Abstract
1. A method for measuring the lipogenesis from [14C]glucose by single fat cells is described: (i) after incubation with `carrier-free' [U-14C]glucose (0·55 μ-mole/ml.), collagenase-isolated fat cells were fixed with osmium tetroxide; (ii) similarly incubated pieces of epididymal fat pads were treated with osmium tetroxide for 90 sec, whereby only the superficial cells are fixed, and the tissue was then disintegrated by shaking with collagenase. The osmium-fixed free cells were washed, sucked into a micropipette, measured under a microscope and assayed individually for 14C-activity.
2. There was a quantitative recovery of 14C-lipid activity from osmium-fixed single cells.
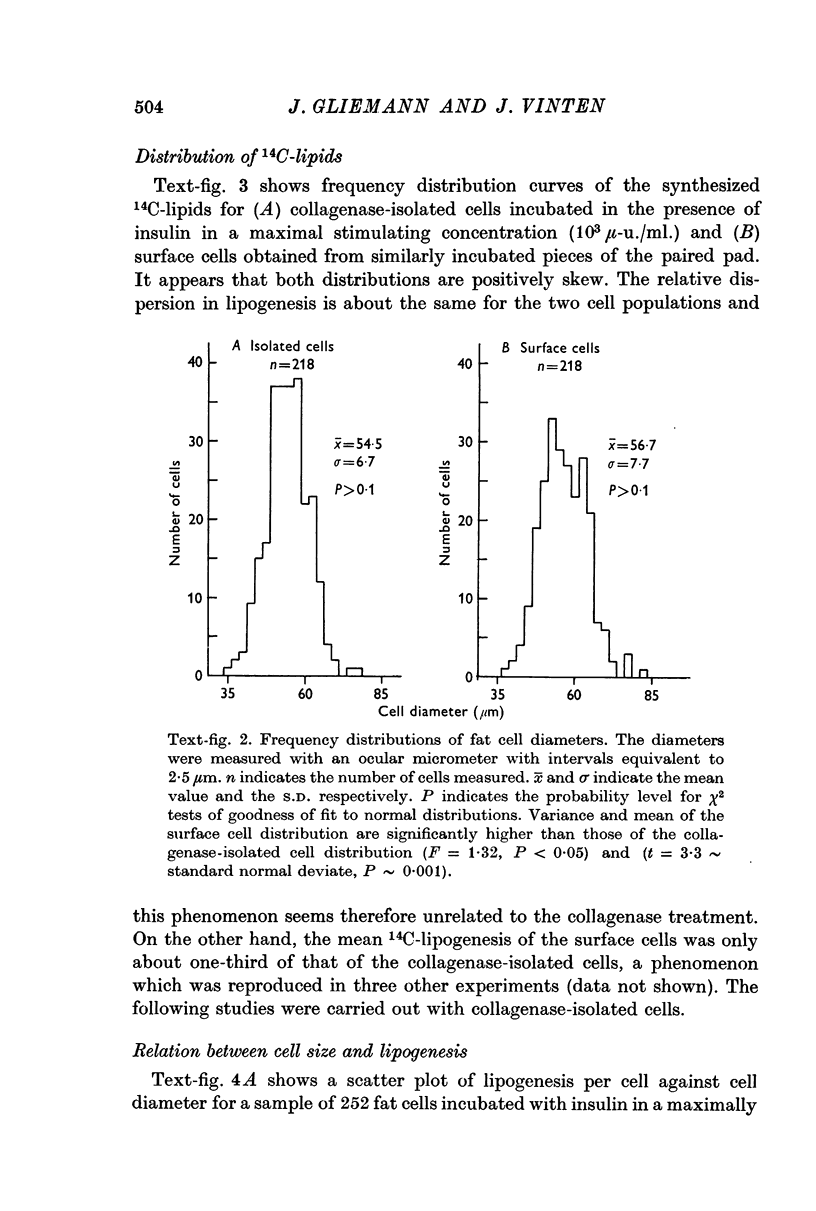
3. Both collagenase-isolated cells and in situ fixed surface cells were normally distributed with respect to diameters (for both cell groups from ad lib. fed rats of ca. 110 g; mean diameter, about 55 μm; S.D. about 7 μm).
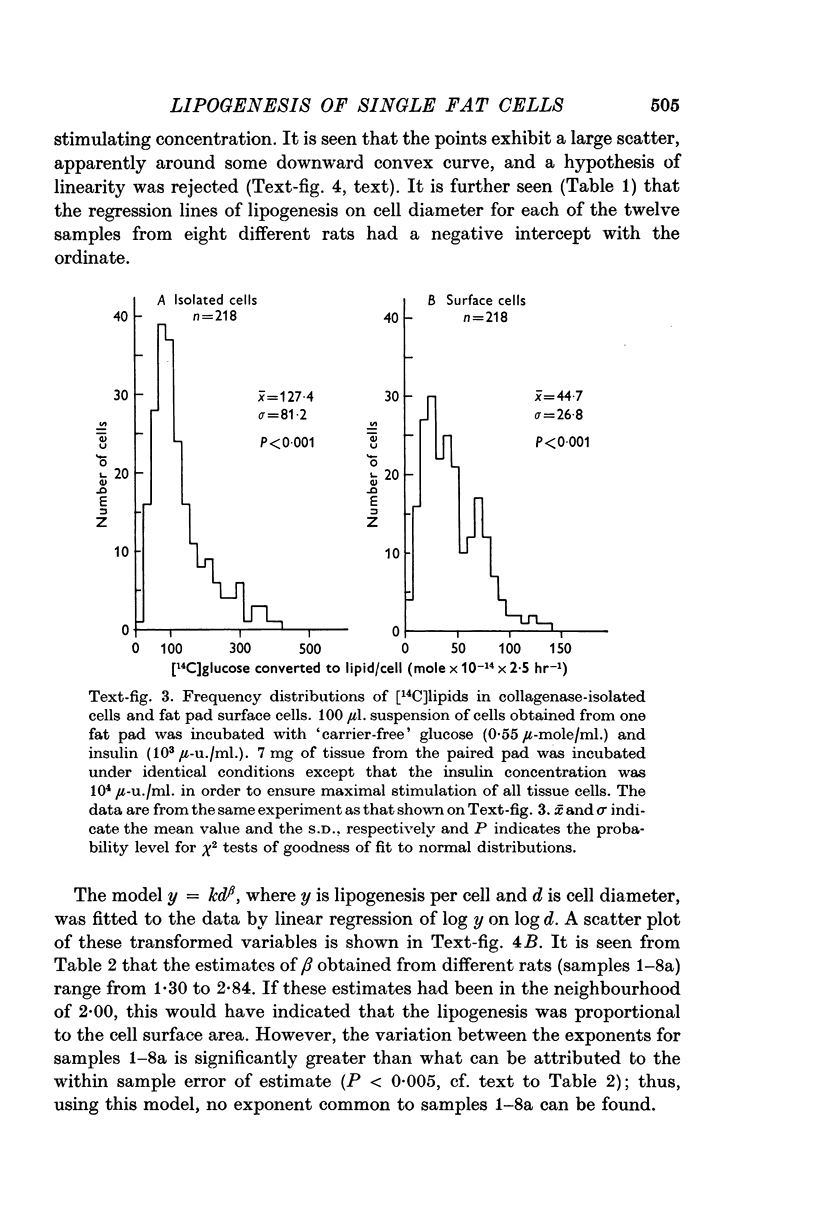
4. Frequency distribution curves (number of fat cells versus 14C-lipogenesis per cell) were asymmetric and very broad (coefficient of variation about 50%) for collagenase-isolated cells incubated with insulin (103 μ-u./ml.). Frequency distribution curves for surface cells obtained from similarly incubated pieces of epididymal fat pads showed a coefficient of variation of the same magnitude, whereas the mean lipogenesis of these cells was only about one third of that of the isolated cells.
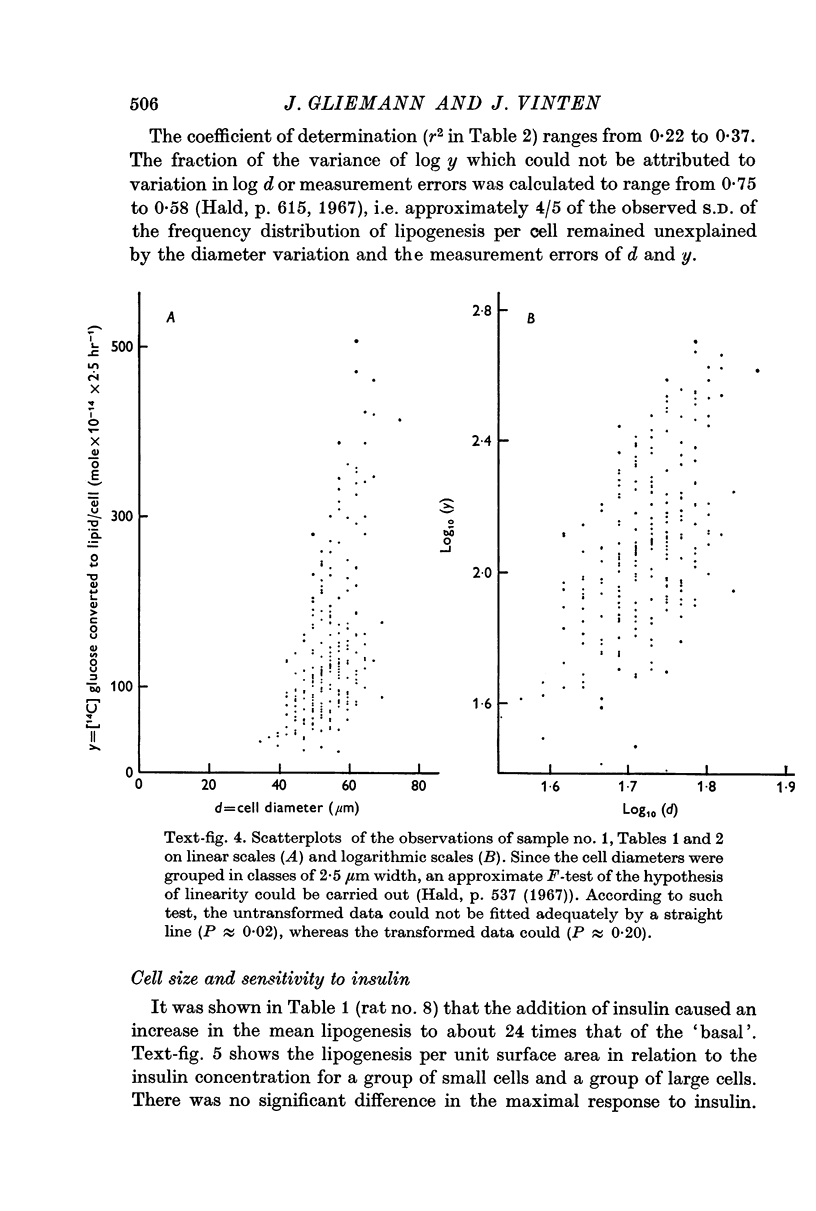
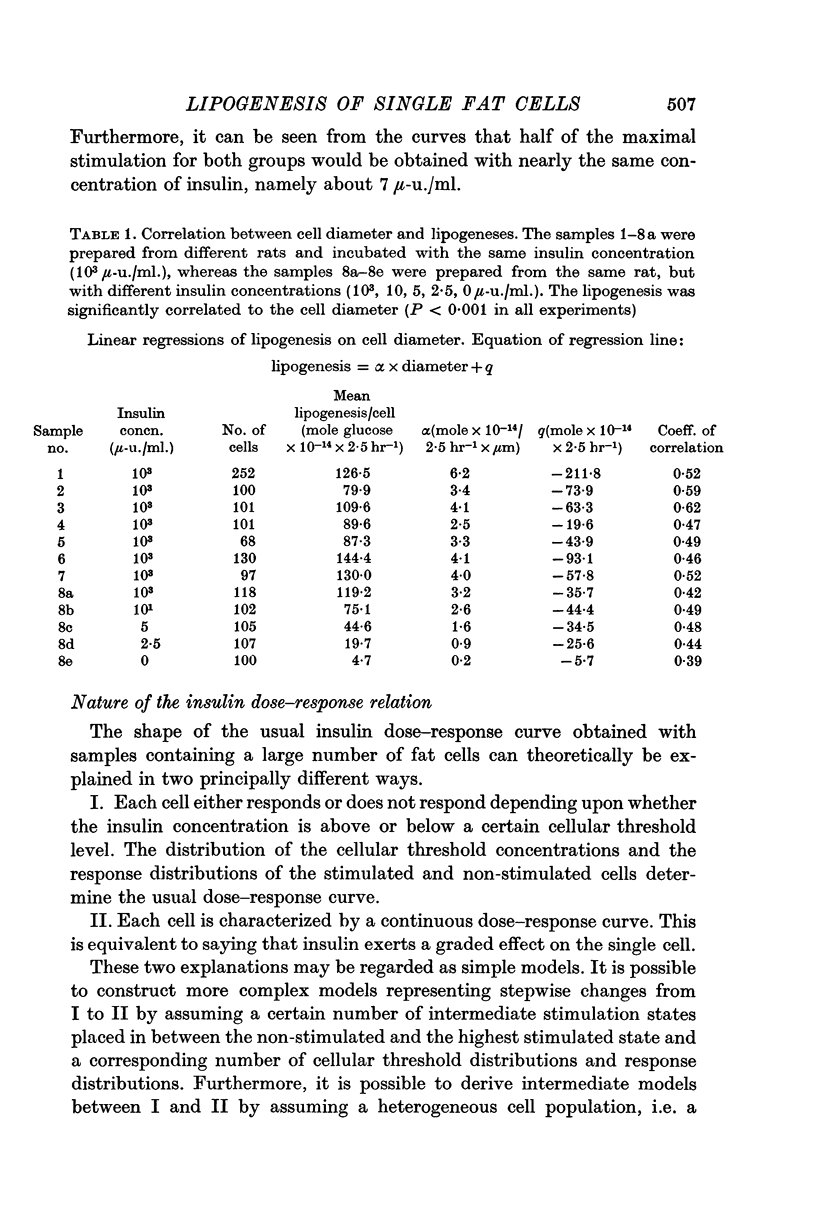
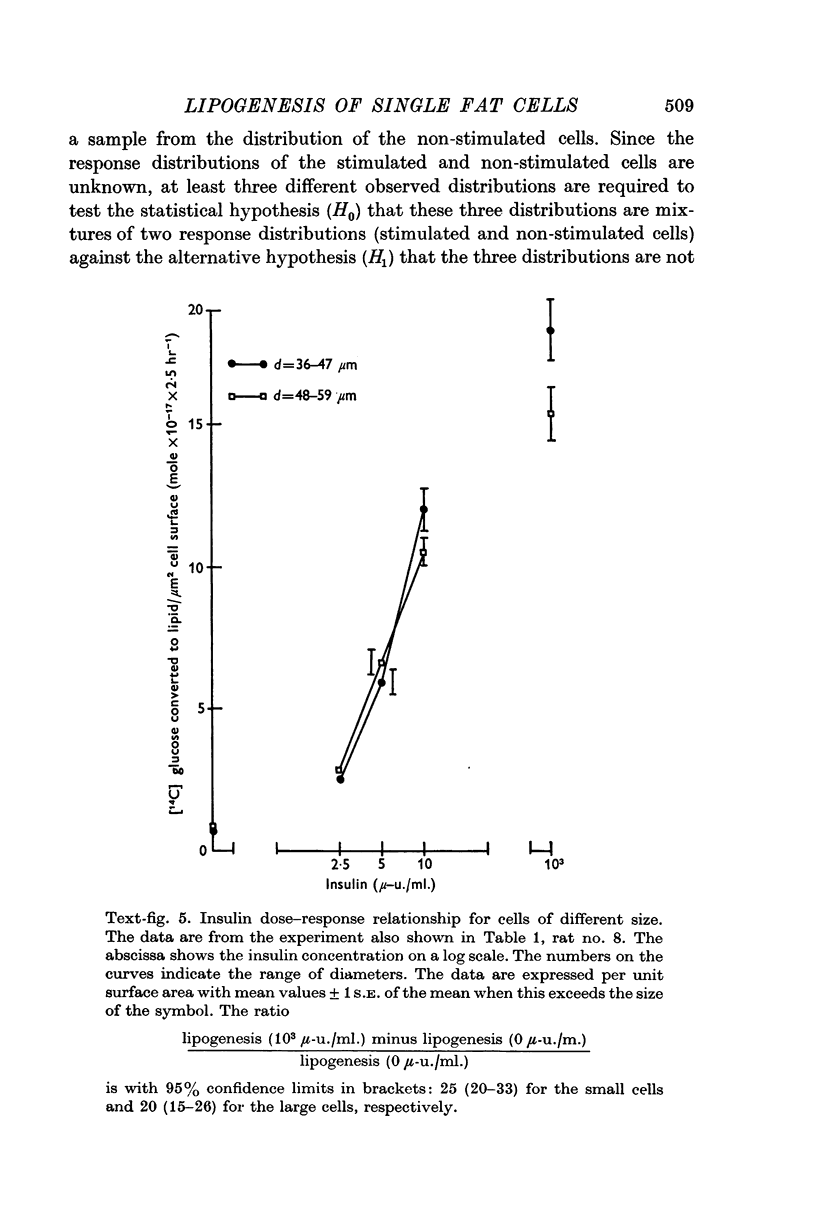
5. Collagenase-isolated cells incubated in the presence of insulin (103 μ-u./ml.) showed a weak but highly significant positive correlation between fat cell diameter and 14C-lipogenesis (eight rats, r about 0·5 and P < 0·001 for each rat). Analysis of the relationship: lipogenesis = k × diameter to the exponent of β showed that the estimates of β varied significantly from rat to rat (range: 1·3-2·9). Similar correlations between cell size and lipogenesis were found both for cells incubated with insulin in various submaximal concentrations and for cells incubated without insulin.
6. Small and large cells from the same rat were equally sensitive to insulin.
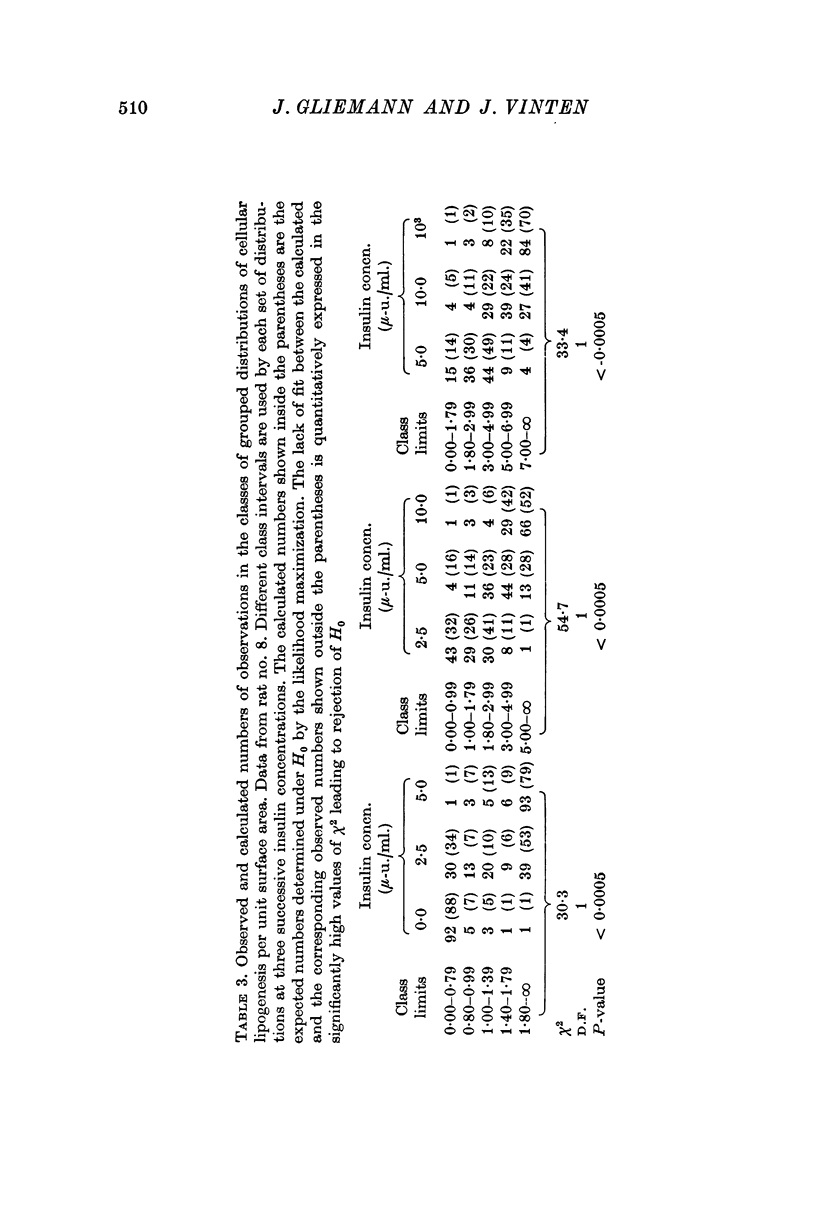
7. Statistical analysis of frequency distribution curves (number of cells versus 14C-lipogenesis per unit surface area) representing cells from the same rat incubated with insulin 0, 2·5, 5, 10, and 103 μ-u./ml., respectively, suggests that insulin exerts a graded influence on the lipogenesis of each fat cell.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björntorp P., Karlsson M. Triglyceride synthesis in human subcutaneous adipose tissue cells of different size. Eur J Clin Invest. 1970 Aug;1(2):112–117. doi: 10.1111/j.1365-2362.1970.tb00607.x. [DOI] [PubMed] [Google Scholar]
- DOLE V. P., MEINERTZ H. Microdetermination of long-chain fatty acids in plasma and tissues. J Biol Chem. 1960 Sep;235:2595–2599. [PubMed] [Google Scholar]
- Di Girolamo M., Mendlinger S., Fertig J. W. A simple method to determine fat cell size and number in four mammalian species. Am J Physiol. 1971 Sep;221(3):850–858. doi: 10.1152/ajplegacy.1971.221.3.850. [DOI] [PubMed] [Google Scholar]
- Fisher R. B., Gilbert J. C. The effect of insulin on the kinetics of pentose permeation of the rat heart. J Physiol. 1970 Sep;210(2):297–304. doi: 10.1113/jphysiol.1970.sp009211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliemann J. Assay of insulin-like activity by the isolated fat cell method. I. Factors influencing the response to crystalline insulin. Diabetologia. 1967 Aug;3(4):382–388. doi: 10.1007/BF02342631. [DOI] [PubMed] [Google Scholar]
- Gliemann J. Glucose metabolism and response to insulin of isolated fat cells and epididymal. fat pads. Acta Physiol Scand. 1968 Apr;72(4):481–491. doi: 10.1111/j.1748-1716.1968.tb03872.x. [DOI] [PubMed] [Google Scholar]
- Greenwood M. R., Johnson P. R., Hirsch J. Relationship of age and cellularity to metabolic activity in C57B mice. Proc Soc Exp Biol Med. 1970 Mar;133(3):944–947. doi: 10.3181/00379727-133-34600. [DOI] [PubMed] [Google Scholar]
- HIRSCH J., FARQUHAR J. W., AHRENS E. H., Jr, PETERSON M. L., STOFFEL W. Studies of adipose tissue in man. A microtechnic for sampling and analysis. Am J Clin Nutr. 1960 Jul-Aug;8:499–511. doi: 10.1093/ajcn/8.4.499. [DOI] [PubMed] [Google Scholar]
- Hirsch J., Gallian E. Methods for the determination of adipose cell size in man and animals. J Lipid Res. 1968 Jan;9(1):110–119. [PubMed] [Google Scholar]
- Hollenberg C. H. The origin and glyceride distribution of fatty acids in rat adipose tissue. J Clin Invest. 1966 Feb;45(2):205–216. doi: 10.1172/JCI105333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanrenaud B. Adipose tissue dynamics and regulation, revisited. Ergeb Physiol. 1968;60:57–140. doi: 10.1007/BFb0107251. [DOI] [PubMed] [Google Scholar]
- Knittle J. L., Hirsch J. Effect of early nutrition on the development of rat epididymal fat pads: cellularity and metabolism. J Clin Invest. 1968 Sep;47(9):2091–2098. doi: 10.1172/JCI105894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salans L. B., Dougherty J. W. The effect of insulin upon glucose metabolism by adipose cells of different size. Influence of cell lipid and protein content, age, and nutritional state. J Clin Invest. 1971 Jul;50(7):1399–1410. doi: 10.1172/JCI106623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salans L. B., Knittle J. L., Hirsch J. The role of adipose cell size and adipose tissue insulin sensitivity in the carbohydrate intolerance of human obesity. J Clin Invest. 1968 Jan;47(1):153–165. doi: 10.1172/JCI105705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith U. Insulin responsiveness and lipid synthesis in human fat cells of different sizes: effect of the incubation medium. Biochim Biophys Acta. 1970 Dec 15;218(3):417–423. doi: 10.1016/0005-2760(70)90004-4. [DOI] [PubMed] [Google Scholar]
- Zinder O., Arad R., Shapiro B. Effect of cell size on the metabolism of isolated fat cells. Isr J Med Sci. 1967 Nov-Dec;3(6):787–791. [PubMed] [Google Scholar]
- Zinder O., Shapiro B. Effect of cell size on epinephrine- and ACTH-induced fatty acid release from isolated fat cells. J Lipid Res. 1971 Jan;12(1):91–95. [PubMed] [Google Scholar]