Abstract
1. A method was developed for locating the rostral part of the medial lemniscus in anaesthetized cats and then exploring it with a micro-electrode selective for single axons. Records were made from 165 axons, all shown histologically to lie in the lemniscus.
2. Almost all lemniscal axons responded at short latency to a shock through surface electrodes over the dorsal columns at C2. The great majority probably belonged to the dorsal column-lemniscal system, though some may have belonged to other (e.g. spinocervicothalamic) systems.
3. Resting discharge was seen in almost all axons in the absence of any stimulation, and must have been generated almost entirely in the relevant relay nuclei, particularly since in many axons it was easily depressed or totally inhibited by appropriately placed mechanical stimulation of skin or a shock to the dorsal columns.
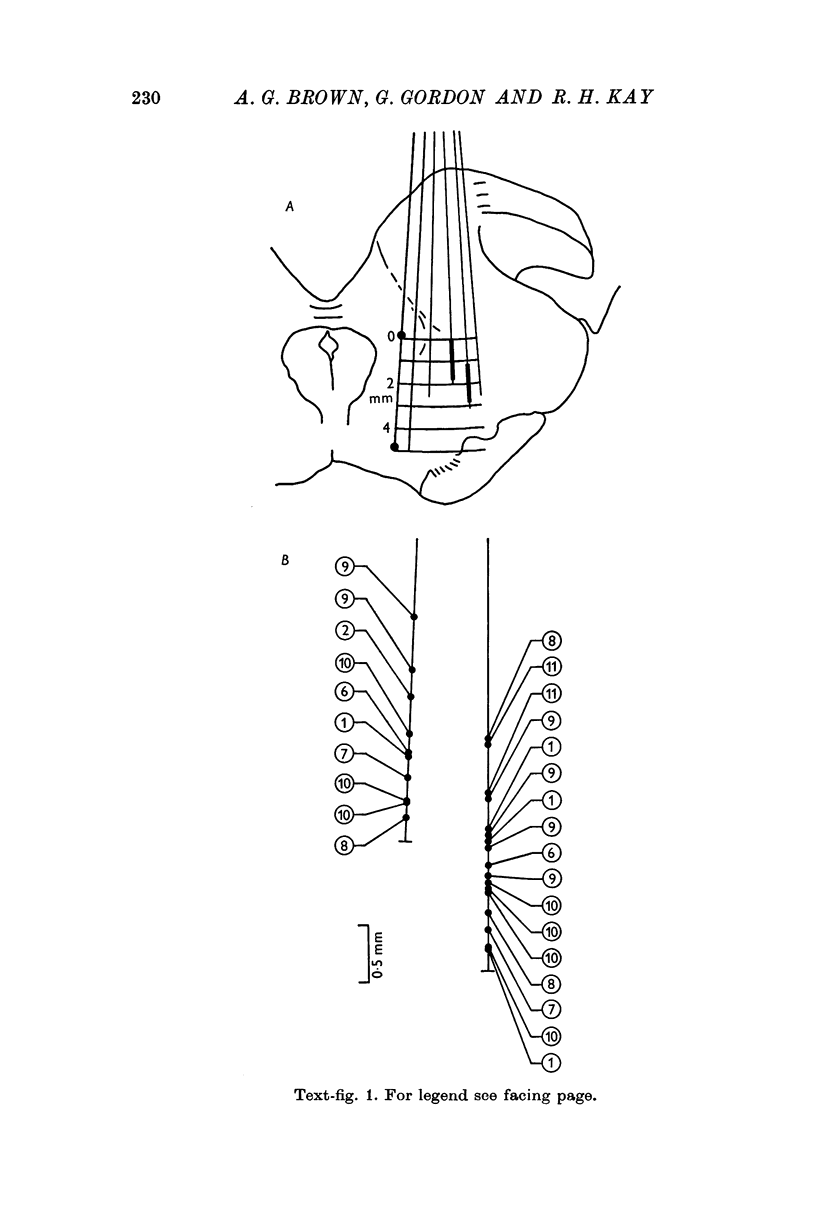
4. For each fibre held for an adequate length of time, the receptive field, if accessible, was classified as accurately as possible. Fifty-two axons were precisely categorized in this way: many more were studied for long enough to yield useful information.
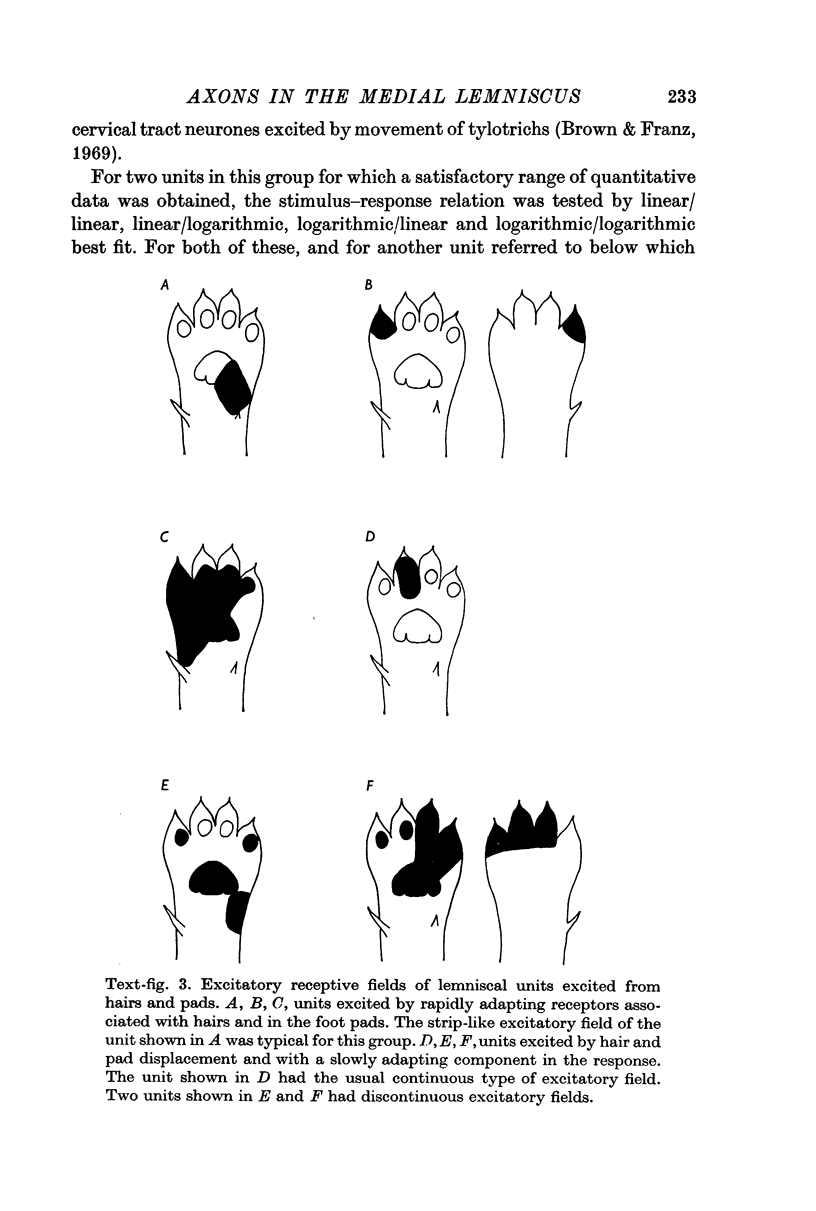
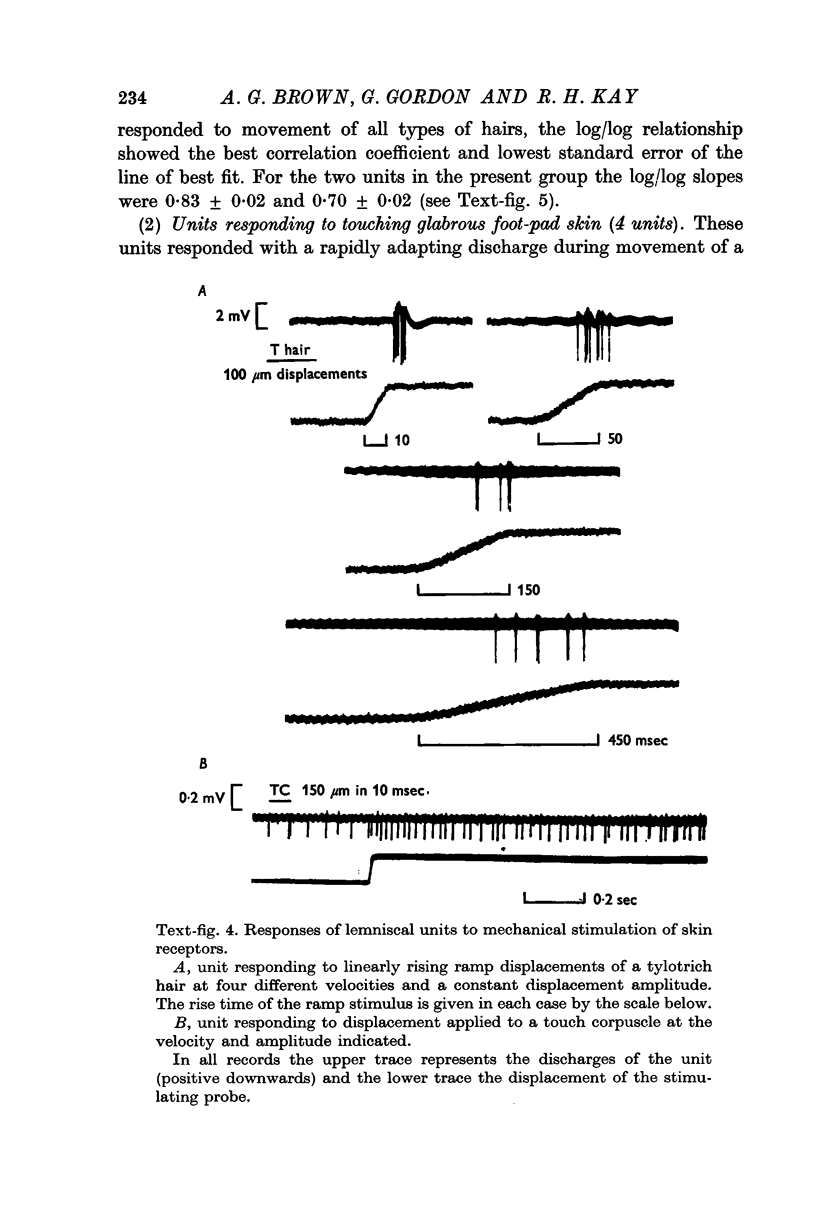
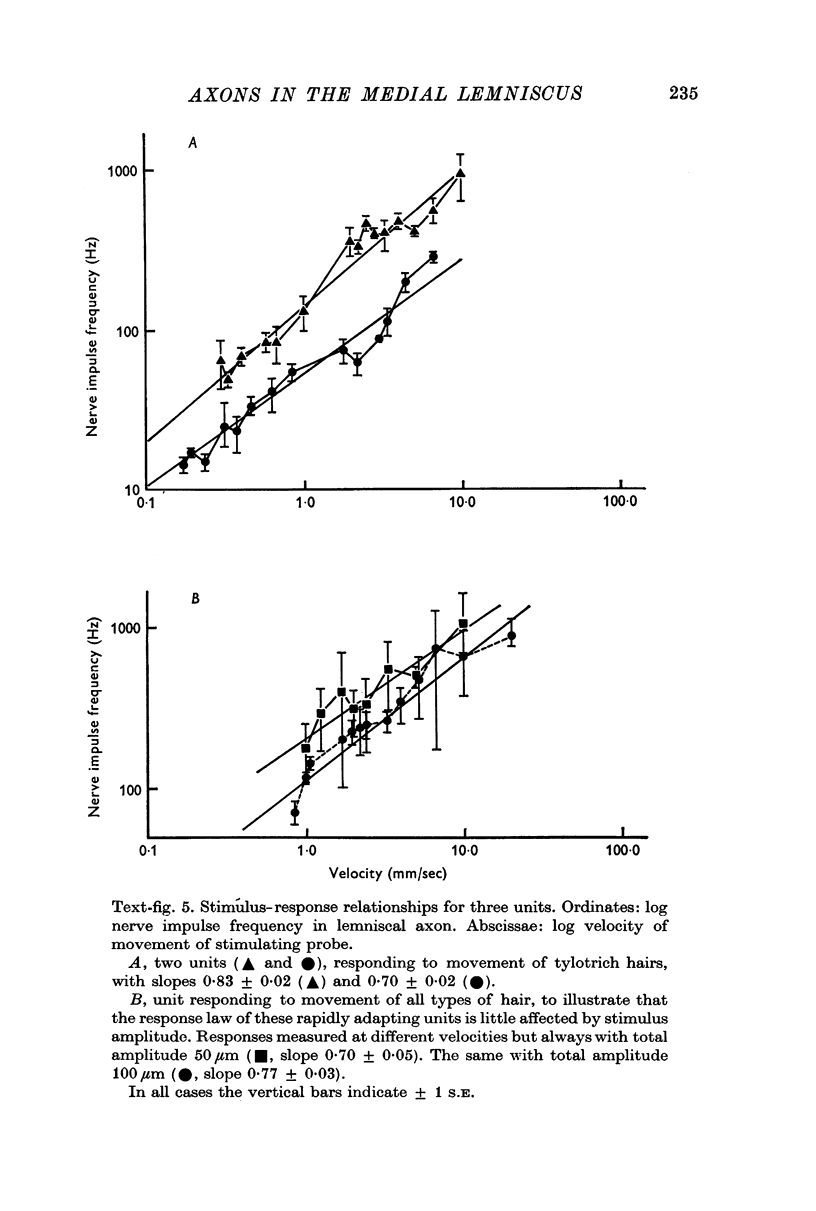
5. One half (twenty-six) of the best categorized axons had receptive fields suggesting excitation by only one type of receptor: fifteen by tylotrich hairs, four by rapidly adapting tactile foot pad receptors, two by claw movement, two by cutaneous touch corpuscles and three by Type II cutaneous receptors. Rigidly held probes driven by electromechanical transducers were used to establish stimulus/response relations. Adjacent or surround inhibition was seen in nearly all these fields, except for the Type II category.
6. The other half (twenty-six) of the best categorized axons showed various degrees of inter-receptive excitatory convergence. Five responded to all types of hair, twelve to hair movement and foot-pad displacement, and nine to hair movement combined with inputs from a variety of slowly adapting receptors in skin or deep tissues, thresholds for the latter ranging from light contact to noxious pressure.
7. Forty axons responded with a slowly adapting discharge to joint movement, some with properties suggesting that their receptors did not lie in the joint capsule itself. The high threshold of most of these axons to dorsal column stimulation suggested that the relevant primary axons lay either deep in the dorsal column or in some other tract.
8. Of axons whose receptive fields were accurately located, 88% lay in forelimb or upper trunk — the remainder in lower trunk, hind limb or tail. The forepaw accounted for 41% of the former group. Axons with receptively `pure' properties tended to lie in central or deep parts of the main lemniscal mass at the level studied. Axons responding to joint movement tended to lie deep in the main mass and in the ventromedial lemniscal bundle. There was some clustering of axons with identical receptive properties.
Full text
PDF

















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown A. G. Cutaneous afferent fibre collaterals in the dorsal columns of the cat. Exp Brain Res. 1968;5(4):293–305. doi: 10.1007/BF00235904. [DOI] [PubMed] [Google Scholar]
- Brown A. G. Effects of descending impulses on transmission through the spinocervical tract. J Physiol. 1971 Dec;219(1):103–125. doi: 10.1113/jphysiol.1971.sp009652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Franz D. N. Responses of spinocervical tract neurones to natural stimulation of identified cutaneous receptors. Exp Brain Res. 1969;7(3):231–249. doi: 10.1007/BF00239031. [DOI] [PubMed] [Google Scholar]
- Brown A. G., Iggo A. A quantitative study of cutaneous receptors and afferent fibres in the cat and rabbit. J Physiol. 1967 Dec;193(3):707–733. doi: 10.1113/jphysiol.1967.sp008390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess P. R., Perl E. R. Myelinated afferent fibres responding specifically to noxious stimulation of the skin. J Physiol. 1967 Jun;190(3):541–562. doi: 10.1113/jphysiol.1967.sp008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess P. R., Petit D., Warren R. M. Receptor types in cat hairy skin supplied by myelinated fibers. J Neurophysiol. 1968 Nov;31(6):833–848. doi: 10.1152/jn.1968.31.6.833. [DOI] [PubMed] [Google Scholar]
- Chambers M. R., Andres K. H., von Duering M., Iggo A. The structure and function of the slowly adapting type II mechanoreceptor in hairy skin. Q J Exp Physiol Cogn Med Sci. 1972 Oct;57(4):417–445. doi: 10.1113/expphysiol.1972.sp002177. [DOI] [PubMed] [Google Scholar]
- Dart A. M., Gordon G. Some properties of spinal connections of the cat's dorsal column nuclei which do not involve the dorsal columns. Brain Res. 1973 Aug 17;58(1):61–68. doi: 10.1016/0006-8993(73)90823-8. [DOI] [PubMed] [Google Scholar]
- GORDON G., JUKES M. G. DUAL ORGANIZATION OF THE EXTEROCEPTIVE COMPONENTS OF THE CAT'S GRACILE NUCLEUS. J Physiol. 1964 Sep;173:263–290. doi: 10.1113/jphysiol.1964.sp007456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington T., Merzenich M. M. Neural coding in the sense of touch: human sensations of skin indentation compared with the responses of slowly adapting mechanoreceptive afferents innvervating the hairy skin of monkeys. Exp Brain Res. 1970;10(3):251–264. doi: 10.1007/BF00235049. [DOI] [PubMed] [Google Scholar]
- Hilton S. M., Torres S. H. Selective hypersensitivity to bradykinin in salivary glands with ligated ducts. J Physiol. 1970 Nov;211(1):37–48. doi: 10.1113/jphysiol.1970.sp009264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänig W., Schmidt R. F., Zimmermann M. Single unit responses and the total afferent outflow from the cat's foot pad upon mechanical stimulation. Exp Brain Res. 1968;6(2):100–115. doi: 10.1007/BF00239165. [DOI] [PubMed] [Google Scholar]
- KUYPERS H. G., HOFFMAN A. L., BEASLEY R. M. Distribution of cortical "feedback" fibers in the nuclei cuneatus and gracilis. Proc Soc Exp Biol Med. 1961 Dec;108:634–637. doi: 10.3181/00379727-108-27019. [DOI] [PubMed] [Google Scholar]
- KUYPERS H. G., TUERK J. D. THE DISTRIBUTION OF THE CORTICAL FIBRES WITHIN THE NUCLEI CUNEATUS AND GRACILIS IN THE CAT. J Anat. 1964 Apr;98:143–162. [PMC free article] [PubMed] [Google Scholar]
- MORIN F., CATALANO J. V. Central connections of a cervical nucleus (nucleus cervicalis lateralis of the cat). J Comp Neurol. 1955 Aug;103(1):17–32. doi: 10.1002/cne.901030104. [DOI] [PubMed] [Google Scholar]
- Mann M. D. Axons of dorsal spinocerebellar tract which respond to activity in cutaneous receptors. J Neurophysiol. 1971 Nov;34(6):1035–1050. doi: 10.1152/jn.1971.34.6.1035. [DOI] [PubMed] [Google Scholar]
- Mountcastle V. B., Talbot W. H., Sakata H., Hyvärinen J. Cortical neuronal mechanisms in flutter-vibration studied in unanesthetized monkeys. Neuronal periodicity and frequency discrimination. J Neurophysiol. 1969 May;32(3):452–484. doi: 10.1152/jn.1969.32.3.452. [DOI] [PubMed] [Google Scholar]
- PERL E. R., WHITLOCK D. G., GENTRY J. R. Cutaneous projection to second-order neurons of the dorsal column system. J Neurophysiol. 1962 May;25:337–358. doi: 10.1152/jn.1962.25.3.337. [DOI] [PubMed] [Google Scholar]
- Petit D., Burgess P. R. Dorsal column projection of receptors in cat hairy skin supplied by myelinated fibers. J Neurophysiol. 1968 Nov;31(6):849–855. doi: 10.1152/jn.1968.31.6.849. [DOI] [PubMed] [Google Scholar]
- Petit D. Postsynaptic fibres in the dorsal columns and their relay in the nucleus gracilis. Brain Res. 1972 Dec 24;48:380–384. doi: 10.1016/0006-8993(72)90194-1. [DOI] [PubMed] [Google Scholar]
- Rustioni A. Non-primary afferents to the nucleus gracilis from the lumbar cord of the ct. Brain Res. 1973 Mar 15;51:81–95. doi: 10.1016/0006-8993(73)90366-1. [DOI] [PubMed] [Google Scholar]
- Uddenberg N. Differential localization in dorsal funiculus of fibres originating from different receptors. Exp Brain Res. 1968;4(4):367–376. doi: 10.1007/BF00235701. [DOI] [PubMed] [Google Scholar]
- Uddenberg N. Functional organization of long, second-order afferents in the dorsal funiculus. Exp Brain Res. 1968;4(4):377–382. doi: 10.1007/BF00235702. [DOI] [PubMed] [Google Scholar]
- Whitsel B. L., Petrucelli L. M., Sapiro G. Modality representation in the lumbar and cervical fasciculus gracilis of squirrel monkeys. Brain Res. 1969 Sep;15(1):67–78. doi: 10.1016/0006-8993(69)90310-2. [DOI] [PubMed] [Google Scholar]