Abstract
1. Pieces of human retina were dissected from eyes enucleated because of malignant tumours. The isolated retinas were perfused by an ionic medium (36° C) and investigated spectrophotometrically.
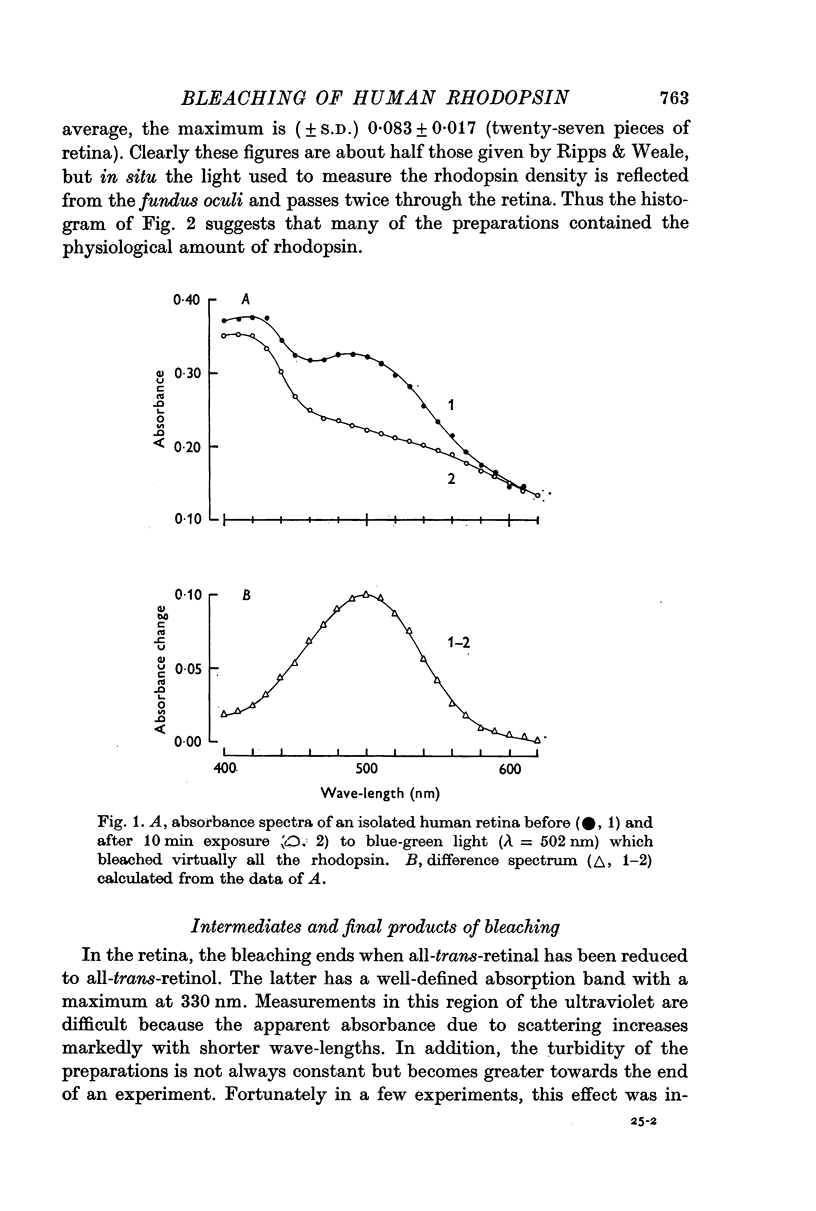
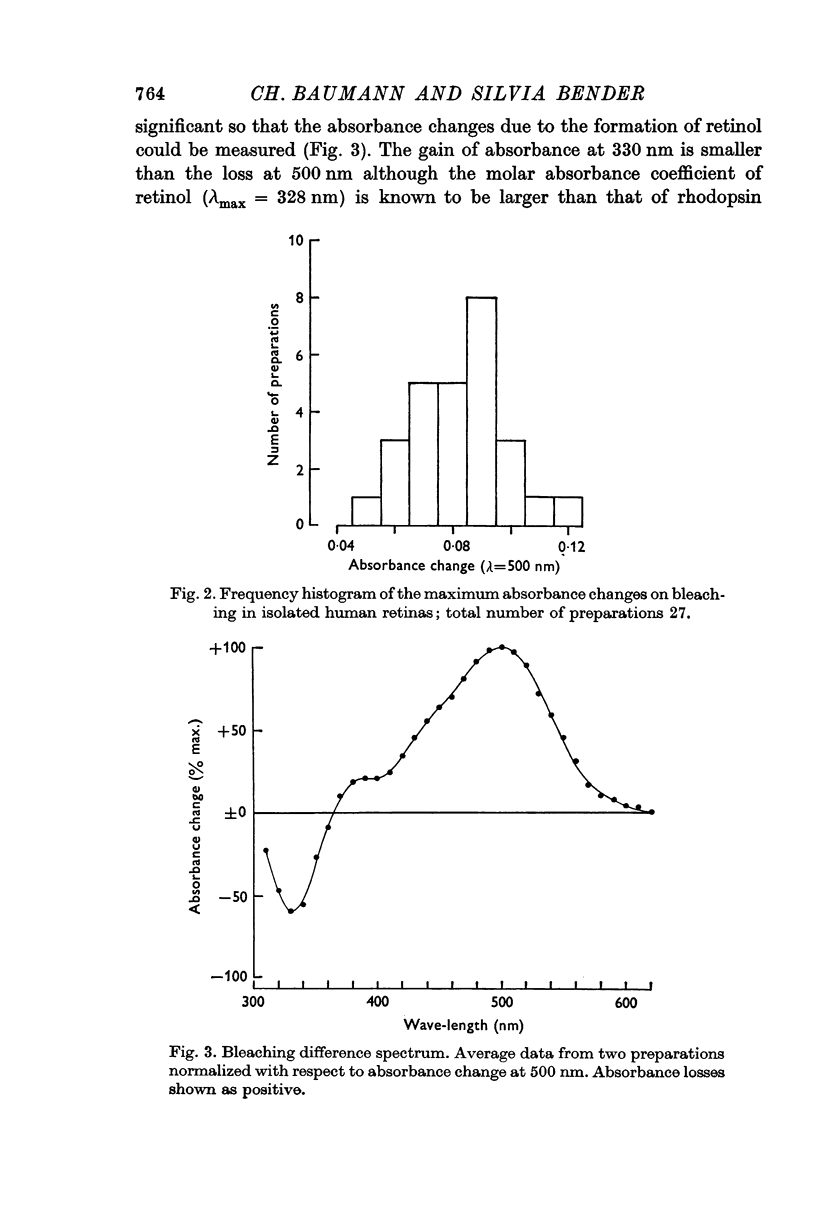
2. Rhodopsin was identified on the basis of its difference spectrum. The maximum absorbance change on bleaching was about 0·1 (λ = 500 nm).
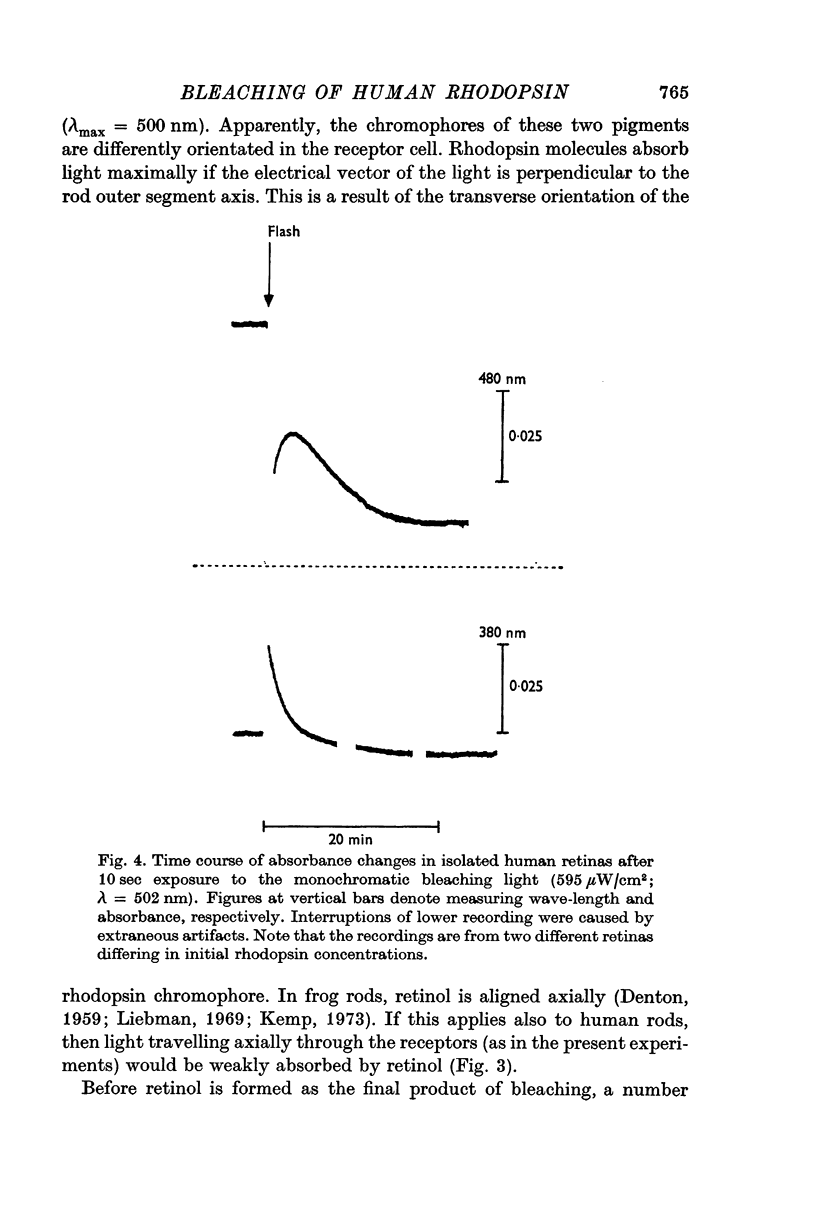
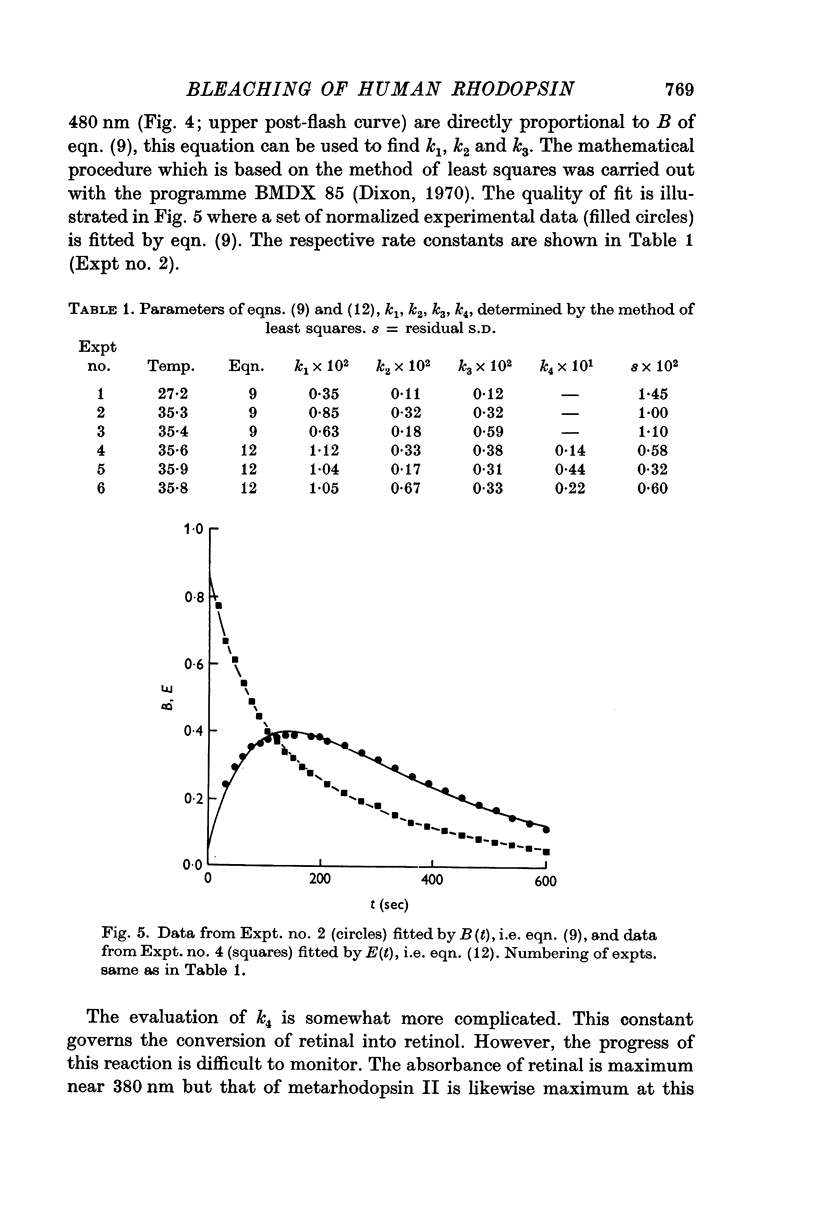
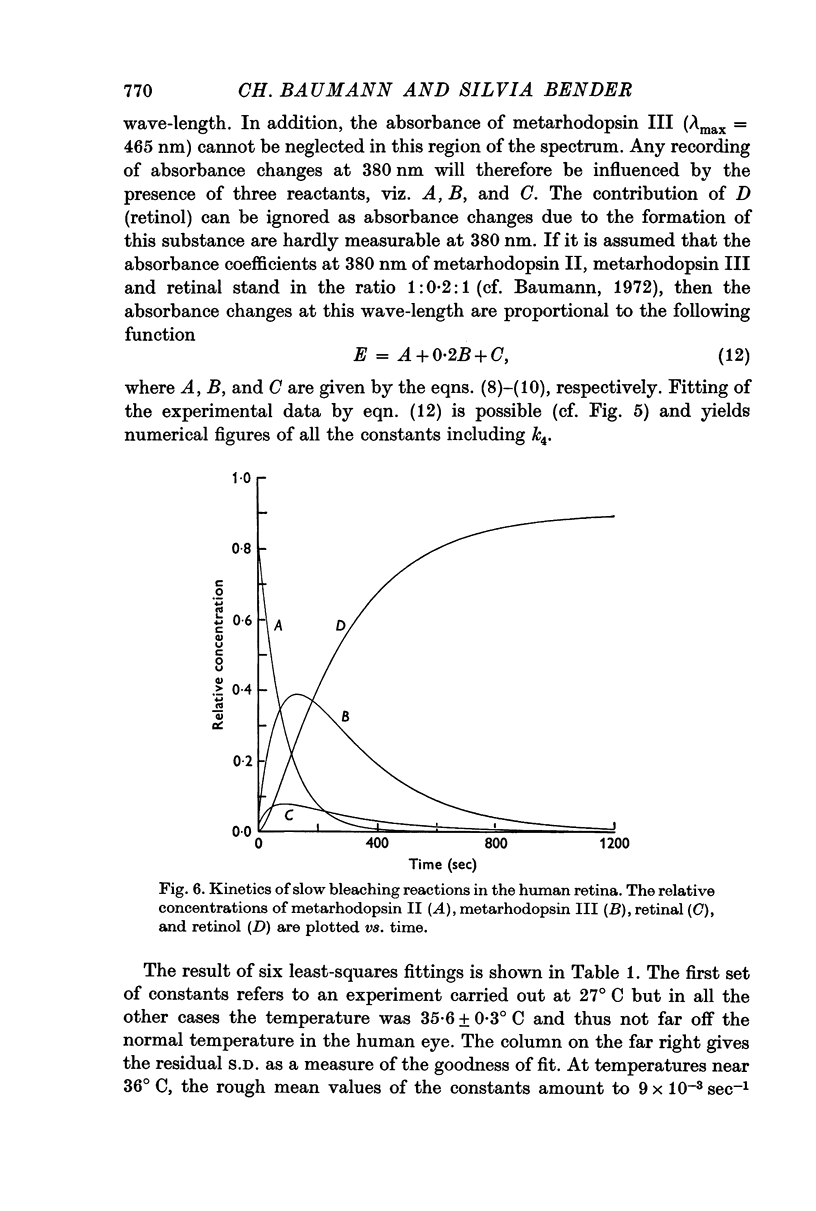
3. The process of bleaching was quantitatively analysed in terms of four slow reactions, viz. (a) conversion of metarhodopsin II into metarhodopsin III, (b) hydrolysis of metarhodopsin II into retinal and opsin, (c) decay of metarhodopsin III to retinal, and (d) reduction of retinal to retinol.
4. First-order rate constants for the reactions in 3 were 9 × 10-3 sec-1 (a), 3 × 10-3 sec-1 (b), 4 × 10-3 sec-1 (c), and 3 × 10-2 sec-1 (d).
5. With two simplified versions of the model summarized in 3 and 4, the description of experimental data was less accurate.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpern M. Rhodopsin kinetics in the human eye. J Physiol. 1971 Sep;217(2):447–471. doi: 10.1113/jphysiol.1971.sp009580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann C. Die Photosensitivität des Sehpurpurs in der isolierten Netzhaut. Vision Res. 1965 Aug;5(7):425–434. doi: 10.1016/0042-6989(65)90050-7. [DOI] [PubMed] [Google Scholar]
- Baumann C. Flash photolysis of rhodopsin in the isolated frog retina. Vision Res. 1970 Sep;10(9):789–798. doi: 10.1016/0042-6989(70)90158-6. [DOI] [PubMed] [Google Scholar]
- Cone R. A., Brown P. K. Spontaneous regeneration of rhodopsin in the isolated rat retina. Nature. 1969 Mar 1;221(5183):818–820. doi: 10.1038/221818a0. [DOI] [PubMed] [Google Scholar]
- DENTON E. J. The contributions of the orientated photosensitive and other molecules to the absorption of whole retina. Proc R Soc Lond B Biol Sci. 1959 Jan 27;150(938):78–94. doi: 10.1098/rspb.1959.0009. [DOI] [PubMed] [Google Scholar]
- Ostroy S. E., Erhardt F., Abrahamson E. W. Protein configuration changes in the photolysis of rhodopsin. II. The sequence of intermediates in thermal decay of cattle metarhodopsin in vitro. Biochim Biophys Acta. 1966 Feb 7;112(2):265–277. doi: 10.1016/0926-6585(66)90326-8. [DOI] [PubMed] [Google Scholar]
- RUSHTON W. A. The difference spectrum and the photosensitivity of rhodopsin in the living human eye. J Physiol. 1956 Oct 29;134(1):11–29. doi: 10.1113/jphysiol.1956.sp005622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripps H., Weale R. A. Flash bleaching of rhodopsin in the human retina. J Physiol. 1969 Jan;200(1):151–159. doi: 10.1113/jphysiol.1969.sp008686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripps H., Weale R. A. Rhodopsin regeneration in man. Nature. 1969 May 24;222(5195):775–777. doi: 10.1038/222775a0. [DOI] [PubMed] [Google Scholar]


