Abstract
PerR is a ferric uptake repressor (Fur) homolog that functions as the central regulator of the inducible peroxide stress response in Bacillus subtilis. PerR has been previously demonstrated to regulate the mrgA, katA, ahpCF, hemAXCDBL, and zosA genes. We now demonstrate that PerR also mediates both the repression of its own gene and that of fur. Whereas PerR-mediated repression of most target genes can be elicited by either manganese or iron, repression of perR and fur is selective for manganese. Genetic studies indicate that repression of PerR regulon genes by either manganese or iron requires PerR and is generally independent of Fur. Indeed, in a fur mutant, iron-mediated repression is enhanced. Unexpectedly, repression of the fur gene by manganese appears to require both PerR and Fur, but only PerR binds to the fur regulatory region in vitro. The fur mutation appears to act indirectly by affecting cellular metal ion pools and thereby affecting PerR-mediated repression. While many components of the perR regulon are strongly induced by hydrogen peroxide, little, if any, induction of fur and perR could be demonstrated. Thus, not all components of the PerR regulon are components of the peroxide stimulon. We suggest that PerR exists in distinct metallated forms that differ in DNA target selectivity and in sensitivity to oxidation. This model is supported by the observation that the metal ion composition of the growth medium can greatly influence the transcriptional response of the various PerR regulon genes to hydrogen peroxide.
Metal ions participate in a myriad of cellular functions, including respiration, enzyme catalysis, and stabilization of protein structure. Intracellular metal ion homeostasis must be maintained not only to reap the benefits of these nutrients but also to protect against toxic effects when metals are in excess (25, 28). A detrimental characteristic of some metal ions, particularly Fe(II), is the ability to react with hydrogen peroxide (H2O2) to produce the damaging hydroxyl radical (˙OH). It is therefore necessary that intracellular levels of both reactive oxygen species (ROS) and Fe(II) be tightly regulated (30, 31).
In Bacillus subtilis, iron uptake is regulated by Fur, a metalloregulatory protein that binds Fe(II) as a corepressor (2, 3). Under iron-replete conditions, Fur represses iron uptake functions, including siderophore biosynthesis and transport genes. In bacteria such as Escherichia coli and Vibrio cholerae, Fur can also bind manganese and repress iron uptake functions even when iron is limiting (15, 22). In contrast, repression of the Fur regulon in B. subtilis is highly selective for iron (3, 5).
The B. subtilis H2O2 stress response is regulated by PerR, one of three Fur homologs in this organism (3). Increased levels of H2O2, either exogenously or endogenously derived, induce katA (catalase), ahpCF (alkylhydroperoxide reductase), hemAXCDBL (heme biosynthesis), zosA (zinc uptake), and mrgA (DNA-binding protein) (1, 6, 8; A. Gaballa and J. D. Helmann, submitted for publication). Like that by other Fur homologs, repression by PerR requires a divalent metal ion. Addition of either Mn(II) or Fe(III) to cultures is sufficient to repress expression of mrgA and katA, two components of the PerR regulon (5, 6). Note that although the form of iron added to cultures is Fe(III), in vivo this is likely to be reduced to Fe(II), which is thought to be the form that interacts with PerR to effect repression. In vitro, purified PerR binds to operator sites overlapping target promoters. The ability of PerR to sense peroxide stress appears to be affected by the identity of the metal cofactor: PerR-Fe dissociates more readily from target operators than does PerR-Mn following exposure to H2O2 (18).
Bacteria have evolved complex mechanisms by which to coordinately regulate metal uptake and oxidative stress responses (30, 31). In E. coli, fur is controlled by the OxyR and the SoxR/S systems and induced by oxidants (34). Induction of Fur may allow the cell to repress iron uptake under conditions of oxidative stress, and the abundant Fur protein may also serve to scavenge free iron inside the cell (34). In contrast to iron, manganous and zinc ions can protect the cell against oxidative stress. We have recently demonstrated that ZosA (YkvW), a Zn(II) uptake ATPase, is controlled by PerR and facilitates increased accumulation of zinc under conditions of peroxide stress (Gaballa and Helmann, submitted).
In this study, we demonstrated that PerR functions as a transcriptional repressor both for its own gene and for fur. Both perR and fur are repressed by Mn(II) in a PerR-dependent fashion but are not repressed by iron. Unexpectedly, neither fur nor perR was significantly induced by H2O2. Since these results differ from those reported previously for other components of the PerR regulon, we herein present a comprehensive comparison of regulation of all PerR regulon genes. We demonstrate (i) that the metal selectivity of repression differs among the components of the PerR regulon, (ii) that both iron-mediated repression and manganese-mediated repression require PerR, and (iii) that the extent of induction by H2O2 is highly variable and is influenced by the metal ion composition of the growth medium. These results are supportive of a model in which PerR can exist in various metallated forms that differ in both DNA target selectivity and sensitivity to H2O2.
MATERIALS AND METHODS
Media and growth conditions.
B. subtilis strains were grown at 37°C in Luria broth (LB) or minimal medium containing 40 mM potassium morpholinepropanesulfonic acid (MOPS) (adjusted to pH 7.4 with KOH), 2 mM potassium phosphate buffer (pH 7.0), glucose (2%, wt/vol), (NH4)2SO4 (2 g/liter), MgSO4 · 7H2O (0.2 g/liter), trisodium citrate · 2H2O (1 g/liter), potassium glutamate (1 g/liter), tryptophan (10 mg/liter), 3 nM (NH4)6Mo7O24, 400 nM H3BO3, 30 nM CoCl2, 10 nM CuSO4, 10 nM ZnSO4, and MnCl2 and FeCl3 added to the concentrations indicated (5). Ampicillin (100 μg ml−1), spectinomycin (150 μg ml−1), or kanamycin (40 μg ml−1) was used for selection of E. coli strains. Erythromycin (1 μg ml−1) and lincomycin (25 μg ml−1; for testing of macrolide-lincosamide-streptogramin B resistance), spectinomycin (100 μg ml−1), kanamycin (10 μg ml−1), neomycin (10 μg ml−1), and chloramphenicol (5 μg ml−1) were used for the selection of various B. subtilis strains.
Bacterial strains, phage, and plasmids.
The B. subtilis strains and phage, E. coli strain, and plasmids used in this study are described in Table 1. SPβ phage are derivatives of SPβc2Δ2 (35) and were constructed by integration of a promoter region-cat-lacZ operon fusion constructed in pJPM122 into strain ZB307A as described previously (29). SPβ-transducing lysates were produced by heat induction from the indicated lysogens.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Characteristics | Reference or source |
|---|---|---|
| B. subtilis | ||
| CU1065 | W168 attSPβ trpC2 | Laboratory stock |
| ZB307A | W168 SPβc2Δ2::Tn917::pSK10Δ6 | 35 |
| HB1000 | ZB307A attSPβ | Laboratory stock |
| HB1122 | ZB307A SPβc2Δ2::Tn917::Φ(mrgA-cat-lacZ) | 6 |
| HB0518 | ZB307A SPβc2Δ2::Tn917::Φ(katA-cat-lacZ) | 3 |
| HB8206 | ZB307A SPβc2Δ2::Tn917::Φ(zosA-cat-lacZ) | A. Gaballa |
| HB1602 | ZB307A SPβc2Δ2::Tn917::Φ(ahpC-cat-lacZ) | 1 |
| HB0539 | ZB307A SPβc2Δ2::Tn917::Φ(perR-cat-lacZ) | This work |
| HB6560 | ZB307A SPβc2Δ2::Tn917::Φ(fur-cat-lacZ) | This work |
| HB0568 | CU1065 SPβc2Δ2::Tn917::Φ(mrgA-cat-lacZ) | This work |
| HB0567 | CU1065 SPβc2Δ2::Tn917::Φ(katA-cat-lacZ) | This work |
| HB8108 | CU1065 SPβc2Δ2::Tn917::Φ(zosA-cat-lacZ) | A. Gaballa |
| HB2083 | CU1065 SPβc2Δ2::Tn917::Φ(ahpC-cat-lacZ) | This work |
| HB1041 | CU1065 hemA-lacZ | 6 |
| HB2062 | CU1065 SPβc2Δ2::Tn917::Φ(perR-cat-lacZ) | This work |
| HB2076 | CU1065 SPβc2Δ2::Tn917::Φ(fur-cat-lacZ) | This work |
| HB0509 | HB1000 perR::spc | 3 |
| HB2078 | CU1065 perR::kan | This work |
| HB2116 | CU1065 perR::kan SPβc2Δ2::Tn917::Φ(mrgA-cat-lacZ) | This work |
| HB2117 | CU1065 perR::kan SPβc2Δ2::Tn917::Φ(katA-cat-lacZ) | This work |
| HB2118 | CU1065 perR::kan SPβc2Δ2::Tn917::Φ(zosA-cat-lacZ) | This work |
| HB2120 | CU1065 perR::kan SPβc2Δ2::Tn917::Φ(ahpC-cat-lacZ) | This work |
| HB2121 | CU1065 perR::kan hemA-lacZ | This work |
| HB2119 | CU1065 perR::kan SPβc2Δ2::Tn917::Φ(perR-cat-lacZ) | This work |
| HB2115 | CU1065 perR::kan SPβc2Δ2::Tn917::Φ(fur-cat-lacZ) | This work |
| HB2501 | CU1065 fur::kan | This work |
| HB2082 | CU1065 fur::kan SPβc2Δ2::Tn917::Φ(mrgA-cat-lacZ) | This work |
| HB2110 | CU1065 fur::kan SPβc2Δ2::Tn917::Φ(katA-cat-lacZ) | This work |
| HB2111 | CU1065 fur::kan SPβc2Δ2::Tn917::Φ(zosA-cat-lacZ) | This work |
| HB2113 | CU1065 fur::kan SPβc2Δ2::Tn917::Φ(ahpC-cat-lacZ) | This work |
| HB2114 | CU1065 fur::kan hemA-lacZ | This work |
| HB2112 | CU1065 fur::kan SPβc2Δ2::Tn917::Φ(perR-cat-lacZ) | This work |
| HB2081 | CU1065 fur::kan SPβc2Δ2::Tn917::Φ(fur-cat-lacZ) | This work |
| HB7503 | CU1065 mntR::kan | 27 |
| HB2079 | CU1065 mntR::kan SPβc2Δ2::Tn917::Φ(fur-cat-lacZ) | This work |
| HB6507 | HB1000 ahpC::Tn10 (spc) | 1 |
| HB6566 | HB1000 ahpC::Tn10 (spc) SPβc2Δ2::Tn917::Φ(fur-cat-lacZ) | This work |
| HB1308 | Spontaneous katA mutant; cured of SPβ | 6 |
| HB6570 | HB1308 SPβc2Δ2::Tn917::Φ(fur-cat-lacZ) | This work |
| E. coli DH5α | Laboratory stock | |
| Plasmids | ||
| pBSKII+ | pBR322 replicon | Stratagene |
| pJPM122 | cat-lacZ operon fusion vector for SPβ | 29 |
| pGEM-cat | pGEM-3zf(+)-cat-1 | 33 |
| pDG780 | pBSKII+ containing kanamycin resistance cassette | 13 |
| pHB6518 | pJPM122 with fur promoter | This work |
| pSK1 | pGEM-cat with 542-bp fragment containing perR | 3 |
| pAFH3 | pBSKII+ with 542-bp fragment containing perR | This work |
| pAFH5 | pJPM122 with perR promoter | This work |
| pMF20 | pGEM-cat with 776-bp fragment containing perR | This work |
| pMF21 | pGEM-cat with perR::kan | This work |
Construction of perR::kan and fur::kan mutant strains.
A fragment containing perR was amplified from B. subtilis CU1065 DNA with forward primer 5′-GCAAGCTTGAGTATATGGGAAT-3′ and reverse primer 5′-GGAATTCGGAAAAGAATTTGATGAGTC-3′ to introduced HindIII and EcoRI sites (underlined). The HindIII-EcoRI-digested fragment was cloned into pGEM-cat, generating plasmid pMF20. The SacI-HincII fragment from pDG780 containing a kanamycin resistance cassette was inserted between SacI and XmaI sites within perR to generate pMF21. CU1065 was transformed with ScaI-digested pMF21 with selection for kanamycin resistance, and transformants were screened for loss of plasmid-borne chloramphenicol resistance to distinguish single-crossover from double-crossover recombination. The resulting CU1065 perR::kan strain was designated HB2078. The presence of perR::kan in the strain was confirmed by PCR and Southern analysis. The isogenic fur mutant was constructed by transformation of CU1065 with chromosomal DNA from HB6543 (fur::kan) (3).
DNA manipulations and sequencing.
Isolation of B. subtilis chromosomal DNA, transformation, and specialized SPβ transductions were done by standard procedures (7). E. coli plasmid DNA and restriction enzyme fragments were isolated with the QIAprep spin miniprep and PCR purification kits, respectively (Qiagen Inc., Chatsworth, Calif.). Restriction endonucleases, DNA ligase, Vent DNA polymerase (New England Biolabs, Beverly, Mass.), Pfu DNA polymerase (Stratagene, La Jolla, Calif.), RNase-free DNase, and avian myeloblastosis virus reverse transcriptase (Promega Corp., Madison, Wis.) were used in accordance with the manufacturers' instructions. DNA sequencing was performed with AmpliTaq-FS DNA polymerase and dye terminator chemistry by the DNA services facility of the Cornell New York State Center for Advanced Technology-Biotechnology.
DNase I footprinting assays.
Purification of B. subtilis Fur and PerR and DNase I protection assays were performed as previously described (2, 18). G+A sequencing ladders were generated as previously described (23), with incubation at 104°C for 20 min. PCRs were used to amplify templates for the footprinting experiments. The primer pairs used were as follows: for perR, 5′-GCAAGCTTGAGTATATGGGAAT-3′ and 5′-GGGGATCCGAGCCATAGAGTTAAC-3′; for fur, 5′-GCGCTGATTTCATCTCTCTTT-3′ and 5′-GGATGAGTGCAGTTGTTTCTTA-3′. Fragments were purified and digested with the appropriate restriction enzymes, and the ends were filled in with [α-32P]dATP and the E. coli polymerase I Klenow fragment (exo−; New England Biolabs).
Primer extension analysis.
For mapping of the fur promoter, HB0509 (perR mutant) cells were grown in LB and total RNA was isolated at the end of logarithmic growth essentially as previously described (10). For the perR promoter, total RNA was isolated from mid-logarithmic-phase cells with an RNAWIZ kit (Ambion). Primer extension reactions were set up as follows. Thirty micrograms of RNA was hybridized to ∼2 pmol of the appropriate end-labeled primer in buffer containing 60 mM NaCl, 50 mM Tris-HCl (pH 7.9), 10 mM dithiothreitol (DTT), and 40 U of RNasin (Promega). Following hybridization, extension buffer (72 mM NaCl, 50 mM Tris-HCl [pH 7.9], 10 mM DTT, 20 mM MgCl2), deoxynucleoside triphosphates, and avian myeloblastosis virus reverse transcriptase were added to the mixture, which was incubated at 37°C for 30 min. The primer extension products were precipitated, resuspended in sequence loading buffer, and loaded onto a 6% acrylamide sequencing gel. A PCR cycle sequencing kit (Epicenter) was used to generate sequencing ladders corresponding to the perR and fur promoter-operator regions.
Northern analysis of fur.
Samples for Northern analysis were prepared as described for the resuspension experiment and collected at 3 h after resuspension. Total RNA was isolated with RNAWIZ reagent (Ambion). A 5-μg sample of total RNA was then separated with a 1% formaldehyde gel, transferred to nylon membrane, and hybridized with radiolabeled probe at 50°C overnight in ULTRAhyb solution (Ambion). Membranes were then washed twice with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) plus 0.1% sodium dodecyl sulfate (SDS), followed by two washes with 0.5× SSC plus 0.1% SDS for 15 min at 50°C. The fur probe was prepared by Sau3AI digestion of the PCR product containing the complete coding region of fur, which contains three internal Sau3AI sites. The digested products were purified and labeled with [α-32P]dATP and the Klenow fragment of DNA polymerase.
Construction of cat-lacZ reporter fusions.
For the fur-cat-lacZ fusion, the promoter region of fur was amplified from HB1000 chromosomal DNA by a PCR with 5′-GATCCTCTAAGCTTTTTTAAAATC-3′ as the forward primer and 5′-ATCAACGGATCCGAACTC-3′ as the reverse primer. The PCR mixture contained 50 μM deoxynucleoside triphosphates, 100 pmol each of the forward and reverse primers, 2 U of Vent DNA polymerase, and Vent buffer in a total volume of 100 μl. The reaction mixtures were subjected to denaturation for 2 min at 94°C, followed by 30 cycles of 10 s at 95°C, 30 s at 50°C, 30 s at 72°C, and a final extension of 5 min at 72°C. The resulting PCR product was cloned into pJPM122 as a HindIII-to-BamHI fragment (underlined sites) to generate pHB6518. The insert was verified by sequencing. pHB6518 was linearized and used to transform ZB307A with selection for neomycin resistance to generate strain HB6560. Phage generated from this strain (SPβ6560) was used to move the fur-cat-lacZ operon fusion into various strain backgrounds as indicated.
For the perR-cat-lacZ fusion, an EcoRV-HincII fragment from pSK1 containing the perR promoter was cloned into the EcoRV site of pBSK, generating pAFH3. The BamHI-HindIII fragment from pAFH3 was cloned into pJPM122 to generate reporter fusions as described above. The mrgA, katA, ahpC, and zosA-cat-lacZ fusions are described elsewhere (1, 3, 6; Gaballa and Helmann, submitted). The DNA fragments used for cat-lacZ fusions contained all of the putative promoters and Per boxes. The fragments used to generate promoter fusions extended from −464 to +47 for mrgA, from −304 to +265 for katA, from −332 to +32 for zosA, from −1326 to +159 for ahpC, from −90 to +97 for perR, and from −120 to +312 for fur (all relative to the start codon). The hemA-lacZ fusion was constructed by Campbell integration of a plasmid containing hemA-lacZ (6). The hemA-lacZ fusion was moved to different backgrounds by transformation with HB1041 (CU1065 hemA-lacZ) chromosomal DNA and selection for chloramphenicol resistance.
β-Gal assays.
For resuspension experiments, overnight cultures of cells grown in LB containing appropriate antibiotics were transferred at a 1:100 dilution into fresh MOPS-buffered minimal medium with 10 μM FeCl3 and 5 μM MnCl2. The overnight cultures were transferred again at a 1:100 dilution into the same minimal medium. The cells were incubated until the optical density at 600 nm was about 0.2. The cells were then washed once with minimal medium with no added FeCl3 or MnCl2, collected by centrifugation, and resuspended in minimal medium either with no added FeCl3 or MnCl2 or with 10 μM FeCl3, 5 μM MnCl2, or both. This time point was designated time zero. Samples were removed for β-galactosidase assay (β-Gal) at the indicated times by the method of Miller as described previously (5, 24). All assays were performed on duplicate samples, and the values were averaged. Glassware was acid washed when possible.
For experiments with the fur and mntR mutant strains (see Fig. 5), overnight cultures of cells grown in LB containing appropriate antibiotics were transferred at a 1:100 dilution into fresh MOPS-buffered minimal medium with 10 μM Fe(III). The overnight cultures were transferred again at a 1:100 dilution into minimal medium with either 1 or 10 μM Fe(III) (as indicated) and the indicated concentration of Mn(II). Cells in mid-log phase were collected for β-Gal assay.
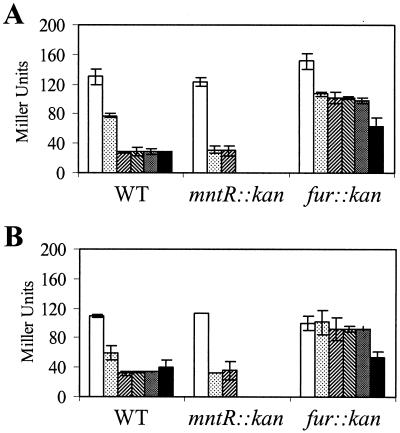
FIG. 5.
Effects of mntR and fur mutations on metalloregulation of the fur gene. Overnight cultures of strains carrying the fur-cat-lacZ reporter fusion were diluted 1:100 into minimal medium with either 1 μM Fe(III) (A) or 10 μM Fe(III) (B) and Mn(II) at 0, 0.1, 1, 10, 100, or 1,000 μM (left to right). Cells were grown to mid-log phase and collected for β-Gal assay. Note that the mntR mutant strain does not grow in concentrations of Mn(II) of 10 μM and greater (27), so no data were obtained for these conditions. WT, wild type.
Peroxide induction experiments.
Cell samples at 2 h after resuspension (see resuspension experiment protocol described above) were transferred into new tubes (prewarmed at 37°C). H2O2 (100 μM) was added, and samples were taken for β-Gal assay after 30 and 60 min.
Polyclonal anti-PerR Ab production and immunoblotting experiments.
Purified PerR was submitted to the Cornell University Animal Research Laboratory for production of rabbit polyclonal antibodies (Ab). Immunodetection was performed with crude extracts by SDS-polyacrylamide gel electrophoresis, followed by electroblotting onto polyvinylidene difluoride membrane (Bio-Rad). Membranes were blocked with 50 mg of nonfat dried milk per ml at 4°C. Anti-PerR Ab was added at a dilution of 1:500 in Tris-buffered saline (TBS) with 0.05% Tween 20. After incubation for 1 h at room temperature, the membrane was washed three times in TBS-Tween 20. Goat anti-rabbit Ab conjugated to alkaline phosphatase (Bio-Rad) was used as the secondary Ab at a concentration of 1:3,000. After incubation for 1 h, the membrane was washed three times in TBS-Tween 20 and once in TBS. The colorimetric signal was visualized by incubation with alkaline phosphatase substrate (Bio-Rad) in development buffer in accordance with the manufacturer's protocols.
RESULTS
Identification of PerR-binding sites in the perR and fur promoter regions.
We have previously identified five operons within the PerR regulon (mrgA, katA, ahpCF, hemAXCDBL, and zosA) (1, 3, 4, 6; Gaballa and Helmann, submitted). To identify additional candidate components of this regulon, we searched the B. subtilis genome with the 15-bp Per box consensus sequence (TTATAATnATTATAA; reference 17) and found additional candidate Per boxes upstream of the perR and fur genes.
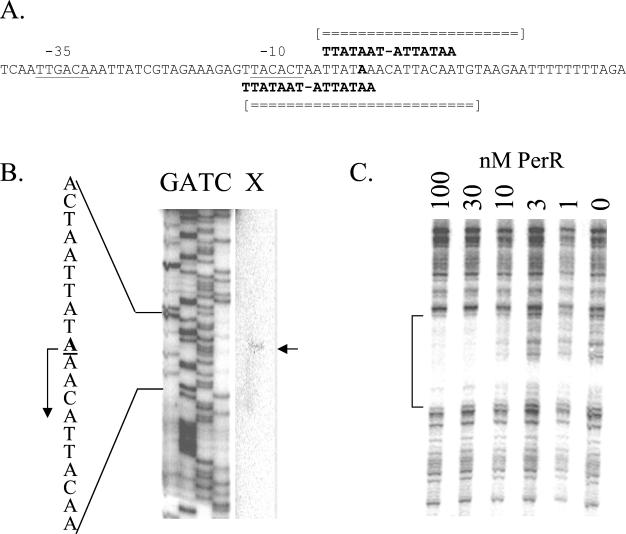
Two overlapping Per boxes are present upstream of the perR gene (Fig. 1A), which is suggestive of a role in autoregulation. By primer extension start site mapping, we determined that transcription initiates from a σA-type promoter at an A residue 45 bp upstream of the start codon (Fig. 1B). The upstream Per box overlaps the −10 consensus sequence. In DNase I footprinting experiments, as little as 10 nM PerR protected an ∼25-bp region surrounding the transcription start site against DNase I digestion (Fig. 1C). This is comparable to the affinity of PerR for other operator regions that have been studied (18).
FIG. 1.
Interaction of PerR with the perR regulatory region. (A) The −35 and −10 regions of the perR promoter are underlined, and the two overlapping Per box elements are indicated. The A residue start site for transcription is in bold. Regions of both DNA strands protected against DNase I digestion by bound PerR protein are indicated by broken double lines. (B) Primer extension mapping of the perR transcriptional start site (arrow) indicates transcript initiation with the A residue indicated in the sequence to the left. (C) DNase I footprint of purified PerR binding to the perR promoter. PerR was added at the concentrations (nanomolar) indicated in the presence of 10 μM Mn(II). Shown are results from footprinting on the bottom strand; however, top-strand analysis was also performed (data not shown). The regions protected against digestion, as determined by alignment with G+A sequencing ladders (data not shown), are summarized in panel A.
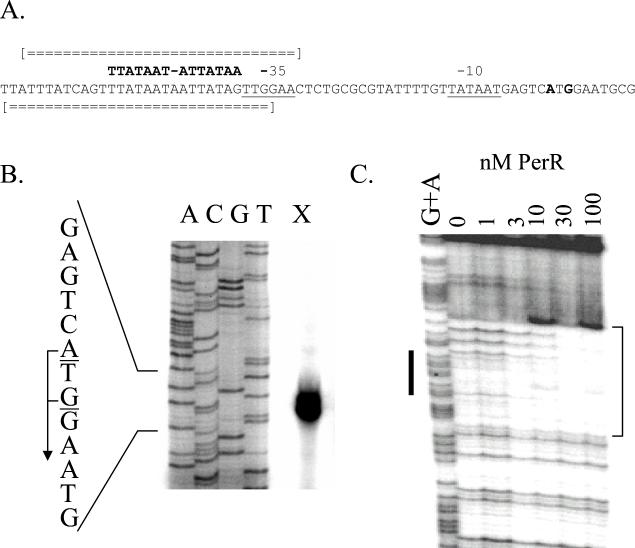
Genome searches also revealed a consensus Per box in the fur regulatory region. The DNA sequence of the fur gene (Fig. 2A) contains a candidate σA-dependent promoter with a 12-of-14 match to the −35 and extended −10 consensus sequences (16). Primer extension analysis, performed with RNA isolated from late-logarithmic-phase (transition phase) cells, identified two transcripts that initiated at A and G residues located 25 and 27 nucleotides upstream from the fur start codon (Fig. 2B). PerR bound tightly to the fur operator; strong protection was again observed with as little as 10 nM PerR (Fig. 2C). These results are consistent with a direct role of PerR as a repressor of the fur gene. However, since Per and Fur boxes are similar in sequence, this region can also be interpreted as a weak (12-of-19) match to the Fur box, raising the possibility that fur is autoregulated. However, no Fur binding was observed, even at a concentration of 100 nM (data not shown). At known Fur-regulated promoters, 10 nM Fur is usually sufficient to saturate binding (N. Baichoo and J. D. Helmann, submitted for publication). These results indicate that Fur does not interact directly with its own promoter and suggest, instead, that Fur is regulated directly by PerR (see below).
FIG. 2.
Interaction of PerR with the fur regulatory region. (A) The −35 and −10 elements of the fur promoter are underlined, and the transcriptional start sites are in bold. The Per box upstream of the −35 element is indicated. Regions of both DNA strands protected against DNase I digestion by bound PerR protein are indicated by broken double lines. (B) Primer extension mapping of the transcription start sites of the fur gene. Transcription initiates at the indicated A and G residues. (C) DNase I footprint of PerR binding to the fur operator region. The results shown are for the bottom strand. Purified PerR was added at the concentrations indicated in the presence of 10 μM Mn(II). The bold line adjacent to the G+A ladder indicates the position of the Per box, and the protected region is bracketed.
Patterns of metalloregulation within the PerR regulon.
All of the components of the PerR regulon that have been described are repressed by addition of manganese to the growth medium, and in some cases, repression by iron has also been observed. We sought to directly compare the metalloregulation of all of the components of the PerR regulon under identical conditions. To monitor expression of perR and fur, we constructed transcriptional fusions between the corresponding regulatory regions and lacZ and integrated the resulting cat-lacZ operon fusions into the SPβ prophage. Similar reporter constructs were used for the other PerR regulon genes, with the exception of the hemA promoter, which was fused to a lacZ reporter gene by plasmid integration (Table 1).
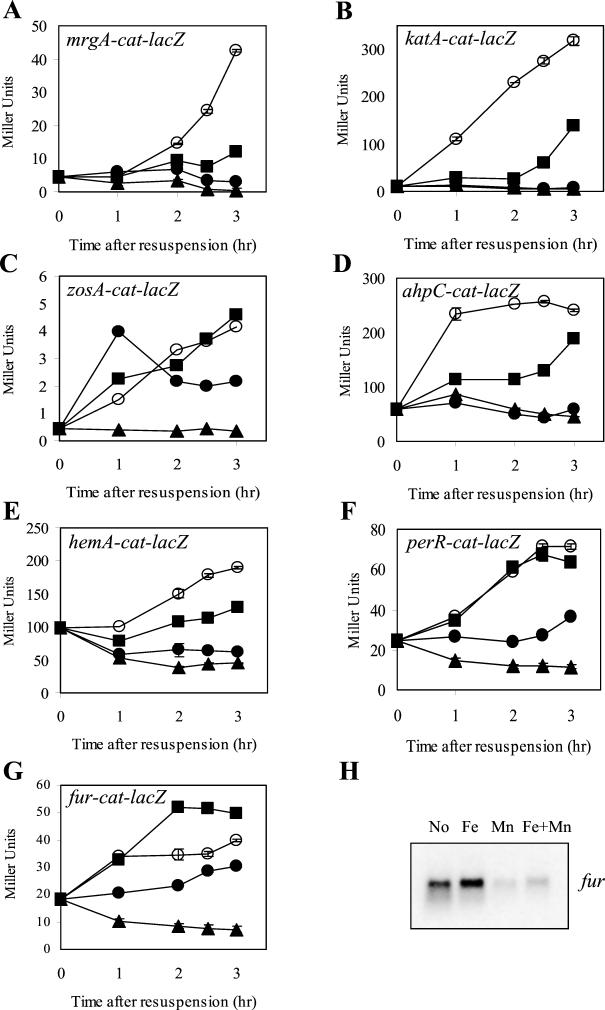
To compare the abilities of Mn(II) and Fe(III) to repress PerR-regulated genes, we used resuspension experiments as previously described (3, 5). In these studies, cells were grown to mid-logarithmic phase, harvested, and resuspended in minimal medium containing various levels of Mn(II) and Fe(III). In confirmation of our previously reported studies (3, 5), expression of an mrgA-cat-lacZ fusion is very low when cells are resuspended in minimal medium containing either 5 μM Mn(II), 10 μM Fe(III), or both. Only when both metal ions are omitted from the medium does gene expression commence (Fig. 3A). This supports the suggestion that either Mn(II) or Fe(II) can function in vivo as a corepressor with PerR. Similar patterns of metalloregulation were observed for the katA (Fig. 3B) and ahpC (Fig. 3D) promoter fusions. Repression of hemA by iron was inefficient but still observable (Fig. 3E).
FIG. 3.
Metal selectivity of gene regulation in resuspension experiments. Cells were resuspended in minimal medium either containing no added Mn(II) or Fe(III) (open circles) or containing 5 μM Mn(II) (filled triangles), 10 μM Fe(III) (filled squares), or both (filled circles). Strains contained mrgA-cat-lacZ (A), katA-cat-lacZ (B), zosA-cat-lacZ (C), ahpC-cat-lacZ (D), hemA-cat-lac (E), perR-cat-lacZ (F), or fur-cat-lacZ (G). Samples were taken at the times indicated and assayed for β-Gal activity. The results shown are representative of at least three independent experiments; error bars represent the standard error of the mean of duplicate samples. Panel H is a Northern blot analysis of the fur transcript from cultures collected 3 h after resuspension in minimal medium containing the indicated metal ion supplementation.
In contrast, expression of the perR-cat-lacZ (Fig. 3F) and fur-cat-lacZ (Fig. 3G) fusions increased after resuspension in medium lacking both metal ions or containing added Fe(III). Only when Mn(II) was present was expression efficiently repressed by PerR. Even addition of 100 μM Fe(III) did not repress gene expression nearly as efficiently as 5 μM Mn(II) (data not shown). A similar pattern was observed for the zosA gene: expression was repressed by Mn(II) but not by iron (Fig. 3C). The manganese selectivity of transcriptional repression was corroborated by additional studies: immunoblotting with anti-PerR Ab demonstrated that cells grown with 5 μM Mn(II) had 2.4-fold lower levels of PerR protein, whereas iron supplementation led to a slight (1.2-fold) increase in PerR (data not shown). Similarly, the effects of metal ions on fur transcription were measured by Northern analysis: Fe(III) supplementation slightly increased mRNA levels, while Mn(II) decreased mRNA levels (Fig. 3H). Repression of zosA is also selective for Mn(II), as judged by Northern analysis (data not shown).
Role of PerR in metalloregulation.
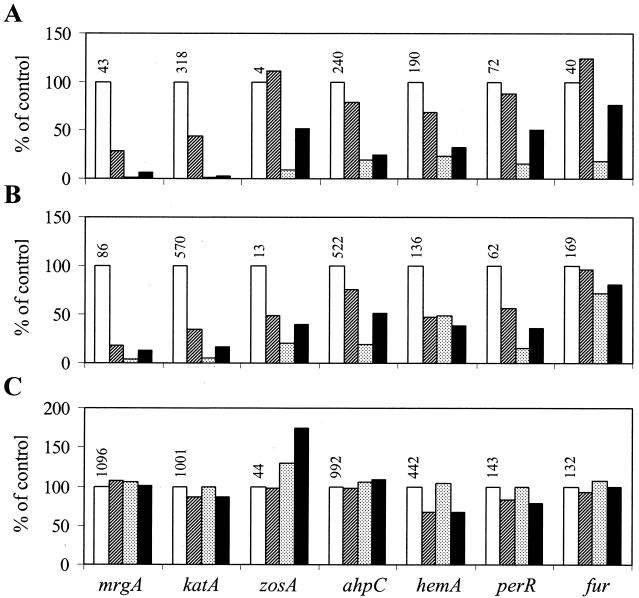
The diverse responses of the various components of the PerR regulon to metal ion supplementation could be due to the combinatorial effects of multiple metalloregulatory proteins. For example, it is possible that PerR mediates Mn(II)-dependent repression while Fur might contribute to the iron-dependent effects. To determine whether the effects of manganese and iron require PerR, Fur, or both, we repeated these studies after transfer of the reporter fusions to perR and fur mutant strains. For this analysis, we compared the levels of β-Gal 3 h after resuspension in minimal medium containing 5 μM Mn(II), 10 μM Fe(III), neither, or both. The results for the wild-type strain (from Fig. 3) are summarized in Fig. 4A. Note that Mn(II) addition led to decreased expression of all of the genes, while iron supplementation had a variable effect.
FIG. 4.
Roles of PerR and Fur in metalloregulation of PerR regulon genes. Resuspension experiments were performed as described in the legend to Fig. 3 with all seven promoter fusions (indicated at the bottom of panel C) in either the wild-type (A) background or the fur (B) or perR (C) mutant strain background. All samples were measured 3 h. after resuspension and normalized to the level in medium lacking manganese and iron supplementation (white bars; absolute values are shown above the bars). The cells were resuspended in minimal medium either containing (from left to right) no added Mn(II) or Fe(III) (white bars) or containing 10 μM Fe(III) (hatched), 5 μM Mn(II) (stippled), or both (black). The data in panel A are the same as those shown for the 3-h time point in Fig. 3.
When this experiment was repeated with the perR mutant strain (Fig. 4C), there was no significant repression of any of the genes by either Mn(II) or Fe(III) addition. Furthermore, comparison of the actual expression levels indicated that all of the PerR-regulated genes are derepressed in the perR mutant background. This suggests that even after resuspension in the unsupplemented (metal-limited) minimal medium, there is still significant PerR-dependent repression of these genes. These data indicate that the effects of both Mn(II) and Fe(III) on expression of PerR regulon components are mediated by PerR.
Effects of perturbing metal ion homeostasis on metalloregulation.
In contrast with perR, the fur mutation had relatively modest effects on the metalloregulation of PerR regulon genes (Fig. 4B). The overall patterns of metal-dependent repression are similar to that of the wild type for nearly all of the PerR regulon components. However, several interesting effects are worth noting. First, in several cases, Fe(III) elicits greater repression in the fur mutant than in the wild type. In the fur mutant, for example, resuspension in the iron-supplemented medium reduces expression of the zosA and perR promoters, which were not iron responsive in the wild type. Second, in most cases, the absolute levels of expression are somewhat higher in the fur mutant background, suggesting that there is a general, nonspecific derepression of these genes in this strain background. Third, the fur mutation also affected the Mn(II)-dependent repression of some genes. This is most apparent for the fur-cat-lacZ fusion, which was strongly repressed by Mn(II) in the wild-type strain (Fig. 4A) but not in either a perR (Fig. 4C) or a fur (Fig. 4B) mutant strain. These effects could all be explained by elevated intracellular iron pools in the fur mutant strain that shift PerR from the manganese-containing form to the iron-containing form, thereby leading to enhanced repression of iron-responsive genes and decreased repression of genes responsive only to Mn(II). Note that manganese and iron have been shown to compete for binding to PerR in in vitro studies (18).
To test the idea that the effects of the fur mutation on the Mn(II)-dependent repression of fur-cat-lacZ might be due to alterations to the intracellular metal ion pools, we measured the transcriptional response of the fur promoter region to various levels of Mn(II) (ranging from 100 nM to 1 mM). Consistent with previous studies of PerR regulon genes, 1 μM Mn(II) was sufficient for complete repression of fur-cat-lacZ in medium containing either 1 μM (Fig. 5A) or 10 μM Fe(III) (Fig 5B). The relatively inefficient repression elicited by 100 nM Mn(II) was enhanced in an mntR mutant strain that is derepressed for Mn(II) uptake (27). In contrast, there was little repression by Mn(II) in the fur mutant strain unless very high concentrations of Mn(II) were added. Since Mn(II) uptake is tightly regulated by MntR, it is difficult to perturb intracellular Mn(II) levels simply by manipulating concentrations in the medium. Experiments with a fur mntR double mutant might address this question, but we have not succeeded in constructing such a strain. Nevertheless, these results are consistent with the idea that PerR responds to intracellular pools of Mn(II) and Fe(II) and that these are affected by mutations that alter metal ion homeostasis systems.
Patterns of H2O2 induction within the PerR regulon.
Many of the genes in the PerR regulon are known to be strongly induced by H2O2, and this transcriptional response has been shown to be modulated by the metal ion content of the growth medium: growth in Mn(II)-supplemented medium greatly reduced the extent of induction, while iron supplementation enhances induction (6, 18). Unexpectedly, we were unable to induce expression of the fur gene with H2O2, paraquat, or cumene hydroperoxide (data not shown). Furthermore, expression of the fur gene was not derepressed in either an ahpC or a katA mutant background (data not shown), conditions that lead to elevated expression of other peroxide-inducible genes (1, 6).
To compare the abilities of all of the PerR-regulated operons to be induced by H2O2 and to systematically investigate the effects of metal ions on induction, we have resuspended cells in minimal medium containing 5 μM Mn(II), 10 μM Fe(III), neither, or both, as shown in Fig. 3. After 2 h of growth, cultures were treated with 100 μM H2O2 (or left untreated) and gene expression was measured after 30 min (Fig. 6) or 60 min (data not shown). As expected, the level of gene expression was lowest in those cells growing in the Mn(II)-supplemented medium and these cells displayed the least response to H2O2 challenge. In contrast, in media supplemented with Fe(III), there was greater induction of the mrgA, katA, and zosA promoters. While qualitatively similar, the absolute level of induction in these resuspension experiments is not as great as that observed previously for the mrgA (4), katA (18), or zosA promoter (Gaballa and Helmann, submitted). Indeed, transcriptional profiling experiments performed with rich medium suggest that mrgA, katA, and zosA can each be fully derepressed by H2O2: the increase in mRNA levels in H2O2-induced cells is comparable to that in a perR mutant (J. D. Helmann et al., unpublished data).
FIG. 6.
Induction of PerR regulon genes by H2O2. Strains containing the indicated reporter fusions were grown in minimal medium with no added Mn(II) or Fe(III) (None) or with 10 μM Fe(III) (Fe), 5 μM Mn(II) (Mn), or both (Fe+Mn) as described in the legend to Fig. 3. At 2 h after resuspension, the cultures were split and 100 μM H2O2 was added to one sample. After growth for another 30 min, cells were harvested for β-Gal assay and induced expression (gray bars) was compared to expression in the absence of H2O2 addition (white bars). Note that the data represented by the white bars are the same as those for the 2.5-h time point in Fig. 3. Experiments were performed twice; error bars represent the standard error of the mean.
In contrast with the mrgA and katA promoters, the ahpC and hemA promoters were only weakly induced by H2O2 treatment and little, if any, induction of the perR and fur promoters was observed. However, these promoters are all repressed by PerR in response to Mn(II). These results demonstrate that PerR regulon components differ both in susceptibility to repression by various metal ions and in the ability to be induced by H2O2. Since PerR-mediated repression can be elicited by manganese, iron, and perhaps other divalent metal ions (5), this leads to a model in which various metallated forms of PerR differ in both DNA target selectivity and reactivity with H2O2. Experiments testing these ideas by the in vitro reconstitution of different forms of PerR have been done (18), and additional studies are in progress.
DISCUSSION
The E. coli Fur protein is the prototype of a large family of metal-dependent repressor proteins. In E. coli and many other gram-negative bacteria, Fur regulates iron uptake functions by repressing gene expression in the presence of Fe(II), which acts as a corepressor (9). B. subtilis contains three Fur homologs that coordinate gene expression in response to iron (Fur), zinc (Zur), or H2O2 (PerR) (3, 11). All three proteins are dimeric, DNA-binding repressors that contain a single Zn(II) atom per monomer, which is thought to play a structural role, and a second regulatory metal ion that acts as a cofactor that is necessary for binding to the target operator sites (2, 18). However, they differ in metal selectivity: Fur is activated by Fe(II), Zur is activated by Zn(II), and PerR is activated by several different metal ions, including Mn(II) and Fe(II).
PerR negatively regulates expression of the peroxide stress stimulon (17). Genes shown to be under direct PerR control include katA (major vegetative catalase), ahpCF (alkyl hydroperoxide reductase), mrgA (Dps-like DNA-binding protein), hemAXCDBL (heme biosynthesis operon), and zosA (zinc uptake system). Here, we extend the PerR regulon to include both perR itself and fur (Fig. 1 and 2). Autoregulation of PerR has also been reported in Staphylococcus aureus (19) and in the homolog CatR from Streptomyces coelicolor (14). Unexpectedly, repression of perR and fur was elicited by addition of Mn(II), but not Fe(III), to the growth medium (Fig. 3). This is consistent with our suggestion that PerR forms different metallated species in the cell that differ in target selectivity (18).
We have systematically compared the metal selectivities of transcriptional repression of all of the PerR regulon components (Fig. 3 and 4). While all of the components of the regulon are repressed by Mn(II), the transcriptional responses to iron vary from repression (mrgA and katA) to a slight induction (fur). Both the iron repression and the manganese repression of mrgA and katA require PerR and are independent of Fur (Fig. 4). This contrasts with the dual regulation of the E. coli mntH gene, which is repressed by Mn(II) through the MntR protein and by Fe(II) through Fur (26). Mutation of the fur gene also affects the metalloregulation of PerR regulon components. In the fur mutant strain, repression elicited by iron is enhanced and the Mn(II)-dependent repression of fur is greatly decreased (Fig. 4). Since Fur does not bind to the fur regulatory region, we suspect that derepression is an indirect effect of the fur mutation. Indeed, the observed derepression can be at least partially overcome with high levels of Mn(II) supplementation (Fig. 5). These findings are consistent with the observations that fur mutant cells accumulate elevated levels of intracellular iron (21; E. Guedon and J. D. Helmann, unpublished data) and that this may alter the distribution of PerR among its various metallated forms. For example, elevated intracellular Fe(II) may supplant Mn(II) in PerR, in effect causing derepression of fur.
The finding that fur is a direct target for PerR repression is reminiscent of the finding that E. coli fur is under the control of the peroxide-sensing transcription factor OxyR, as well as the superoxide response system SoxRS (34). However, unlike the situation in E. coli, transcription of B. subtilis fur is not peroxide inducible. Similar regulation has been observed in S. aureus: PerR represses fur, yet fur is not inducible by H2O2 (19). Interestingly, PerR repression in S. aureus is also elicited by Mn(II) but not by iron. The physiological relevance of regulating fur expression in response to intracellular Mn(II) levels is not clear.
The molecular mechanism by which PerR senses H2O2 is not clear, but we have proposed that it likely involves disulfide bond formation between two Cys residues postulated to serve as ligands for the regulatory metal ion (18). This model is supported by the observation that growth of cells with Mn(II) reduces the H2O2 induction of target genes, whereas growth with Fe(III) increases induction (Fig. 6). It is envisioned that these changes in medium composition affect the identity of the regulatory metal ion bound to PerR and thereby affect redox activity. Indeed, biochemical studies demonstrate that binding of PerR to its target operator regions in vitro is sensitive to H2O2 but can be restored by thiol-reducing agents such as DTT (18). Further, the identity of the regulatory metal ion cofactor influences the sensitivity of PerR to oxidants: the iron-containing form is quite sensitive to H2O2, while addition of Mn(II) reduces this sensitivity (18). Thus, we favor a model in which one or more of the redox-active cysteine residues also serves as ligand to the regulatory metal ion. An alternative model postulates that peroxide sensing involves disulfide bond formation between Cys residues that normally serve as ligands to the Zn(II) ion (14). This mechanism, analogous to the regulatory mechanism controlling Hsp33 activity (12, 20), was suggested for the S. coelicolor PerR ortholog CatR (14).
While it is tempting to speculate that the identity of the metal ion cofactor determines the relative sensitivity of different PerR species to H2O2, the correlation between metal specificity of repression and peroxide inducibility is imperfect. This model is supported by the observations that repression of both mrgA and katA by PerR can be elicited by iron and that both genes are strongly induced by H2O2. In contrast, repression of both fur and perR appears to be selective for Mn(II) and neither gene can be strongly induced by H2O2. However, the zosA gene is also selectively repressed by Mn(II) (although iron can serve as a repressor at least in a fur mutant strain) but this gene is strongly induced by H2O2 (Fig. 6 and data not shown).
The results reported here lead to two important conclusions about the PerR regulon. First, we demonstrated that not all components of the PerR regulon are inducible by peroxide. While the inability to induce fur with H2O2 was initially interpreted as resulting from the Mn(II) selectivity of gene repression, other factors may also be at play. Second, we demonstrated that the metalloregulation of different PerR regulon genes is distinct: some can be repressed by either manganese or iron, while others are manganese specific. It is interesting that the metal selectivity of PerR also varies between species: in S. aureus, PerR is selective for Mn(II) (19), while the Campylobacter jejuni ortholog responds to iron (32). In ongoing biochemical studies, we are attempting to generate different metallated forms of PerR for a direct comparison of DNA target selectivity and peroxide reactivity in vitro.
Acknowledgments
We thank Joe Calvo and members of the Helmann laboratory for helpful comments on the manuscript.
This work was supported by a grant from the National Science Foundation (MCB-9983656).
REFERENCES
- 1.Bsat, N., L. Chen, and J. D. Helmann. 1996. Mutation of the Bacillus subtilis alkyl hydroperoxide reductase (ahpCF) operon reveals compensatory interactions among hydrogen peroxide stress genes. J. Bacteriol. 178:6579-6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bsat, N., and J. D. Helmann. 1999. Interaction of Bacillus subtilis Fur (ferric uptake repressor) with the dhb operator in vitro and in vivo. J. Bacteriol. 181:4299-4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bsat, N., A. Herbig, L. Casillas-Martinez, P. Setlow, and J. D. Helmann. 1998. Bacillus subtilis contains multiple Fur homologues: identification of the iron uptake (Fur) and peroxide regulon (PerR) repressors. Mol. Microbiol. 29:189-198. [DOI] [PubMed] [Google Scholar]
- 4.Chen, L., and J. D. Helmann. 1995. Bacillus subtilis MrgA is a Dps(PexB) homologue: evidence for metalloregulation of an oxidative stress gene. Mol. Microbiol. 18:295-300. [DOI] [PubMed] [Google Scholar]
- 5.Chen, L., L. P. James, and J. D. Helmann. 1993. Metalloregulation in Bacillus subtilis: isolation and characterization of two genes differentially repressed by metal ions. J. Bacteriol. 175:5428-5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, L., L. Keramati, and J. D. Helmann. 1995. Coordinate regulation of Bacillus subtilis peroxide stress genes by hydrogen peroxide and metal ions. Proc. Natl. Acad. Sci. USA 92:8190-8194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cutting, S. M., and P. B. VanderHorn. 1990. Genetic analysis, p. 27-74. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley & Sons, Ltd., Chichester, United Kingdom.
- 8.Dowds, B. C. A. 1994. The oxidative stress response in Bacillus subtilis. FEMS Microbiol. Lett. 124:255-264. [DOI] [PubMed] [Google Scholar]
- 9.Escolar, L., J. Perez-Martin, and V. de Lorenzo. 1999. Opening the iron box: transcriptional metalloregulation by the Fur protein. J. Bacteriol. 181:6223-6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fredrick, K., and J. D. Helmann. 1997. RNA polymerase sigma factor determines start-site selection but is not required for upstream promoter element activation on heteroduplex (bubble) templates. Proc. Natl. Acad. Sci. USA 94:4982-4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaballa, A., and J. D. Helmann. 1998. Identification of a zinc-specific metalloregulatory protein, Zur, controlling zinc transport operons in Bacillus subtilis. J. Bacteriol. 180:5815-5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graumann, J., H. Lilie, X. Tang, K. A. Tucker, J. H. Hoffmann, J. Vijayalakshmi, M. Saper, J. C. Bardwell, and U. Jakob. 2001. Activation of the redox-regulated molecular chaperone Hsp33-a two-step mechanism. Structure 9:377-387. [DOI] [PubMed] [Google Scholar]
- 13.Guérout-Fleury, A.-M., K. Shazand, N. Frandsen, and P. Stragier. 1995. Antibiotic-resistance cassettes for Bacillus subtilis. Gene 167:335-336. [DOI] [PubMed] [Google Scholar]
- 14.Hahn, J. S., S. Y. Oh, K. F. Chater, Y. H. Cho, and J. H. Roe. 2000. H2O2-sensitive fur-like repressor CatR regulating the major catalase gene in Streptomyces coelicolor. J. Biol. Chem. 275:38254-38260. [DOI] [PubMed] [Google Scholar]
- 15.Hantke, K. 1987. Selection procedure for deregulated iron transport mutants (fur) in Escherichia coli K-12: fur not only affects iron metabolism. Mol. Gen. Genet. 210:135-139. [DOI] [PubMed] [Google Scholar]
- 16.Helmann, J. D. 1995. Compilation and analysis of Bacillus subtilis σA-dependent promoter sequences: evidence for extended contact between RNA polymerase and upstream promoter DNA. Nucleic Acids Res. 23:2351-2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herbig, A., and J. D. Helmann. 2002. Metal ion uptake and oxidative stress, p. 405-414. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives, 2nd ed. ASM Press, Washington, D.C.
- 18.Herbig, A. F., and J. D. Helmann. 2001. Roles of metal ions and hydrogen peroxide in modulating the interaction of the Bacillus subtilis PerR peroxide regulon repressor with operator DNA. Mol. Microbiol. 41:849-859. [DOI] [PubMed] [Google Scholar]
- 19.Horsburgh, M. J., M. O. Clements, H. Crossley, E. Ingham, and S. J. Foster. 2001. PerR controls oxidative stress resistance and iron storage proteins and is required for virulence in Staphylococcus aureus. Infect. Immun. 69:3744-3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jakob, U., M. Eser, and J. C. Bardwell. 2000. Redox switch of hsp33 has a novel zinc-binding motif. J. Biol. Chem. 275:38302-38310. [DOI] [PubMed] [Google Scholar]
- 21.Keyer, K., and J. A. Imlay. 1996. Superoxide accelerates DNA damage by elevating free-iron levels. Proc. Natl. Acad. Sci. USA 93:13635-13640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lam, M. S., C. M. Litwin, P. A. Carroll, and S. B. Calderwood. 1994. Vibrio cholerae fur mutations associated with loss of repressor activity: implications for the structural-functional relationships of Fur. J. Bacteriol. 176:5108-5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu, S. T., and G. F. Hong. 1998. Three-minute G + A specific reaction for DNA sequencing. Anal. Biochem. 255:158-159. [DOI] [PubMed] [Google Scholar]
- 24.Miller, J. H. 1972. Experiments in molecular genetics, p. 352-355. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 25.O'Halloran, T. V. 1993. Transition metals in control of gene expression. Science 261:715-725. [DOI] [PubMed] [Google Scholar]
- 26.Patzer, S. I., and K. Hantke. 2001. Dual repression by Fe2+-Fur and Mn2+-MntR of the mntH gene, encoding an NRAMP-like Mn2+ transporter in Escherichia coli. J. Bacteriol. 183:4806-4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Que, Q., and J. D. Helmann. 2000. Manganese homeostasis in Bacillus subtilis is regulated by MntR, a bifunctional regulator related to the diphtheria toxin repressor family of proteins. Mol. Microbiol. 35:1454-1468. [DOI] [PubMed] [Google Scholar]
- 28.Silver, S. 1998. Genes for all metals—a bacterial view of the periodic table. The 1996 Thom Award lecture. J. Ind. Microbiol. Biotechnol. 20:1-12. [DOI] [PubMed] [Google Scholar]
- 29.Slack, F. J., J. P. Mueller, and A. L. Sonenshein. 1993. Mutations that relieve nutritional repression of the Bacillus subtilis dipeptide permease operon. J. Bacteriol. 175:4605-4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Storz, G., and J. A. Imlay. 1999. Oxidative stress. Curr. Opin. Microbiol. 2:188-194. [DOI] [PubMed] [Google Scholar]
- 31.Touati, D. 2000. Iron and oxidative stress in bacteria. Arch. Biochem. Biophys. 373:1-6. [DOI] [PubMed] [Google Scholar]
- 32.van Vliet, A. H., M. L. Baillon, C. W. Penn, and J. M. Ketley. 1999. Campylobacter jejuni contains two fur homologs: characterization of iron-responsive regulation of peroxide stress defense genes by the PerR repressor. J. Bacteriol. 181:6371-6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Youngman, P. 1990. Use of transposons and integrational vectors for mutagenesis and construction of gene fusions in Bacillus species, p. 221-266. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley & Sons, Ltd., Chichester, United Kingdom.
- 34.Zheng, M., B. Doan, T. D. Schneider, and G. Storz. 1999. OxyR and SoxRS regulation of fur. J. Bacteriol. 181:4639-4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zuber, P., and R. Losick. 1987. Role of AbrB in Spo0A- and Spo0B-dependent utilization of a sporulation promoter in Bacillus subtilis. J. Bacteriol. 169:2223-2230. [DOI] [PMC free article] [PubMed] [Google Scholar]