Abstract
1. Intracellular recordings of membrane potentials have been made in vitro from the exocrine acinar cells of the mouse pancreas using glass micro-electrodes.
2. The mean membrane potential of the acinar cells during superfusion with Krebs-Henseleit solution was -39·2 mV. Increasing [K]o tenfold decreased the membrane potential by 28 mV when [K]o was above 10 mM. This depolarization was not affected by atropine (1·4 × 10-6 M). Strophanthin-G (10-3 M) slowly depolarized the cells at about 10 mV hr-1.
3. Brief exposure to acetylcholine (ACh), 5·5 × 10-5 M, or pancreozymin resulted in a short lasting depolarization of the acinar cells. Atropine (1·4 × 10-6 M) blocked the depolarizing action of ACh but not that of pancreozymin. Adrenaline (5·5 × 10-5 M) or cyclic AMP (10-3-10-4 M) did not influence the membrane potential.
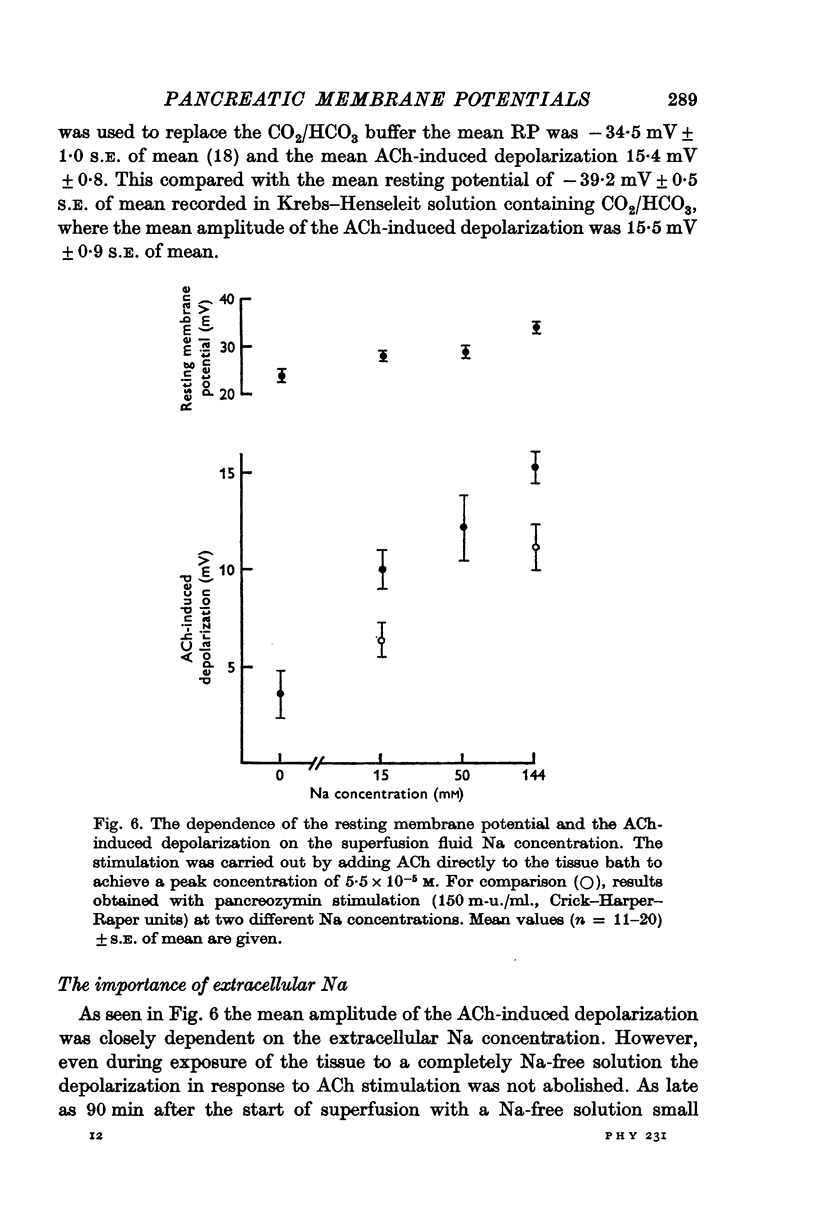
4. The amplitude of the ACh-induced depolarization was not dependent on the presence of CO2/HCO3 in the bathing fluid, but it was closely dependent on the extracellular Na concentration. However, ACh was still able to evoke a small depolarization even after prolonged exposure of the tissue to a Na-free solution.
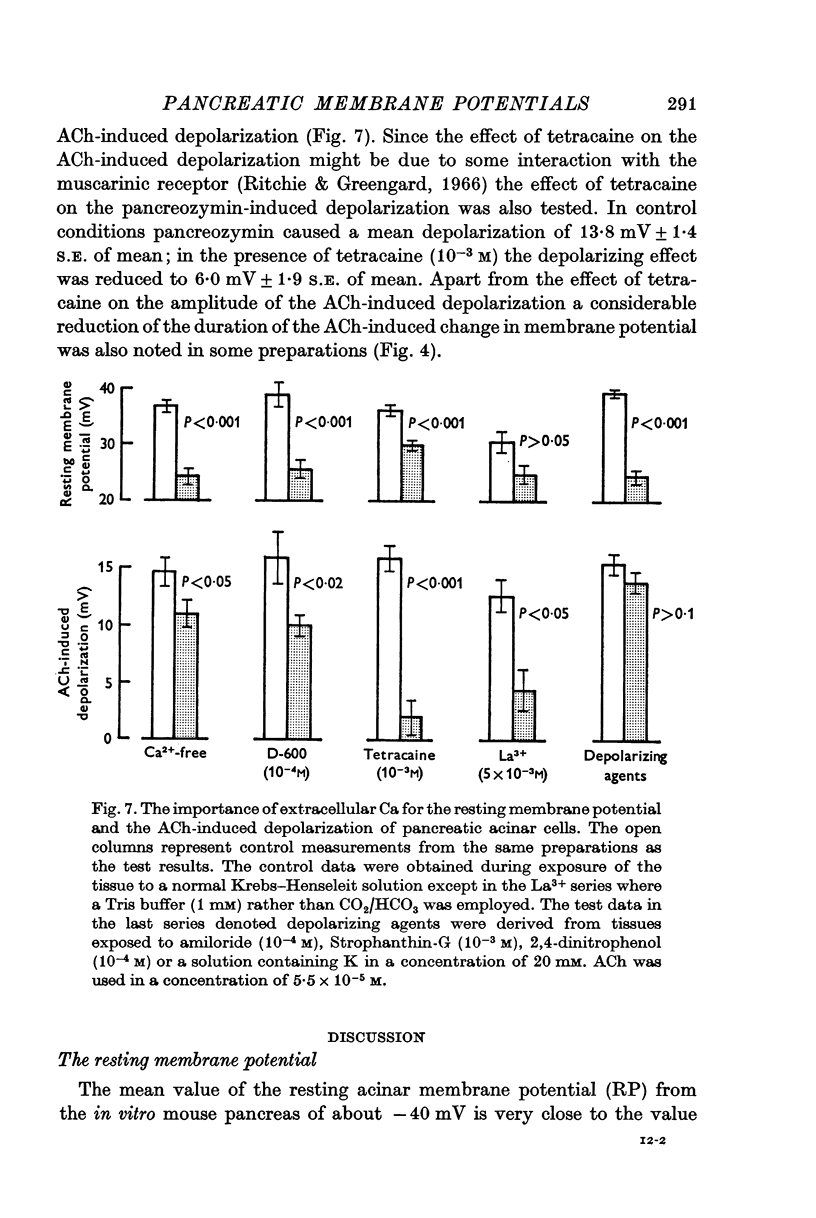
5. During exposure of the tissue to a Ca-free solution the resting membrane potential was decreased and the ACh-induced depolarization was significantly reduced. Some substances which are known in other tissues to inhibit membrane Ca2+ currents, i.e. La3+, D-600 and tetracaine, were able to reduce, but never abolish, the ACh-induced depolarization.
6. These results suggest that the effect of ACh on the pancreatic acinar cell is to increase the permeability of the membrane to commonly occurring ions with a consequent Na-influx and a small Ca-influx.
Full text
PDF

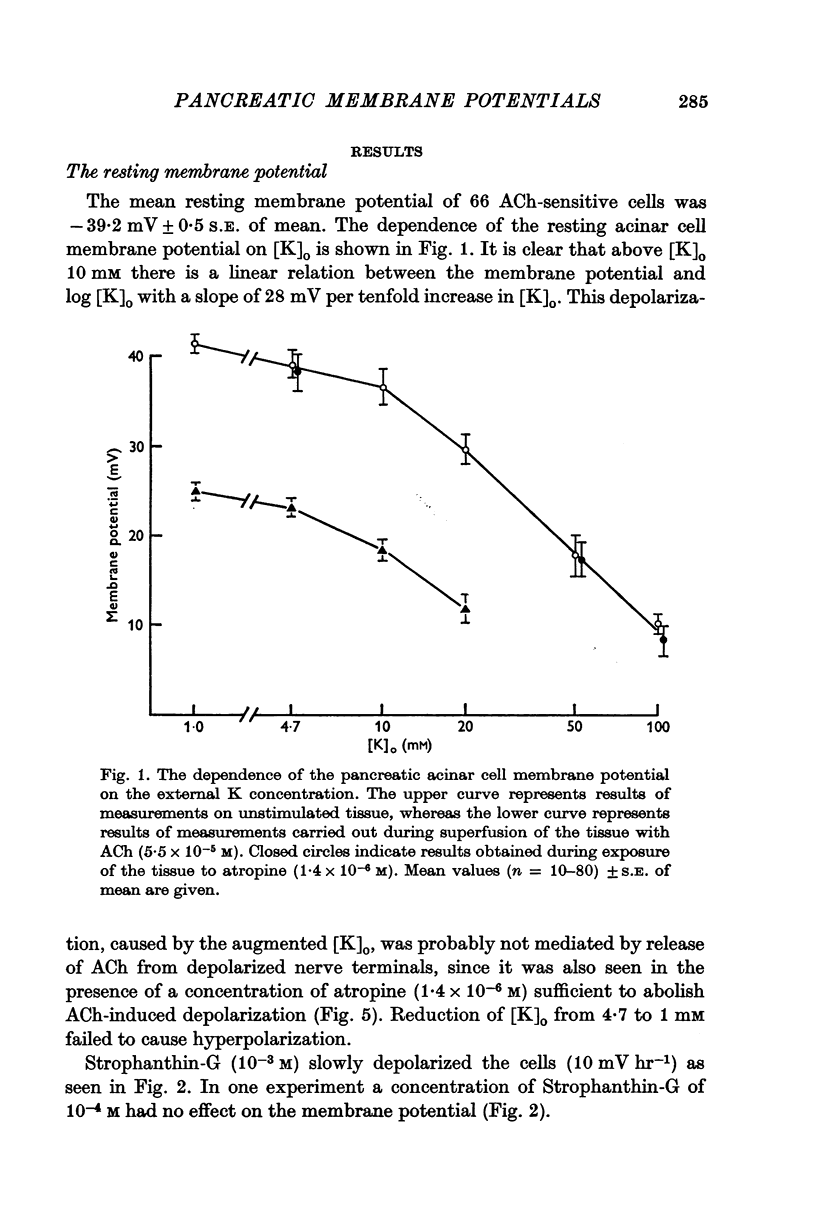
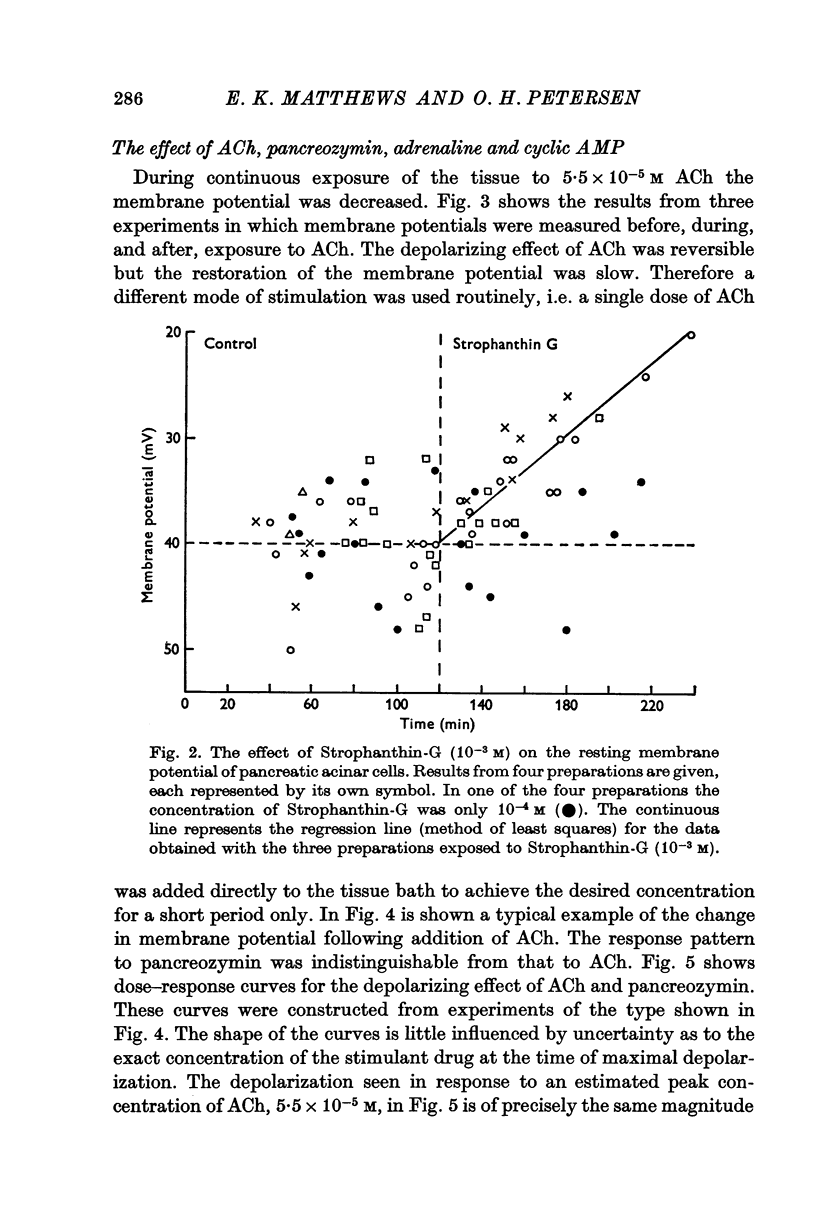
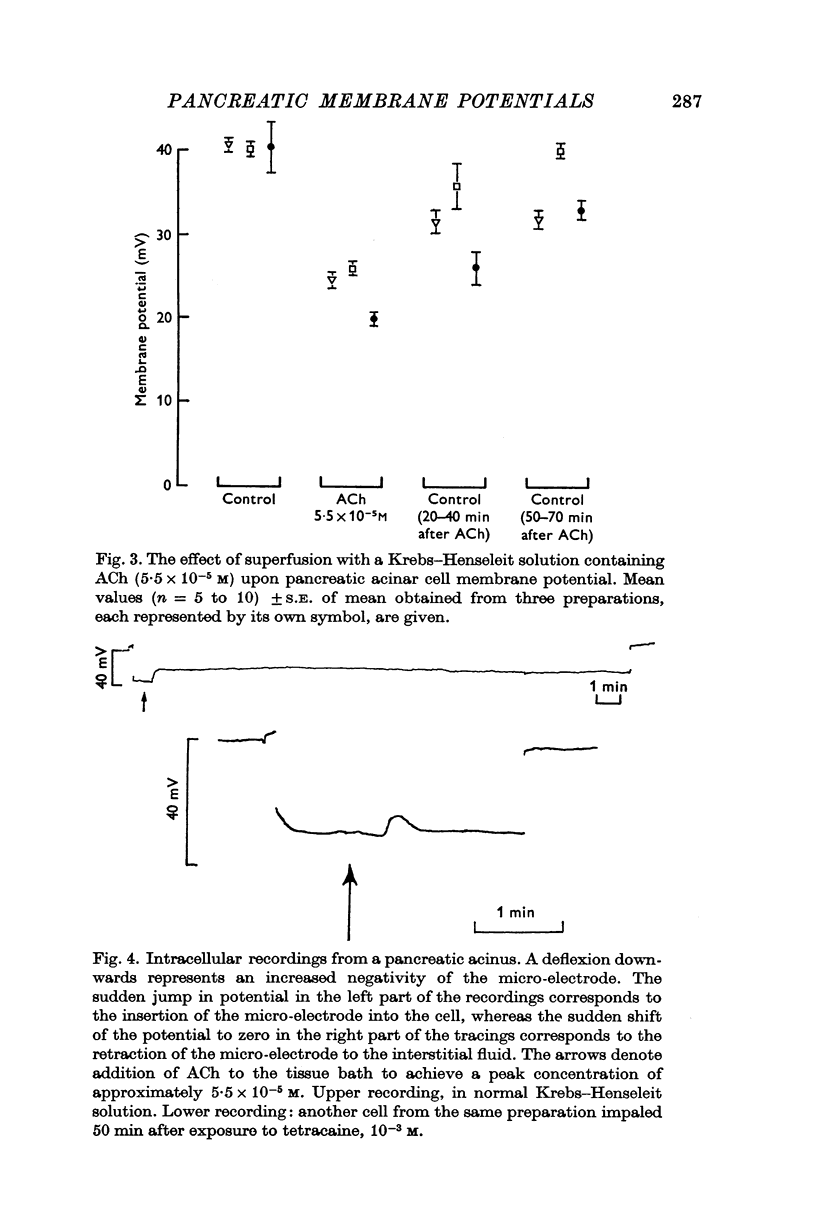
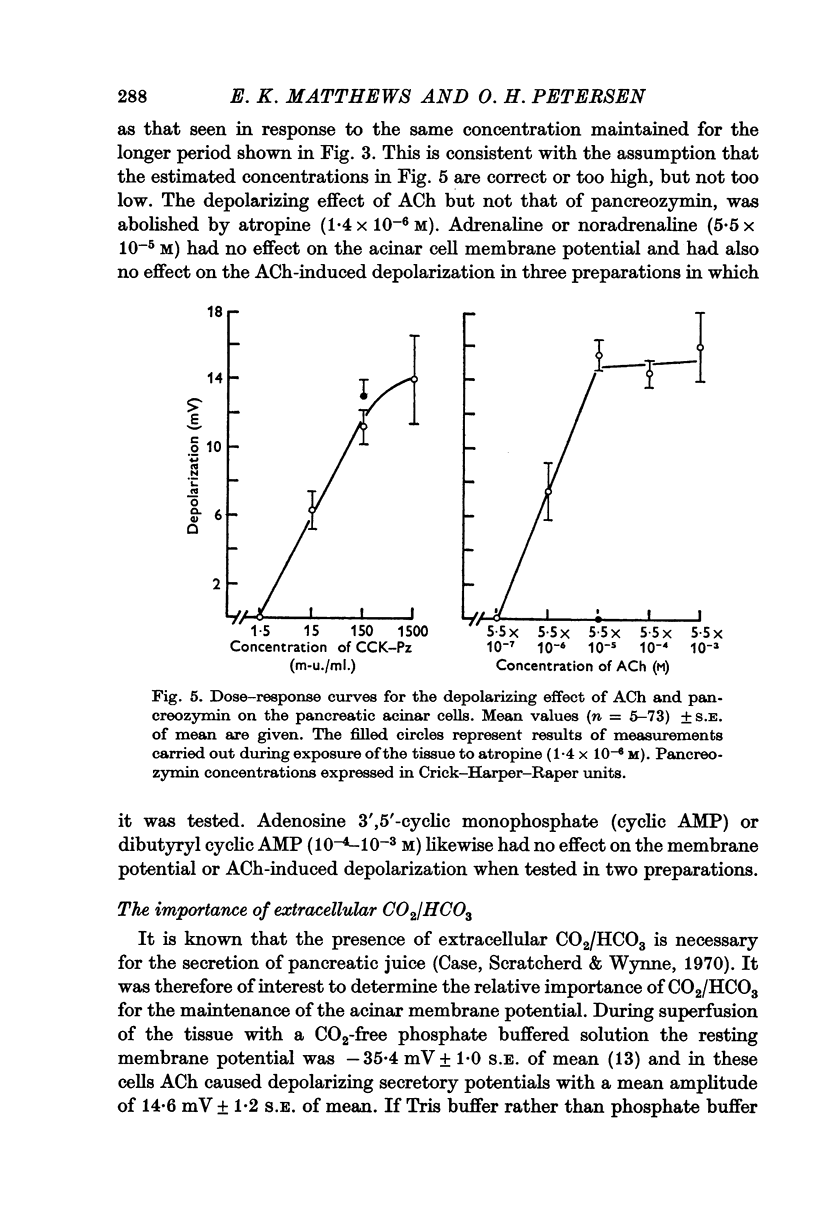





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benz L., Eckstein B., Matthews E. K., Williams J. A. Control of pancreatic amylase release in vitro: effects of ions, cyclic AMP, and colchicine. Br J Pharmacol. 1972 Sep;46(1):66–67. doi: 10.1111/j.1476-5381.1972.tb06849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R. M., Scratcherd T. The actions of dibutyryl cyclic adenosine 3',5'-monophosphate and methyl xanthines on pancreatic exocrine secretion. J Physiol. 1972 Jun;223(3):649–667. doi: 10.1113/jphysiol.1972.sp009867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R. M., Scratcherd T., Wynne R. D. The origin and secretion of pancreatic juice bicarbonate. J Physiol. 1970 Sep;210(1):1–15. doi: 10.1113/jphysiol.1970.sp009193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean P. M., Matthews E. K. Electrical activity in pancreatic islet cells: effect of ions. J Physiol. 1970 Sep;210(2):265–275. doi: 10.1113/jphysiol.1970.sp009208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean P. M., Matthews E. K. Pancreatic acinar cells: measurement of membrane potential and miniature depolarization potentials. J Physiol. 1972 Aug;225(1):1–13. doi: 10.1113/jphysiol.1972.sp009926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Kanno T., Sampson S. R. Influence of the ionic environment on the membrane potential of adrenal chromaffin cells and on the depolarizing effect of acetylcholine. J Physiol. 1967 Jul;191(1):107–121. doi: 10.1113/jphysiol.1967.sp008239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Kanno T. The effect of amethocaine on acetylcholine-induced depolarization and catecholamine secretion in the adrenal chromaffin cell. Br J Pharmacol Chemother. 1967 Aug;30(3):612–619. doi: 10.1111/j.1476-5381.1967.tb02167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. The effect of sodium ions on neuromuscular transmission. J Physiol. 1952 Sep;118(1):73–87. doi: 10.1113/jphysiol.1952.sp004773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlhardt M., Bauer B., Krause H., Fleckenstein A. New selective inhibitors of the transmembrane Ca conductivity in mammalian myocardial fibres. Studies with the voltage clamp technique. Experientia. 1972 Mar 15;28(3):288–289. doi: 10.1007/BF01928693. [DOI] [PubMed] [Google Scholar]
- MORRILL G. A., KABACK H. R., ROBBINS E. EFFECT OF CALCIUM ON INTRACELLULAR SODIUM AND POTASSIUM CONCENTRATIONS IN PLANT AND ANIMAL CELLS. Nature. 1964 Nov 14;204:641–642. doi: 10.1038/204641a0. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Matthews E. K. The effect of pancreozymin and acetylcholine on the membrane potential of the pancreatic acinar cells. Experientia. 1972 Sep 15;28(9):1037–1038. doi: 10.1007/BF01918657. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Poulsen J. H., Thorn N. A. Secretory potentials, secretory rate and water permeability of the duct system in the cat submandibular gland during perfusion with calcium-free Locke's solution. Acta Physiol Scand. 1967 Oct-Nov;71(2):203–210. doi: 10.1111/j.1748-1716.1967.tb03726.x. [DOI] [PubMed] [Google Scholar]
- Petersen O. H. Some factors influencing stimulation-induced release of potassium from the cat submandibular gland to fluid perfused through the gland. J Physiol. 1970 Jun;208(2):431–447. doi: 10.1113/jphysiol.1970.sp009129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O. H. The dependence of the transmembrane salivary secretory potential on the external potassium and sodium concentration. J Physiol. 1970 Sep;210(1):205–215. doi: 10.1113/jphysiol.1970.sp009204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderstap A. S., Bonting S. L. Cyclic AMP and enzyme secretion by the isolated rabbit pancreas. Pflugers Arch. 1969;313(1):62–70. doi: 10.1007/BF00586329. [DOI] [PubMed] [Google Scholar]
- Ritchie J. M., Greengard P. On the mode of action of local anesthetics. Annu Rev Pharmacol. 1966;6:405–430. doi: 10.1146/annurev.pa.06.040166.002201. [DOI] [PubMed] [Google Scholar]
- SCHNEYER L. H., SCHNEYER C. A. Electrolyte and inulin spaces of rat salivary glands and pancreas. Am J Physiol. 1960 Oct;199:649–652. doi: 10.1152/ajplegacy.1960.199.4.649. [DOI] [PubMed] [Google Scholar]
- Schulz I., Yamagata A., Weske M. Micropuncture studies on the pancreas of the rabbit. Pflugers Arch. 1969;308(3):277–290. doi: 10.1007/BF00586559. [DOI] [PubMed] [Google Scholar]
- Williams J. A. Origin of transmembrane potentials in non-excitable cells. J Theor Biol. 1970 Aug;28(2):287–296. doi: 10.1016/0022-5193(70)90056-1. [DOI] [PubMed] [Google Scholar]
- van Breemen C., De Weer P. Lanthanum inhibition of 45Ca efflux from the squid giant axon. Nature. 1970 May 23;226(5247):760–761. doi: 10.1038/226760a0. [DOI] [PubMed] [Google Scholar]


