Full text
PDF































Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander S., Rowan D., Millar W., Scott R. Treatment of urinary incontinence by electric pessary. A report of 18 patients. Br J Urol. 1970 Apr;42(2):184–190. doi: 10.1111/j.1464-410x.1970.tb10020.x. [DOI] [PubMed] [Google Scholar]
- Anderson N. C., Jr Voltage-clamp studies on uterine smooth muscle. J Gen Physiol. 1969 Aug;54(2):145–165. doi: 10.1085/jgp.54.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anlezark G. M., Arbuthnott G. W., Christie J. E., Crow T. J. Proceedings: Electrical self-stimulation with electrodes in the region of the interpeduncular nucleus. J Physiol. 1973 Oct;234(2):103P–104P. [PubMed] [Google Scholar]
- Arbuthnott G., Fuxe K., Ungerstedt U. Central catecholamine turnover and self-stimulation behaviour. Brain Res. 1971 Apr 2;27(2):406–413. doi: 10.1016/0006-8993(71)90272-1. [DOI] [PubMed] [Google Scholar]
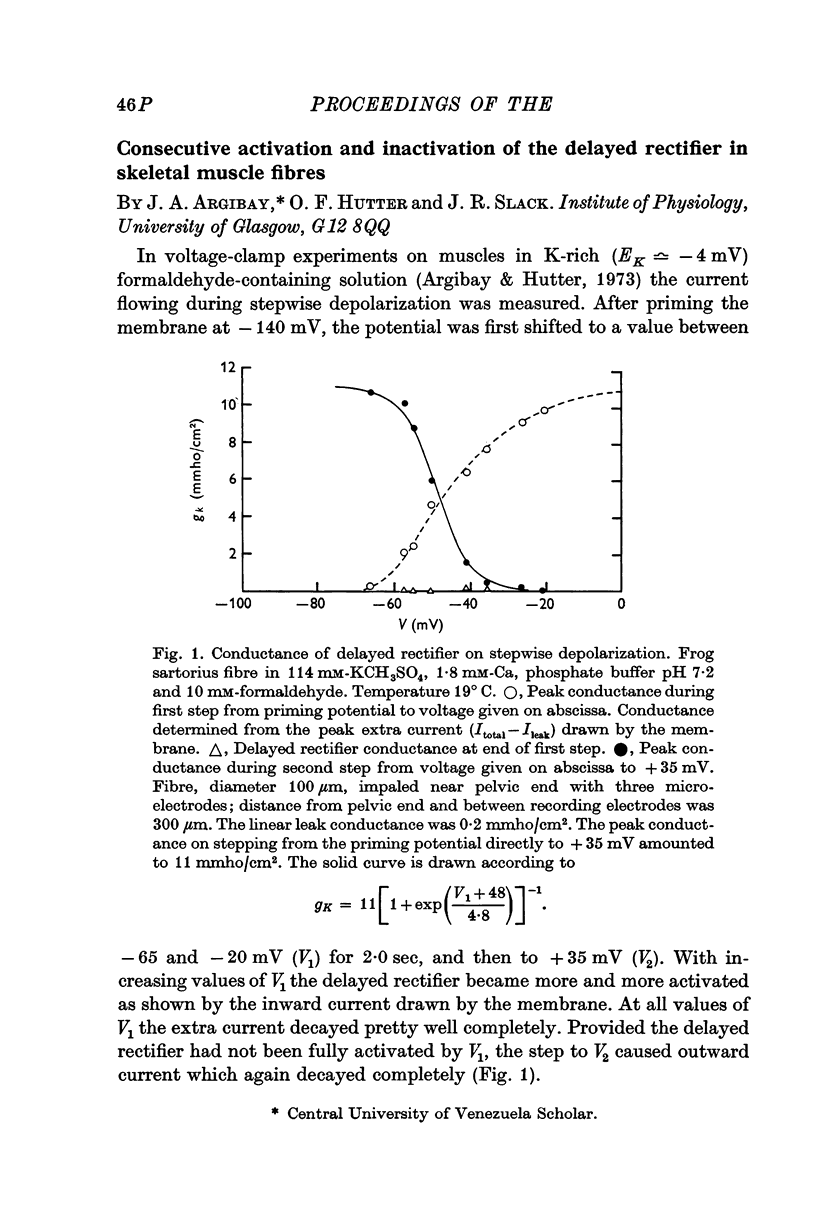
- Argibay J. A., Hutter O. F. Voltage-clamp experiments on the inactivation of the delayed potassium current in skeletal muscle fibres. J Physiol. 1973 Jul;232(1):41P–43P. [PubMed] [Google Scholar]
- Armstrong C. M. Interaction of tetraethylammonium ion derivatives with the potassium channels of giant axons. J Gen Physiol. 1971 Oct;58(4):413–437. doi: 10.1085/jgp.58.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALIS J. U., CONEN P. E. THE ROLE OF ALVEOLAR INCLUSION BODIES IN THE DEVELOPING LUNG. Lab Invest. 1964 Oct;13:1215–1229. [PubMed] [Google Scholar]
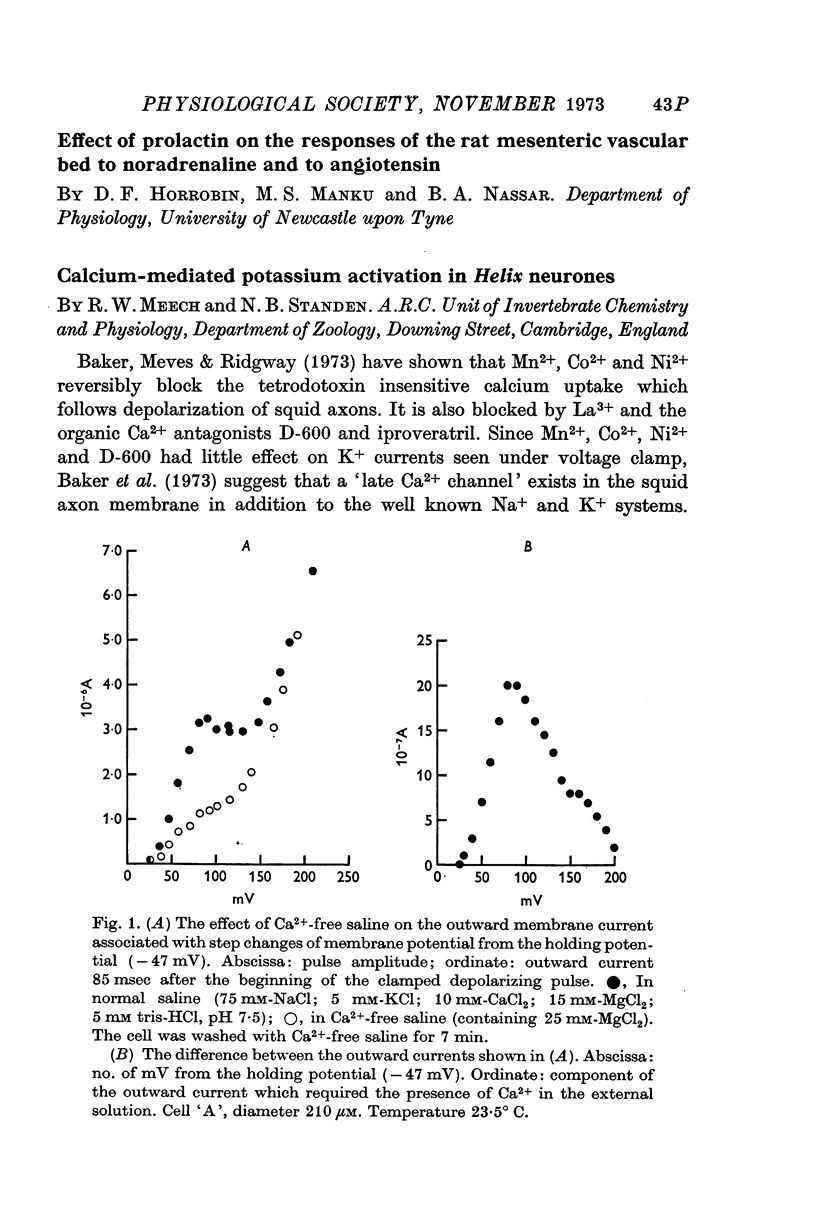
- Baker P. F., Hodgkin A. L., Ridgway E. B. Depolarization and calcium entry in squid giant axons. J Physiol. 1971 Nov;218(3):709–755. doi: 10.1113/jphysiol.1971.sp009641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Meves H., Ridgway E. B. Effects of manganese and other agents on the calcium uptake that follows depolarization of squid axons. J Physiol. 1973 Jun;231(3):511–526. doi: 10.1113/jphysiol.1973.sp010246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baños G., Daniel P. M., Moorhouse S. R., Pratt O. E. The influx of amino acids into the brain of the rat in vivo: the essential compared with some non-essential amino acids. Proc R Soc Lond B Biol Sci. 1973 Feb 27;183(1070):59–70. doi: 10.1098/rspb.1973.0004. [DOI] [PubMed] [Google Scholar]
- Baños G., Daniel P. M., Pratt O. E. Inhibition of entry of L-arginine into the brain of the rat, in vivo, by L-lysine or L-ornithine. J Physiol. 1971;214 (Suppl):24P–25P. [PubMed] [Google Scholar]
- Baños G., Daniel P. M., Pratt O. E. Saturation of a shared mechanism which transports L-arginine and L-lysine into the brain of the living rat. J Physiol. 1974 Jan;236(1):29–41. doi: 10.1113/jphysiol.1974.sp010420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamy D., Lamming P. A., Stevenson A. Daily patterns of feeding and excretion in rats confined to metabolism cages: strain and age differences. Lab Anim. 1970 Oct;4(2):215–225. doi: 10.1258/002367770781071572. [DOI] [PubMed] [Google Scholar]
- Bihler I., Sawh P. C. Effects of diphenylhydantoin on the transport of Na + and K + and the regulation of sugar transport in muscle in vitro. Biochim Biophys Acta. 1971 Oct 12;249(1):240–251. doi: 10.1016/0005-2736(71)90101-5. [DOI] [PubMed] [Google Scholar]
- Black J. W., Duncan W. A., Durant C. J., Ganellin C. R., Parsons E. M. Definition and antagonism of histamine H 2 -receptors. Nature. 1972 Apr 21;236(5347):385–390. doi: 10.1038/236385a0. [DOI] [PubMed] [Google Scholar]
- Blakemore C., Donaghy M. J., Maffei L., Movshon J. A., Rose D., Van Sluyters R. C. Evidence that nitrous oxide can maintain anaesthesia after induction with barbiturates. J Physiol. 1974 Mar;237(2):39P–41P. [PubMed] [Google Scholar]
- Boyd G. W., Landon J., Peart W. S. Radioimmunoassay for determining plasms-levels of angiotensin II in man. Lancet. 1967 Nov 11;2(7524):1002–1005. doi: 10.1016/s0140-6736(67)90284-x. [DOI] [PubMed] [Google Scholar]
- Brooks R. E. Ultrastructure of the physostomatous swimbladder of Rainbow trout (Salmo gairdneri). Z Zellforsch Mikrosk Anat. 1970;106(4):473–483. doi: 10.1007/BF00340286. [DOI] [PubMed] [Google Scholar]
- Burger H. G., Fink G., Lee V. W. Luteinizing hormone releasing factor in ultrafiltrates of blood collected from the pituitary stalk of ovariectomized rats and rats subjected to electrical stimulation of the preoptic area. J Endocrinol. 1972 Aug;54(2):227–237. doi: 10.1677/joe.0.0540227. [DOI] [PubMed] [Google Scholar]
- Caldwell K. P. The treatment of incontinence by electronic implants. Hunterian Lecture delivered at the Royal College of Surgeons of England on 8th December 1966. Ann R Coll Surg Engl. 1967 Dec;41(6):447–459. [PMC free article] [PubMed] [Google Scholar]
- Chapman R. A. The onic dependence of the strength and spontaneous relations of the potassium contracture induced in the heart of the frog Rana pipiens. J Physiol. 1973 Jun;231(2):209–232. doi: 10.1113/jphysiol.1973.sp010229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman R. A., Tunstall J. The dependence of the contractile force generated by frog auricular trabeculae upon the external calcium concentration. J Physiol. 1971 May;215(1):139–162. doi: 10.1113/jphysiol.1971.sp009462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claret M., Coraboeuf E. Membrane potential of perfused and isolated rat liver. J Physiol. 1970 Sep;210(2):137P–138P. [PubMed] [Google Scholar]
- Claret M., Mazet J. L. Ionic fluxes and permeabilities of cell membranes in rat liver. J Physiol. 1972 Jun;223(2):279–295. doi: 10.1113/jphysiol.1972.sp009847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Prediction of repetitive firing behaviour from voltage clamp data on an isolated neurone soma. J Physiol. 1971 Feb;213(1):31–53. doi: 10.1113/jphysiol.1971.sp009366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow T. J., Arbuthnott G. W. Function of catecholamine-containing neurones in mammalian central nervous system. Nat New Biol. 1972 Aug 23;238(86):245–246. doi: 10.1038/newbio238245a0. [DOI] [PubMed] [Google Scholar]
- Curwain B. P., Holton P., Spencer J. Evidence that the inhibitory effect of burimamide on gastric secretion is not due to decreased gastric mucosal blood flow. J Physiol. 1973 Apr;230(1):33P–34P. [PubMed] [Google Scholar]
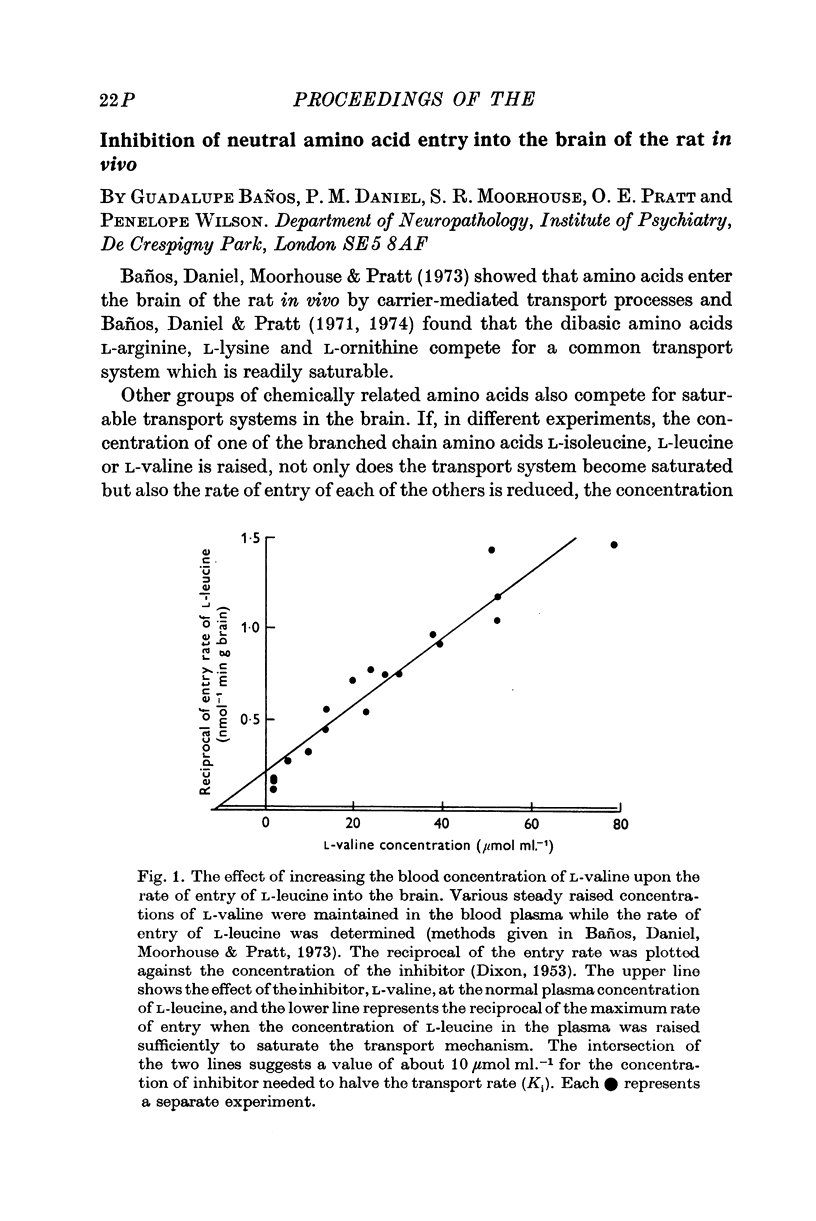
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
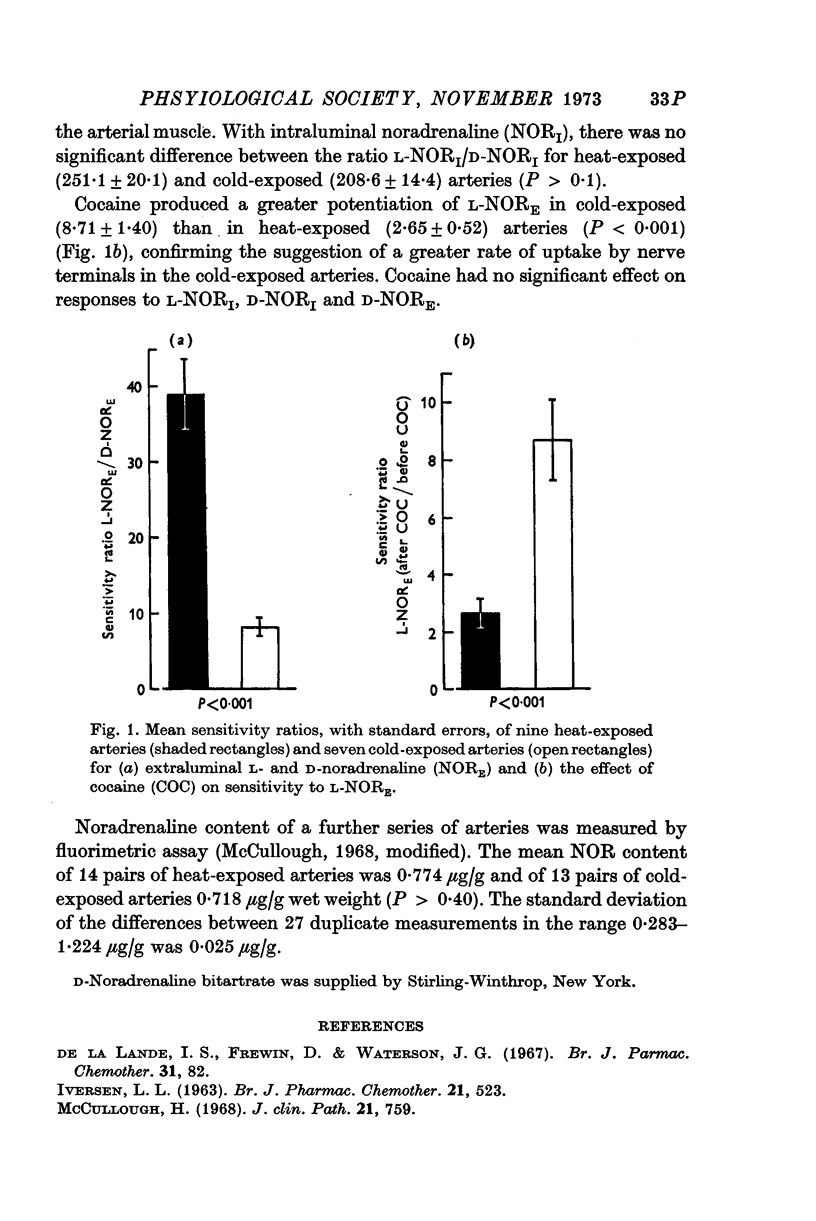
- De la Lande I. S., Frewin D., Waterson J. G. The influence of sympathetic innervation on vascular sensitivity to noradrenaline. Br J Pharmacol Chemother. 1967 Sep;31(1):82–93. doi: 10.1111/j.1476-5381.1967.tb01979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreifuss J. J., Kelly J. S. Recurrent inhibition of antidromically identified rat supraoptic neurones. J Physiol. 1972 Jan;220(1):87–103. doi: 10.1113/jphysiol.1972.sp009696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORDA O., MCILWAIN H. Anticonvulsants on electrically stimulated metabolism of separated mammalian cerebral cortex. Br J Pharmacol Chemother. 1953 Jun;8(2):225–229. doi: 10.1111/j.1476-5381.1953.tb00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formby B. The in vivo and in vitro effect of diphenylhydantoin and phenobarbitone on K+ activated phosphohydrolase and (Na+, K+)-activated ATPase in particulate membrane fractions from rat brain. J Pharm Pharmacol. 1970 Feb;22(2):81–85. doi: 10.1111/j.2042-7158.1970.tb08396.x. [DOI] [PubMed] [Google Scholar]
- Frontali N., Häggendal J. Noradrenaline and dopamine content in the brain of the cockroach Periplaneta americana. Brain Res. 1969 Jul;14(2):540–542. doi: 10.1016/0006-8993(69)90134-6. [DOI] [PubMed] [Google Scholar]
- Gauer O. H., Henry J. P., Behn C. The regulation of extracellular fluid volume. Annu Rev Physiol. 1970;32:547–595. doi: 10.1146/annurev.ph.32.030170.002555. [DOI] [PubMed] [Google Scholar]
- Gibbons W. R., Fozzard H. A. High potassium and low sodium contractures in sheep cardiac muscle. J Gen Physiol. 1971 Nov;58(5):483–510. doi: 10.1085/jgp.58.5.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S. The rat anococcygeus muscle and its response to nerve stimulation and to some drugs. Br J Pharmacol. 1972 Jul;45(3):404–416. doi: 10.1111/j.1476-5381.1972.tb08097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluck L., Kulovich M. V., Eidelman A. I., Cordero L., Khazin A. F. Biochemical development of surface activity in mammalian lung. IV. Pulmonary lecithin synthesis in the human fetus and newborn and etiology of the respiratory distress syndrome. Pediatr Res. 1972 Feb;6(2):81–99. doi: 10.1203/00006450-197202000-00002. [DOI] [PubMed] [Google Scholar]
- Goldman L., Schauf C. L. Quantitative description of sodium and potassium currents and computed action potentials in Myxicola giant axons. J Gen Physiol. 1973 Mar;61(3):361–384. doi: 10.1085/jgp.61.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENRY J. P., GAUER O. H., REEVES J. L. Evidence of the atrial location of receptors influencing urine flow. Circ Res. 1956 Jan;4(1):85–90. doi: 10.1161/01.res.4.1.85. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. Potassium contractures in single muscle fibres. J Physiol. 1960 Sep;153:386–403. doi: 10.1113/jphysiol.1960.sp006541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
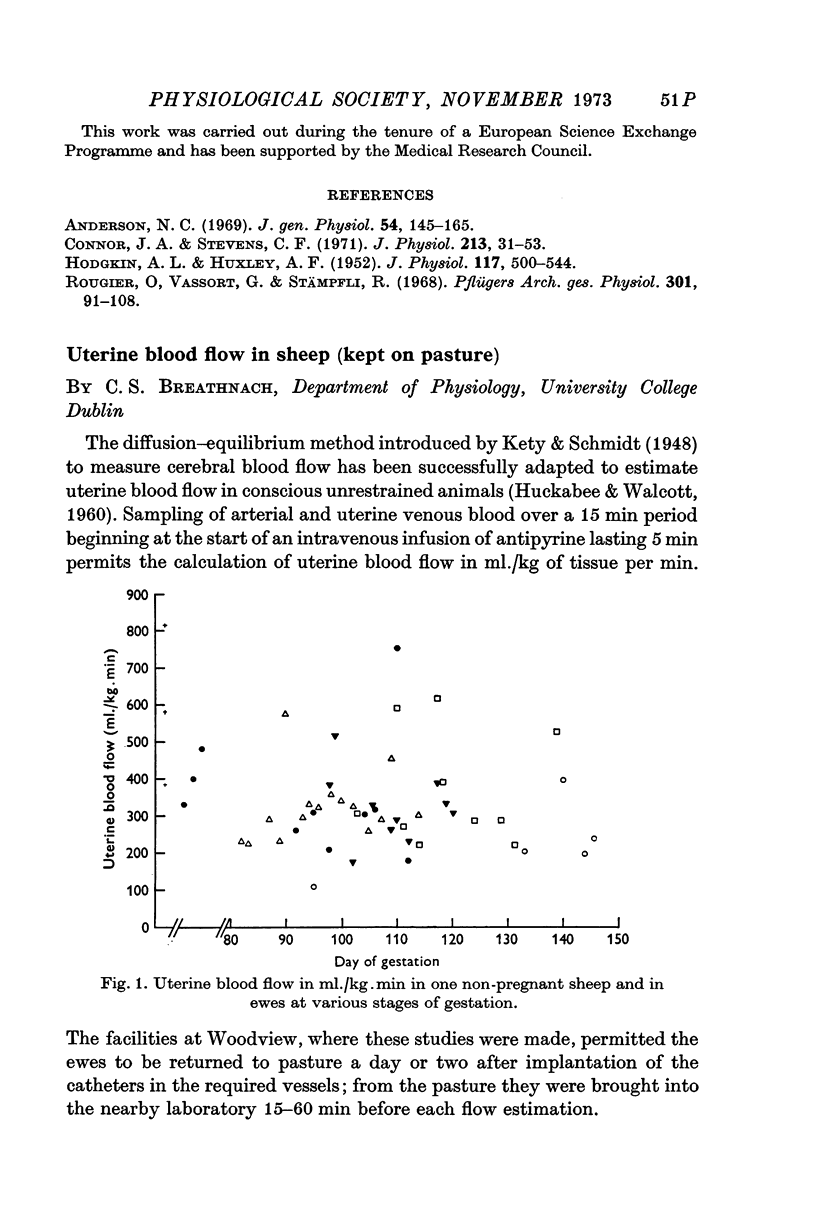
- HUCKABEE W. E., WALCOTT G. Determination of organ blood flow using 4-aminantipyrine. J Appl Physiol. 1960 Nov;15:1139–1143. doi: 10.1152/jappl.1960.15.6.1139. [DOI] [PubMed] [Google Scholar]
- Harper A. A., Reed J. D., Smy J. R. Gastric blood flow in anaesthetized cats. J Physiol. 1968 Feb;194(3):795–807. doi: 10.1113/jphysiol.1968.sp008435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkinson B. R., Lightwood R. Electrical treatment of incontinence. Br J Surg. 1967 Sep;54(9):802–805. doi: 10.1002/bjs.1800540915. [DOI] [PubMed] [Google Scholar]
- Horowicz P., Gage P. W., Eisenberg R. S. The role of the electrochemical gradient in determining potassium fluxes in frog striated muscle. J Gen Physiol. 1968 May;51(5 Suppl):193S+–193S+. [PubMed] [Google Scholar]
- Hughes G. M. Ultrastructure of the lung of Neoceratodus and Lepidosiren in relation to the lung of other vertebrates. Folia Morphol (Praha) 1973;21(2):155–161. [PubMed] [Google Scholar]
- Hutter O. F. Potassium conductance of skeletal muscle treated with formaldehyde. Nature. 1969 Dec 20;224(5225):1215–1217. doi: 10.1038/2241215a0. [DOI] [PubMed] [Google Scholar]
- Hébert F., Fouron J. C., Boileau J. C., Biron P. Pulmonary fate of vasoactive peptides in fetal, newborn, and adult sheep. Am J Physiol. 1972 Jul;223(1):20–23. doi: 10.1152/ajplegacy.1972.223.1.20. [DOI] [PubMed] [Google Scholar]
- IVERSEN L. L. THE UPTAKE OF NORADRENALINE BY THE ISOLATED PERFUSED RAT HEART. Br J Pharmacol Chemother. 1963 Dec;21:523–537. doi: 10.1111/j.1476-5381.1963.tb02020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Wright M. J. Evidence for "sustained" and "transient" neurones in the cat's visual cortex. Vision Res. 1974 Jan;14(1):133–136. doi: 10.1016/0042-6989(74)90128-x. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Wright M. J. The outer disinhibitory surround of the retinal ganglion cell receptive field. J Physiol. 1972 Oct;226(2):511–544. doi: 10.1113/jphysiol.1972.sp009996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kety S. S., Schmidt C. F. THE NITROUS OXIDE METHOD FOR THE QUANTITATIVE DETERMINATION OF CEREBRAL BLOOD FLOW IN MAN: THEORY, PROCEDURE AND NORMAL VALUES. J Clin Invest. 1948 Jul;27(4):476–483. doi: 10.1172/JCI101994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakides C., Levin R. J. Endometrial mechanisms involved in the electrical activity associated with the secretion of uterine fluid. J Physiol. 1973 Feb;229(1):17P–18P. [PubMed] [Google Scholar]
- Kyriakides C., Levin R. J. The effects of ions on the transuterine endometrial potential difference. J Physiol. 1971 Jan;212(2):41P–42P. [PubMed] [Google Scholar]
- LUTTGAU H. C., NIEDERGERKE R. The antagonism between Ca and Na ions on the frog's heart. J Physiol. 1958 Oct 31;143(3):486–505. doi: 10.1113/jphysiol.1958.sp006073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledsome J. R., Linden R. J. The role of left atrial receptors in th diuretic response to left atrial distension. J Physiol. 1968 Sep;198(2):487–503. doi: 10.1113/jphysiol.1968.sp008619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin R. J., Edwards F. The transuterine endometrial potential difference, its variation during the oestrous cycle and its relation to uterine secretion. Life Sci. 1968 Oct 1;7(19):1019–1036. doi: 10.1016/0024-3205(68)90208-7. [DOI] [PubMed] [Google Scholar]
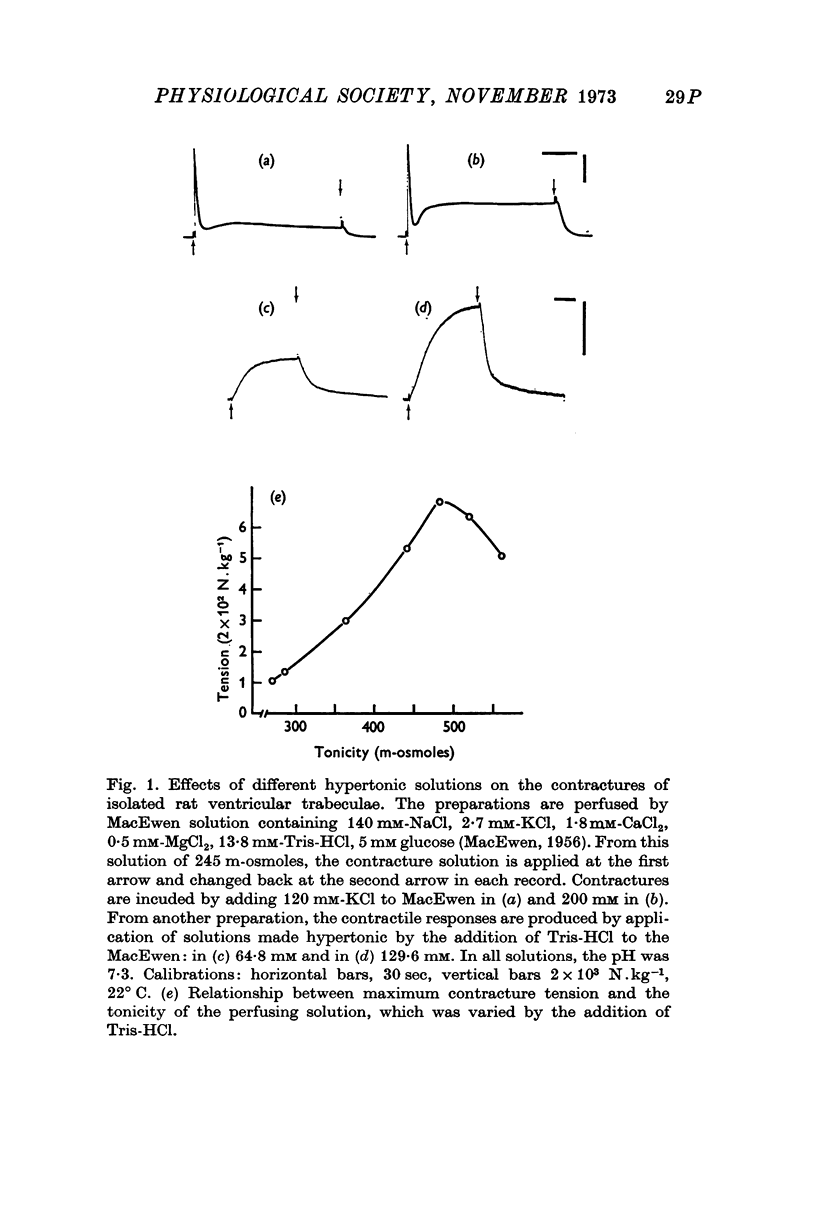
- MCEWEN L. M. The effect on the isolated rabbit heart of vagal stimulation and its modification by cocaine, hexamethonium and ouabain. J Physiol. 1956 Mar 28;131(3):678–689. doi: 10.1113/jphysiol.1956.sp005493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay D. M., MacKay V. Orientation-sensitive after-effects or dichoptically presented colour and form. Nature. 1973 Apr 13;242(5398):477–479. doi: 10.1038/242477a0. [DOI] [PubMed] [Google Scholar]
- McCullough H. Semi-automated method for the differential determination of plasma catecholamines. J Clin Pathol. 1968 Nov;21(6):759–763. doi: 10.1136/jcp.21.6.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W. Intracellular calcium injection causes increased potassium conductance in Aplysia nerve cells. Comp Biochem Physiol A Comp Physiol. 1972 Jun 1;42(2):493–499. doi: 10.1016/0300-9629(72)90128-4. [DOI] [PubMed] [Google Scholar]
- Molinoff P. B., Axelrod J. Distribution and turnover of octopamine in tissues. J Neurochem. 1972 Jan;19(1):157–163. doi: 10.1111/j.1471-4159.1972.tb01265.x. [DOI] [PubMed] [Google Scholar]
- Molinoff P. B., Landsberg L., Axelrod J. An enzymatic assay for octopamine and other beta-hydroxylated phenylethylamines. J Pharmacol Exp Ther. 1969 Dec;170(2):253–261. [PubMed] [Google Scholar]
- Morad M., Rolett E. L. Relaxing effects of catecholamines on mammalian heart. J Physiol. 1972 Aug;224(3):537–558. doi: 10.1113/jphysiol.1972.sp009912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison J. F. Splanchnic slowly adapting mechanoreceptors with punctate receptive fields in the mesentery and gastrointestinal tract of the cat. J Physiol. 1973 Sep;233(2):349–361. doi: 10.1113/jphysiol.1973.sp010311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss R. L., Dyball R. E., Cross B. A. Excitation of antidromically identified neurosecretory cells of the paraventricular nucleus by oxytocin applied iontophoretically. Exp Neurol. 1972 Jan;34(1):95–102. doi: 10.1016/0014-4886(72)90190-2. [DOI] [PubMed] [Google Scholar]
- Nathanson J. A., Greengard P. Octopamine-sensitive adenylate cyclse: evidence for a biological role of octopamine in nervous tissue. Science. 1973 Apr 20;180(4083):308–310. doi: 10.1126/science.180.4083.308. [DOI] [PubMed] [Google Scholar]
- Nett T. M., Akbar A. M., Niswender G. D., Hedlund M. T., White W. F. A radioimmunoassay for gonadotropin-releasing hormone (Gn-RH) in serum. J Clin Endocrinol Metab. 1973 May;36(5):880–885. doi: 10.1210/jcem-36-5-880. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Barker J. L. The pharmacology of recurrent inhibition in the supraoptic neurosecretory system. Brain Res. 1971 Dec 24;35(2):501–511. doi: 10.1016/0006-8993(71)90491-4. [DOI] [PubMed] [Google Scholar]
- Pattle R. E., Schock C., Dirnhuber P., Creasey J. M. Lamellar transformation of lung mitochondria under conditions of stress. Nature. 1972 Dec 22;240(5382):468–469. doi: 10.1038/240468a0. [DOI] [PubMed] [Google Scholar]
- Petrík P. The ultrastructure of the chicken lung in the final stages of embryonic development. Folia Morphol (Praha) 1967;15(2):176–186. [PubMed] [Google Scholar]
- Podleski T., Changeux J. P., Blumenthal R., Kasai M. Conformational transitions in the course of membrane excitation. In: Molecular properties of drug receptors. Ciba Found Symp. 1970:197–228. [PubMed] [Google Scholar]
- Reed J. D., Smy J. R. Mechanisms relating gastric acid secretion and mucosal blood flow during gastrin and histamine stimulation. J Physiol. 1971 Dec;219(3):571–585. doi: 10.1113/jphysiol.1971.sp009678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson H. A., Steele J. E. Activation of insect nerve cord phosphorylase by octopamine and adenosine 3',5'-monophosphate. J Neurochem. 1972 Jun;19(6):1603–1606. doi: 10.1111/j.1471-4159.1972.tb05105.x. [DOI] [PubMed] [Google Scholar]
- Rougier O., Vassort G., Stämpfli R. Voltage clamp experiments on frog atrial heart muscle fibres with the sucrose gap technique. Pflugers Arch Gesamte Physiol Menschen Tiere. 1968;301(2):91–108. doi: 10.1007/BF00362729. [DOI] [PubMed] [Google Scholar]
- Russell W. J. Nitrous oxide--is it an adequate anaesthetic? J Physiol. 1973 May;231(1):20P–21P. [PubMed] [Google Scholar]
- Schock C., Pattle R. E., Creasey J. M. Methods for electron microscopy of the lamellated osmiophilic bodies of the lung. J Microsc. 1973 Apr;97(3):321–330. doi: 10.1111/j.1365-2818.1973.tb03787.x. [DOI] [PubMed] [Google Scholar]
- Sorokin S P. A morphologic and cytochemical study on the great alveolar cell. J Histochem Cytochem. 1966 Dec;14(12):884–897. doi: 10.1177/14.12.884. [DOI] [PubMed] [Google Scholar]
- Swanson P. D., Crane P. O. Diphenylhydantoin and movement of radioactive sodium into electrically stimulated cerebral slices. Biochem Pharmacol. 1972 Nov 1;21(21):2899–2905. doi: 10.1016/0006-2952(72)90214-6. [DOI] [PubMed] [Google Scholar]
- Trimper C. E., Lumbers E. R. The renin-angiotensin system in foetal lambs. Pflugers Arch. 1972;336(1):1–10. doi: 10.1007/BF00589136. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. Stereotaxic mapping of the monoamine pathways in the rat brain. Acta Physiol Scand Suppl. 1971;367:1–48. doi: 10.1111/j.1365-201x.1971.tb10998.x. [DOI] [PubMed] [Google Scholar]
- Valtin H., Sawyer W. H., Sokol H. W. Neurohypophysial principles in rats homozygous and heterozygous for hypothalamic diabetes insipidus (Brattleboro strain). Endocrinology. 1965 Oct;77(4):701–706. doi: 10.1210/endo-77-4-701. [DOI] [PubMed] [Google Scholar]
- Walker R. J., Ramage A. G., Woodruff G. N. The presence of octopamine in the brain of Helix aspersa and its action on specific snail neurones. Experientia. 1972 Oct 15;28(10):1173–1174. doi: 10.1007/BF01946151. [DOI] [PubMed] [Google Scholar]
- Woodbury D. M., Kemp J. W. Pharmacology and mechanisms of action of diphenylhydantoin. Psychiatr Neurol Neurochir. 1971 Mar-Apr;74(2):91–115. [PubMed] [Google Scholar]
- Worthington W. C. Blood samples from the pituitary stalk of the rat: method of collection and factors determining volume. Nature. 1966 May 14;210(5037):710–712. doi: 10.1038/210710a0. [DOI] [PubMed] [Google Scholar]