Abstract
1. The receptive field properties of visual cortical neurones were investigated in kittens that had been subjected to either unilateral or bilateral labyrinthectomy shortly after birth.
2. Two kittens were reared in a normal visual environment. Another two were reared in the dark with recurrent exposures to vertically oriented black and white stripes, which in normal kittens is known to bias the distribution of receptive field orientations.
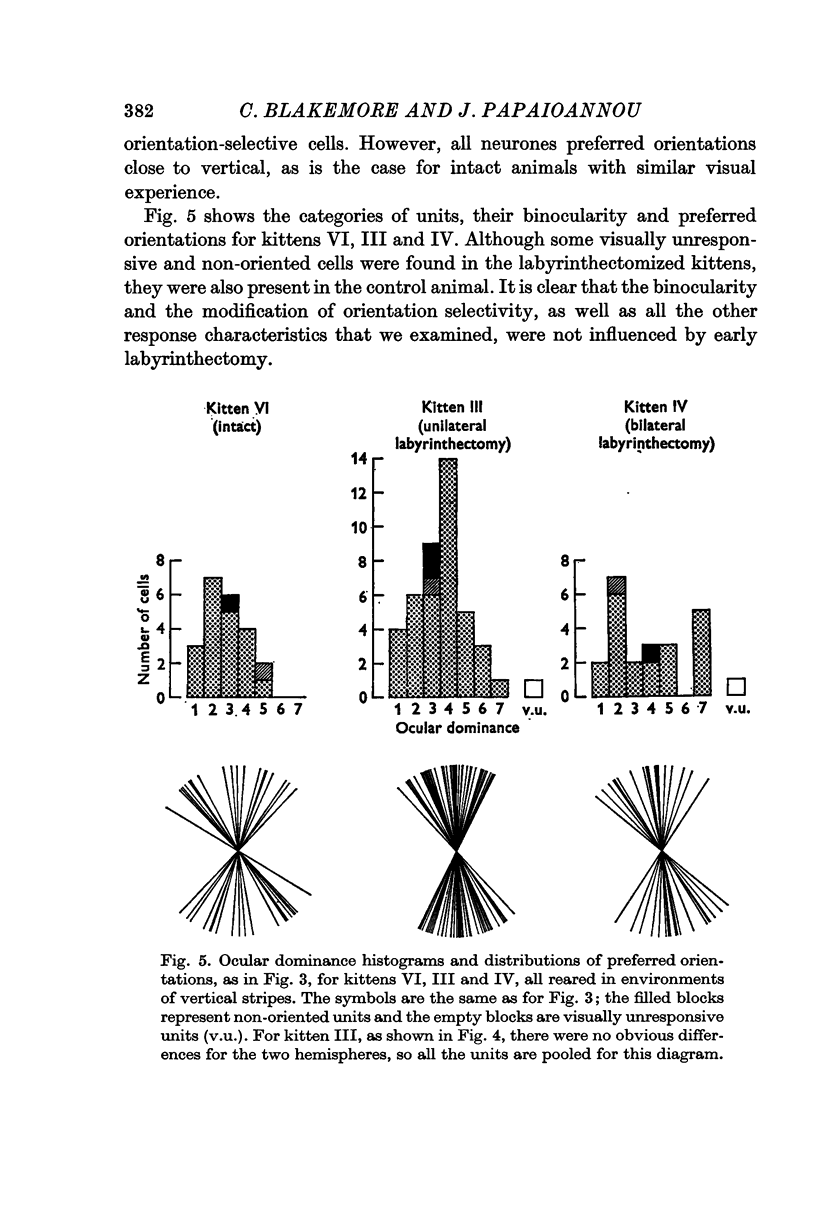
3. For both normally reared and stripe-reared labyrinthectomized kittens, no differences were detected in cell types, preferred orientations, binocularity, columnar organization, or any other neuronal properties, compared with similarly reared intact kittens.
4. The failure to detect deficits in visual development after labyrinthectomy is discussed in relation to other reports of vestibular influences on the visual system of the adult cat.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlow H. B., Pettigrew J. D. Lack of specificity of neurones in the visual cortex of young kittens. J Physiol. 1971 Oct;218 (Suppl):98P–100P. [PubMed] [Google Scholar]
- Blakemore C., Cooper G. F. Development of the brain depends on the visual environment. Nature. 1970 Oct 31;228(5270):477–478. doi: 10.1038/228477a0. [DOI] [PubMed] [Google Scholar]
- Blakemore C., Fiorentini A., Maffei L. A second neural mechanism of binocular depth discrimination. J Physiol. 1972 Nov;226(3):725–749. doi: 10.1113/jphysiol.1972.sp010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denney D., Adorjani C. Orientation specificity of visual cortical neurons after head tilt. Exp Brain Res. 1972;14(3):312–317. doi: 10.1007/BF00816165. [DOI] [PubMed] [Google Scholar]
- Fishman M. C., Michael P. Integration of auditory information in the cat's visual cortex. Vision Res. 1973 Aug;13(8):1415–1419. doi: 10.1016/0042-6989(73)90002-3. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. RECEPTIVE FIELDS OF CELLS IN STRIATE CORTEX OF VERY YOUNG, VISUALLY INEXPERIENCED KITTENS. J Neurophysiol. 1963 Nov;26:994–1002. doi: 10.1152/jn.1963.26.6.994. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch H. V., Spinelli D. N. Modification of the distribution of receptive field orientation in cats by selective visual exposure during development. Exp Brain Res. 1971 Jun 29;12(5):509–527. doi: 10.1007/BF00234246. [DOI] [PubMed] [Google Scholar]
- Hirsch H. V., Spinelli D. N. Visual experience modifies distribution of horizontally and vertically oriented receptive fields in cats. Science. 1970 May 15;168(3933):869–871. doi: 10.1126/science.168.3933.869. [DOI] [PubMed] [Google Scholar]
- Horn G., Stechler G., Hill R. M. Receptive fields of units in the visual cortex of the cat in the presence and absence of bodily tilt. Exp Brain Res. 1972;15(2):113–132. doi: 10.1007/BF00235577. [DOI] [PubMed] [Google Scholar]
- Horn G. The effect of somaesthetic and photic stimuli on the activity of units in the striate cortex of unanaesthetized, unrestrained cats. J Physiol. 1965 Jul;179(2):263–277. doi: 10.1113/jphysiol.1965.sp007661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Binocular interaction in striate cortex of kittens reared with artificial squint. J Neurophysiol. 1965 Nov;28(6):1041–1059. doi: 10.1152/jn.1965.28.6.1041. [DOI] [PubMed] [Google Scholar]
- Jacobson M. Development of specific neuronal connections. Science. 1969 Feb 7;163(3867):543–547. doi: 10.1126/science.163.3867.543. [DOI] [PubMed] [Google Scholar]
- Levick W. R. Another tungsten microelectrode. Med Biol Eng. 1972 Jul;10(4):510–515. doi: 10.1007/BF02474199. [DOI] [PubMed] [Google Scholar]
- Marchiafava P. L., Pompeiano O. Enhanced excitability of intra-geniculate optic tract endings produced by vestibular volleys. Arch Ital Biol. 1966 Dec;104(4):459–479. [PubMed] [Google Scholar]
- Morrell F. Visual system's view of acoustic space. Nature. 1972 Jul 7;238(5358):44–46. doi: 10.1038/238044a0. [DOI] [PubMed] [Google Scholar]
- Papaioannou J. N. Changes in the light-evoked discharges from lateral geniculate nucleus neurones in the cat, induced by caloric labyrinthine stimulation. Exp Brain Res. 1973 Mar 29;17(1):10–17. doi: 10.1007/BF00234560. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. J Neurophysiol. 1965 Nov;28(6):1029–1040. doi: 10.1152/jn.1965.28.6.1029. [DOI] [PubMed] [Google Scholar]


