Abstract
Acetone carboxylase is the key enzyme of bacterial acetone metabolism, catalyzing the condensation of acetone and CO2 to form acetoacetate. In this study, the acetone carboxylase of the purple nonsulfur photosynthetic bacterium Rhodobacter capsulatus was purified to homogeneity and compared to that of Xanthobacter autotrophicus strain Py2, the only other organism from which an acetone carboxylase has been purified. The biochemical properties of the enzymes were virtually indistinguishable, with identical subunit compositions (α2β2γ2 multimers of 85-, 78-, and 20-kDa subunits), reaction stoichiometries (CH3COCH3 + CO2 + ATP→CH3COCH2COO− + H+ + AMP + 2Pi), and kinetic properties (Km for acetone, 8 μM; kcat = 45 min−1). Both enzymes were expressed to high levels (17 to 25% of soluble protein) in cells grown with acetone as the carbon source but were not present at detectable levels in cells grown with other carbon sources. The genes encoding the acetone carboxylase subunits were identified by transposon mutagenesis of X. autotrophicus and sequence analysis of the R. capsulatus genome and were found to be clustered in similar operons consisting of the genes acxA (β subunit), acxB (α subunit), and acxC (γ subunit). Transposon mutagenesis of X. autotrophicus revealed a requirement of σ54 and a σ54-dependent transcriptional activator (AcxR) for acetone-dependent growth and acetone carboxylase gene expression. A potential σ54-dependent promoter 122 bp upstream of X. autotrophicus acxABC was identified. An AcxR gene homolog was identified 127 bp upstream of acxA in R. capsulatus, but this activator lacked key features of σ54-dependent activators, and the associated acxABC lacked an apparent σ54-dependent promoter, suggesting that σ54 is not required for expression of acxABC in R. capsulatus. These studies reveal a conserved strategy of ATP-dependent acetone carboxylation and the involvement of transcriptional enhancers in acetone carboxylase gene expression in gram-negative acetone-utilizing bacteria.
In addition to its importance as an industrial solvent, acetone is a major fermentation product of certain anaerobic bacteria (19, 51), an intermediate in the microbial metabolism of propane and isopropanol (7, 32, 49), and one of the ketone bodies produced under ketogenic conditions (i.e., fasting or diabetes) in mammals. Acetone is known to undergo metabolic transformations in mammals, where the physiological importance is not fully understood (4, 25), and in diverse microbes which are capable of growth using acetone as the primary source of carbon and energy (20). The mammalian metabolism of acetone is believed to be mediated largely by cytochrome P450 isozyme 2E1, sequentially producing acetol and methylglyoxal as gluconeogenic intermediates (8, 11, 28). The carbon atoms originating from acetone are incorporated into glucose in starved mice, suggesting that acetone may be an intermediate in the only mammalian pathway allowing net synthesis of glucose from fatty acids (4, 25, 26, 30).
Two distinct transformations have been proposed as the first step of acetone metabolism in microorganisms: an O2-dependent hydroxylation reaction producing acetol, a reaction analogous to that proposed for cytochrome P450 2E1 in mammals (32, 47, 49), and a CO2-dependent carboxylation to produce acetoacetate (9, 14, 22, 24, 38, 39, 41, 43). While in vivo and in vitro studies have provided evidence supporting the proposed pathways (20), attempts to characterize acetone-degrading enzymes at the molecular level have met with limited success. To date, the only microbial acetone-utilizing enzyme that has been purified to homogeneity in an active state is the acetone carboxylase of Xanthobacter autotrophicus strain Py2 (formerly Xanthobacter sp. strain Py2), a gram-negative aerobic bacterium (42). This enzyme is inducible and is expressed at high levels (∼25% of soluble protein) in cells grown with acetone or isopropanol as the sole added source of carbon (42, 43). The enzyme is a multimeric (α2β2γ2) protein that catalyzes the MgATP-dependent carboxylation of acetone according to the reaction CH3COCH3 + CO2 + ATP→CH3COCH2COO− + H+ + AMP + 2Pi. As the stoichiometry of the reaction shows, acetone carboxylation requires the hydrolysis of both the γ and β phosphodiester bonds of ATP. At present, the mechanism of catalysis of this novel enzyme, including the central role of phosphodiester bond hydrolysis in the process, remains unclear. In addition, it is not clear whether other uncharacterized bacterial acetone carboxylases function by a similar or distinct mechanism of action. To shed light on these important questions, we set out to identify the genes encoding the subunits of the acetone carboxylase from X. autotrophicus via a genetic approach and to purify and characterize an acetone carboxylase from a different bacterium. For these comparative studies, we selected Rhodobacter capsulatus, a purple nonsulfur photosynthetic bacterium capable of growth with acetone under anaerobic photoheterotrophic conditions (33). Low levels of acetone carboxylase activity have previously been demonstrated in cell extracts of this bacterium, although further attempts to purify the enzyme in an active state were unsuccessful (6).
In the present work the conditions for the purification of acetone carboxylase from R. capsulatus in a fully active state are reported. By genome screening and molecular genetics, the genes encoding the acetone carboxylase subunits and associated transcriptional activators in both R. capsulatus and X. autotrophicus were identified. The combined biochemical and genetic characterization of these two systems reveals a highly conserved strategy of bacterial acetone carboxylation. In addition, the unique genetic footprint of the acetone carboxylase systems is used to identify a similar system in ulcer-causing bacterium Helicobacter pylori, an organism not previously studied with regard to acetone metabolism.
MATERIALS AND METHODS
16S rRNA analysis.
Full-length 16S rRNA gene sequence analysis of X. autotrophicus strain Py2 was performed by MIDI Labs (Newark, Del.). Sequence alignment of the 1,480-bp gene provided the closest match to X. autotrophicus (0.44% genetic difference). This value lies within the confidence range for making a species level match for the strain.
Growth of bacteria and preparation of cell extracts.
R. capsulatus strain B10 (ATCC 33303) was grown photoheterotrophically at 30°C in a 45-liter-capacity glass vessel with mineral salts medium (46) containing 50 mM acetone as the carbon source. Illumination was provided by two 600-W tungsten-halogen lamps contained within a water-jacketed tube submersed in the fermentor vessel. The cultures were harvested after reaching an A600 between 2.5 and 4.0 by tangential-flow filtration with a Pellicon system (Millipore Corp.) and stored at −80°C. Frozen cell paste (121 g) was resuspended in an equal volume of buffer A (25 mM MOPS [morpholinepropanesulfonic acid; pH 7.6], 1 mM dithiothreitol) with 0.2 mg of lysozyme/ml and DNase I. The cell suspension was passed twice through a French pressure cell at 110,000 kPa and 4°C. The lysate was clarified by centrifugation (105,000 × g for 1 h at 4°C).
Purification of acetone carboxylase from R. capsulatus.
Purification procedures were performed at 4°C using buffer B (25 mM MOPS [pH 7.6], 1 mM dithiothreitol, 5% glycerol) unless otherwise noted. The supernatant of the cell extract was diluted with 1.5 volumes of buffer B and applied to a 5.0- by 13.2-cm column of DEAE-Sepharose equilibrated with buffer B at a linear flow rate of 24 cm/h. The column was washed with 390 ml of buffer B, followed by 650 ml of buffer B containing 100 mM KCl. Bound protein was fractionated with a 1,360-ml linear gradient of from 100 to 200 mM KCl. The fractions containing acetone carboxylase activity eluted at an average KCl concentration of 160 mM. These were pooled and concentrated by ultrafiltration using a YM100 membrane (Amicon). The concentrated sample was applied in three separate portions to a 2.6- by 95-cm Sephacryl S-300 gel filtration column equilibrated in buffer B containing 150 mM KCl at a linear flow rate of 11.3 cm/h. The active fractions were pooled, diluted 10-fold with 10 mM MOPS (pH 7.2)-5% glycerol-1 mM dithiothreitol, and applied to a 2.5- by 16-cm column of Macro-Prep ceramic hydroxyapatite (Bio-Rad) at a linear flow rate of 73 cm/h. The column was washed with 114 ml of the same buffer, and acetone carboxylase was eluted with a 1,370-ml linear gradient from 0 to 100 mM potassium phosphate. The purified acetone carboxylase was concentrated to 15.2 mg/ml and dialyzed against buffer B.
Enzyme assays.
Fixed-time point and continuous coupled spectrophotometric assays of acetone carboxylase activity were performed as described previously (42) except that the concentration of potassium acetate was 40 mM rather than 80 mM. Acetone, acetoacetate, inorganic phosphate, pyrophosphate, AMP, and ADP were quantified by analytical procedures described previously (42). Other ketones were evaluated as possible substrates for acetone carboxylase at concentrations of 3 mM. After a 60-min incubation period, the substrate remaining and product(s) formed were assayed by using the same procedures described for acetone.
Protein characterization.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (12% total gel; 2.7% cross-linker running gel) was performed in accordance with the Laemmli procedure. Electrophoresed proteins were visualized by staining with Coomassie blue. The apparent molecular masses of polypeptides based on SDS-PAGE migration were determined by comparison with Rf values of molecular weight standard proteins. The standards used were myosin (200 kDa), β-galactosidase (116.2 kDa), phosphorylase b (97.4 kDa), bovine serum albumin (66.2 kDa), ovalbumin (45 kDa), carbonic anhydrase (31 kDa), soybean trypsin inhibitor (21.5 kDa), lysozyme (14.4 kDa), and aprotinin (6.5 kDa). Polypeptide molecular weights were also determined by matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry (MS) performed by the Medical College of Wisconsin's Protein/Nucleic Acid Shared Facility in linear mode with an acceleration voltage of 25 kV. The relative subunit proportions were determined by integrated scanning densitometry of SDS-PAGE gels with an Alpha Imager gel scanner and software. The native molecular weight was estimated by size exclusion chromatography using a 1.6- by 53.5-cm Sephacryl S-300 column, which was developed at 12 cm/h with 25 mM MOPS (pH 7.6) containing 0.2 M KCl. The column was calibrated with apoferritin (443 kDa), β-amylase (200 kDa), alcohol dehydrogenase (150 kDa), and bovine serum albumin (66 kDa). The acetone carboxylase from X. autotrophicus Py2 was purified as described previously (42). The three subunits of the acetone carboxylases from R. capsulatus and X. autotrophicus Py2 were separated for N-terminal sequencing by SDS-PAGE. The polypeptides were electroblotted onto polyvinylidene difluoride, excised, and submitted for sequencing by Edman degradation at Midwest Analytical. Multielement metal analysis was performed by inductively coupled plasma mass spectrometry at the Utah State University Utah Veterinary Diagnostic Laboratory. Total-amino-acid analysis was performed by the Medical College of Wisconsin's Protein/Nucleic Acid Shared Facility. Protein concentrations were determined by a modified biuret assay (13) using bovine serum albumin as the protein standard.
Data analysis.
Kinetic constants (Km and Vmax) were calculated by fitting initial rate data to the Michaelis-Menten equation as described by Cleland (15) with SigmaPlot software.
Transposon mutagenesis and isolation of acetone-negative mutants.
Transposon mutagenesis was carried out using transposon delivery vector pRL27 as described elsewhere (R. A. Larsen, M. M. Wilson, A. M. Guss, and W. W. Metcalf, unpublished data). Kanamycin-resistant mutants were screened for loss of growth on acetone by replica plating from MOPS minimal medium (35) containing 1% glucose to MOPS minimal medium containing either 40 mM acetone or 10% (vol/vol gas phase) propylene. Acetone-negative mutants were identified as those which were unable to grow on acetone but were still able to grow on propylene. For selected mutants, the transposon insertion site was cloned and sequenced with transposon-specific sequencing primers. The double-stranded sequence of acxRABC was completed with primers designed to sequences that were obtained with transposon-specific primers.
R. capsulatus gene sequences.
Sequences of R. capsulatus proteins were obtained by accessing the genome sequence (50), which is available on-line through WIT (What Is That), a World Wide Web-based system for retrieval and analysis of gene sequences (www.mcs.anl.gov/compbio). R. capsulatus sequence data are currently accessible via WIT at the Capsulapedia website at the following addresses: http://rhodo.img.cas.cz and http://igweb.integratedgenomics.com/Igwit/.
Multiple sequence alignments.
Sequence similarities were identified with the Basic Local Alignment Search Tool (BLAST). Multiple sequence alignments were performed with NPS@:Multalin, version 5.4.1 (16, 17).
Nucleotide sequence accession numbers.
X. autotrophicus strain Py2 gene sequences have been deposited with the GenBank data bank under the following accession no.: acxR, acxA, acxB, and acxC, AY055852; rpoN, AY055375.
RESULTS
Assay of acetone carboxylase in cell extracts of R. capsulatus strain B10.
Low rates of acetone carboxylation have been reported by Birks and Kelly for cell extracts of acetone-grown R. capsulatus and Rhodomicrobium vannielii when ATP, Mg2+, and coenzyme A (CoA) or acetyl-CoA were included in the assays (6). In this previous study, R. capsulatus extracts were fractionated by ion-exchange chromatography with recovery of some activity in fractions enriched in polypeptides of 70 and 85 kDa by SDS-PAGE. However, the rates of acetone carboxylation observed were much too low to be of physiological significance, and further attempts to purify the carboxylase activity were not successful (6).
The assays developed for the purification of the acetone carboxylase from X. autotrophicus were applied to cell extracts of acetone-grown R. capsulatus strain B10 in order to determine whether the enzyme was amenable to in vitro studies. Using this assay, we were routinely able to obtain acetone carboxylation rates in the range of the corresponding in vivo rates (0.03 to 0.08 U/mg). Typical rates in cell extracts were about 0.04 U/mg, a number directly comparable to that measured in cell extracts of X. autotrophicus (reported to be 0.050 U/mg) (42). The addition of a monovalent ion, e.g., K+ or NH4+, at concentrations of 20 to 80 mM was required for optimal acetone carboxylase activity, while CoA and acetyl-CoA had no effect on acetone carboxylase activity. The activity in cell extracts was not sensitive to oxygen and was stable for several days at 4°C. Furthermore, enzyme activity was recovered after conventional chromatographic separations under the conditions described in Materials and Methods.
Purification and characterization of R. capsulatus acetone carboxylase.
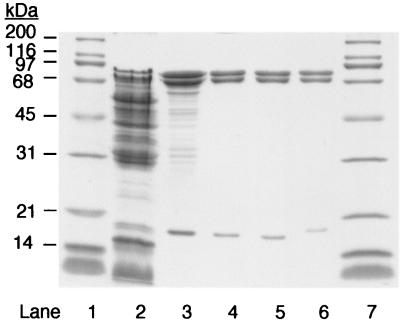
Acetone carboxylase from the soluble fraction of cell extracts was purified 5.9-fold to apparent homogeneity with a recovery of 36% and specific activity for acetone carboxylation of 0.240 U mg−1 of protein (Table 1). As shown in Fig. 1, three polypeptides, with apparent molecular masses of 82, 70, and 19 kDa by SDS-PAGE, were associated with acetone carboxylation activity. This banding profile is nearly identical to that of the three-subunit acetone carboxylase from X. autotrophicus (compare Fig. 1, lanes 5 and 6). The subunits of the acetone carboxylase are clearly visible in unfractionated cell extracts and, based on the purification, represent approximately 17% of soluble cellular protein in acetone-grown cells. Metal analysis revealed the presence of Mn, Zn, and Fe associated with purified acetone carboxylase, with Mn present at the highest level (Table 2). The metal ions associated with the enzyme added to 1 mol of M2+/mol of αβγ protomer, while the metal complement added to 1.5 mol/mol of αβγ protomer for the acetone carboxylase from X. autotrophicus. As summarized in Tables 2 and 3, the acetone carboxylases of R. capsulatus and X. autotrophicus are remarkably similar from a biochemical standpoint in all areas examined.
TABLE 1.
Purification of acetone carboxylase from R. capsulatus B10
| Step | Volume (ml) | Total protein (mg) | Total activity (U)a | Sp act (U/mg) | Purifi- cation (fold) | Recovery (%) |
|---|---|---|---|---|---|---|
| Cell extract | 224 | 2,464 | 100.3 | 0.041 | 1.0 | 100 |
| DEAE-Sepharose | 367 | 488 | 59.9 | 0.123 | 3.0 | 60 |
| Sephacryl S-300 | 98 | 226 | 51.8 | 0.230 | 5.6 | 52 |
| Hydroxyapatite | 10 | 152 | 36.4 | 0.240 | 5.9 | 36 |
Activity assays were performed using a mixture consisting of 0.1 to 0.4 mg of protein, 2 mM MgATP, 40 mM CO2 plus KHCO3, 3 mM acetone, 8 mM MgCl2, and 40 mM potassium acetate and with an ATP-regenerating system consisting of 7 mM phosphoenolpyruvate, 6 U of pyruvate kinase, and 10 U of adenylate kinase. One unit of activity is defined as 1 μmol of acetone degraded per min at 30°C.
FIG. 1.
SDS-PAGE analysis of R. capsulatus acetone carboxylase. Lanes 1 and 7, molecular weight standards (1 μg each); lane 2, cell extract (16.5 μg); lane 3, DEAE-Sepharose fraction (7.5 μg); lane 4, Sephacryl S-300 fraction (2 μg); lane 5, hydroxyapatite fraction (2 μg); lane 6, purified acetone carboxylase from X. autotrophicus (2 μg).
TABLE 2.
Biochemical and kinetic properties of acetone carboxylases from R. capsulatus B10 and X. autotrophicus, Py2
| Acetone carboxylase source | Biochemical properties
|
Kinetic propertiesa
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Native molecular mass (kDA) | Native configuration | Molar ratio/α2β2γ2 of metal:
|
M2+ stoichiometry (mol of M2+/mol of αβγ) | Organic cofactors | Level of expression (% of soluble proteins) | Acetone
|
Acetone without CO2
|
ATP
|
CO2
|
|||||||
| Fe | Mn | Zn | Km (apparent) | Vmax | Km (apparent) | Vmax | Km (apparent) | Vmax | Km (apparent) | Vmax | ||||||
| R. capsulatus B10 | 361 ± 52c | α2β2γ2 | 0.14 | 1.5 | 0.31 | 0.98 | NDe | 17 | 8.2 ± 0.5 μM | 0.49 ± 0.01 | 3.2 ± 0.2 μM | 0.605 ± 0.008 | 98 ± 15 μM | 0.47 ± 0.02 | 2.3 ± 0.2 nM | 0.291 ± 0.007 |
| X. autotrophicus Py2b | 353d | α2β2γ2 | 0.7 | 1.3 | 1.0 | 1.50 | ND | 25 | 7.8 ± 0.8 μM | 0.49 ± 0.02 | 7.7 ± 0.5 μM | 0.62 ± 0.01 | 122 ± 14 μM | 0.46 ± 0.02 | 4.2 ± 0.7 nM | 0.23 ± 0.01 |
The kinetic constants for acetone and ATP were determined spectrophotometrically as described previously (42) and have units of micromoles of phosphate produced per minute per milligram. Two phosphates are produced per acetone consumed under turnover conditions (see the reaction in the introduction), so the rate of phosphate consumption is twice the rate of acetoacetate formation when CO2 is present. Since ATP hydrolysis is uncoupled from acetone carboxylation with acetone present, it was not possible to determine kinetic constants for CO2 by the spectrophotometric assay. Instead, these measurements were determined by monitoring rates of acetone degradation by gas chromatography, and hence the values have units of micromoles of acetone degraded per minute per milligram.
Data are from Sluis and Ensign (42).
As determined by gel Filtration (Sephacryl S-300).
As determined by high-pressure liquid chromatography gel filtration (Ultraspherogel TSK 3000SW).
ND, none detected.
TABLE 3.
Biochemical properties of subunits of acetone carboxylases from R. capsulatus B10 and X. autotrophicus Py2
| Acetone carboxylase source | Biochemical properties of subunit:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| α
|
β
|
γ
|
|||||||
| Molecular mass (kDA) by:
|
Relative molar ratiob | Molecular mass (kDA) by:
|
Relative molar ratio | Molecular mass (kDA) by:
|
Relative molar ratio | ||||
| MALDI-TOF (MS) | SDS-PAGE | MALDI-TOF (MS) | SDS-PAGE | MALDI-TOF (MS) | SDS-PAGE | ||||
| R. capsulatus B10 | 85.2 | 82 | 1.0 | 78.6 | 70 | 1.1 | 19.5 | 19 | 1.2 |
| X. autotrophicus Py2a | 85.3 | 79 | 1.0 | 78.3 | 68 | 1.0 | 19.6 | 23 | 1.2 |
Data are from Sluis and Ensign (42).
Determined by integrated scanning densitometry.
ATP hydrolysis products, reaction stoichiometries, and kinetic constants.
ATP was the only high-energy compound capable of supporting acetone carboxylation with R. capsulatus acetone carboxylase (no detectable activity was observed with CTP, ITP, GTP, XTP, or pyrophosphate). As shown in Fig. 2A, acetone carboxylation resulted in the production of inorganic phosphate and AMP as the primary nucleotide hydrolysis products, with the same overall stoichiometry observed for the acetone carboxylase of X. autotrophicus (see the reaction in the introduction). As shown in Fig. 2B, in the absence of CO2, R. capsulatus acetone carboxylase catalyzed the acetone-dependent hydrolysis of ATP to produce ADP, AMP, and inorganic phosphate at rates comparable to that observed under normal turnover conditions (Fig. 2A). This feature has also been observed for the acetone carboxylase of X. autotrophicus. Finally, the kinetic characterization of R. capsulatus acetone carboxylase provided Km and Vmax values very similar to those obtained for X. autotrophicus acetone carboxylase (Table 2). The kinetic characterization demonstrates that acetone carboxylase exhibits a fairly high affinity for acetone (Km = 8 μM) but a relatively low turnover number for catalysis (45 min−1, calculated on the basis of the αβγ protomeric unit). The relatively low turnover of the enzyme is offset by the very high level of expression of the enzyme during growth with acetone as the carbon source (17 to 25% of soluble protein), providing in vivo rates of acetone carboxylation that support growth with the observed doubling times of 4 to 10 h.
FIG. 2.
Time course of acetone carboxylase-catalyzed acetone degradation and product formation. Assays were performed as described in Materials and Methods using a mixture containing 0.20 mg of purified acetone carboxylase, 20 mM ATP, 40 mM CO2 plus KHCO3, and 2 μmol of acetone. Each time point is an average of measurements on duplicate vials, which varied at most by 5% for each time point. (A) Assays performed in the presence of 50 mM CO2 plus KHCO3. (B) Assays performed in the absence of CO2 and KHCO3. Symbols: □, acetone; ▪, acetoacetate; ○, inorganic phosphate; ▵, AMP; ⋄, ADP; •, pyrophosphate.
Identification of the genes encoding R. capsulatus acetone carboxylase by genome screening.
The physiological, biochemical, and kinetic characterization of the R. capsulatus and X. autotrophicus acetone carboxylases suggests that the enzymes are highly conserved. The genetic characterization of the enzymes was pursued in order to confirm this at the molecular level. The majority of the R. capsulatus genome has been sequenced and generously made available for public access by Vlcek and coworkers (50). Accordingly, the N-terminal sequences of the acetone carboxylase subunits of R. capsulatus were obtained and used to identify potential candidate open reading frames (ORFs) from the genome sequence. As shown in Table 4, three adjacent ORFs in the R. capsulatus genome encode proteins with properties that correspond to those of the α, β, and γ subunits of the acetone carboxylase. Thus, these genes, which were not previously assigned definitive functions, encode the three subunits of the acetone carboxylase.
TABLE 4.
Identification and characterization of acetone carboxylase genes and gene products
| Bacterium and parameter | Result for subunit:
|
||
|---|---|---|---|
| α | β | γ | |
| R. capsulatus B10 | |||
| N terminus (Edman degradation) | LNAPTAIRGIV | LDREKTRSVQ | AYTKAKIKDLV |
| N terminus (deduced) | MNAPTAIRGIVR | MPLDREKTRSVQ | MAYTKAKIKDLV |
| Mr (deduced) | 85,319 | 78,936 | 19,639 |
| No. of amino acids | 769 | 717 | 167 |
| Gene designationa | RRC02651 | RRC02652 | RRC02650 |
| X. autotrophicus Py2 | |||
| N terminus (Edman degradation) | NDb (blockedc) | ND (blocked) | AYTXAKIVDLVDG |
| N terminus (deduced) | MNVTVDQSTLAG | MNVPVGHLRNVQ | MAYTRSKIVDLVDG |
| Mr (deduced) | 86,342 | 78,509 | 19,774 |
| No. of amino acids | 776 | 717 | 168 |
| Gene designationd | acxB | acxA | acxC |
| H. pylori 26695 | |||
| N terminus (Edman degradation) | ND | ND | ND |
| N terminus (deduced) | MANLLKNGKTL | MKDARVQVMGID | MVMSKYTQEQI |
| Mr (deduced) | 86,511 | 78,532 | 19,915 |
| No. of amino acids | 765 | 713 | 168 |
| Gene designatione | HP0696 | HP0695 | HP0697 |
| H. pylori J99 | |||
| N terminus (Edman degradation) | ND | ND | ND |
| N terminus (deduced) | MANLLKNGKTLK | MKDARVQVMGID | MSKYTQEQIKNL |
| Mr (deduced) | 86,607 | 78,332 | 19,670 |
| No. of amino acids | 765 | 712 | 166 |
| Gene designatione | jhp0632 | jhp0633 | jhp0631 |
| Alignment data | |||
| % Residues conserved (all four) | 61.3 | 62.7 | 49.7 |
Identification of the genes encoding X. autotrophicus Py2 acetone carboxylase by transposon mutagenesis.
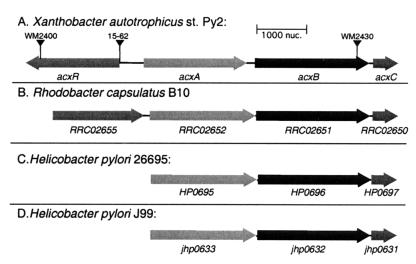
A highly efficient transposon mutagenesis system for the isolation and characterization of genes in X. autotrophicus has recently been developed (R. A. Larsen, M. M. Wilson, A. M. Guss, and W. W. Metcalf, unpublished data). A transposon delivery vector is used for the random insertion into the X. autotrophicus genome of a transposable element that confers kanamycin resistance, followed by replica plating to identify the phenotype of interest (in this case, strains which are acetone negative). The transposon and associated genomic DNA were then excised and sequenced to identify the target gene. This procedure was used to isolate five acetone-negative mutants, which were subjected to further phenotypic and molecular characterization. One of these mutants contained an insertion within a gene, designated acxB, whose translated product has a high degree of sequence identity (84.3%) to that of RRC02652 of R. capsulatus, i.e., the α subunit of the acetone carboxylase. Sequencing in both directions from the site of transposon insertion showed that the β and γ subunit genes (acxA and acxC, respectively) are adjacent to acxB with the same orientations and order as the genes in R. capsulatus (compare Fig. 3A and B). The β and γ subunits from the two organisms have a striking degree of identity as well: 84.2% for AcxA and the product of RRC02651 and 69.6% for AcxC and the product of RRC02650.
FIG. 3.
Acetone carboxylase operons. Inverted triangles, positions of the insertional mutations in acxR and acxB. The products of acxA, RRC02652, HP0695, and jhp0633 are acetone carboxylase beta subunit homologs; the products of acxB, RRC02651, HP0696, and jhp0632 are acetone carboxylase alpha subunit homologs; the products of acxC, RRC02650, HP0697, and jhp0631 are acetone carboxylase gamma subunit homologs; the products of acxR and RRCO2652 are the putative transcriptional activators of X. autotrophicus and R. capsulatus acetone carboxylases, respectively.
Characterization of acetone carboxylase genes and identification of a putative acetone carboxylase in H. pylori by BLAST analysis.
An analysis of sequences in the database revealed the presence of ORFs with the same organization as the acetone carboxylase genes within the genomes of the two strains of H. pylori that have been sequenced (3, 48) (Fig. 3). As shown in Table 4, the striking similarities between the hypothetical proteins encoded by these genes and the acetone carboxylase subunits of X. autotrophicus and R. capsulatus strongly suggest that the H. pylori genes encode an identical acetone carboxylase system. This idea is highlighted by the 50 to 62% identities present in the alignments of all four sequences (Table 4). Presumably, H. pylori is yet another bacterium capable of acetone metabolism, although this has not, to our knowledge, been investigated to date.
No other three-ORF operons with these characteristic features were identified in any of the other sequenced bacterial genomes available at present. When analyzed by BLAST, the α and β subunits of the acetone carboxylases showed significant identity to some proteins within the superfamily of cycloamidohydrolases (27, 34). Specifically, the α and β subunits show 15 to 30% identity when aligned against proteins belonging to the hydantoinase subfamily of the cycloamidohydrolases. The products of H. pylori ORFs HP0695 and HP0696 were originally designated putative hydantoin utilization protein A and N-methylhydantoinase based on this identity. Hydantoinases catalyze the hydrolysis of the cyclic amide bond of substituted pyrimidines and hydantoins to form the corresponding N-carbamyl amino acids (34, 45). Hydantoinases use zinc to facilitate substrate hydrolysis, and one member of this family, i.e., N-methylhydantoinase, requires ATP for substrate hydrolysis (34, 45). The identity between the α and β subunits of the acetone carboxylase and the hydantoinase proteins suggests that the proteins may be evolutionarily related. However, it is difficult to rationalize this identity based on the distinct functions of the enzymes. Additionally, the γ subunits of the acetone carboxylases did not show significant identity to hydantoinases or any other proteins in the database.
Involvement of a σ54-dependent transcriptional activator in acetone metabolism by X. autotrophicus Py2.
Of the five characterized acetone-negative X. autotrophicus mutants obtained by transposon mutagenesis, one contained an insertion in a gene encoding a structural component of acetone carboxylase (i.e., acxB, as discussed above). Two mutants contained insertions in the gene encoding the probable RNA polymerase σ54 (rpoN) subunit (73% identity with rpoN of Azorhizobium caulinodans; 55% identity with rpoN of Bradyrhizobium japonicum). Of the two remaining mutants, one contained an insertion in a gene encoding a putative σ54-dependent transcriptional activator of 68,271 Mr (∼30% identity with members of this family), while the second mutation was 10 bp upstream of the same gene (i.e., after the ribosome binding site). All of the acetone-negative mutants retained the ability to grow using propylene, a growth substrate whose metabolic pathway leads to the formation of acetoacetate, the same product that is formed from acetone carboxylation (2). By sequencing the regions flanking the putative σ54-dependent transcriptional activator, the position of this protein with respect to the acetone carboxylase operon was determined. As shown in Fig. 3, the gene for the putative activator, acxR, is located 496 bp upstream of acxA and is divergently transcribed.
An analysis of the primary sequence of AcxR revealed the presence of features unique to σ54-dependent transcriptional activators (52), including the distinctive “switch I” motif (consensus SELFGXXXGAFTGA; Fig. 4). To obtain further evidence for the involvement of σ54 in the regulation of acetone metabolism, we looked for the presence of the signature −24/−12 promoter element required for σ54 holoenzyme transcription initiation (5). Characteristic features of the −24/−12 promoter are the presence of GG and GC doublets spaced 10 bp apart, with the consensus being TGGCACGNNNNTTGC (5). A good match with sequence TGGCACGCATCCTGC (conserved elements in boldface) was identified 122 bp upstream of acxA.
FIG. 4.
Multiple sequence alignment of regulators of ketone metabolism. X. Py2 AcxR, acetone carboxylase regulator in X. autotrophicus (accession no. AY055852); R.c. RRC02655, putative acetone carboxylase regulator in R. capsulatus; B.s. AcoR, acetoin metabolism regulator in B. subtilis (accession no. H69581). Residues shaded in black are identical in all three proteins; residues shaded in grey are identical in two of the three proteins; residues in boldface are conserved as IV, LM, FY, or NDQE in all three sequences. Switch I motifs are boxed.
BLAST analysis of the R. capsulatus genome revealed the presence of an ORF encoding a putative transcriptional activator located 127 bp upstream of the acetone carboxylase operon (Fig. 3). This putative activator (ORF designation, RRC02655) is similar in size (67,595 Mr), and is 33.8% identical, to AcxR. However, as shown in Fig. 4, this protein lacks the characteristic switch I motif of the σ54-dependent activators. Instead, the central domain is more reminiscent of enhancer-binding proteins that function with the housekeeping σ70-RNA polymerase (36). R. capsulatus is one of the bacteria shown to regulate gene expression in this manner, i.e., regulation of nitrogen metabolism by activator NtrC (10, 36). No sequence with similarity to the consensus σ54-dependent promoter was present upstream of the R. capsulatus acetone carboxylase operon, providing further evidence that σ54 is not involved in transcription of the operon.
Analysis of the H. pylori genomes did not reveal any ORFs encoding putative transcriptional activators in similar proximity to the acetone carboxylase operon. A BLAST analysis of the H. pylori genomes indicated that the only σ54-dependent transcriptional activator in these bacteria is FlgR, which is encoded by the ORFs HP0703 and JHP0631 in strains 26695 and J99, respectively. FlgR has previously been shown to be a response regulator for the expression of flagellar basal body and hook genes (44). There are no consensus σ54-dependent promoter sequences upstream of the putative acxABC operon of either H. pylori strain; thus, it is unlikely that FlgR or σ54 is involved in regulation of this operon in H. pylori.
DISCUSSION
In 1950 Siegel reported that acetone was capable of supporting the growth of the purple nonsulfur photosynthetic bacterium Rhodopseudomonas gelatinosa and provided evidence that the metabolism of acetone was initiated by carboxylation to acetoacetate (41). In subsequent years, diverse other bacteria have been shown to grow with acetone, but it was not until 1997 that the first acetone-metabolizing enzyme was purified and characterized (42). In the present work, the enzymes and genes involved in acetone carboxylation in R. capsulatus, a photosynthetic bacterium closely related to the one described by Siegel in 1950, are identified, characterized, and compared to those of X. autotrophicus strain Py2. These studies reveal a highly conserved strategy of acetone metabolism for these two distinct bacteria. The unique genetic footprint of the acetone carboxylase operon (Fig. 3) suggests the presence of similar systems in the two strains of H. pylori whose genomes have been sequenced (3, 48). These results highlight the power of genome screening in the identification and characterization of novel catabolic activities, an approach that should prove useful for characterizing the genes and enzymes of other acetone-utilizing microbes.
Studies of diverse aerobic and anaerobic acetone-utilizing bacteria suggest that carboxylation is a common strategy for the initiation of microbial acetone catabolism, although there is some evidence that hydroxylation may be an alternative strategy for some bacteria (20). To date, the acetone carboxylases of X. autotrophicus and R. capsulatus B10 are the only bacterial acetone-utilizing enzymes that have been purified to homogeneity in an active state. A GTP-dependent acetone carboxylase activity from acetone-grown Rhodococcus rhodochrous strain B276, a hydrocarbon-oxidizing actinomycete, has been partially purified, and has been found to have a similar three-subunit structure (apparent molecular masses of 85, 74, and 16 kDa by SDS-PAGE) (14). Curiously, ATP was incapable of supporting acetone carboxylation by this enzyme, while CTP, ITP, and XTP were capable of supporting rates up to 50% of that observed with GTP (14). However, the in vitro rates observed for this enzyme were only ∼10% of the corresponding in vivo rates, and substantial enzyme activity was lost during purification, suggesting that this enzyme may have additional cofactor requirements beyond those identified in the initial studies (14). In vivo and in vitro studies of strict anaerobes that grow with acetone, e.g., sulfate reducers, denitrifiers, and fermenters, support a similar CO2-dependent pathway, but all attempts to reconstitute acetone carboxylase activity in vitro for these strains have been unsuccessful (22-24, 37-39). Thus, it remains to be determined whether the putative acetone carboxylases of these organisms are structurally and functionally similar to those of X. autotrophicus, R. capsulatus, and Rhodococcus rhodochrous. At present, genome sequences for the other known acetone-utilizing bacteria are not available, and no genes linked to acetone metabolism have been identified by cloning or mutational analysis, so there is no basis for making predictions based on sequence searches and alignments.
The subunit composition, primary structure, and reaction stoichiometry of the acetone carboxylase enzymes distinguish them from all other carboxylases characterized to date. Particularly intriguing is the formation of AMP and inorganic phosphate as the products of ATP hydrolysis in the course of the reaction (see the reaction in the introduction). We had hoped that the primary structure of the acetone carboxylases would reveal insights into their mechanism of action and how ATP hydrolysis supports the reaction. Unfortunately, the sequences do not provide any obvious clues. The only enzymes that have significant identity with the acetone carboxylase subunits are hydantoinases, microbial enzymes that catalyze the hydrolysis of the cyclic amide bonds of substituted pyrimidines (12, 31, 34, 45). An examination of the substrates, products, possible intermediates, and plausible mechanisms of the two classes of enzymes does not reveal any common theme to suggest why they might be related. Possibly, the enzymes evolved from a common ancestor, with the conserved portions representing structural rather than catalytic domains of the divergent proteins. Alternatively, there may be some common theme to the mechanisms of these transformations, e.g., stabilization of a similar transition state intermediate or conservation of general acids or bases that are not presently apparent from the limited mechanistic information available.
The acetone carboxylases in both X. autotrophicus and R. capsulatus are expressed at very high levels (17 to 25% of soluble protein) upon exposure to acetone but are not expressed at detectable levels when the bacteria are grown with conventional carbon sources. Importantly, neither system appears to be subject to catabolite repression, as the presence of other carbon sources (e.g., acetate, succinate, or glucose for X. autotrophicus and malate for R. capsulatus) does not affect acetone carboxylase expression. The identification of putative transcriptional activator proteins encoded by genes immediately adjacent to and upstream of the acx operons of X. autotrophicus and R. capsulatus (Fig. 3 and 4) suggests a likely mechanism for controlling expression of the operons. For AcxR from X. autotrophicus, the activator presumably functions as a σ54-dependent enhancer-binding protein based on the presence of conserved motifs characteristic of the central activator domain of this class of transcriptional activators (Fig. 4) (52). The assignment of AcxR as a σ54-dependent enhancer is further strengthened by the requirement of σ54 for acetone carboxylase expression and the identification of a σ54 promoter upstream of the Acx operon. Enhancer-binding proteins are distinguished from conventional transcriptional activators (e.g., the cyclic AMP receptor protein) by binding to target DNA sequences that may be a large distance away from, and either upstream or downstream of, the promoter (52). DNA looping allows the formation of a productive complex between the activator and the RNA polymerase holoenzyme for transcriptional initiation (52). Enhancer-binding proteins are modular proteins, with the N-terminal portions typically forming the signal reception domain (52). Interestingly, σ54-dependent enhancer-binding protein AcoR controls the expression of genes for acetoin (3-hydroxy-2-butanone) metabolism in some bacteria (1, 21, 29). AcoR in Bacillus subtilis is very similar in size (67 kDa) to AcxR and the product of RRC02655 and shares the conserved motifs expected for a σ54-dependent activator within the central activation domain (Fig. 4, conserved elements from position 370 to 470 in the numbering scheme) (1). The large sizes of these regulators relative to those of other enhancer-binding proteins, due in part to the large N-terminal domain, suggest that they function by direct binding of the inducer molecule, as opposed to some other members of this family, which are response regulators of two-component systems (52). Acetoin and acetone are both small molecules with a ketone functional group, and it is conceivable that similar binding sites for the ketone group are present within the N-terminal domains of the proteins (Fig. 4). Interestingly, 2-butanone (acetoin lacking the hydroxyl group) is an inducer of and substrate for X. autotrophicus acetone carboxylase, and X. autotrophicus will grow with 2-butanone via acetone carboxylase-catalyzed formation of 2-ketovalerate as the initial product (M. K. Sluis and S. A. Ensign, unpublished results).
The putative transcriptional activator from R. capsulatus lacks the characteristic switch I motif within the central activation domain of the promoter (Fig. 4), and there is no −24/−12 promoter sequence upstream of the acx operon. This suggests that the R. capsulatus activator facilitates transcription via an interaction with σ70 rather than σ54. Interestingly, one of the prototype enhancer-binding transcriptional activator proteins is NtrC, which regulates nitrogen metabolism by an interaction with σ70 in R. capsulatus (10, 36, 52). Thus, for R. capsulatus there is a defined precedent for using the housekeeping σ70 in conjunction with cis-acting DNA elements to regulate gene expression.
The tentative identification of an acetone carboxylase operon in both strains of H. pylori that have been sequenced is intriguing for a number of reasons. Helicobacter is in a different subclass (epsilon subclass) of the proteobacteria from X. autotrophicus and Rhodobacter (alpha subclass) and has a quite different physiology and morphology. H. pylori is pathogenic to humans, with colonization of the gastric mucosa leading in some cases to gastritis and peptic ulcers (18). While X. autotrophicus and Rhodobacter are nutritionally versatile, facultatively autotrophic, free-living organisms that are easily isolated from the environment, H. pylori has no known nonhuman reservoir and has complex nutritional requirements (40). To our knowledge, acetone metabolism has not been investigated for H. pylori. In addition, it is not clear what levels of acetone H. pylori might experience in the gastric mucosa or whether ketogenic conditions (e.g., poor diet, fasting, diabetes) are predisposing factors for H. pylori colonization. While there are clear regulatory elements located adjacent to the acx operons of X. autotrophicus and R. capsulatus, no such regulatory elements could be identified in H. pylori (Fig. 3). Thus, the physiological role of acetone metabolism might be quite different in H. pylori than it is in X. autotrophicus or R. capsulatus, where the operons are up-regulated to very high levels, allowing growth with acetone and CO2 as the sole carbon sources.
Acknowledgments
We thank Timothy Hoover for useful discussions and for his careful reading of the manuscript. We thank Robert Haselkorn and Jan Paces for generously allowing us access to the R. capsulatus genome. We are grateful to Michael Madigan for providing the R. capsulatus strain (B10) used in these studies.
This work was supported by National Institutes of Health grant GM51805 to S.A.E. and by National Science Foundation grant MCB 987459 and by a Searle Scholars Award from the Chicage Community Trust to W.W.M.
REFERENCES
- 1.Ali, N. O., J. Bignon, G. Rapoport, and M. Debarbouille. 2001. Regulation of the acetoin catabolic pathway is controlled by sigma L in Bacillus subtilis. J. Bacteriol. 183:2497-2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen, J. R., D. D. Clark, J. G. Krum, and S. A. Ensign. 1999. A role for coenzyme M (2-mercaptoethanesulfonic acid) in a bacterial pathway of aliphatic epoxide carboxylation. Proc. Natl. Acad. Sci. USA 96:8432-8437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alm, R. A., L.-S. L. Ling, D. T. Moir, B. L. King, E. D. Brown, P. C. Doig, D. R. Smith, B. Noonan, B. C. Guild, B. L. DeJonge, G. Carmel, P. J. Tummino, A. Caruso, M. Uria-Nickelsen, D. M. Mills, C. Ives, R. Gibson, D. Merberg, S. D. Mills, Q. Jiang, D. E. Taylor, G. F. Vovis, and T. J. Trust. 1999. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397:176-180. [DOI] [PubMed] [Google Scholar]
- 4.Argilés, J. P. 1986. Has acetone a role in the conversion of fat to carbohydrate in mammals? Trends Biochem. Sci. 11:61-65. [Google Scholar]
- 5.Barrios, H., B. Valderrama, and E. Morett. 1999. Compilation and analysis of sigma 54-dependent promoter sequences. Nucleic Acids Res. 27:4305-4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birks, S. J., and D. J. Kelly. 1997. Assay and properties of acetone carboxylase, a novel enzyme involved in acetone-dependent growth and CO2 fixation in Rhodobacter capsulatus and other photosynthetic and denitrifying bacteria. Microbiology 143:755-766. [DOI] [PubMed] [Google Scholar]
- 7.Blevins, W. T., and J. J. Perry. 1972. Metabolism of propane, N-propylamine, and propionate by hydrocarbon-utilizing bacteria. J. Bacteriol. 112:513-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bondoc, F. Y., Z. Bao, W. Hu, F. J. Gonzalez, Y. Wang, C. S. Yang, and J. Hong. 1999. Acetone catabolism by cytochrome P450 2E1: studies with CYP2E1-null mice. Biochem. Pharmacol. 58:461-463. [DOI] [PubMed] [Google Scholar]
- 9.Bonnet-Smits, E. M., L. A. Robertson, J. P. Van Dijken, E. Senior, and J. G. Kuenen. 1988. Carbon dioxide fixation as the initial step in the metabolism of acetone by Thiosphaera pantotropha. J. Gen. Microbiol. 134:2281-2289. [Google Scholar]
- 10.Bowman, W. C., and R. G. Kranz. 1998. A bacterial ATP-dependent, enhancer binding protein that activates the housekeeping RNA polymerase. Genes Dev. 12:1884-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, L. S., M. J. Lee, J. Y. Hong, W. Q. Huang, E. Wang, and C. S. Yang. 1994. Relationship between cytochrome P450 2E1 and acetone catabolism in rats as studied with diallyl sulfide as an inhibitor. Biochem. Pharmacol. 48:2199-2205. [DOI] [PubMed] [Google Scholar]
- 12.Chien, H. R., Y. L. Jih, W. Y. Yang, and W. H. Hsu. 1998. Identification of the open reading frame for the Pseudomonas putida D-hydantoinase gene and expression of the gene in Escherichia coli. Biochim. Biophys. Acta 1395:68-77. [DOI] [PubMed] [Google Scholar]
- 13.Chromy, V., J. Fischer, and V. Kulhanek. 1974. Re-evaluation of EDTA-chelated biuret reagent. Clin. Chem. 20:1362-1363. [PubMed] [Google Scholar]
- 14.Clark, D. D., and S. A. Ensign. 1999. Evidence for an inducible nucleotide-dependent acetone carboxylase in Rhodococcus rhodochrous B276. J. Bacteriol. 181:2752-2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cleland, W. W. 1979. Statistical analysis of enzyme kinetic data. Methods Enzymol. 63:103-138. [DOI] [PubMed] [Google Scholar]
- 16.Combet, C., C. Blanchet, C. Geourjon, and G. Deléage. 2000. NPS@:Network protein sequence analysis. Trends Biochem. Sci. 25:147-150. [DOI] [PubMed] [Google Scholar]
- 17.Corpet, F. 1988. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 16:10881-10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cover, T. L., and M. J. Blaser. 1992. Helicobacter pylori and gastroduodenal disease. Annu. Rev. Med. 43:135-145. [DOI] [PubMed] [Google Scholar]
- 19.Davies, R., and M. Stephenson. 1941. Studies on the acetone-butyl-alcohol fermentation. I. Nutritional and other factors involved in the preparation of active suspensions of Clostridium acetobutylicum (Weizmann). Biochem. J. 35:1320-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ensign, S. A., F. J. Small, J. R. Allen, and M. K. Sluis. 1998. New roles for CO2 in the microbial metabolism of aliphatic epoxides and ketones. Arch. Microbiol. 169:179-187. [DOI] [PubMed] [Google Scholar]
- 21.Huang, M., F. B. Oppermann-Sanio, and A. Steinbuchel. 1999. Biochemical and molecular characterization of the Bacillus subtilis acetoin catabolic pathway. J. Bacteriol. 181:3837-3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janssen, P. H., and B. Schink. 1995. 14CO2 exchange with acetoacetate catalyzed by dialyzed cell-free extracts of the bacterial strain BunN grown with acetone and nitrate. Eur. J. Biochem. 228:677-682. [DOI] [PubMed] [Google Scholar]
- 23.Janssen, P. H., and B. Schink. 1995. Catabolic and anabolic enzyme activities and energetics of acetone metabolism of the sulfate-reducing bacterium Desulfococcus biacutus. J. Bacteriol. 177:277-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janssen, P. H., and B. Schink. 1995. Metabolic pathways and energetics of the acetone-oxidizing, sulfate-reducing bacterium. Desulfobacterium cetonicum. Arch. Microbiol. 163:188-194. [DOI] [PubMed] [Google Scholar]
- 25.Kalapos, M. P. 1999. Possible physiological roles of acetone metabolism in humans. Med. Hypotheses 53:236-242. [DOI] [PubMed] [Google Scholar]
- 26.Kalapos, M. P., J. Mandl, G. Bánhegyi, F. Antoni, and T. Garzo. 1994. Net glucose formation from acetone in isolated murine hepatocytes. The effect of different pretreatments of mice. Int. J. Biochem. 26:1069-1079. [DOI] [PubMed] [Google Scholar]
- 27.Kim, G. J., and H. S. Kim. 1998. Identification of the structural similarity in the functionally related amidohydrolases acting on the cyclic amide ring. Biochem. J. 330:295-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koop, D. R., and J. P. Casazza. 1985. Identification of ethanol-inducible P-450 isozyme 3a as the acetone and acetol monooxygenase of rabbit microsomes. J. Biol. Chem. 260:13607-13612. [PubMed] [Google Scholar]
- 29.Kruger, N., and A. Steinbuchel. 1992. Identification of acoR, a regulatory gene for the expression of genes essential for acetoin catabolism in Alcaligenes eutrophus H16. J. Bacteriol. 174:4391-4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Landau, B. R., and H. Brunengraber. 1987. The role of acetone in the conversion of fat to carbohydrate. Trends Biochem. Sci. 12:113-114. [Google Scholar]
- 31.LaPointe, G., S. Viau, D. LeBlanc, N. Robert, and A. Morin. 1994. Cloning, sequencing, and expression in Escherichia coli of the d-hydantoinase gene from Pseudomonas putida and distribution of homologous genes in other microorganisms. Appl. Environ. Microbiol. 60:888-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lukins, H. H., and J. W. Foster. 1963. Methyl ketone metabolism in hydrocarbon-utilizing mycobacteria. J. Bacteriol. 85:1074-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Madigan, M. T. 1990. Photocatabolism of acetone by nonsulfur purple bacteria. FEMS Microbiol. Lett. 71:281-286. [Google Scholar]
- 34.May, O., A. Habenicht, R. Mattes, C. Syldatk, and M. Siemann. 1998. Molecular evolution of hydantoinases. Biol. Chem. 379:743-747. [PubMed] [Google Scholar]
- 35.Neidhardt, F. C., P. L. Bloch, and D. F. Smith. 1974. Culture medium for enterobacteria. J. Bacteriol. 119:736-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Osuna, J., X. Soberon, and E. Morett. 1997. A proposed architecture for the central domain of the bacterial enhancer-binding proteins based on secondary structure prediction and fold recognition. Protein Sci. 6:543-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Platen, H., P. H. Janssen, and B. Schink. 1994. Fermentative degradation of acetone by an enrichment culture in membrane-separated culture devices and in cell suspensions. FEMS Microbiol. Lett. 122:27-32. [DOI] [PubMed] [Google Scholar]
- 38.Platen, H., and B. Schink. 1989. Anaerobic degradation of acetone and higher ketones by newly isolated denitrifying bacteria. J. Gen. Microbiol. 135:883-891. [DOI] [PubMed] [Google Scholar]
- 39.Platen, H., and B. Schink. 1990. Enzymes involved in anaerobic degradation of acetone by a denitrifying bacterium. Biodegradation 1:243-251. [DOI] [PubMed] [Google Scholar]
- 40.Reynolds, D. J., and C. W. Penn. 1994. Characteristics of Helicobacter pylori growth in a defined medium and determination of its amino acid requirements. Microbiology 140:2649-2656. [DOI] [PubMed] [Google Scholar]
- 41.Siegel, J. M. 1950. The metabolism of acetone by the photosynthetic bacterium Rhodopseudomonas gelatinosa. J. Bacteriol. 60:595-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sluis, M. K., and S. A. Ensign. 1997. Purification and characterization of acetone carboxylase from Xanthobacter strain Py2. Proc. Natl. Acad. Sci. USA 94:8456-8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sluis, M. K., F. J. Small, J. R. Allen, and S. A. Ensign. 1996. Involvement of an ATP-dependent carboxylase in a CO2-dependent pathway of acetone metabolism by Xanthobacter strain Py2. J. Bacteriol. 178:4020-4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spohn, G., and V. Scarlato. 1999. Motility of Helicobacter pylori is coordinately regulated by the transcriptional activator FlgR, an NtrC homolog. J. Bacteriol. 181:593-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Syldatk, C., O. May, J. Altenbuchner, R. Mattes, and M. Siemann. 1999. Microbial hydantoinases--industrial enzymes from the origin of life? Appl. Microbiol. Biotechnol. 51:293-309. [DOI] [PubMed] [Google Scholar]
- 46.Tayeh, M. A., and M. T. Madigan. 1987. Malate dehydrogenase in phototrophic purple bacteria: purification, molecular weight, and quaternary structure. J. Bacteriol. 169:4196-4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor, D. G., P. W. Trudgill, R. E. Cripps, and P. R. Harris. 1980. The microbial metabolism of acetone. J. Gen. Microbiol. 118:159-170. [Google Scholar]
- 48.Tomb, J.-F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. A. Dougherty, K. Nelson, J. Quackenbush, L. Zhou, E. F. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, H. G. Khalak, A. Glodek, K. McKenney, L. M. Fitzegerald, N. Lee, M. D. Adams, E. K. Hickey, D. E. Berg, J. D. Gocayne, T. R. Utterback, J. D. Peterson, J. M. Kelley, P. D. Karp, H. O. Smith, C. M. Fraser, and J. C. Venter. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539-547. [DOI] [PubMed] [Google Scholar]
- 49.Vestal, J. R., and J. J. Perry. 1969. Divergent metabolic pathways for propane and propionate utilization by a soil isolate. J. Bacteriol. 99:216-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vlcek, C., V. Paces, N. Maltsev, J. Paces, R. Haselkorn, and M. Fonstein. 1997. Sequence of a 189-kb segment of the chromosome of Rhodobacter capsulatus SB1003. Proc. Natl. Acad. Sci. USA 94:9384-9388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Westheimer, F. H. 1969. Acetoacetate decarboxylase from Clostridium acetobutylicum. Methods Enzymol. 14:231-241. [Google Scholar]
- 52.Xu, H., and T. R. Hoover. 2001. Transcriptional regulation at a distance in bacteria. Curr. Opin. Microbiol. 4:138-144. [DOI] [PubMed] [Google Scholar]