites are located at the 3′ ends of two genes coding for predicted proteins with functions unrelated to those of chaperones. This suggests that H. pylori HspR may regulate the expression of genes encoding proteins with diverse functions. The control of transcription by HspR depends on the binding of this protein to large operators mapping to different positions surrounding the promoters (17). In addition to this regulation, the possibility that another transcriptional repressor, a homolog of the B. subtilis HrcA protein (16) encoded by the hrcA-grpE-dnaK locus, might also be involved in controlling stress response has been postulated (8, 17). Evidences that chaperone gene transcription in this system responds to an increase in salinity and temperature elevation to 42°C, but not to 45°C, have been recently reported (8, 17).
In this work we determined in detail the temporal pattern of transcriptional response for the three HspR-dependent promoters to different environmental stresses. A temperature shift to 42°C causes a typical heat shock response at the Pgro, Phrc, and Pcbp promoters characterized by a fast and strong induction of transcription and a subsequent shutoff phase (Fig. 2). The initial induction is a true consequence of HspR-mediated derepression of transcription initiation, as stabilities of the different mRNAs were found to remain unchanged after temperature upshift with respect to the nonstressed situation. The absolute differences in mRNA stabilities between the three promoters appear, however, to influence the onset and temporal pattern of the adaptation phase of transcription: the Pcbp promoter, which transcribes the mRNA with the shortest half-life of the three heat shock promoters (6 min) (Fig. 3), shows the earliest onset of the shutoff phase of transcription at about 10 min after temperature upshift while the Phrc and Pgro promoters, transcribing mRNAs with significantly longer half-lives (45 and >90 min, respectively), appear to enter this phase only at later stages or not at all, respectively. Despite these differences in mRNA stabilities, however, it is reasonable to assume that at the level of transcriptional initiation, HspR-dependent repression is restored on all three promoters shortly after the initial induction of transcription. Likely, at this stage of the heat shock response, the HspR repressor begins to regain its active, repression-competent conformation, possibly aided by the action of one or more specialized molecular chaperones. In support of this hypothesis is the recent finding that the DnaK chaperone of S. coelicolor can act as a corepressor together with the HspR protein (1). In this model, a DnaK-HspR complex efficiently represses transcription under non-heat shock conditions while a sudden increase in temperature leads to the transient sequestration of DnaK by accumulating unfolded proteins, thereby freeing the HspR repressor, which thus becomes inefficient in DNA binding and transcriptional repression. The increased amounts of DnaK protein produced after this induction of transcription then lead to efficient refolding of denatured proteins and restoration of the HspR-DnaK complex, which reestablishes transcriptional repression. Although in H. pylori no indications for the existence of such a complex have been found, it is likely that DnaK or another molecular chaperone participates in regulation of the DNA binding activity of HspR. In support of this hypothesis is the fact that puromycin treatment of H. pylori cells leads to a strong and irreversible induction of transcription at all three HspR-regulated promoters (Fig. 5). This induction could be interpreted as a result of the permanent sequestration of DnaK by the puromycin-induced aberrant proteins, and the consequent irreversible loss of DNA binding activity by the HspR repressor. Under physiological conditions DnaK would therefore represent the intracellular stress sensor, which mediates the appropriate transcriptional response according to the level of misfolded proteins present in the cytoplasm. All extracellular stresses, which produce aberrant or misfolded proteins in the cytoplasm, should therefore elicit a heat shock response at the HspR-dependent promoters via titration of the DnaK protein. As shown in Fig. 4, environmental stresses different from temperature changes can indeed induce a transcriptional response at the HspR-regulated promoters. The Pcbp and Pgro promoters are strongly induced by the addition of 300 mM NaCl to the growth medium, and the kinetics of induction correspond to the ones observed under heat shock conditions.
In contrast to the Pcbp and Pgro promoters, transcription from the Phrc promoter is insensitive to osmotic shock treatment, possibly indicating that this stimulus is not sufficient to destabilize the bound protein from this operator. In support of this hypothesis is the finding that HspR binds to the Phrc-associated operator with two- and fourfold-higher affinities than the Pcbp- and Pgro-associated operators, respectively (17). Alternatively, a more complex mechanism of transcriptional control might be operating on the Phrc promoter, possibly involving HrcA, the repressor encoded by the first gene of the dnaK operon. HspR and HrcA might act as corepressors on this promoter, and this dual control might lead to a differentiation of the transcriptional response according to the environmental stimulus perceived. The involvement of a second repressor, though, also implies a second negative modulator of the heat shock response. In B. subtilis it has been shown that the GroEL chaperonin functions as a negative regulator of heat shock gene expression (13, 14). In this system, GroEL is necessary for the HrcA repressor protein to function efficiently as a repressor of the dnaK operon by maintaining HrcA in a properly folded state. We speculate that an HrcA-unknown chaperone and the HspR-DnaK systems in H. pylori are required to fine-tune chaperone gene transcription in response to a sudden change in the environmental growth condition.
Acknowledgments
We thank C. Mallia for editing the manuscript and G. Corsi for artwork.
This work has been supported partially by EU-TMR grant FMRX-CT980164, Chiron, MURST, and University of Bologna. I.D. is the recipient of an EU-TMR fellowship (FMRX-CT980164).
REFERENCES
- 1.Bucca, G., A. M. Brassington, H. J. Schonfeld, and C. Smith. 2000. The HspR regulon of Streptomyces coelicolor: a role for the DnaK chaperone as a transcriptional co-repressordagger. Mol. Microbiol. 38:1093-1103. [DOI] [PubMed] [Google Scholar]
- 2.Bucca, G., G. Farina, A. M. Puglia, and C. P. Smith. 1995. The dnaK operon of Streptomyces coelicolor encodes a novel heat shock protein which binds to the promoter region of the operon. Mol. Microbiol. 17:663-674. [DOI] [PubMed] [Google Scholar]
- 3.Delany, I., G. Spohn, R. Rappuoli, and V. Scarlato. 2002. In vitro selection of high affinity HspR-binding sites within the genome of Helicobacter pylori. Gene 283:63-69. [DOI] [PubMed] [Google Scholar]
- 4.Dunn, B. E., R. M. Roop II, C.-C. Sung, S. A. Sharma, G. I. Perez-Perez, and M. J. Blaser. 1992. Identification and purification of a cpn60 heat shock protein homolog from Helicobacter pylori. Infect. Immun. 60:1946-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunn, B. E., N. B. Vakil, B. G. Schneider, M. M. Miller, J. B. Zitzer, T. Peutz, and S. H. Phadnis. 1997. Localization of Helicobacter pylori urease and heat shock protein in human gastric biopsies. Infect. Immun. 65:1181-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans, D. J., D. G. Evans, L. Engstrand, and D. Graham. 1992. Urease-associated heat shock protein of Helicobacter pylori. Infect. Immun. 60:2125-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grandvalet, C., P. Servant, and P. Mazodier. 1997. Disruption of hspR, the repressor gene of the dnaK operon in Streptomyces albus G. Mol. Microbiol. 23:77-84. [DOI] [PubMed] [Google Scholar]
- 8.Homuth, G., S. Domm, D. Kleiner, and W. Schumann. 2000. Transcriptional analysis of major heat shock genes of Helicobacter pylori. J. Bacteriol. 182:4257-4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huesca, M., S. Borgia, P. Hoffman, and C. Lingwood. 1996. Acidic pH changes receptor binding specificity of Helicobacter pylori: a binary adhesion model in which surface heat shock (stress) proteins mediate sulfatide recognition in gastric colonization. Infect. Immun. 64:2643-2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kansau, I., F. Guillain, J.-M. Thiberge, and A. Labigne. 1996. Nickel binding and immunological properties of the C-terminal domain of the Helicobacter pylori GroES homologue (HspA). Mol. Microbiol. 22:1013-1023. [DOI] [PubMed] [Google Scholar]
- 11.Macario, A. J. L., M. Lange, B. K. Ahring, and E. C. De Macario. 1999. Stress genes and proteins in the archaea. Microbiol. Mol. Biol. Rev. 63:923-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macchia, G., A. Massone, D. Burroni, A. Covacci, S. Censini, and R. Rappuoli. 1993. The Hsp60 protein of Helicobacter pylori: structure and immune response in patients with gastroduodenal diseases. Mol. Microbiol. 9:645-652. [DOI] [PubMed] [Google Scholar]
- 13.Mogk, A., G. Homuth, C. Scholz, L. Kim, F. X. Schimd, and W. Schumann. 1997. The GroE chaperonin machine is a major modulator of the CIRCE heat shock regulon of Bacillus subtilis. EMBO J. 16:4579-4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Narberhaus, F. 1999. Negative regulation of bacterial heat shock genes. Mol. Microbiol. 31:1-8. [DOI] [PubMed] [Google Scholar]
- 15.Phadnis, S. H., M. H. Parlow, M. Levy, D. Ilver, J. B. Caulkins, J. B. Connors, and B. E. Dunn. 1996. Surface localization of Helicobacter pylori urease and heat shock protein homolog requires bacterial autolysis. Infect. Immun. 64:905-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schulz, A., and W. Schumann. 1996. hrcA, the first gene of the Bacillus subtilis dnaK operon encodes a negative regulator of class I heat shock genes. J. Bacteriol. 178:1088-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spohn, G., and V. Scarlato. 1999. The autoregulatory HspR repressor protein governs chaperone gene transcription in Helicobacter pylori. Mol. Microbiol. 34:663-674. [DOI] [PubMed] [Google Scholar]
- 18.Spohn, G., and V. Scarlato. 1999. Motility of Helicobacter pylori is coordinately regulated by the transcriptional activator FlgR, an NtrC homolog. J. Bacteriol. 181:593-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suerbaum, S., C. Josenhans, and A. Labigne. 1993. Cloning and genetic characterization of the Helicobacter pylori and Helicobacter mustelae flagellin genes and construction of H. pylori flaA- and flaB-negative mutants by electroporation-mediated allelic exchange. J. Bacteriol. 175:3278-3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suerbaum, S., J.-M. Thiberge, I. Kansau, R. L. Ferrero, and A. Labigne. 1994. Helicobacter pylori hspA-hspB heat shock gene cluster: nucleotide sequence, expression, putative function and immunogenicity. Mol. Microbiol. 14:959-974. [DOI] [PubMed] [Google Scholar]
- 21.Tomb, J. F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, et al. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539-547. [DOI] [PubMed] [Google Scholar]
- 22.Ueguchi, C., M. Kakeda, H. Yamada, and T. Mizuno. 1994. An analogue of the DnaJ molecular chaperone in Escherichia coli. Proc. Natl. Acad. Sci. USA 91:1054-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wetzstein, M., U. Völker, J. Dedio, S. Löbau, U. Zuber, M. Schiesswohl, C. Herget, M. Hecker, and W. Schumann. 1992. Cloning, sequencing, and molecular analysis of the dnaK locus from Bacillus subtilis. J. Bacteriol. 174:3300-3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiang, Z., S. Censini, P. F. Bayeli, J. L. Telford, N. Figura, R. Rappuoli, and A. Covacci. 1995. Analysis of expression of CagA and VacA virulence factors in 43 strains of Helicobacter pylori reveals that clinical isolates can be divided into two major types and that CagA is not necessary for expression of the vacuolating cytotoxin. Infect. Immun. 63:94-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yokota, K., Y. Hira, M. Haque, S. Hayashi, H. Isogai, T. Suguyama, E. Nagamachi, Y. Tsukada, N. Fujii, and K. Oguma. 1994. Heat shock protein produced by Helicobacter pylori. J. Med. Microbiol. 45:270-277. [DOI] [PubMed] [Google Scholar]
- 26.Yuan, G., and S.-L. Wong. 1995. Regulation of groE expression in Bacillus subtilis: the involvement of the σA-like promoter and the roles of the inverted repeat sequence (CIRCE). J. Bacteriol. 177:5427-5433. [DOI] [PMC free article] [PubMed] [Google Scholar]