Abstract
Alignment of the Pseudomonas aeruginosa ferric pyoverdine receptor, FpvA, with similar ferric-siderophore receptors revealed that the mature protein carries an extension of ca. 70 amino acids at its N terminus, an extension shared by the ferric pseudobactin receptors of P. putida. Deletion of fpvA from the chromosome of P. aeruginosa reduced pyoverdine production in this organism, as a result of a decline in expression of genes (e.g., pvdD) associated with the biosynthesis of the pyoverdine peptide moiety. Wild-type fpvA restored pvd expression in the mutant, thereby complementing its pyoverdine deficiency, although a deletion derivative of fpvA encoding a receptor lacking the N terminus of the mature protein did not. The truncated receptor was, however, functional in pyoverdine-mediated iron uptake, as evidenced by its ability to promote pyoverdine-dependent growth in an iron-restricted medium. These data are consistent with the idea that the N-terminal extension plays a role in FpvA-mediated pyoverdine biosynthesis in P. aeruginosa.
Iron is an essential cofactor for many microbial enzymes and, as such, is required for growth of most bacteria (20). This need for iron is, however, complicated by the low solubility and, thus, bioavailability of this element in nature (20, 45). Many bacteria overcome this problem by synthesizing high-affinity iron-chelating molecules termed siderophores that, together with cell surface receptors specific for the iron-siderophore complexes, serve to provide the organism with iron under the most nutritionally dilute conditions (7). Significantly, pathogenic organisms also encounter an iron-limited environment in the host, where siderophore-mediated iron uptake plays an equally important role in growth and, thus, pathogenesis of many disease-causing bacteria (21, 55).
Pseudomonas aeruginosa is an opportunistic human pathogen associated with infections of compromised individuals (15). The organism produces two known siderophores, pyoverdine (11) and pyochelin (10), and can utilize a number of siderophores produced by other microorganisms (9, 38, 52), which likely explains the large number of ferric-siderophore receptor homologues identified in the recently completed genome sequence (66). Production of pyoverdine has been documented in vivo (22), consistent both with the in vivo induction of pyoverdine biosynthetic genes (24) and a demonstrated role for this siderophore in promoting in vivo growth and pathogenesis (41, 48, 67). Pyoverdine is, by far, the superior iron chelator in aqueous medium (62), exhibiting a stability constant of 1024 at pH 7 (40). A mixed hydroxymate-catecholate siderophore, pyoverdine is characterized by a conserved hydroxquinoline chromophore bound to an amino acid tail of variable length and composition (8). The latter appears to be responsible for the strain specificity of pyoverdine utilization in P. aeruginosa (42). Two gene clusters involved in pyoverdine biosynthesis have been identified, which are responsible for synthesis of the chromophore (pvc) (64, 65) and peptide (pvd) (13, 25, 35, 56, 69) moieties of the siderophore, respectively. The fpvA gene (51) encoding the ferric pyoverdine receptor (50) also maps to the pvd gene cluster (37).
Pyoverdine production occurs in response to iron limitation, and its production decreases with increasing iron availability (39). This iron-regulated expression is mediated by a homologue of the Fur repressor protein shown to regulate iron-siderophore systems in Escherichia coli (4, 54). Fur only indirectly regulates pyoverdine biosynthesis in P. aeruginosa, via the alternate sigma factor PvdS (74), which positively regulates several pyoverdine biosynthetic genes (12, 36, 44) but is itself regulated by Fur (12, 36, 44, 47). More recently, a LysR-type transcriptional activator of the pvc genes, PtxR, has been described in P. aeruginosa (23, 65) and is itself regulated by PvdS (71).
An interesting feature of FpvA is its N-terminal extension that may be unique to certain receptors involved in signal transduction. Both the FecA ferric dicitrate receptor of E. coli (53, 73) and the PupB ferric pseudobactin BN7/8 receptor of Pseudomonas putida WCS358 (33) share this extension and are involved in receptor-dependent expression of the corresponding receptor genes (26, 34). Such receptor-dependent signaling of gene expression involves an atypical two-component regulatory system, including a cytoplasmic membrane-associated component (FecR and PupR) and a cytoplasmic alternate sigma factor of the extracytoplasmic factor family (FecI and PupI) (2, 34, 70). Signaling involves the novel N termini of these receptors (32, 34), which has, in the case of FecA, been shown to physically associate with the cytoplasmic membrane component FecR (14), which itself interacts with FecI (14, 63). Ligand binding to the outer membrane receptor ultimately activates or releases the alternate sigma factors, which then stimulate receptor gene expression (6). In the present study, we assessed the significance of FpvA vis-à-vis expression of genes associated with pyoverdine-mediated iron uptake and noted that pyoverdine production and expression of pyoverdine biosynthetic genes are reduced in fpvA knockouts. As expected, FpvA-mediated pyoverdine production requires the N-terminal extension of the protein.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 1. Plasmid pJSS4, a pRK415 derivative carrying the wild-type fpvA gene in the same orientation as the resident lac promoter of this vector, was constructed by releasing the gene on a ca. 4.7-kb SphI fragment from pPVR2 and, following polishing of the ends with Klenow fragment, cloning it into PstI-restricted and Klenow-treated pRK415. Plasmid JSS5 is a pRK415 derivative carrying a truncated fpvA (dubbed fpvA*) gene that encodes a receptor that retains the signal sequence but lacks 64 of the first 66 amino acids (V3 to Q66) of the mature protein. Initially, the 3′ end of the gene was recovered on a 2.4-kb EcoRI-PstI fragment from pPVR2 and cloned into EcoRI-PstI-restricted pRK415 to yield pJSS3. To eliminate the sequence encoding V3 to Q66 of the mature FpvA protein, the 5′ end of fpvA was then amplified by PCR in two parts, one upstream of the region to be deleted and one downstream. The upstream region (450 bp), which encompassed the promoter, ribosome-binding site, start codon, and signal sequence, was amplified with Vent polymerase (New England Biolabs, Inc., Mississauga, Ontario, Canada) and primers JS12 (5′-GTCACTGCAGAATTCTCAATGC-CTGGCTCGAAGCGCGAC-3′; PstI site underlined and EcoRI site in bold) and JS11 (5′-CACGAAGCTTTTCCTGCGCCTGGACATATC-3′; HindIII site underlined). The downstream region (700 bp) was amplified with Vent polymerase and primers JS13 (5′-CTGGAAGCTTGGCAATGCGATAACCATCAG-3′; HindIII site underlined) and Shen2 (5′-GGCCCTTGAATTCATGGGTAGGT-3′; EcoRI site underlined). Both reaction mixtures contained 50 ng of pPVR2 template DNA, 40 pmol of each primer, 0.2 mM each deoxynucleoside triphosphate, 2 mM MgSO4, 10% (vol/vol) dimethyl sulfoxide, and 2 U of Vent DNA polymerase in 1× thermal reaction buffer (New England Biolabs). The mixtures were heated for 2 min at 94°C and then subjected to 30 cycles of 1 min at 94°C, 30 s at 57°C, and 2 min at 72°C. Following purification of the PCR products (with a QIAquick PCR purification kit [Qiagen, Inc., Mississauga, Ontario, Canada]) and restriction digestion with PstI and HindIII (upstream fragment) or HindIII and EcoRI (downstream fragment), the appropriately deleted 5′ end of fpvA was reconstructed following a three-piece ligation with PstI-EcoRI-restricted pRK415. The 5′ end of fpvA containing the deletion was then liberated on a 1.1-kb EcoRI fragment, which was cloned into EcoRI-restricted pJSS3. One plasmid in which the 5′ and 3′ ends of fpvA* were in the proper orientation, pJSS5, expressed a FpvA protein of the expected size, as confirmed by Western immunoblotting. Nucleotide sequencing of fpvA* confirmed its proper construction.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| P. aeruginosa | ||
| K767 | Wild-type PAO1 | |
| K1660 | K767 ΔfpvA | This study |
| K1661 | K1660 attB::fpvAa | This study |
| K1662 | K1660 attB::fpvA∗a,b | This study |
| E. coli | ||
| DH5α | endA hsdR17 supE44 thi-1 recA1 gyrA relA1 Δ(lacZYA-argF)U169 deoR [Φ80dlacΔ(lacZ)M15] | (3) |
| MM294 | supE448 rfbD1 spoT1 thi-1 endA1 hsdR17 pro | (59) |
| Plasmids | ||
| pAK1900 | E. coli-P. aeruginosa shuttle cloning vector; Apr/Cbr | A. Kropinski, Queen's University |
| pPVR2 | pAK1900::fpvA | (51) |
| pEX18Tc | Broad-host-range gene replacement vector; Tcr | (27) |
| pJSS2 | pEX18Tc::ΔfpvA | This study |
| pRK2013 | Broad-host-range helper/mobilization vector; Kmr | (16) |
| pRK415 | Broad-host-range cloning vector; Tcr | (30) |
| pJSS4 | pRK415::fpvA | This study |
| pJSS5 | pRK415::fpvA∗b | This study |
| mini-CTX1 | P. aeruginosa chromosome integration vector; Tcr | (28) |
| pJSS6 | Mini-CTX1::fpvA | This study |
| pJSS8 | Mini-CTX1::fpvA∗b | This study |
| pMP190 | Broad-host-range, low-copy-number lacZ fusion vector; Cmr Smr | (60) |
| pMP190::pvdD | pMP190::pvdD-lacZ; Cmr | (57) |
Carries the fpvA genes in the chromosome at the attB site.
5′ deletion of the fpvA gene, corresponding to 64 of the first 66 amino acids of the mature protein.
The ΔfpvA derivative of PAO1 strain K767, dubbed K1660, was constructed with gene replacement vector pEX18Tc, in which sequences upstream and downstream of fpvA were cloned. Sequences upstream of the fpvA coding region were amplified by PCR with primers JS7 (5′-CAGCGAACCGCTCCATCTG-3′; anneals ca. 750 bp upstream of fpvA start codon) and JS8 (5′-CGGATCCAGGACTGAGACCGTG-3′; BamHI site underlined; anneals ca. 20 bp upstream of fpvA start codon). Sequences downstream of fpvA were amplified with primers JS9 (5′-GCGTTCTGCTTCTCGGCTAC-3′; anneals ca. 35 bp downstream of the fpvA stop codon) and JS10 (5′-GCAACCTGGCGATGGATG-3′; anneals ca. 750 bp downstream of the fpvA stop codon). Reaction mixture formulations and amplification parameters were as described above. The upstream fragment was then purified with the QIAquick-spin PCR Purification Kit (Qiagen, Inc.) and cloned into pCR-Blunt II-TOPO (Invitrogen Corp., Carlsbad, Calif.) with the Zero Blunt PCR Cloning Kit and a protocol supplied by the manufacturer. Following sequencing of the insert to ensure that no errors had been introduced by PCR, the 700-bp upstream fragment was released from the TOPO vector by digestion with EcoRI and BamHI and cloned into EcoRI-BamHI-restricted pEX18Tc to yield pJSS1. The downstream PCR product was similarly purified, cloned into pCR-Blunt II-TOPO, and sequenced. This fragment was then released by digestion with BamHI and PstI and cloned into BamHI-PstI-restricted pJSS1 to yield pJSS2. ΔfpvA-carrying pJSS2 was then introduced into E. coli strain S17-1 and mobilized into wild-type P. aeruginosa PAO1 strain K767 via conjugation as previously described (49). Cells of K767 carrying pJSS2 in the chromosome were selected on tetracycline and chloramphenicol (25 μg/ml; to counterselect the donor E. coli). Following selection on Luria-Bertani (LB) agar containing 10% (wt/vol) sucrose, sucrose-resistant colonies were screened for the presence of the fpvA deletion by PCR with primers JS7 and JS10. Loss of FpvA in K1660 was ultimately confirmed by Western immunoblotting (see below).
Introduction of wild-type or truncated fpvA into the chromosome of strain K1660 was achieved with the mini-CTX1 vector and a protocol developed by Hoang and coworkers (28). The wild-type gene was first recovered on a 4.3-kb PstI fragment from plasmid pPVR2 and cloned into PstI-restricted mini-CTX1. The resultant vector, pJSS6, was introduced into E. coli S17-1 and mobilized into K1660 via conjugation (49). Following selection on tetracycline and chloramphenicol (25 μg/ml; counterselection), fpvA was recovered in the chromosome (at the φCTX attB site) in the absence of plasmid backbone sequences with recombinase-encoding plasmid pFLP2. This plasmid was mobilized from E. coli S17-1 as described above with selection on carbenicillin. Tetracycline-sensitive, carbenicillin-resistant colonies that had lost pJSS6 plasmid backbone sequences from the chromosome were then streaked onto LB agar containing 10% (wt/vol) sucrose to recover those that had subsequently lost pFLP2. Several putative fpvA-carrying derivatives of K1600 were screened for restoration of FpvA production by Western immunoblotting.
Similarly, fpvA* was cloned into mini-CTX1, although by a two-step procedure. Initially, the 5′ end of fpvA, including the start codon, signal sequence, ribosome-binding site, and promoter regions, were recovered on a 450-bp EcoRI-HindIII fragment from pJSS5 and cloned into EcoRI-HindIII-restricted mini-CTX1 to yield pJSS7. A 3.1-kb HindIII fragment of pJSS4 carrying an fpvA gene encoding sequences beyond the first 66 amino acids of mature FpvA was then cloned into HindIII-restricted, alkaline phosphatase-treated pJSS7. Restriction analysis identified those plasmids that carried the 3′ end of fpvA in the proper orientation with respect to the 5′ end of the truncated gene, one of which was saved and designated pJSS8. This plasmid was then mobilized from E. coli S17-1, and a fpvA*-carrying K1660 derivative (dubbed K1662) was selected exactly as described above for K1661. Expression of the truncated FpvA* protein in K1662 was confirmed by Western immunoblotting.
Growth conditions.
LB broth (1% [wt/vol] Difco Tryptone, 0.5% [wt/vol] Difco yeast extract, 0.5% [wt/vol] NaCl) was used as the rich medium throughout. The iron-deficient succinate minimal medium used has been described previously (39).
Bacteria were cultured at 37°C with shaking (200 rpm). Strains were maintained on LB agar, although succinate minimal cultures were always inoculated with bacteria previously grown on succinate minimal agar. The following antibiotics were included in growth media as required: tetracycline (P. aeruginosa, 100 μg/ml; E. coli, 10 μg/ml), carbenicillin (400 μg/ml), ampicillin (100 μg/ml), kanamycin (50 μg/ml), and chloramphenicol (P. aeruginosa, 150 μg/ml; E. coli, 30 μg/ml).
DNA methods.
Standard protocols were used for restriction endonuclease digestions, ligations, treatment of DNA with Klenow fragment or calf intestinal alkaline phosphatase, and agarose gel electrophoresis (58). The alkaline lysis method (58) or a plasmid midi kit (Qiagen Inc.) was used to isolate plasmids from E. coli DH5α. DNA fragments used for cloning were extracted from agarose gels with Prep-A-Gene (Bio-Rad Laboratories, Richmond, Calif.) as recommended by the manufacturer. E. coli cells were made competent by the CaCl2 method (58) or, when supercompetent cells of E. coli were required, the method of Inoue et al. (29). Oligonucleotides for use in PCRs were chemically synthesized by Cortec DNA Services Inc., Kingston, Ontario, Canada. Nucleotide sequencing with universal or custom primers was also carried out by Cortec DNA Services Inc.
Growth assay.
P. aeruginosa grown overnight in iron-deficient succinate minimal medium was subcultured into the same medium supplemented with ethylene diamine di(ο-hydroxyphenyl)acetic acid (EDDHA; 1.5 to 3 mg/ml) to a final A600 of 0.1. In some experiments, pyoverdine (100 μg/ml) (38) was also included in the growth medium.
Pyoverdine assays.
Overnight cultures of P. aeruginosa grown in iron-deficient succinate minimal medium were harvested by centrifugation, washed twice with the same medium, and subcultured into the same medium to a final A600 of 0.1. Pyoverdine production was then assessed by measuring the A405 of 1-ml aliquots of cell-free culture supernatants (40) taken hourly during growth at 37°C. Alternatively, 1-ml cell-free samples were recovered and hydroxamate nitrogen of pyoverdine was measured by a modification of a previously described protocol (18). Values for both assays were normalized to A600 to provide a measure of per-cell pyoverdine production.
β-Galactosidase assay.
P. aeruginosa bacteria carrying pMP190 and its derivatives were grown overnight in iron-deficient succinate minimal medium supplemented with chloramphenicol. Following subculture into the same medium lacking chloramphenicol with or without FeCl3 (100 μM), cells were grown to late log phase before being harvested and assayed for β-galactosidase activity as described previously (43).
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western immunoblotting.
Whole-cell extracts (31) were subjected to sodium dodecyl sulfate-(10% [wt/vol]) polyacrylamide gel electrophoresis (61) and Western immunoblotting (75) with an FpvA-specific rabbit polyclonal antiserum (51).
RT-PCR.
RNA was prepared from strains of P. aeruginosa subcultured in succinate minimal medium with or without iron (200 μM FeCl3) supplementation to an A600 of ca. 0.7 with a Qiagen RNeasy mini kit (Qiagen) in accordance with the manufacturer's instructions. Reverse transcription (RT)-PCR was performed on 1 to 100 ng of RNA with a Qiagen OneStep RT-PCR kit (Qiagen) and primer pairs specific for pvcA (5′-CATCGAACAGGTCCAGTTGCC-3′ and 5′-ATCCTCACCAGGTCGCCGAACA-3′), pvdD (5′-GAAAGCGCGGCCTACCATA-3′ and 5′-ATCGTCGTCTCGACCTGCCGAG-3′), and rpsL (5′-GCAACTATCAACCAG-3′ and 5′-GCTGTGCTCTTGCAG-3′). Reaction mixtures were heated for 30 min at 50°C, followed by 15 min at 95°C and then 30 cycles of 45 s at 94°C, 45 s at 58°C, and 60 s at 72°C before finishing with 10 min at 72°C. RT-PCR products were stored at 4°C until visualized by agarose gel electrophoresis. RT-free PCRs were carried out on all RNA templates to control against genomic DNA contamination.
RESULTS AND DISCUSSION
Role of FpvA in pyoverdine production.
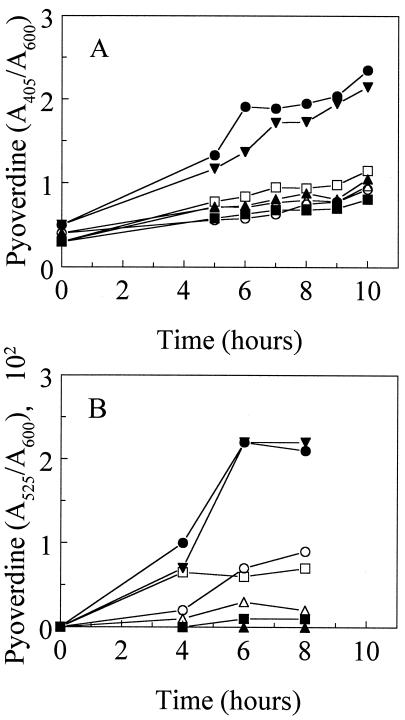
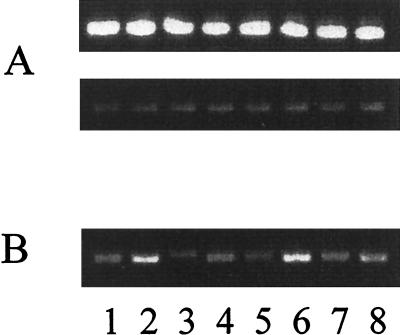
Alignment of FpvA with homologous ferric-siderophore receptors (Fig. 1) demonstrated that the mature FpvA protein, like the ferric pseudobactin receptors of P. putida, possesses an extension at the N terminus of ca. 70 amino acids that is not shared by other ferric-siderophore receptors. In the case of the PupB ferric pseudobactin BN7/BN8 receptor, this extension plays a role in PupB-mediated signal transduction associated with upregulation of pupB gene expression (34). To assess the possible involvement, therefore, of FpvA in receptor-dependent expression of fpvA, the entirety of the fpvA coding region was deleted from the chromosome of wild-type P. aeruginosa PAO1 strain K767. Intriguingly, the resultant strain, K1660, was, unlike its parent, nonfluorescent on iron-deficient minimal succinate plates, suggesting that it was deficient in pyoverdine biosynthesis. Examination of pyoverdine production as a function of growth in an iron-limited medium confirmed the pyoverdine deficiency of the FpvA-deficient K1660 strain (Fig. 2). As expected, then, K1660 also failed to grow in iron-limited minimal medium supplemented with the nonmetabolizable iron chelator EDDHA (Fig. 3), a phenotype typical of P. aeruginosa with defects in pyoverdine production or utilization. Two loci, pvd and pvc, are known to be associated with pyoverdine biosynthesis in P. aeruginosa (65, 69). Using an available pvdD-lacZ fusion vector, loss of fpvA in K1660 was correlated with an inconsistent decline in pvdD expression of twofold or less (as a measure of expression of genes of the pvd locus, which are expected to be coregulated [12, 36, 44]) (data not shown). Beare and colleagues reported a similar ca. 2.5-fold decline in expression in another pvd gene, pvdF, in an FpvA knockout, also with a lacZ reporter (P. A. Beare et al., Pseudomonas 2001, Brussels, Belgium, abstr. PS146, 2001). With RT-PCR, however, it was clear that the FpvA-deficient strain indeed exhibited reduced pvdD expression during growth under iron limitation relative to its FpvA+ parent (Fig. 4B, c.f. lanes 2 and 4). The FpvA-producing wild-type strain produced surprisingly modest levels of pvdD under iron-limiting conditions (Fig. 4, compare lane 2 with lane 1), likely reflecting the fact that RNA for RT-PCR was harvested from cells early during growth in an iron-limiting medium (at an optical density at 600 nm of 0.7), when they would be only moderately iron limited. The identification of pvdD transcripts in wild-type cells (K767) growing under iron-replete conditions (Fig. 4, lane 1), although perhaps unexpected, was consistent with the earlier demonstration that L-broth-grown (i.e., iron-replete) cells of P. aeruginosa produce some FpvA receptor protein (and other iron-regulated receptors) (46). Thus , P. aeruginosa apparently expresses these iron-regulated genes at low levels under iron-replete conditions and methods such as RT-PCR are sensitive enough to detect this.
FIG. 1.
Multiple-sequence alignment of FpvA and its homologues. Proteins related to FpvA were identified with BLAST (1) (http://www.ncbi.nlm.nih.gov/BLAST/), and the mature sequences (putative signal peptidase cleavage sites were identified by the method of Nielsen et al. [46] [http://www.cbs.dtu.dk/services/SignalP/]) were aligned with CLUSTALW (68) (http://www.ebi.ac.uk/clustalw/). Only the N termini are shown. FptA, P. aeruginosa Fe(III)-pyochelin receptor (accession number P42512); PbuA, P. putida M114 Fe(III)-pseudobactin M114 receptor (accession number Q08017); FhuE, E. coli receptor for Fe(III)-coprogen, -ferrioxamine B, and -rhodotorulic acid (accession number P16869); FauA, Bordetella pertussis Fe(III)-alcaligin receptor (accession number AAD26430); PupA, P. putida WCS358 Fe(III)-pseudobactin 358 receptor (accession number P25184); FpvA, P. aeruginosa Fe(III)-pyoverdine receptor (accession number P48632); PupB, P. putida WCS358 Fe(III)-pseudobactin BN7/BN8 receptor (accession number P38047); PA2466, probable P. aeruginosa TonB-dependent receptor (accession number D83337).
FIG. 2.
Pyoverdine production by P. aeruginosa strains K767 (•), K1660 (▴), K1660(pRK415) (□), K1660(pJSS4) (○), K1660(pJSS5) (▪), K1661 (▾), and K1662 (▵) as a function of growth in iron-deficient succinate minimal medium. Pyoverdine levels were estimated by measuring the A405 of the cell-free culture supernatant (A) and hydroxamate nitrogen at A520 (B). Values are normalized with respect to culture density, so as to provide a measure of per-cell pyoverdine production. All strains grew equally well in the minimal medium, plateauing at an A600 of ca. 1.4 after 7 h of growth. The results are representative of three independent experiments run on different days. Note that data points for K1662 in panel A are masked by data points of other strains in the lower part of the graph.
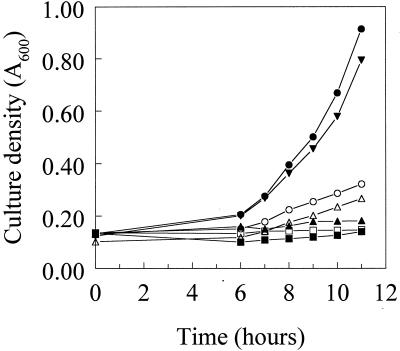
FIG. 3.
Growth of P. aeruginosa strains K767 (•), K1660 (▴), K1660(pRK415) (□), K1660(pJSS4) (○), K1660(pJSS5) (▪), K1661 (▾), and K1662 (▵) in EDDHA-supplemented (3 mg/ml) succinate minimal medium. The results are representative of three independent experiments run on different days.
FIG. 4.
rpsL (A) and pvdD (B) expression in P. aeruginosa cultured under iron-sufficient (odd-numbered lanes) and iron-deficient (even-numbered lanes) conditions measured by RT-PCR of total RNA (24 ng, top of panel A; 4 ng, bottom of panel A; 4 ng, panel B) isolated from strains K767 (lanes 1 and 2), K1660 (lanes 3 and 4), K1661 (lanes 5 and 6), and K1662 (lanes 7 and 8).
In contrast to that of pvdD, expression of the pvc genes did not appear to be altered in K1660 relative to its wild-type parent strain under conditions of iron limitation (data not shown), although the very weak expression of pvcA detected likely precluded detection of modest changes in pvc gene expression. In any case, FpvA control of pyoverdine biosynthesis occurs via the peptide biosynthetic genes at least.
Interestingly, introduction of the cloned wild-type fpvA gene (on plasmid pJSS4) into K1660 failed to restore pyoverdine production (Fig. 2) or growth on EDDHA-supplemented (i.e., iron-restricted) minimal medium (Fig. 3) despite the restored production of FpvA in the mutant (Fig. 5, lane 4). Still, introduction of this gene into the chromosome of K1660 (at the φCTX attB site with the mini-CTX1 vector of Hoang et al. [28], yielding strain K1661), which similarly restored FpvA production (Fig. 5, lane 5), did restore pyoverdine production (Fig. 2) and growth in EDDHA-supplemented medium (Fig. 3). Not unexpectedly, the restored pyoverdine production paralleled an increase in pvdD gene expression (Fig. 4B, lane 6). One explanation for the failure of the plasmid-borne fpvA gene to complement the pyoverdine and growth deficiencies of K1660 is that the presence of multiple copies of this gene, in particular its upstream promoter region, might have titrated out a positive regulator associated with expression of both fpvA and genes of pyoverdine biosynthesis. Still, a previous report showed that plasmid-borne fpvA was able to complement the growth defect of an FpvA-deficient mutant (31), although the fpvA mutant in this instance was constructed by insertional inactivation and no gene sequences were deleted. It was possible, therefore, that any growth restoration seen resulted from recombination between chromosomal fpvA sequences and the plasmid-borne gene. Screening of a P. aeruginosa gene library for sequences able to restore pyoverdine biosynthesis and growth (on EDDHA) of pJSS4-harboring K1660, in an attempt to identify such a regulatory gene, have so far failed.
FIG. 5.
Western immunoblot showing FpvA production in P. aeruginosa strains K767 (lane 1), K1660 (lane 2), K1660(pRK415) (lane 3), K1660(pJSS4) (lane 4), K1661 (lane 5), K1660(pJSS5) (lane 6), and K1662 (lane 7) grown in iron-limited succinate minimal medium. Whole-cell extracts were prepared from stationary-phase cells and probed with antibodies to FpvA following electrophoresis and electroblotting as described in Materials and Methods.
Intriguingly, the only ferric-siderophore receptors to be previously characterized with respect to their involvement in signal transduction are FecA and PupB. Unlike, FpvA, however, these receptors transport, and thus respond to, siderophores not produced by the organism itself. Presumably, this provides a way for the cell to sense the presence of these siderophores, upregulating the transport genes only when they are likely to be of use. The need to couple pyoverdine biosynthesis to receptor utilization, presumably of the ferrated siderophore, is less clear. P. aeruginosa is capable of producing two siderophores, although only one is likely needed at any given time. Perhaps the coupling of siderophore uptake via FpvA to pyoverdine biosynthesis is a way of ensuring that pyoverdine is effective at chelating and, thus, providing iron to the organism in a particular environment before investing energy in producing this siderophore. With two endogenous and potentially multiple heterologous siderophores to choose from, this would ensure that the cell invests in pyoverdine only when it is prudent to do so. In the presence, for example, of enterobactin (which is typically a better chelator than pyoverdine [19]) or under conditions in which pyochelin is the better chelator, the resultant failure of pyoverdine to acquire iron would, through its failure to interact productively with FpvA, ensure that energy is not wasted synthesizing a siderophore that is ineffective at acquiring iron. This mirrors what is seen with respect to FpvA expression, inasmuch as strains deficient in pyoverdine production exhibit reduced synthesis of this protein (17). The observation here that the levels of the N-terminally truncated FpvA* protein were noticeably reduced relative to those of wild-type FpvA (Fig. 5, compare lanes 6 and 7 with lanes 4 and 5) is therefore consistent with the reduction in pyoverdine seen in FpvA*-expressing P. aeruginosa. That pyoverdine-deficient strain K1660(pJSS4) produced wild-type levels of FpvA (Fig. 5, lane 4) likely reflects the multicopy nature of the plasmid-borne fpvA gene in this instance and promotion of fpvA gene expression from the resident lac promoter of pJSS4 parent plasmid pRK415, both of which would provide some escape from the usual pyoverdine controls.
Involvement of the N terminus in FpvA-mediated pyoverdine production.
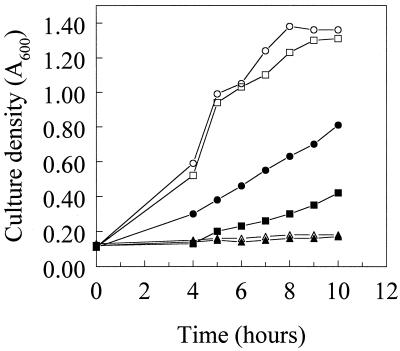
The involvement of FecA and PupB in signal transduction is intimately tied to the unique N-terminal extensions of these receptors (32, 34). To asses, then, the importance of the N terminus of FpvA vis-à-vis the receptor's involvement in pyoverdine biosynthesis, a gene encoding a truncated version of FpvA lacking 64 of the first 66 amino acids (V3 to Q66) of the mature protein (dubbed FpvA*) was constructed. Like wild-type FpvA-encoding pJSS4, FpvA*-encoding pJSS5 did not promote enhanced pyoverdine production by K1660 (Fig. 2) or growth of K1660 in the presence of EDDHA (Fig. 3), although the truncated protein was expressed (Fig. 5, lane 6). Unlike K1661, however, where introduction of the wild-type fpvA gene at the φCTX attB site of K1660 complemented the growth and pyoverdine production defects, the fpvA* gene of K1662 did not promote enhanced pyoverdine production (Fig. 2) or growth in the presence of EDDHA (Fig. 3). As expected, FpvA* was also unable to restore wild-type pvdD expression levels in K1662 (Fig. 4B, compare lane 8 with lanes 2 and 6). Nonetheless, the truncated protein was produced in K1662 (Fig. 5, lane 7) and this strain did demonstrate pyoverdine-enhanced growth in EDDHA-supplemented medium, something also seen in wild-type PAO1 strain K767 but not in FpvA-deficient stain K1660 (Fig. 6). Growth of P. aeruginosa in the presence of EDDHA is typically dependent upon the presence of a functional ferric-pyoverdine uptake system and is thus a good measure of pyoverdine-mediated iron acquisition. Thus, FpvA* is presumably competent for transport but defective with regard to its role in pyoverdine biosynthesis. An N-terminally truncated FecA receptor was similarly competent for ferric dicitrate transport but unable to induce fecA gene expression in this case (32). It is likely, therefore, that FpvA, specifically its N terminus, plays a role in a signal transduction process leading to activation of the pvd genes of pyoverdine biosynthesis. PvdS is known to activate pvdD gene expression (12), suggesting that this extracytoplasmic sigma factor mediates the influence of FpvA on pyoverdine biosynthesis. Interestingly, homologues of fecIR/pupIR have been identified in the pvd locus (PA2387 and PA2388), as revealed by the recently completed P. aeruginosa genome sequence (66). Deletion of these genes does not, however, impact pyoverdine production by P. aeruginosa (A. Redly, unpublished data).
FIG. 6.
Influence of exogenously added pyoverdine on growth of P. aeruginosa strains K767 (circles), K1660 (triangles), and K1662 (squares) in EDDHA-supplemented (1.5 mg/ml) succinate minimal medium. Filled symbols, no pyoverdine added; open symbols, 100 μg of pyoverdine added per ml.
While FpvA appears to be unique among ferric-siderophore receptors in terms of its role in regulating siderophore biosynthesis, PupA, the receptor for the endogenous pseudobactin of P. putida WCS358, also possesses an N-terminal extension (Fig. 1) and is thus anticipated to play some role in the regulation of gene expression. While this remains to be elucidated, the results of a previous study demonstrating pseudobactin 358-dependent expression of a pseudobactin 358 biosynthetic gene was suggestive of receptor (i.e., PupA) involvement, since utilization of the siderophore was probably needed for this upregulation (72). The involvement, if any, of PupA in pseudobactin 358 synthesis was not, however, examined in this study. Still, an earlier study revealed no difference in siderophore production by PupA-deficient mutants compared to the wild type (5), suggesting that pseudobactin biosynthesis in P. putida WCS358, unlike pyoverdine biosynthesis in P. aeruginosa, has no receptor involvement.
Acknowledgments
This work was supported by an operating grant from the Canadian Institutes of Health Research (formerly the Medical Research Council of Canada) to K.P. K.P. is a Canadian Cystic Fibrosis Foundation (CCFF) Scholar.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. S. Alejandro, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST ands PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angerer, A., S. Enz, M. Ochs, and V. Braun. 1995. Transcriptional regulation of ferric citrate transport in Escherichia coli K-12: Fecl belongs to a new subfamily of sigma 70-type factors that respond to extracytoplasmic stimuli. Mol. Microbiol. 18:163-174. [DOI] [PubMed] [Google Scholar]
- 3.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1992. Short protocols in molecular biology, 2nd ed. John Wiley & Sons, Inc., New York, N.Y.
- 4.Barton, H. A., Z. Johnson, A. I. Vasil, and M. L. Vasil. 1996. Ferric uptake regulator mutants of Pseudomonas aeruginosa with distinct alterations in the iron-dependent repression of exotoxin A and siderophores in aerobic and microaerobic environments. Mol. Microbiol. 21:1001-1017. [DOI] [PubMed] [Google Scholar]
- 5.Bitter, W., J. D. Marugg, L. A. de Weger, J. Tommassen, and P. J. Weisbeek. 1991. The ferric-pseudobactin receptor PupA of Pseudomonas putida: homology to TonB-dependent Escherichia coli receptors and specificity of the protein. Mol. Microbiol. 5:647-655. [DOI] [PubMed] [Google Scholar]
- 6.Braun, V. 1997. Surface signaling: novel transcription initiation mechanism starting from the cell surface. Arch. Microbiol. 167:325-331. [DOI] [PubMed] [Google Scholar]
- 7.Braun, V., and H. Killmann. 1999. Bacterial solutions to the iron-supply problem. Trends Biochem Sci 24:104-109. [DOI] [PubMed] [Google Scholar]
- 8.Budzikiewicz, H. 1993. Secondary metabolites from fluorescent pseudomonads. FEMS Microbiol. Rev. 104:209-228. [DOI] [PubMed] [Google Scholar]
- 9.Cornelis, P., N. Moguilevsky, J. F. Jacques, and P. Masson. 1987. Study of the siderophores and receptors in different clinical isolates of Pseudomonas aeruginosa, p. 290-306. In G. Doering, I. A. Holder, and K. Botzenhart (ed.), Basic research and clinical aspects of Pseudomonas aeruginosa. S. Karger, Basel, Switzerland. [DOI] [PubMed]
- 10.Cox, C. D. 1980. Iron uptake with ferripyochelin and ferric citrate by Pseudomonas aeruginosa. J. Bacteriol. 142:581-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox, C. D., and P. Adams. 1985. Siderophore activity of pyoverdin for Pseudomonas aeruginosa. Infect. Immun. 48:130-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cunliffe, H. E., T. R. Merriman, and I. L. Lamont. 1995. Cloning and characterization of pvdS, a gene required for pyoverdine synthesis in Pseudomonas aeruginosa: PvdS is probably an alternate sigma factor. J. Bacteriol. 177:2744-2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enard, C., A. Diolez, and D. Expert. 1988. Systemic virulence of Erwinia chrysanthemi 3937 requires a functional iron assimilation system. J. Bacteriol. 170:2419-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Enz, S., S. Mahren, U. H. Stroeher, and V. Braun. 2000. Surface signaling in ferric citrate transport gene induction: interaction of the FecA, FecR, and FecI regulatory proteins. J. Bacteriol. 182:637-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fick, R. B., Jr. 1993. Pseudomonas aeruginosa the opportunist: pathogenesis and disease. CRC Press, Inc., Boca Raton, Fla.
- 16.Figurski, D. H., and E. R. Helinski. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. USA 76:1648-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gensberg, K., K. Hughes, and A. W. Smith. 1992. Siderophore-specific induction of iron uptake in Pseudomonas aeruginosa. J. Gen. Microbiol. 138:2381-2387. [DOI] [PubMed] [Google Scholar]
- 18.Gillam, A. H., A. G. Lewis, and R. J. Andersen. 1981. Quantitative determination of hydroxamic acids. Anal. Chem. 53:841-844. [Google Scholar]
- 19.Griffiths, E. 1987. The iron-uptake systems of pathogenic bacteria, p. 69-137. In J. J. Bullen and E. Griffiths (ed.), Iron and infection. John Wiley & Sons, New York, N.Y.
- 20.Griffiths, E. 1999. Iron in biological systems, p. 1-26. In J. J. Bullen and E. Griffiths (ed.), Iron and infection: molecular, physiological and clinical aspects. John Wiley & Sons, Inc., New York, N.Y.
- 21.Griffiths, E., and P. Williams. 1999. The iron-uptake systems of pathogenic bacteria, fungi and protozoa, p. 87-212. In J. J. Bullen and E. Griffiths (ed.), Iron and infection: molecular, physiological and clinical aspects. John Wiley & Sons, Inc., New York, N.Y.
- 22.Haas, B., J. Kraut, J. Marks, S. C. Zanker, and D. Castignetti. 1991. Siderophore presence in sputa of cystic fibrosis patients. Infect. Immun. 59:3997-4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamood, A. N., J. A. Colmer, U. A. Ochsner, and M. L. Vasil. 1996. Isolation and characterization of a Pseudomonas aeruginosa gene, ptxR, which positively regulates exotoxin A production. Mol. Microbiol. 21:97-110. [DOI] [PubMed] [Google Scholar]
- 24.Handfield, M., D. E. Lehoux, F. Sanschagrin, M. J. Mahan, D. E. Woods, and R. C. Levesque. 2000. In vivo-induced genes in Pseudomonas aeruginosa. Infect. Immun. 68:2359-2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hantke, K. 1983. Identification of an iron uptake system specific for coprogen and rhodotorulic acid in Escherichia coli K12. Mol. Gen. Genet. 191:301-306. [DOI] [PubMed] [Google Scholar]
- 26.Harle, C., I. Kim, A. Angerer, and V. Braun. 1995. Signal transfer through three compartments: transcription initiation of the Escherichia coli ferric citrate transport system from the cell surface. EMBO J. 14:1430-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoang, T. T., R. R. Karkhoff-Schweizer, A. J. Kutchma, and H. P. Schweizer. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77-86. [DOI] [PubMed] [Google Scholar]
- 28.Hoang, T. T., A. J. Kutchma, A. Becher, and H. P. Schweizer. 2000. Integration-proficient plasmids for Pseudomonas aeruginosa: site-specific integration and use for engineering of reporter and expression strains. Plasmid 43:59-72. [DOI] [PubMed] [Google Scholar]
- 29.Inoue, H., H. Nojima, and H. Okayama. 1991. High efficiency transformation of Escherichia coli with plasmids. Gene 96:23-28. [DOI] [PubMed] [Google Scholar]
- 30.Keen, N. T., S. Tamaki, D. Kobayashi, and D. Trollinger. 1988. Improved broad-host-range plasmids for DNA cloning in Gram-negative bacteria. Gene 70:191-197. [DOI] [PubMed] [Google Scholar]
- 31.Kilburn, L., K. Poole, J.-M. Meyer, and S. Neshat. 1998. Insertion mutagenesis of the ferric pyoverdine receptor FpvA of Pseudomonas aeruginosa: identification of permissive sites and a region important for ligand binding. J. Bacteriol. 180:6753-6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim, I., A. Stiefel, S. Plantoer, A. Angerer, and V. Braun. 1997. Transcription induction of the ferric citrate transport genes via the N-terminus of the FecA outer membrane protein, the Ton system and the electrochemical potential of the cytoplasmic membrane. Mol. Microbiol. 23:333-344. [DOI] [PubMed] [Google Scholar]
- 33.Koster, M., J. van de Vossenberg, J. Leong, and P. J. Weisbeek. 1993. Identification and characterization of the pupB gene encoding an inducible ferric-pseudobactin receptor of Pseudomonas putida WCS358. Mol. Microbiol. 8:591-601. [DOI] [PubMed] [Google Scholar]
- 34.Koster, M., W. van Klompenburg, W. Bitter, J. Leong, and P. Weisbeek. 1994. Role for the outer membrane ferric siderophore receptor PupB in signal transduction across the bacterial cell envelope. EMBO J. 13:2805-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lehoux, D. E., F. Sanschagrin, and R. C. Levesque. 2000. Genomics of the 35-kb pvd locus and analysis of novel pvdIJK genes implicated in pyoverdine biosynthesis in Pseudomonas aeruginosa. FEMS Microbiol. Lett. 190:141-146. [DOI] [PubMed] [Google Scholar]
- 36.Leoni, L., A. Ciervo, N. Orsi, and P. Visca. 1996. Iron-regulated transcription of the pvdA gene in Pseudomonas aeruginosa: effect of Fur and PvdS on promoter activity. J. Bacteriol. 178:2299-2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Merriman, T. R., M. E. Merriman, and I. L. Lamont. 1995. Nucleotide sequence of pvdD, a pyoverdine biosynthetic gene from Pseudomonas aeruginosa: PvdD has similarity to peptide synthetases. J. Bacteriol. 177:252-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyer, J.-M. 1992. Exogenous siderophore-mediated iron uptake in Pseudomonas aeruginosa: possible involvement of porin OprF in iron translocation. J. Gen. Microbiol. 138:951-958. [DOI] [PubMed] [Google Scholar]
- 39.Meyer, J.-M., and M. A. Abdallah. 1978. The fluorescent pigment of Pseudomonas fluorescens: biosynthesis, purification and physiochemical properties. J. Gen. Microbiol. 107:319-328. [Google Scholar]
- 40.Meyer, J.-M., F. Halle, D. Hohnadel, P. Lemanceau, and H. Ratefiarivelo. 1987. Siderophores of Pseudomonas—biological properties, p. 189-205. In G. Winkelmann, D. van der Helm, and J. B. Neilands (ed.), Iron transport in microbes, plants and animals. VCH Verlagsgesellschaft mbH, Weinheim, Germany.
- 41.Meyer, J.-M., A. Neely, A. Stintzi, C. Georges, and I. A. Holder. 1996. Pyoverdine is essential for virulence of Pseudomonas aeruginosa. Infect. Immun. 64:518-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meyer, J. M. 2000. Pyoverdines: pigments, siderophores and potential taxonomic markers of fluorescent Pseudomonas species. Arch. Microbiol. 174:135-142. [DOI] [PubMed] [Google Scholar]
- 43.Miller, J. H. 1992. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor Laboratory Press, Plainview, N.Y.
- 44.Miyazaki, H., H. Kato, T. Nakazawa, and M. Tsuda. 1995. A positive regulatory gene, pvdS, for expression of pyoverdin biosynthetic genes in Pseudomonas aeruginosa PAO. Mol. Gen. Genet. 248:17-24. [DOI] [PubMed] [Google Scholar]
- 45.Neilands, J. B., K. Konopka, B. Schwyn, M. Coy, R. T. Francis, B. H. Paw, and A. Bagg. 1987. Comparative biochemistry of microbial iron assimilation, p. 3-33. In G. Winkelmann, D. van der Helm, and J. B. Neilands (ed.), Iron transport in microbes, plants and animals. VCH Verlagsgesellschaft mbH, Weinheim, Germany.
- 46.Nielsen, H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10:1-6. [DOI] [PubMed] [Google Scholar]
- 46a.Nouwens, A. S., S. J. Cordwell, M. R. Larsen, M. P. Molloy, M. Gillings, M. D. Willcox, and B. J. Walsh. 2000. Complementing genomics with proteomics: the membrane subproteome of Pseudomonas aeruginosa PAO1. Electrophoresis 21:3797-3809. [DOI] [PubMed] [Google Scholar]
- 47.Ochsner, U. A., Z. Johnson, I. L. Lamont, H. E. Cunliffe, and M. L. Vasil. 1996. Exotoxin A production in Pseudomonas aeruginosa requires the iron-regulated pvds gene encoding an alternative sigma factor. Mol. Microbiol. 21:1019-1028. [DOI] [PubMed] [Google Scholar]
- 48.Poole, K., C. Dean, D. Heinrichs, S. Neshat, K. Krebes, L. Young, and L. Kilburn. 1996. Siderophore-mediated iron transport in Pseudomonas aeruginosa, p. 371-383. In T. Nakazawa, K. Furukawa, D. Haas, and S. Silver (ed.), Molecular biology of pseudomonads. American Society for Microbiology, Washington, D.C.
- 49.Poole, K., D. E. Heinrichs, and S. Neshat. 1993. Cloning and sequence analysis of an EnvCD homologue in Pseudomonas aeruginosa: regulation by iron and possible involvement in the secretion of the siderophore pyoverdine. Mol. Microbiol. 10:529-544. [DOI] [PubMed] [Google Scholar]
- 50.Poole, K., S. Neshat, and D. Heinrichs. 1991. Pyoverdine-mediated iron transport in Pseudomonas aeruginosa: involvement of a high-molecular-mass outer membrane protein. FEMS Microbiol. Lett. 78:1-5. [PubMed] [Google Scholar]
- 51.Poole, K., S. Neshat, K. Krebes, and D. E. Heinrichs. 1993. Cloning and nucleotide sequence analysis of the ferripyoverdine receptor gene fpvA of Pseudomonas aeruginosa. J. Bacteriol. 175:4597-4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poole, K., L. Young, and S. Neshat. 1990. Enterobactin-mediated iron transport in Pseudomonas aeruginosa. J. Bacteriol. 172:6991-6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pressler, U., H. Staudenmaier, L. Zimmermann, and V. Braun. 1988. Genetics of the iron dicitrate transport system of Escherichia coli. J. Bacteriol. 170:2716-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prince, R. W., C. D. Cox, and M. L. Vasil. 1993. Coordinate regulation of siderophore and exotoxin A production: molecular cloning and sequencing of the Pseudomonas aeruginosa fur gene. J. Bacteriol. 175:2589-2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ratledge, C., and L. G. Dover. 2000. Iron metabolism in pathogenic bacteria. Annu. Rev. Microbiol. 54:881-941. [DOI] [PubMed] [Google Scholar]
- 56.Rogers, H. J. 1973. Iron-binding catechols and virulence in Escherichia coli. Infect. Immun. 7:445-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rombel, I. T., B. J. McMorran, and I. L. Lamont. 1995. Identification of a DNA sequence motif required for expression of iron-regulated genes in pseudomonads. Mol. Gen. Genet. 246:519-528. [DOI] [PubMed] [Google Scholar]
- 58.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 59.Schleif, R. 1987. The l-arabinose operon, p. 1473-1481. In F. Neidhardt, J. Ingraham, K. B. Low, B. Magasanik, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium, vol. 2. American Society for Microbiology, Washington, D.C.
- 60.Spaink, H. P., R. J. H. Okker, C. A. Wijffelman, E. Pees, and B. J. J. Lugtenberg. 1987. Promoters in the nodulation region of the Rhizobium leguminosarum Sym plasmid pRL1J1. Plant Mol. Biol. 9:27-39. [DOI] [PubMed] [Google Scholar]
- 61.Srikumar, R., T. Kon, N. Gotoh, and K. Poole. 1998. Expression of Pseudomonas aeruginosa multidrug efflux pumps MexA-MexB-OprM and MexC-MexD-OprJ in a multidrug-sensitive Escherichia coli strain. Antimicrob. Agents Chemother. 42:65-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sriyosachati, S., and C. D. Cox. 1986. Siderophore-mediated iron acquisition from transferrin by Pseudomonas aeruginosa. Infect. Immun. 52:885-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stiefel, A., S. Mahren, M. Ochs, P. T. Schindler, S. Enz, and V. Braun. 2001. Control of the ferric citrate transport system of Escherichia coli: mutations in region 2.1 of the FecI extracytoplasmic-function sigma factor suppress mutations in the FecR transmembrane regulatory protein. J. Bacteriol. 183:162-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stintzi, A., P. Cornelis, D. Hohnadel, J.-M. Meyer, C. Dean, K. Poole, S. Kourambas, and V. Krishnapillai. 1996. Novel pyoverdine biosynthesis gene(s) of Pseudomonas aeruginosa PAO. Microbiology 142:1181-1190. [DOI] [PubMed] [Google Scholar]
- 65.Stintzi, A., Z. Johnson, M. Stonehouse, U. Ochsner, J. M. Meyer, M. L. Vasil, and K. Poole. 1999. The pvc gene cluster of Pseudomonas aeruginosa: role in synthesis of the pyoverdine chromophore and regulation by PtxR and PvdS. J. Bacteriol. 181:4118-4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, I. T. Paulsen, J. Reizer, M. H. Saier, R. E. W. Hancock, S. Lory, and M. V. Olson. 200l. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 67.Takase, H., H. Nitanai, K. Hoshino, and T. Otani. 2000. Impact of siderophore production on Pseudomonas aeruginosa infections in immunosuppressed mice. Infect. Immun. 68:1834-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thompson, J. D., D. J. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsuda, M., H. Miyazaki, and T. Nakazawa. 1995. Genetic and physical mapping of genes involved in pyoverdin production in Pseudomonas aeruginosa PAO. J. Bacteriol. 177:423-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Van Hove, B., H. Staudenmaier, and V. Braun. 1990. Novel two-component transmembrane transcription control: regulation of iron dicitrate transport in Escherichia coli K-12. J. Bacteriol. 172:6749-6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vasil, M. L., U. A. Ochsner, Z. Johnson, J. A. Colmer, and A. N. Hamood. 1998. The Fur-regulated gene encoding the alternative sigma factor, PvdS, is required for the iron-dependent expression of the LysR-type regulator, PtxR, in Pseudomonas aeruginosa. J. Bacteriol. 180:6784-6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Venturi, V., C. Ottevanger, M. Bracke, and P. Weisbeek. 1995. Iron regulation of siderophore biosynthesis and transport in Pseudomonas putida WCS358: involvement of a transcriptional activator and of the Fur protein. Mol. Microbiol. 15:1081-1093. [DOI] [PubMed] [Google Scholar]
- 73.Wagegg, W., and V. Braun. 1981. Ferric citrate transport in Escherichia coli requires outer membrane receptor protein FecA. J. Bacteriol. 145:156-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wilson, M. J., and I. L. Lamont. 2000. Characterization of an ECF sigma factor protein from Pseudomonas aeruginosa. Biochem. Biophys. Res. Commun. 273:578-583. [DOI] [PubMed] [Google Scholar]
- 75.Zhao, Q., X.-Z. Li, A. Mistry, R. Srikumar, L. Zhang, O. Lomovskaya, and K. Poole. 1998. Influence of the TonB energy-coupling protein on efflux-mediated multidrug resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 42:2225-2231. [DOI] [PMC free article] [PubMed] [Google Scholar]