Abstract
Objective
Relatively little is known about how excess body mass affects adolescents’ capacity to perform sustained exercise. We hypothesized that most of the difficulty that severely overweight adolescents have with sustained exercise occurs because the metabolic costs of moving excess mass result in use of a high proportion of their total oxygen reserve.
Methods
We compared results from a maximal cycle ergometry fitness test in 129 severely overweight adolescents who had BMIs of 41.5 ± 9.7 kg/m2 and ages of 14.5 ± 1.8 years (range: 12.1–17.8 years) and 34 nonoverweight adolescents who had BMIs of 20.1 ± 2.9 kg/m2 and ages of 14.5 ± 1.5 years (range: 12.0–18.1 years). Oxygen uptake (V̇o2) was compared at 3 times: during a 4-minute period of unloaded cycling (ULV̇o2), at the lactate thresh-old estimated by gas exchange (LTV̇o2), and at maximal exertion (V̇o2 max). Heart rate was obtained at rest and at V̇o2 max. Participants also completed a 12-minute walk/run performance test to obtain distance traveled (D12) and heart rate.
Results
Absolute LTV̇o2 and V̇o2 max and LTV̇o2 as a percentage of V̇o2 max were not different in overweight and nonoverweight adolescents during the cycle test. However, absolute ULV̇o2 was significantly greater in overweight adolescents: ULV̇o2 accounted for 35 ± 8% of V̇o2 max (and 63 ± 15% of LTV̇o2) in overweight adolescents but only 20 ± 5% of V̇o2 max (and 39 ± 12% of LTV̇o2) in nonoverweight adolescents. Resting heart rate before initiating the cycle test was significantly greater in overweight than nonoverweight adolescents (94 ± 14 vs 82 ± 15 beats per minute). However, maximal heart rate during the cycle test was significantly lower in overweight adolescents (186 ± 13 vs 196 ± 11 beats per minute). During the walk/run test, mean D12 was significantly shorter for overweight than for nonoverweight adolescents (1983 ± 323 vs 1159 ± 194 m). D12 was negatively related to BMI SDS (r = −0.81) and to ULV̇o2 (r = −0.98).
Discussion
Overweight and nonoverweight adolescents had similar absolute V̇o2 at the lactate threshold and at maximal exertion, suggesting that overweight adolescents are more limited by the increased cardiorespiratory effort required to move their larger body mass through space than by cardiorespiratory deconditioning. The higher percentage of oxygen consumed during sub-maximal exercise indicates that overweight adolescents are burdened by the metabolic cost of their excess mass. Their greater oxygen demand during an unloaded task predicted poorer performance during sustained exercise. Exercise prescriptions for overweight adolescents should account for the limited exercise tolerance imposed by excess body mass, focusing on activities that keep demands below lactate threshold so that exercise can be sustained.
ABBREVIATIONS: V̇o2 max, maximum oxygen uptake; SDS, SD score; ULV̇o2, unloaded oxygen uptake;; LTV̇o2, oxygen uptake at the lactate threshold; V̇o2 max, oxygen uptake at maximal exertion; HRR, heart rate reserve; RPE, rating of perceived exertion; bpm, beats per minute; D12, distance achieved at 12 minutes during walk/run test; ANCOVA, analysis of covariance
Overweight during childhood has been identified as a major health problem in the United States.1–4 Pediatric overweight commonly presages adult obesity5 and is associated with the development of weight-related comorbid conditions and increased morbidity.6–8
Decreased physical activity and a more sedentary lifestyle have been implicated as important factors in the development of pediatric obesity.9–11 Despite that aerobic exercise is widely used in the management of pediatric overweight, relatively few studies have examined the impact of excess adiposity on exercise fitness or functional performance in children and adolescents.12–17
Increased fat mass is associated with decreased exercise performance in overweight children and adolescents.12–17 However, the cause of this limited exercise tolerance remains in question. Several investigators, primarily those who have studied moderately overweight and obese children and adolescents, have reported similar maximal oxygen uptake (V̇o2 max) relative to body weight compared with normal-weight control subjects. These studies have concluded that exercise intolerance in overweight children and adolescents is attributable primarily to the increased metabolic demands of carrying an excess load, rather than a true decrease in cardiorespiratory fitness.15,18–20 However, some studies have reported that overweight individuals have significantly decreased maximal heart rate; heart rate reserve, which is defined as maximal heart rate minus resting heart rate15,16,21; and myocardial work efficiency.22 Whether these differences significantly influence fitness and performance is unclear. Obesity-related changes in exercise catecholamine response,15,16,21 myocardial metabolism, and left ventricular morphology22 may be associated with these findings.
We hypothesized that the primary factor limiting severely overweight adolescents when they undertake sustained exercise would be the increased metabolic cost resulting from having to move a greater mass and that there would also be a smaller but still important effect of diminished cardiorespiratory reserve. We predicted that differences in the energy costs of exercise in overweight and nonoverweight adolescents would be demonstrable most clearly during submaximal exercise tasks: compared with nonoverweight adolescents, overweight adolescents would use a larger percentage of their total cardiorespiratory reserve when performing a submaximal task such as “unloaded” pedaling on a stationary bicycle. We also hypothesized that the increased oxygen uptake during unloaded tasks would predict a poorer outcome on a functional performance test.
METHODS
Participants
We studied 129 severely overweight black and white adolescents who were recruited for a weight loss study23 and 34 healthy, age- and height-matched nonoverweight volunteer adolescents (30 with BMI <85th percentile and 4 with BMI between 85th and 94th percentile) who were recruited for participation in a voluntary study for healthy adolescents (Table 1). Overweight participants were in good general health but were required to have BMI ≥95th percentile for age, gender, and race24 and at least 1 obesity-related comorbid condition (primarily hyperinsulinemia and dyslipidemia). Overweight adolescents had ages of 14.5 ± 1.8 years (range: 12.1–17.8 years), BMIs of 41.5 ± 9.7 kg/m2 (range: 27.2–73.6 kg/m2), and BMI SD scores (SDSs) for age and gender25 of 5.3 ± 2.4 and were studied before they attempted weight loss. The nonoverweight adolescents (age: 14.5 ± 1.5 years; range: 12.0–18.1 years; BMI: 20.1 ± 2.9 kg/m2; BMI SDS: −0.1 ± 0.8) were required to have BMIs of <95th percentile for age and gender and consisted of adolescents without a history of high athletic achievement. For both groups, participants were excluded when they had used any anorexiants within the past 6 months; were pregnant; had major pulmonary, hepatic, or cardiac disorders; or had lost >3% of body weight over the past 2 months. All participants were recruited from the greater Washington, DC, metropolitan area by newspaper advertisements, by fliers posted in local commercial venues, and (in the case of overweight adolescents) through physician referrals.
TABLE 1.
Participant Characteristics
| Variable | Nonoverweight (n = 34)* |
Overweight (n = 129)* |
||
|---|---|---|---|---|
| Boys (n = 15) | Girls (n = 19) | Boys (n = 45) | Girls (n = 84) | |
| Age, y | 14.9 ± 2.0 | 14.3 ± 1.6 | 14.2 ± 1.4 | 14.6 ± 1.5 |
| Race, % | ||||
| Non-Hispanic black | 47 | 37 | 53 | 62 |
| Non-Hispanic white | 53 | 63 | 47 | 38 |
| Weight, kg | 59.1 ± 13.8 | 50.6 ± 9.6 | 118.4 ± 29.4† | 105.0 ± 23.0† |
| Height, cm | 1.69 ± 0.10 | 1.60 ± 0.06 | 1.66 ± 0.09 | 1.63 ± 0.06 |
| BMI, kg/m2 | 20.6 ± 3.4 | 19.7 ± 2.5 | 42.5 ± 8.8† | 39.3 ± 8.1† |
| BMI SDS | 0.31 ± 0.87 | ± 0.26 ± 0.40 | 6.99 ± 3.09† | 4.92 ± 2.36† |
| Fat-free mass, kg | 48.6 ± 11.2 | 38.8 ± 6.0 | 60.4 ± 15.7† | 54.9 ± 9.4† |
| Body fat, % | 17.3 ± 9.9 | 22.4 ± 6.3 | 49.4 ± 4.9† | 47.4 ± 5.9† |
| Breast stage | — | 4 (2–5) | — | 5 (3–5)‡ |
| Testicular volume, mL | 16.2 ± 6.4 | — | 11.1 ± 7.4‡ | — |
| Pubic hair stage | 4 (1–5) | 5 (3–5) | 4 (1–5) | 5 (2–5) |
Mean ± SD except for breast and pubic hair Tanner stage, for which median and range (in parentheses) are reported.
P < .001 versus nonoverweight of the same gender.
P < .05 versus nonoverweight of the same gender.
Each participant was seen twice at the National Institutes of Health Warren Grant Magnuson Clinical Center, once for a maximal cycle ergometry test and once on a separate day for a 12-minute walk/run test. Before exercise testing, each participant was evaluated with a medical history, physical examination, and 12-lead electrocardiogram. Height was recorded as the average of 3 measurements using a stadiometer (Holtain Ltd, Crymmyck, Wales) calibrated before each height to the nearest 1 mm. Weight was obtained using a calibrated digital scale to the nearest 0.1 kg. All participants were free of significant musculoskeletal injury as determined by a physician, and American Heart Association guidelines for exercise testing26 were observed. Participants’ parents provided signed consent statements (and adolescents gave their written assent) for all studies under a protocol approved by the institutional review board of the National Institute of Child Health and Human Development, National Institutes of Health.
Cycle Ergometry Testing Procedure
Cycle ergometry was performed as described previously.14 Briefly, before the test, each participant was familiarized with the cycle ergometer (Ergoline 800; SensorMedics, Yorba Linda, CA) and instructed to maintain pedaling cadence at 60 to 65 revolutions per minute. Cadence was monitored electronically and verified by a member of the research staff. Exercise began with a 4-minute “warm-up” with no additional resistance applied to the pedals (unloaded exercise), followed by continuously increasing work loads of 15 to 20 Watts/min until the participant could no longer continue or could no longer maintain the prescribed pedaling cadence. Participants were encouraged to exercise to the limit of their tolerance. Predicted maximal power was used to select the rate of workload increase for each participant.27 Expired gas exchange was measured breath by breath during exercise using a metabolic cart (Sensormedics Vmax, Yorba Linda, CA). Oxygen uptake during the 4-minute warm-up period of unloaded exercise (ULV̇o2) was calculated as the average oxygen uptake during the last minute of unloaded cycling. Lactate threshold estimated by gas exchange (LTV̇o2) was determined using the V-slope method.28 Maximal oxygen uptake during exercise and exercise-induced respiratory quotient were defined as the 20-second average of values achieved at the end of exercise.
Heart rate was measured by a 12-lead electrocardiogram before and during exercise, and the highest heart rate achieved during the last minute of exercise was defined as the maximal heart rate. Resting heart rate was obtained when the patient had been seated comfortably in a chair for at least 5 minutes before the onset of exercise. The heart rate reserve (HRR), a measure of the ability to increase cardiac function in response to increasing work loads, was measured by subtracting the resting heart rate from the maximal heart rate. Blood pressure was measured every 3 minutes during exercise. Peak exercise rating of perceived exertion (RPE) was measured within the first minute of exercise recovery using the 20-point Borg Rating of Perceived Exertion Scale.29 Participants who met at least 2 of the 4 following criteria during cycle ergometry were considered to have achieved a maximal V̇o2 test and reached their V̇o2 max: (1) maximal heart rate of ≥185 beats per minute (bpm), (2) respiratory quotient of ≥1.02, (3) RPE of 18–20, and (4) achievement of an oxygen plateau.30,31 Attainment of an oxygen plateau was defined as a ≤2.0 mL/kg per min change in oxygen uptake during the last minute of exercise.
Walk/Run Testing Procedure
A single 12-minute walk/run test was performed as previously described.14 Briefly, before exercise, resting blood pressure and heart rate were measured while standing. Participants then were instructed to walk and/or run to cover as much distance as possible in 12 minutes and were encouraged throughout to give their best effort. A hallway that was 47 m in length and 1.8 m in width was used as the course. Heart rate was measured and recorded at baseline (standing) and at 3, 6, 9, and 12 minutes during the walk/run test using a heart rate telemeter with a wristwatch receiver (Polar Vantage NV*; Lake Success, NY). The heart rate achieved at 12 minutes during the walk/run test was defined as the peak heart rate. Total distance (in meters) traveled at 12 minutes (D12) was measured using a measuring wheel (model MM34; Rolatape Corp, Spokane, WA). At the end of the test, each participant was asked to rate his or her effort throughout the entire test on a 0-to-10 scale, with 0 being defined as “no effort” and 10 defined as “greatest effort possible.”
Body Composition
Body composition was assessed after an overnight fast by air displacement plethysmography as described previously.32 Measurements were taken with all participants wearing minimal clothing (either underwear or a tight-fitting bathing suit) and a swim cap. Thoracic gas volume was measured during tidal breathing and during exhalation against a mechanical obstruction. Percentage of body fat was determined from body density using the standard 2-compartment model calculated from the Siri equation.32–34
Statistical Analysis
Data were analyzed on a Macintosh PowerPC using StatView 4.5 and SuperAnova 1.11 software (Abacus Concepts, Inc, Berkeley, CA). Simple and multiple linear regression analyses were used to determine the relationships between variables of interest for continuous variables. Analysis of covariance (ANCOVA) and t tests were used when appropriate for categorical variables. For multiple regression and ANCOVA analyses, pubertal stage was never a significant predictor once chronological age was included in the models. Contingency table analysis with continuity correction was used for categorical variables. Nonparametric Mann-Whitney tests were used for ordered, noncontinuous variables such as RPE or Tanner pubertal stage. P < .05 was used to define statistical significance after applying the Bonferroni-Hochberg35 approach for multiple comparisons. Analyses that excluded the 4 adolescents with BMIs in the 85th to 95th percentiles did not change the direction or significance of any of the relationships described.
RESULTS
Overweight and nonoverweight adolescents were not different in their age, race, or height but (by definition) had significant differences in weight, BMI, and percentage body fat (Table 1). Breast development was also somewhat more advanced in overweight girls (P = .031, χ2 test).
Cycle Ergometry Test
Data from unloaded cycling were obtained from all participants (Table 2). The average ULV̇o2 was significantly greater in overweight than nonover-weight adolescents (P < .0001; Table 2). Eighty-one percent of overweight and 97% of nonoverweight adolescents demonstrated an inflection point in the relationship between V̇co2 and V̇o2 allowing determination of LTV̇o2. Mean LTV̇o2 was not different in overweight and nonoverweight adolescents (P = .72). V̇o2 max analyses were restricted to the 74% of the overweight adolescents and 88% of the nonover-weight adolescents who reached at least 2 of the criteria used to determine the presence of maximal effort during the cycle ergometry test (Fig 1). Among those who achieved V̇o2 max, there was no difference in absolute V̇o2 max between overweight and nonoverweight adolescents (P = .18). However, an ANCOVA including gender, race, age, height, and lean body mass (or body fat mass) found that overweight adolescents had significantly lower adjusted (least squares) mean V̇o2 max (P = .0025). Similarly, a multiple regression analysis predicting V̇o2 max found significant independent contributions for gender (P < .001), race (P = .0063), height (P = .0016), age (P = .05), lean body mass (P = .007), and body fat mass (P = .021).
TABLE 2.
Results of Cycle Ergometry and Walk/Run Tests
| Variable | Nonoverweight (n = 34), Mean ± SD (Range) | Overweight (n = 129), Mean ± SD (Range) | P |
|---|---|---|---|
| Cycle test | |||
| ULV̇o2, mL/min | 396 ± 122 (242–753) | 696 ± 220 (386–1550) | <.0001 |
| LTV̇o2, mL/min | 1069 ± 357 (492–2108) | 1090 ± 272 (556–1816) | .72 |
| V̇o2 max, mL/min | 2067 ± 571 (1151–3383) | 1942 ± 398 (1238–3313) | .18 |
| Peak heart rate, bpm | 196 ± 11 (172–211) | 186 ± 13 (159–212) | <.0001 |
| RPE | 17 ± 2 (13–20) | 17 ± 2 (13–20) | .74 |
| Walk/run test | |||
| D12, m | 1983 ± 323 (1435–2644) | 1159 ± 194 (564–1601) | .0001 |
| Peak heart rate, bpm | 197 ± 17 (145–218) | 175 ± 18 (110–221) | <.0001 |
| RPE | 7 ± 2 (2–10) | 8 ± 1 (4–10) | .06 |
Shown are the results of cycle ergometry and walk/run tests. For the cycle test, oxygen uptake was measured during ULV̇o2, at LTV̇o2, and at V̇o2 max, and peak exercise RPE was measured by using a 20-point scale. For the 12-minute walk/run test, RPE was measured on a 10-point scale.
Fig 1.
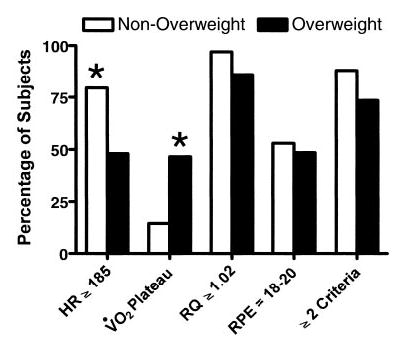
Attainment of criteria for maximal cycle ergometry test. See text for definitions of individual criteria. HR, heart rate; RQ respiratory quotient. Participants were determined to have achieved maximal exertion when ≥2 criteria for the test were met. *P ≤ .001, nonoverweight versus overweight adolescents.
ULV̇o2 accounted for 35 ± 8% of V̇o2 max in the overweight group but only 20 ± 5% of V̇o2 max in the nonoverweight group (P < .0001; Fig 2A). When analyzed as a percentage of the LTV̇o2, the ULV̇o2 accounted for 63 ± 15% of LTV̇o2 for the overweight adolescents but only 39 ± 12% of LTV̇o2 for the nonoverweight adolescents (P < .0001; Fig 2B).
Fig 2.
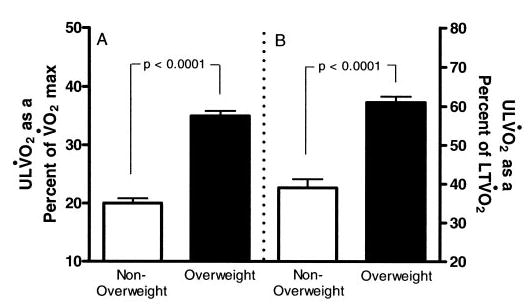
ULV̇o2 as a percentage of V̇o2 max (A) and LTV̇o2 (B) in nonoverweight and overweight adolescents.
BMI SDS was positively associated with ULV̇o2 (r = 0.76; P < .0001; Fig 3A). BMI SDS was also positively associated with V̇o2 max in an analysis that was restricted to overweight adolescents (r = 0.41; P < .0001), but BMI SDS was not significantly associated with V̇o2 max when examined in non-overweight adolescents (r = 0.27; P = .16). In univariate analyses, a continuous positive relationship existed between ULV̇o2 and both lean mass (r = 0.69; P < .0001; Fig 4A) and fat mass (r = 0.78; P < .0001; Fig 4B); however, V̇o2 max, a measure of fitness, was positively associated with lean mass (r = 0.44; P < .0001; Fig 4C) but not with fat mass (r = 0.09; P = .34; Fig 4D).
Fig 3.
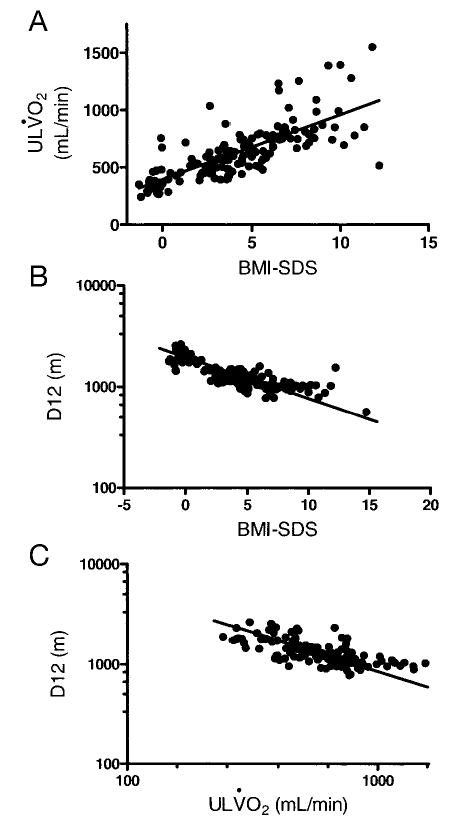
Univariate relationships of BMI SDS with ULV̇o2 (r = −0.81; P < .0001) (A), BMI SDS with D12 (r = 0.76, P < .0001) (B), and ULV̇o2 with D12 (r = −0.98; P < .0001) (C).
Fig 4.
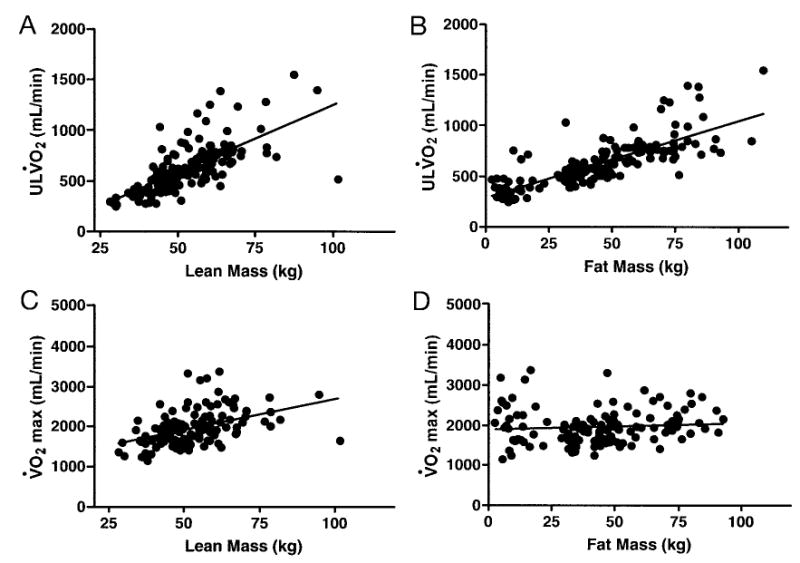
Univariate relationships between ULV̇o2 and lean mass (A; r = 0.69; P < .0001) and fat mass (B; r = 0.78; P < .0001). Univariate relationships between V̇o2 max and lean mass (C; r = 0.44; P < .0001) and fat mass (D; r = 0.09; P =.34).
Heart rate was measured both at rest and at the maximal point of exercise during the cycle test. Resting heart rate before initiating the cycle test was significantly greater in overweight than nonover-weight adolescents (94 ± 14 vs 82 ± 15 bpm; P =.0002; Fig 5A). However, maximal heart rate during the cycle test was significantly lower in overweight adolescents (P < .0001; Table 2, Fig 5A). The HRR was negatively related to BMI SDS (r = −0.53; P < .0001; Fig 5B). In a multivariate model that accounted for age, gender, race, height, lean mass, and fat mass, HRR was an independent predictor of V̇o2 max (P =.0028) and fat mass was no longer a significant predictor of V̇o2 max. The model that contained HRR increased the explained variance in V̇o2 max from 46% to 54% (P < .005).
Fig 5.
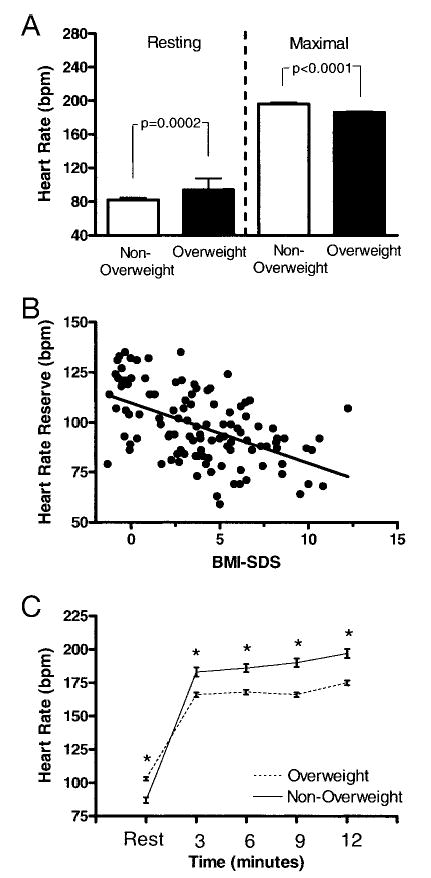
A, Resting and maximal HR during the cycle test. B, Univariate relationship between BMI SDS and cycle test HRR, defined as maximal minus resting HR. C, HR during the walk/run test. *P < .0001.
Walk/Run Test
For the walk/run test, the average distance traveled by the overweight adolescents was significantly less than that of the nonoverweight adolescents (P < .0001; Table 2). BMI SDS was negatively associated with the walk/run distance achieved (r = −0.81; P < .0001; Fig 3B). Although overweight adolescents started with a higher average resting heart rate (103 ± 15 vs 87 ± 12 bpm; P < .0001), nonoverweight adolescents achieved higher mean heart rates at 3, 6, 9, and 12 minutes than overweight adolescents (P < .0001; Fig 5C). Fat mass was the best predictor of D12 (r = −0.79; P < .0001), although HRR as measured during the cycle test was also found to be positively associated with D12 (r = 0.65; P < .0001). In a multivariate analysis that included age, gender, race, height, lean mass, and fat mass, HRR for the walk/run test was an independent predictor of D12. Inclusion of HRR in the model significantly increased the explained variance from 72% to 78% (P < .001)
Relationships Between Cycle Ergometry and Walk/Run Tests
The ULV̇o2 was significantly associated with performance during the walk/run test (r = −0.98; P < .0001; Fig 3C). Absolute V̇o2 max was not a strong univariate predictor of performance on the walk/run test (r = 0.19; P = .04). However, in a multivariate analysis that included age, gender, race, height, lean body mass, body fat mass, V̇o2 max, and HRR from the walk/run test, both V̇o2 max (P = .0003) and HRR (P = .0003) were significant predictors of D12.
DISCUSSION
We studied the fitness and performance of overweight and nonoverweight adolescents, finding that overweight and nonoverweight adolescents have similar absolute (mL O2/minute) cardiorespiratory fitness but that the functional impairment observed in overweight adolescents is significantly associated with the increased energy demands needed to move their excess body weight. The additional metabolic cost of excess adiposity in overweight adolescents was demonstrated most clearly during unloaded cycling. For overweight adolescents, simply moving their lower limbs induced significantly greater absolute oxygen uptake and led to consumption of a significantly larger proportion of their cardiorespiratory reserve. In addition, the greater metabolic cost of unloaded cycling was shown to predict a poorer performance during the functional performance evaluation walk/run test. Part of the metabolic cost of motion in significantly overweight adolescents likely is attributable to decreased mechanical efficiency.
Although several previous studies have found no significant differences in absolute V̇o2 max or LTV̇o2 in overweight and nonoverweight adolescents and adults,16,36,37 numerous different methods have been used to scale V̇o2 max to account for the larger size of overweight individuals. Weight in kilograms, fat mass, fat-free mass, BMI, and height all have been used as normalization measures.14,15,36,38 Age and pubertal stage, because of the muscle mass gained during puberty, are other factors that may contribute uniquely in adolescents. Depending on how the V̇o2 max is scaled, studies have shown that overweight individuals attain lower,14,36,37 higher,15,16 or similar39 oxygen uptake at maximal exercise, when compared with normal-weight control subjects. In this study, absolute values of oxygen uptake during maximal exercise were not different in nonoverweight and overweight adolescents. However, V̇o2 max was significantly decreased in the overweight group when lean body mass or fat mass was taken into account, which suggests the possibility of deconditioning and/or changes in cardiorespiratory function in severely overweight adolescents.
In the present study, maximal heart rate was reduced in overweight adolescents. Other reports have suggested that both deconditioning and obesity may reduce maximal heart rate.21,27,39,40,41 Because of their higher resting heart rate and lower maximal heart rate, overweight adolescents had a significantly lower cycle test HRR. HRR was an independent predictor of V̇o2 max in multiple regression models and was significantly related to fitness and performance in the walk/run test, again suggesting that cardiorespiratory function was affected in the overweight adolescents in the current study. One possible explanation for the difference in our findings compared with some previous pediatric studies that did not find evidence for altered cardiorespiratory function in overweight adolescents during exercise is the severity of the participants’ obesity in the present investigation. Another possible explanation is the presence of comorbidities such as hyperinsulinemia in study participants. Petersen et al22 found that changes in myocardial metabolism (increased fatty acid uptake and oxidation) were associated with insulin resistance in adult women and that myocardial work efficiency was related to BMI. Others have described higher resting heart rate but decreased maximal heart rate in overweight adults,38,40 which may be attributable to decreased maximal catecholamine responses.21,41 To our knowledge, this is the first report of a significant relationship between HRR and fitness and performance in overweight adolescents. Additional study, including detailed measurement of cardiac function, is needed to clarify whether cardiac impairment (or the initial stages of impairment) may exist in severely overweight adolescents.
The increased oxygen cost of activity in overweight individuals is known to affect performance. Maffeis et al17 studied energy expenditure of obese and nonobese adolescents during walking and running and found that obese adolescents had an increase in absolute energy expenditure at each speed measured. The authors concluded that rather than a metabolic defect, the greater rate of energy expenditure in children was attributable to excess load from increased body weight.17 Hulens et al36 found that during submaximal cycling, obese women had a larger absolute oxygen uptake, which accounted for a significantly greater proportion of their total oxygen reserve, than nonobese women. Here, we report that overweight and nonoverweight adolescents had similar oxygen uptake at their apparent lactate threshold and at the maximal point of exercise but that the oxygen uptake during unloaded cycling in the overweight group was significantly greater. To undertake the simple task of pedaling a cycle with no resistance, the overweight adolescents were expending a greater proportion of their maximal oxygen utilization than the nonoverweight adolescents. This increased energy expenditure may be even more significant during weight-bearing activity, such as walking or running.
A significant correlation was found between the ULV̇o2 and D12, suggesting that the greater energy cost to pedal an unloaded cycle translated to a deficiency in the functional performance walk/run test. One study of 6 trained subjects who were asked to walk on a treadmill with 25- and 40-kg backpacks found that heavier loads resulted in increased energy costs over time.19 Similarly, the exercise intolerance of overweight adolescents that we observed could be attributed to increased energy demands as a result of the extra load from excess adiposity. Our study demonstrated that the negative correlation between D12 and BMI was a continuous phenomenon: the greater the BMI, the more severe the functional impairment.
The applicability of the results of this study to the general population may be limited by the severe, symptomatic obesity of the participants, who likely displayed more significant physical limitations than might be observed had the study been restricted to moderately overweight adolescents. However, we believe that studies of severely overweight adolescents are particularly useful in describing the progressive relationship between overweight and exercise intolerance. Another limitation is that all participants recruited were black or white and between 12 and 17 years of age; therefore, these findings may not be generalizable to younger children or to adolescents of other races or ethnicities.
In summary, a large part of the exercise intolerance observed in overweight adolescents seems to be the related to the increased metabolic demands of moving excess mass. Cardiac constraints may also play a role and warrant closer, more detailed evaluation. The clinical implication of understanding how increased load from excess body mass affects exercise tolerance is important for exercise prescriptions for overweight adolescents. Exercise prescriptions may require careful planning so as to decrease overall body mass in addition to targeting cardiorespiratory conditioning. Although weight-bearing exercise should be used when possible, some severely overweight adolescents will require prescriptions for non–weight-bearing exercise to provide a sustainable exercise work intensity that is below the lactate threshold.
Acknowledgments
This study was supported by National Institute of Child Health and Human Development, National Institutes of Health grant ZO1-HD-00641 (to Dr Yanovski). Dr Yanovski and Mr Drinkard are commissioned officers in the US Public Health Service, Department of Health and Human Services.
Footnotes
No conflict of interest declared.
References
- 1.Freedman DS, Srinivasan SR, Valdez RA, Williamson DF, Berenson GS. Secular increases in relative weight and adiposity among children over two decades: the Bogalusa Heart Study. Pediatrics. 1997;99:420–426. doi: 10.1542/peds.99.3.420. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999–12000. JAMA. 2002;288:1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 3.Troiano RP, Flegal KM. Overweight children and adolescents: description, epidemiology, and demographics. Pediatrics. 1998;101(3 pt 2):497–504. [PubMed] [Google Scholar]
- 4.Must A, Strauss RS. Risks and consequences of childhood and adolescent obesity. Int J Obes Relat Metab Disord. 1999;23(suppl 2):S2–S11. doi: 10.1038/sj.ijo.0800852. [DOI] [PubMed] [Google Scholar]
- 5.Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997;337:869–873. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- 6.Must A, Jacques PF, Dallal GE, Bajema CJ, Dietz WH. Long-term morbidity and mortality of overweight adolescents. A follow-up of the Harvard Growth Study of 1922 to 1935. N Engl J Med. 1992;327:1350–1355. doi: 10.1056/NEJM199211053271904. [DOI] [PubMed] [Google Scholar]
- 7.Kuczmarski RJ, Flegal KM, Campbell SM, Johnson CL. Increasing prevalence of overweight among US adults. The National Health and Nutrition Examination Surveys, 1960 to 1991. JAMA. 1994;272:205–211. doi: 10.1001/jama.272.3.205. [DOI] [PubMed] [Google Scholar]
- 8.Must A. Morbidity and mortality associated with elevated body weight in children and adolescents. Am J Clin Nutr. 1996;63(suppl):445S–447S. doi: 10.1093/ajcn/63.3.445. [DOI] [PubMed] [Google Scholar]
- 9.Schlicker SA, Borra ST, Regan C. The weight and fitness status of United States children. Nutr Rev. 1994;52:11–17. doi: 10.1111/j.1753-4887.1994.tb01348.x. [DOI] [PubMed] [Google Scholar]
- 10.Gortmaker SL, Must A, Sobol AM, Peterson K, Colditz GA, Dietz WH. Television viewing as a cause of increasing obesity among children in the United States, 1986–11990. Arch Pediatr Adolesc Med. 1996;150:356–362. doi: 10.1001/archpedi.1996.02170290022003. [DOI] [PubMed] [Google Scholar]
- 11.Goran MI. Measurement issues related to studies of childhood obesity: assessment of body composition, body fat distribution, physical activity, and food intake. Pediatrics. 1998;101:505–518. [PubMed] [Google Scholar]
- 12.Epstein LH, Coleman KJ, Myers MD. Exercise in treating obesity in children and adolescents. Med Sci Sports Exerc. 1996;28:428–435. doi: 10.1097/00005768-199604000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Epstein LH, Goldfield GS. Physical activity in the treatment of childhood overweight and obesity: current evidence and research issues. Med Sci Sports Exerc. 1999;31(suppl):S553–S559. doi: 10.1097/00005768-199911001-00011. [DOI] [PubMed] [Google Scholar]
- 14.Drinkard B, McDuffie J, McCann S, Uwaifo GI, Nicholson J, Yanovski JA. Relationships between walk/run performance and cardiorespiratory fitness in adolescents who are overweight. Phys Ther. 2001;81:1889–1896. [PubMed] [Google Scholar]
- 15.Goran M, Fields DA, Hunter GR, Herd SL, Weinsier RL. Total body fat does not influence maximal aerobic capacity. Int J Obes Relat Metab Disord. 2000;24:841–848. doi: 10.1038/sj.ijo.0801241. [DOI] [PubMed] [Google Scholar]
- 16.Salvadori A, Fanari P, Fontana M, et al. Oxygen uptake and cardiac performance in obese and normal subjects during exercise. Respiration. 1999;66:25–33. doi: 10.1159/000029333. [DOI] [PubMed] [Google Scholar]
- 17.Maffeis C, Schutz Y, Schena F, Zaffanello M, Pinelli L. Energy expenditure during walking and running in obese and nonobese prepubertal children. J Pediatr. 1993;123:193–199. doi: 10.1016/s0022-3476(05)81688-9. [DOI] [PubMed] [Google Scholar]
- 18.Maffeis C, Schena F, Zaffanello M, Zoccante L, Schutz Y, Pinelli L. Maximal aerobic power during running and cycling in obese and non-obese children. Acta Paediatr. 1994;83:113–116. doi: 10.1111/j.1651-2227.1994.tb12965.x. [DOI] [PubMed] [Google Scholar]
- 19.Epstein Y, Rosenblum J, Burstein R, Sawka MN. External load can alter the energy cost of prolonged exercise. Eur J Appl Physiol. 1988;57:243–247. doi: 10.1007/BF00640670. [DOI] [PubMed] [Google Scholar]
- 20.Rowland TW. Effects of obesity on aerobic fitness in adolescent females. Am J Dis Child. 1991;145:764–768. [PubMed] [Google Scholar]
- 21.Gustafson AB, Farrell PA, Kalkhoff RK. Impaired plasma catecholamine response to submaximal treadmill exercise in obese women. Metabolism. 1990;39:410–417. doi: 10.1016/0026-0495(90)90257-d. [DOI] [PubMed] [Google Scholar]
- 22.Peterson LR, Herrero P, Schechtman KB, et al. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation. 2004;109:2191–2196. doi: 10.1161/01.CIR.0000127959.28627.F8. [DOI] [PubMed] [Google Scholar]
- 23.McDuffie JR, Calis KA, Uwaifo GI, et al. Three-month tolerability of orlistat in adolescents with obesity-related comorbid conditions. Obes Res. 2002;10:642–650. doi: 10.1038/oby.2002.87. [DOI] [PubMed] [Google Scholar]
- 24.Must A, Dallal GE, Dietz WH. Reference data for obesity: 85th and 95th percentiles of body mass index (wt/ht2) and triceps skinfold thickness. Am J Clin Nutr. 1991;53:839–846. doi: 10.1093/ajcn/53.4.839. [published correction appears in Am J Clin Nutr. 1991;54:773] [DOI] [PubMed] [Google Scholar]
- 25.Frisancho AR. Anthropometric Standards for the Assessment of Growth and Nutritional Status 4th ed. Ann Arbor, MI: University of Michigan Press; 1999
- 26.Washington RL, Bricker JT, Alpert BS, et al. Guidelines for exercise testing in the pediatric age group. From the Committee on Atherosclerosis and Hypertension in Children, Council on Cardiovascular Disease in the Young, the American Heart Association. Circulation. 1994;90:2166–2179. doi: 10.1161/01.cir.90.4.2166. [DOI] [PubMed] [Google Scholar]
- 27.Wasserman K, Hansen JE, Sue DY, Casaburi R, Whipp BJ. Principles of Exercise Testing and Interpretation 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1999
- 28.Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol. 1986;60:2020–2027. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- 29.Chen MJ, Fan X, Moe ST. Criterion-related validity of the Borg ratings of perceived exertion scale in healthy individuals: a meta-analysis. J Sports Sci. 2002;20:873–899. doi: 10.1080/026404102320761787. [DOI] [PubMed] [Google Scholar]
- 30.Rowland TW. Developmental Exercise Physiology Champaign, IL: Human Kinetics; 1996
- 31.Donnelly JE, Jakicic J, Roscoe M, Jacobsen DJ, Israel RG. Criteria to verify attainment of maximal exercise tolerance test with obese females. Diabetes Res Clin Pract. 1990;10(suppl 1):S283–S286. doi: 10.1016/0168-8227(90)90177-u. [DOI] [PubMed] [Google Scholar]
- 32.Nicholson JC, McDuffie JR, Bonat SH, et al. Estimation of body fatness by air displacement plethysmography in African American and white children. Pediatr Res. 2001;50:467–473. doi: 10.1203/00006450-200110000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Siri W. Body composition from fluid spaces and density: analysis of methods. In: Brozek J, Honschel A, eds. Techniques for Measuring Body Composition Washington, DC: National Academy of Sciences/National Research Council; 1961:223–224
- 34.Fields DA, Goran MI, McCrory MA. Body-composition assessment via air-displacement plethysmography in adults and children: a review. Am J Clin Nutr. 2002;75:453–467. doi: 10.1093/ajcn/75.3.453. [DOI] [PubMed] [Google Scholar]
- 35.Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75:800–803. [Google Scholar]
- 36.Babb TG, Korzick D, Meador M, Hodgson JL, Buskirk ER. Ventilatory response of moderately obese women to submaximal exercise. Int J Obes. 1991;15:59–65. [PubMed] [Google Scholar]
- 37.Hulens M, Vansant G, Lysens R, Claessens AL, Muls E. Exercise capacity in lean versus obese women. Scand J Med Sci Sports. 2001;11:305–309. doi: 10.1034/j.1600-0838.2001.110509.x. [DOI] [PubMed] [Google Scholar]
- 38.Ozcelik O, Aslan M, Ayar A, Kelestimur H. Effects of body mass index on maximal work production capacity and aerobic fitness during incremental exercise. Physiol Res. 2004;53:165–170. [PubMed] [Google Scholar]
- 39.Salvadori A, Fanari P, Mazza P, Agosti R, Longhini E. Work capacity and cardiopulmonary adaptation of the obese subject during exercise testing. Chest. 1992;101:674–679. doi: 10.1378/chest.101.3.674. [DOI] [PubMed] [Google Scholar]
- 40.O’Connor F, Fleg JL, Gerstenblith G, et al. Effect of body fat on exercise hemodynamics in sedentary older men. Aging (Milano) 1994;6:257–265. doi: 10.1007/BF03324251. [DOI] [PubMed] [Google Scholar]
- 41.Salvadori A, Fanari P, Giacomotti E, et al. Kinetics of catecholamines and potassium, and heart rate during exercise testing in obese subjects. Heart rate regulation in obesity during exercise. Eur J Nutr. 2003;42:181–187. doi: 10.1007/s00394-003-0409-3. [DOI] [PubMed] [Google Scholar]


