Abstract
1. Spectrophotometric measurements, using a rapid mixing and stopped flow technique, have been made of the rate at which CO displaces O2 from its combination with haemoglobin.
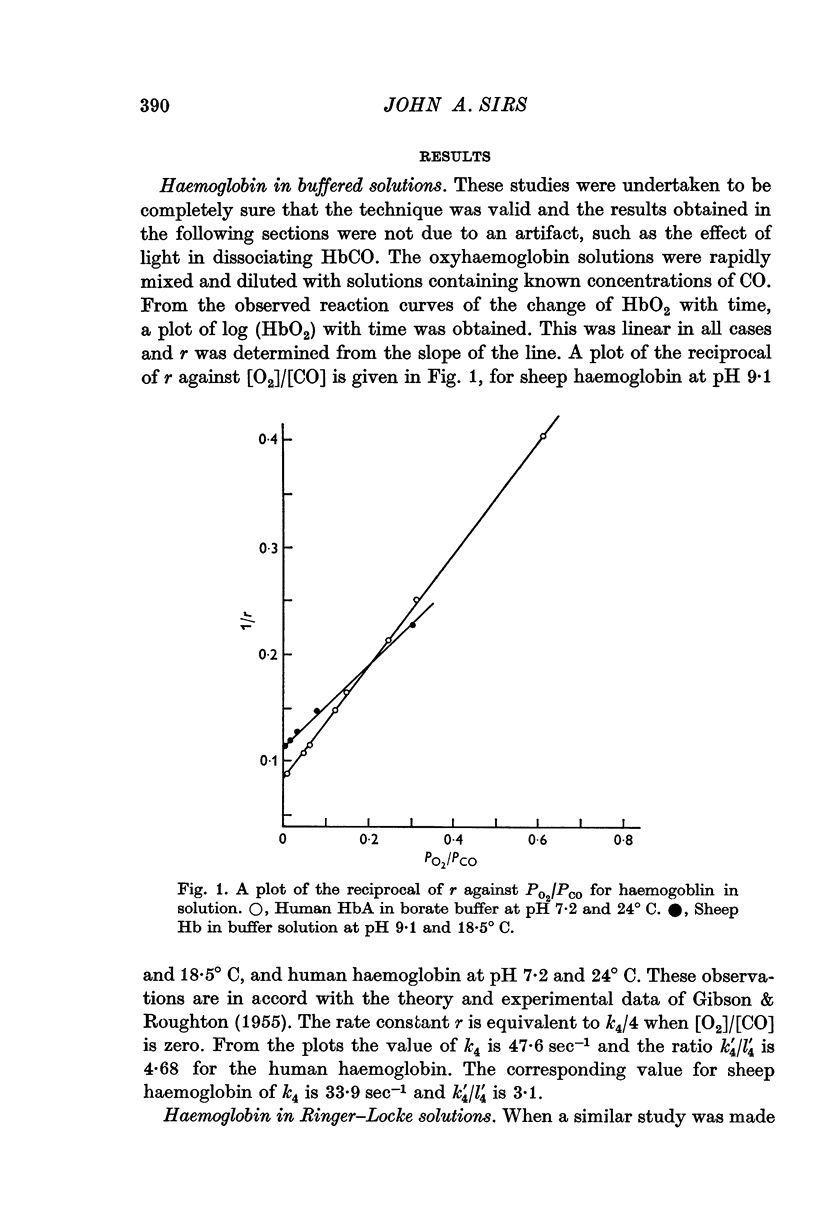
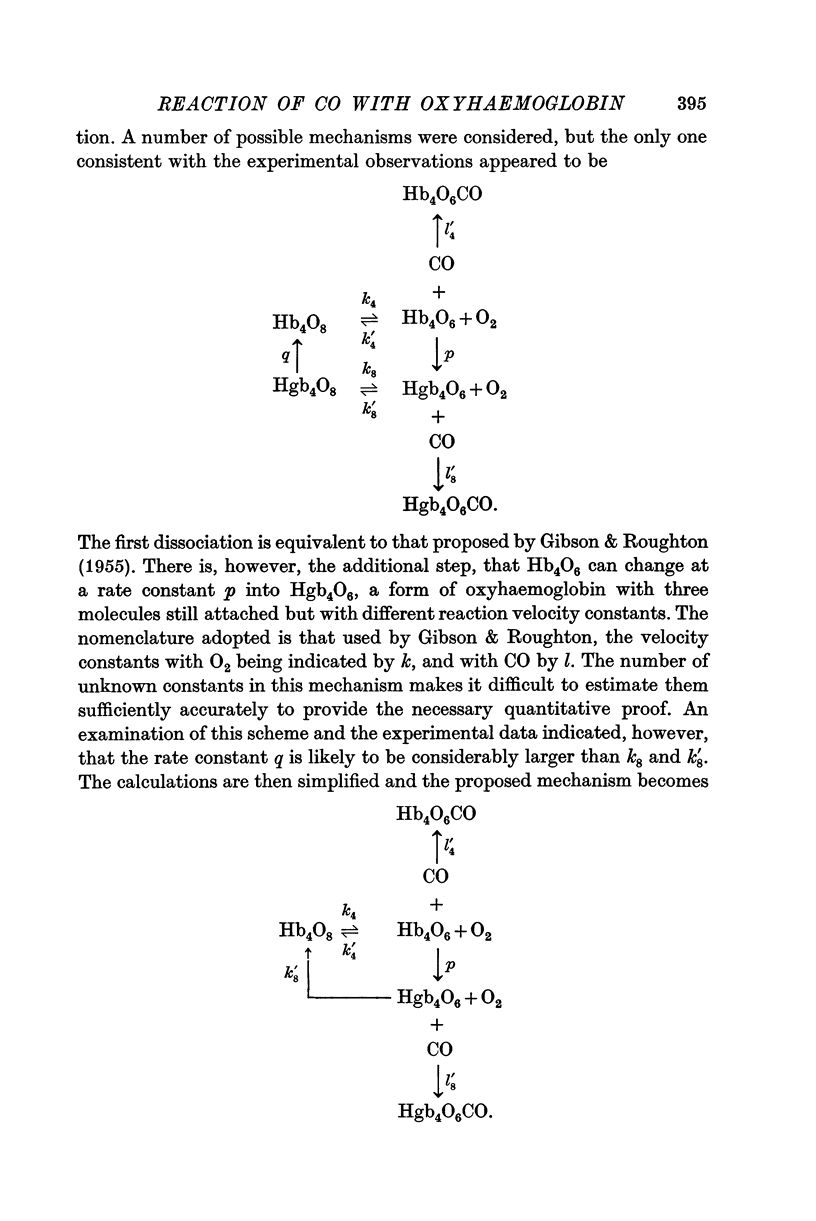
2. In haemoglobin solutions, buffered at pH 7·2 and 9·1, the reaction proceeds by a unimolecular dissociation as proposed by Gibson & Roughton (1955). In a Ringer—Locke solution, equilibrated with a PCO2 of 3 cmHg and at pH 7·4, the reaction of HbO2 with CO is a two-stage process, with a transition from one form of Hb4O6 to another.
3. An investigation of the reaction between CO and HbO2 in erythrocytes, suspended in Ringer—Locke solution, indicates that the rate is determined by the chemical reaction and this also is a two-stage process.
4. The transition is probably associated with the reaction of CO2 with Hb4O6, following the dissociation from fully saturated oxyhaemoglobin of an oxygen molecule. It alters the relative velocity constants of the reactions of O2 and CO with Hb4O6 by 100:1.
5. The implications of these proposals of the equilibria of haemoglobin with CO and O2 are discussed. The difference between the sigmoid equilibria curves at high HbO2 and HbCO values can be explained as due to the different reaction pathways.
Full text
PDF


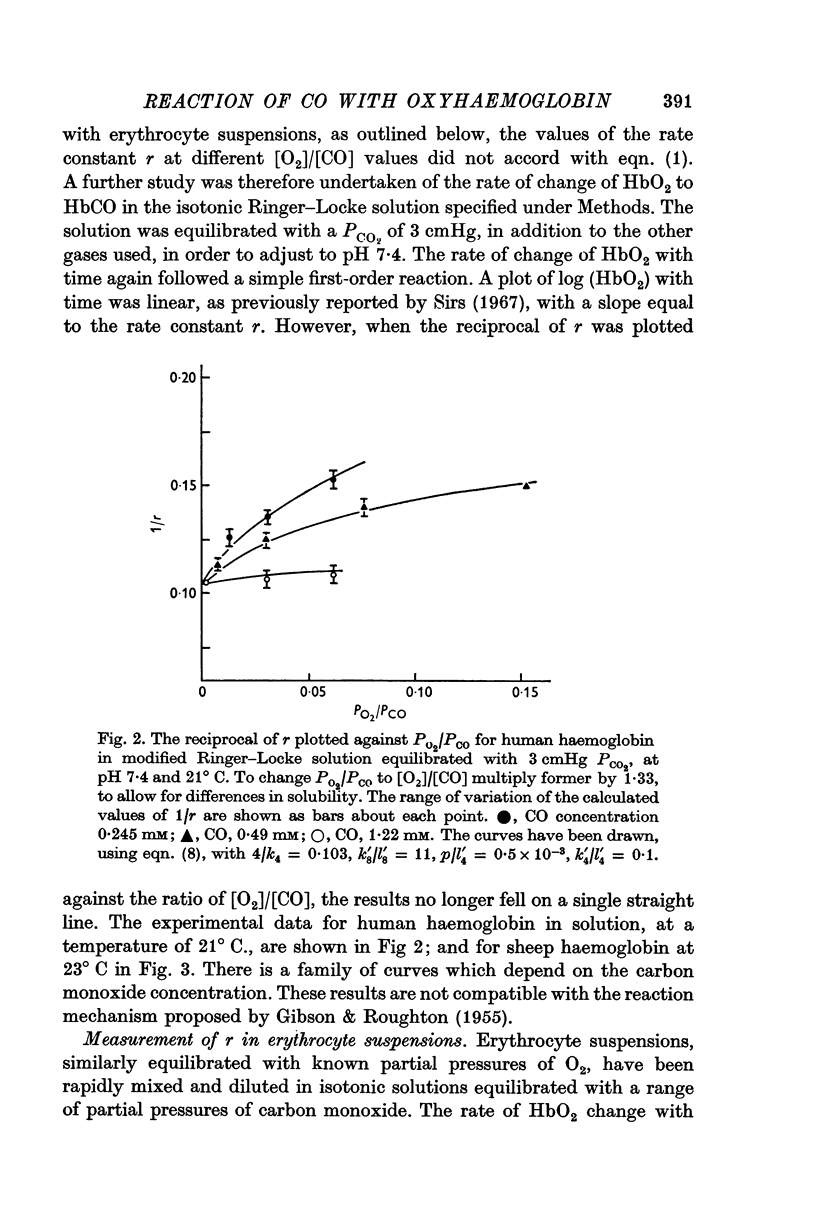







Selected References
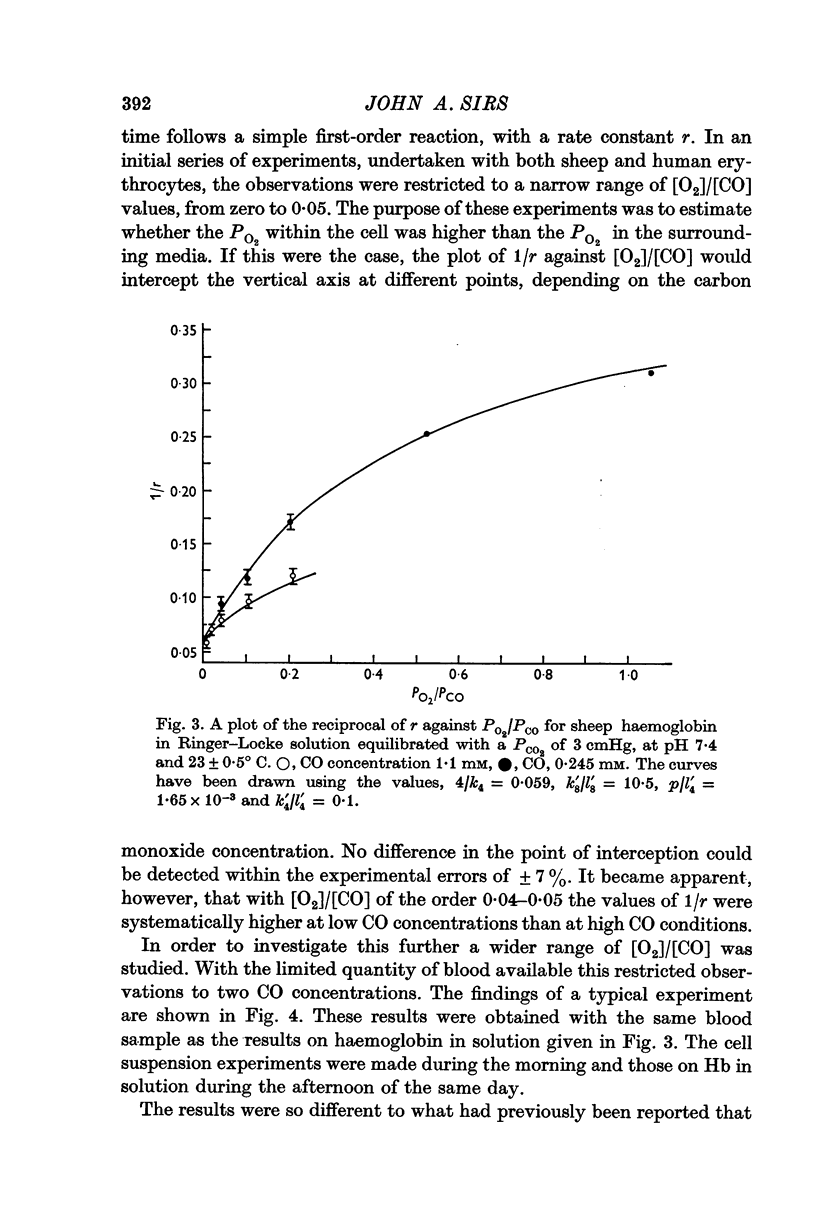
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN T. A., ROOT W. S. Partition of carbon monoxide and oxygen between air and whole blood of rats, dogs and men as affected by plasma pH. J Appl Physiol. 1957 Mar;10(2):186–190. doi: 10.1152/jappl.1957.10.2.186. [DOI] [PubMed] [Google Scholar]
- GIBSON Q. H., ROUGHTON F. J. The kinetics of dissociation of the first oxygen molecule from fully saturated oxyhaemoglobin in sheep blood solutions. Proc R Soc Lond B Biol Sci. 1955 Mar 15;143(912):310–334. doi: 10.1098/rspb.1955.0014. [DOI] [PubMed] [Google Scholar]
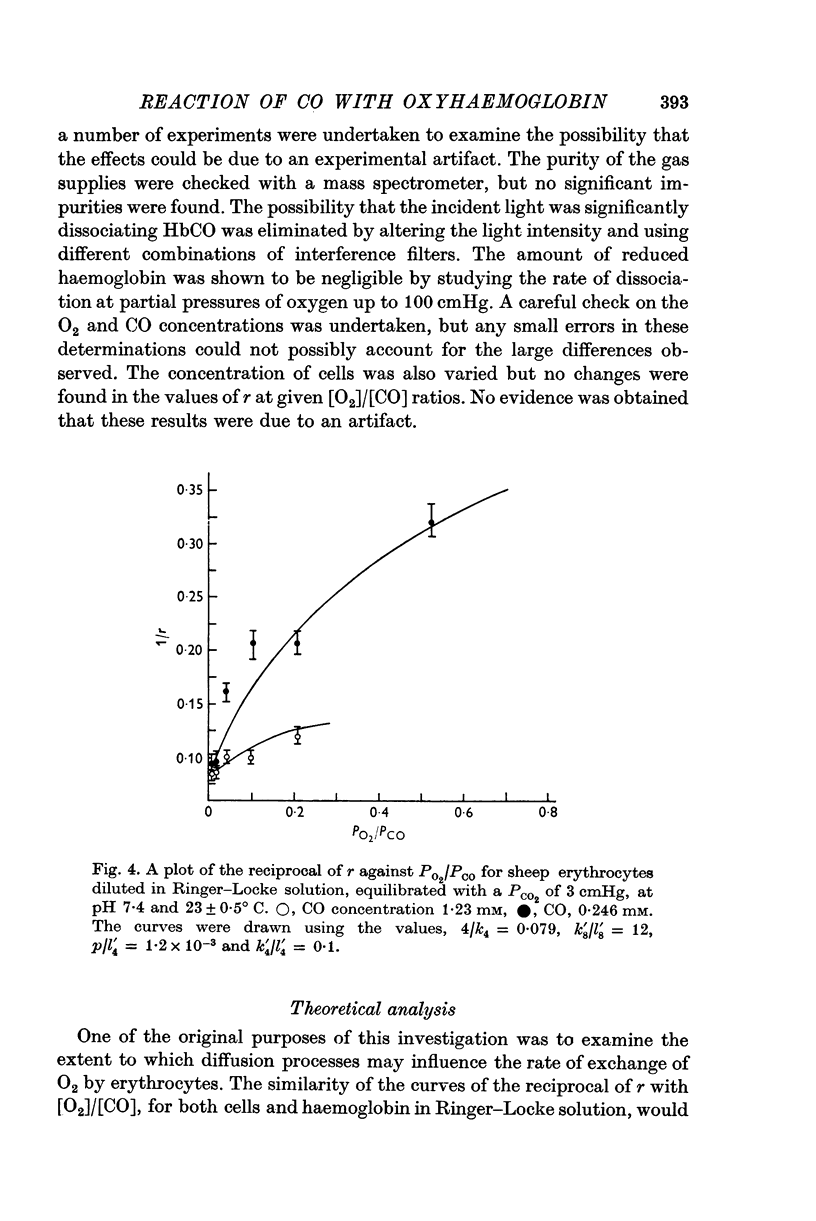
- Haldane J., Smith J. L. The Absorption of Oxygen by the Lungs. J Physiol. 1897 Nov 20;22(3):231–258. doi: 10.1113/jphysiol.1897.sp000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland R. A. Rate at which CO replaces O2 from O2Hb in red cells of different species. Respir Physiol. 1969 Jun;7(1):43–63. doi: 10.1016/0034-5687(69)90068-1. [DOI] [PubMed] [Google Scholar]
- JOELS N., PUGH L. G. The carbon monoxide dissociation curve of human blood. J Physiol. 1958 Jun 18;142(1):63–77. doi: 10.1113/jphysiol.1958.sp005999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perutz M. F. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970 Nov 21;228(5273):726–739. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- ROUGHTON F. J., FORSTER R. E., CANDER L. Rate at which carbon monoxide replaces oxygen from combination with human hemoglobin in solution and in the red cell. J Appl Physiol. 1957 Sep;11(2):269–276. doi: 10.1152/jappl.1957.11.2.269. [DOI] [PubMed] [Google Scholar]
- ROUGHTON F. J. The equilibrium between carbon monoxide and sheep haemoglobin at very high percentage saturations. J Physiol. 1954 Nov 29;126(2):359–383. doi: 10.1113/jphysiol.1954.sp005215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodkey F. L., O'Neal J. D., Collison H. A. Oxygen and carbon monoxide equilibria of human adult hemoglobin at atmospheric and elevated pressure. Blood. 1969 Jan;33(1):57–65. [PubMed] [Google Scholar]
- Rossi-Bernardi L., Roughton F. J. The specific influence of carbon dioxide and carbamate compounds on the buffer power and Bohr effects in human haemoglobin solutions. J Physiol. 1967 Mar;189(1):1–29. doi: 10.1113/jphysiol.1967.sp008152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIRS J. A., ROUGHTON F. J. Stopped-flow measurements of CO and O2 uptake by hemoglobin in sheep erythrocytes. J Appl Physiol. 1963 Jan;18:158–165. doi: 10.1152/jappl.1963.18.1.158. [DOI] [PubMed] [Google Scholar]
- Sirs J. A. The egress of oxygen from human HbO(2) in solution and in the erythrocyte. J Physiol. 1967 Apr;189(3):461–473. doi: 10.1113/jphysiol.1967.sp008179. [DOI] [PMC free article] [PubMed] [Google Scholar]


