Abstract
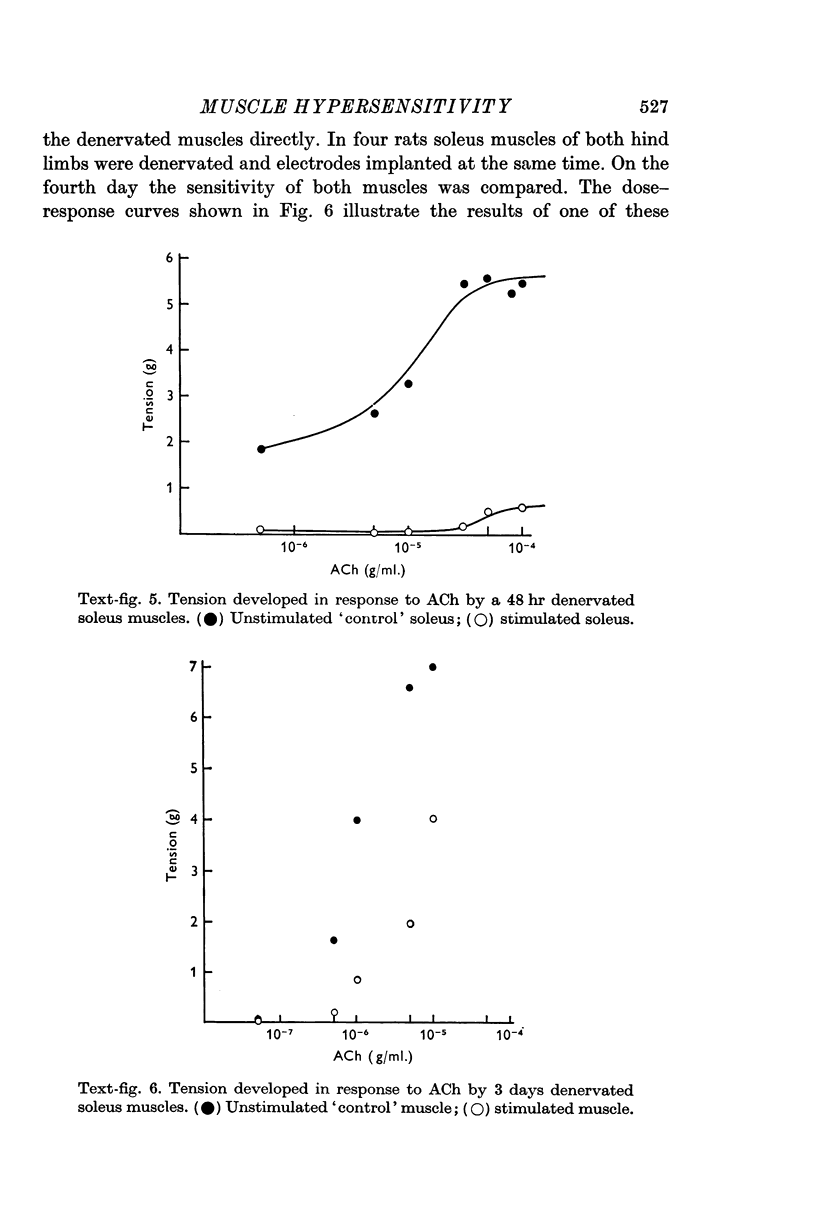
1. Innervated adult skeletal muscle is sensitive to acetylcholine at the end-plate region only. After denervation the entire muscle membrane becomes chemosensitive. The period of greatest increase in sensitivity in rat soleus muscles following section of the sciatic nerve in the thigh is between 48 and 72 hr post-operatively.
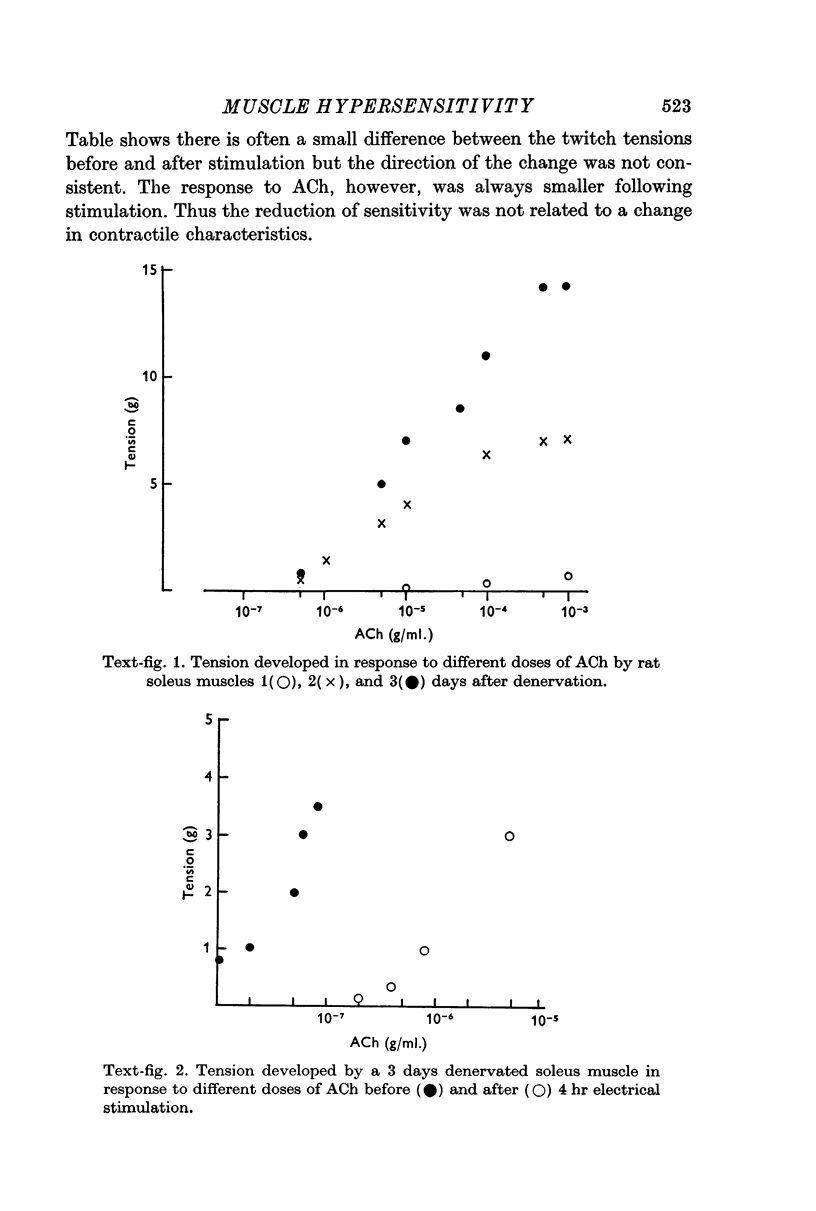
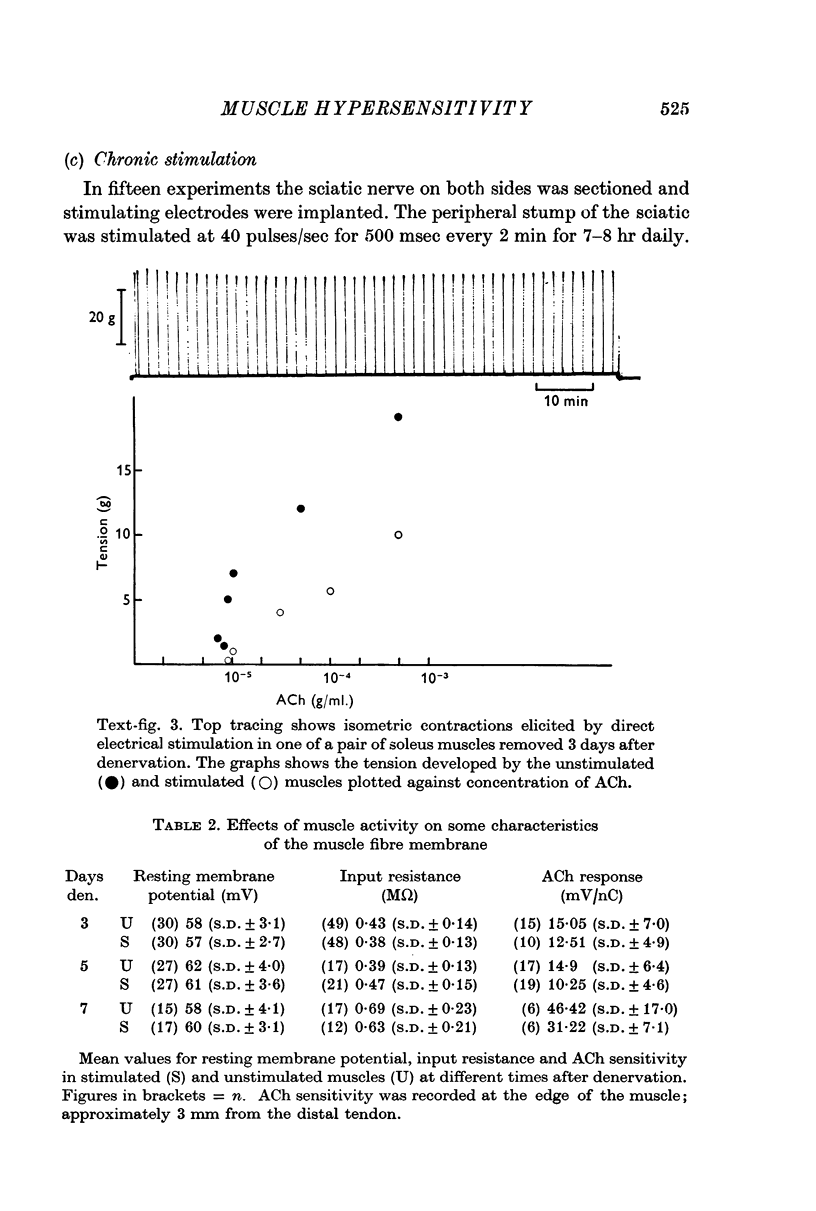
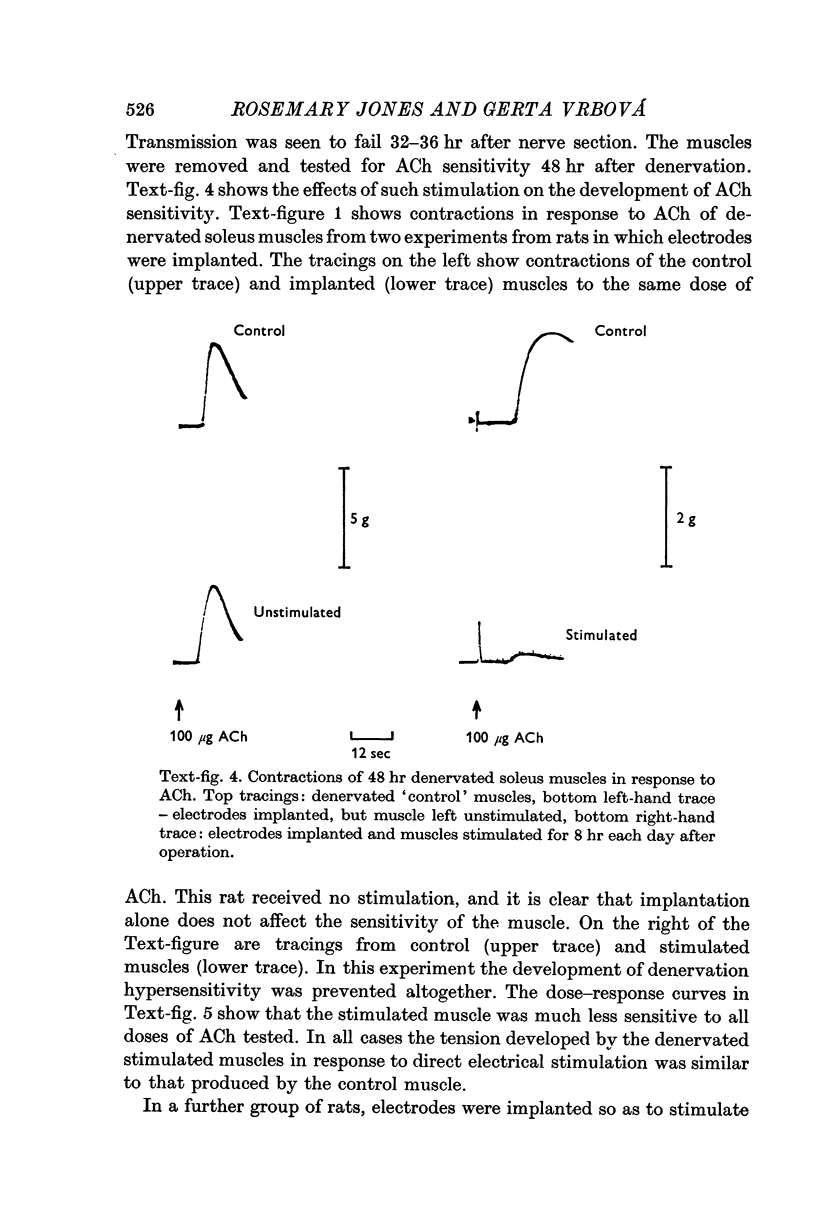
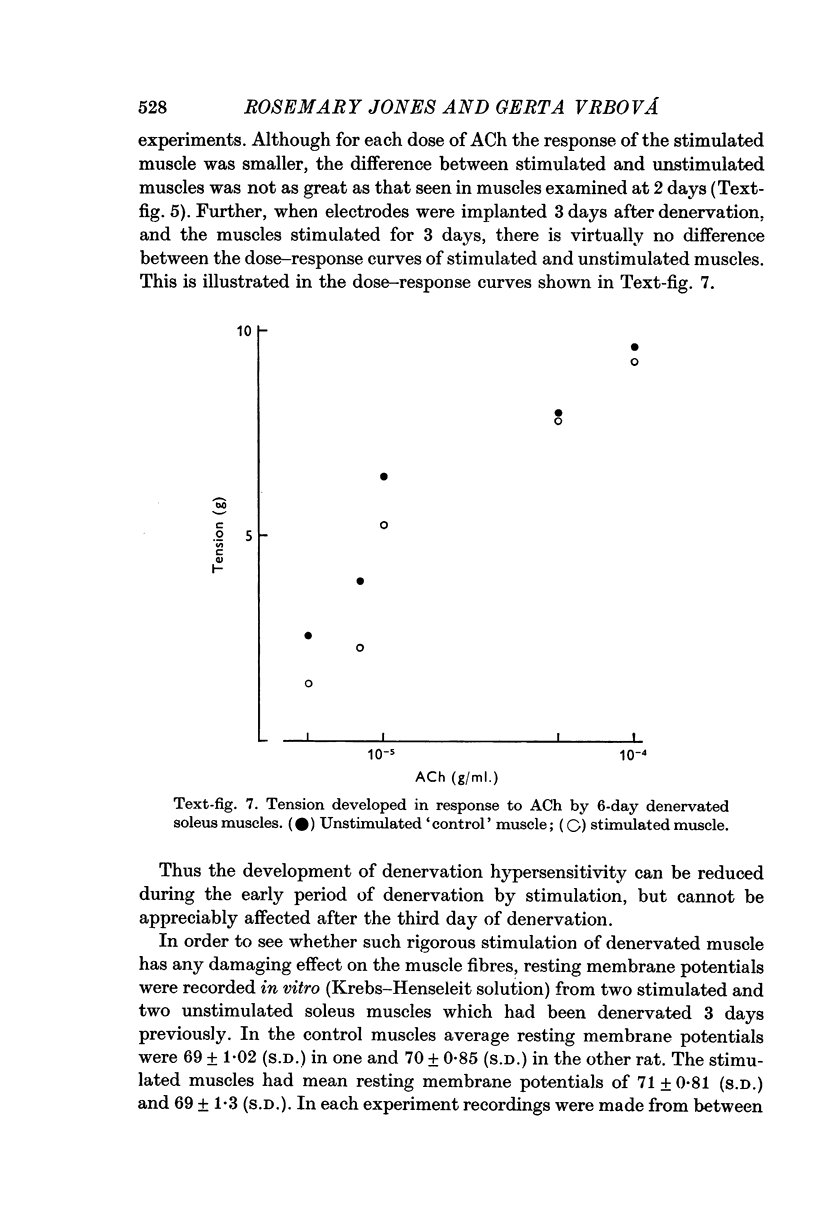
2. Direct electrical stimulation was found to prevent the onset of the development of denervation hypersensitivity during the first 2-3 days after nerve section. Thereafter, electrical stimulation only reduced the sensitivity of denervated muscles to acetylcholine (ACh).
3. The period of greatest increase in sensitivity follows loss of transmission and degeneration of the nerve terminals. Once this degeneration is under way, electrical stimulation is no longer as effective in preventing the development of denervation hypersensitivity.
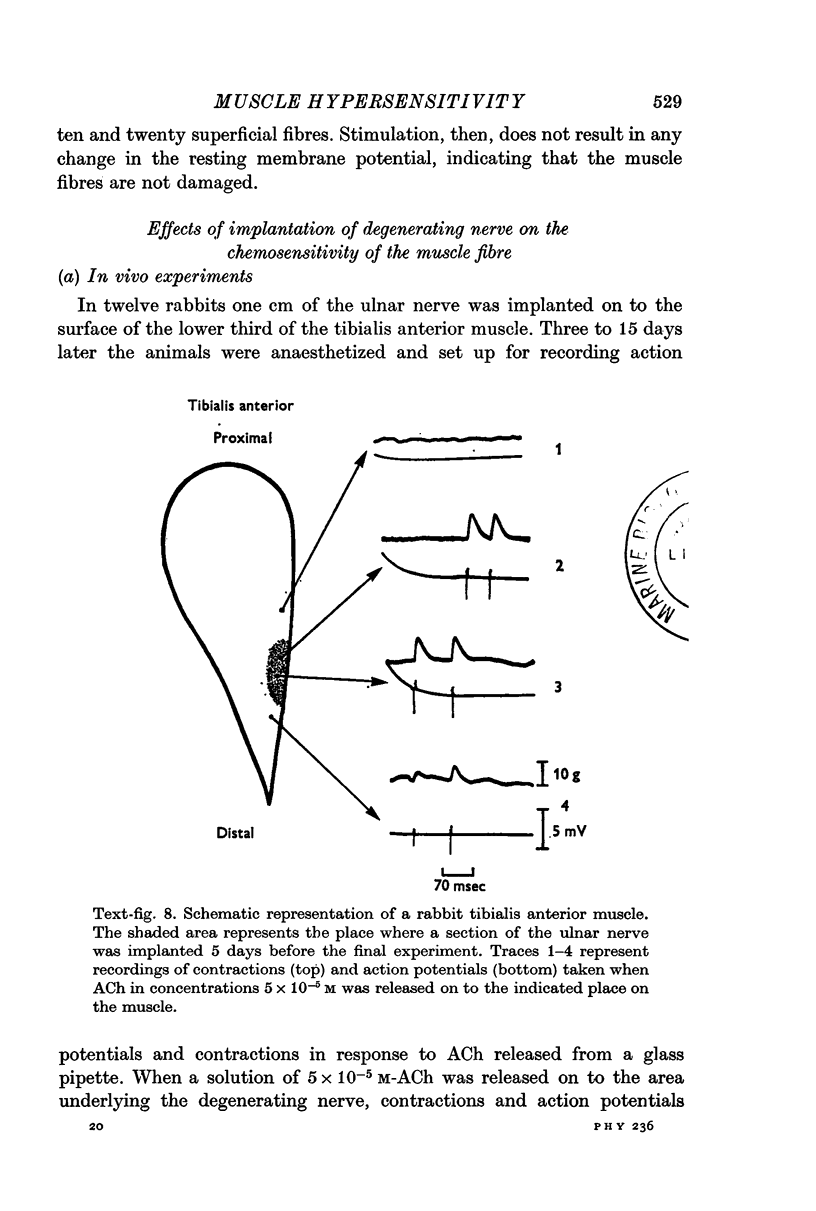
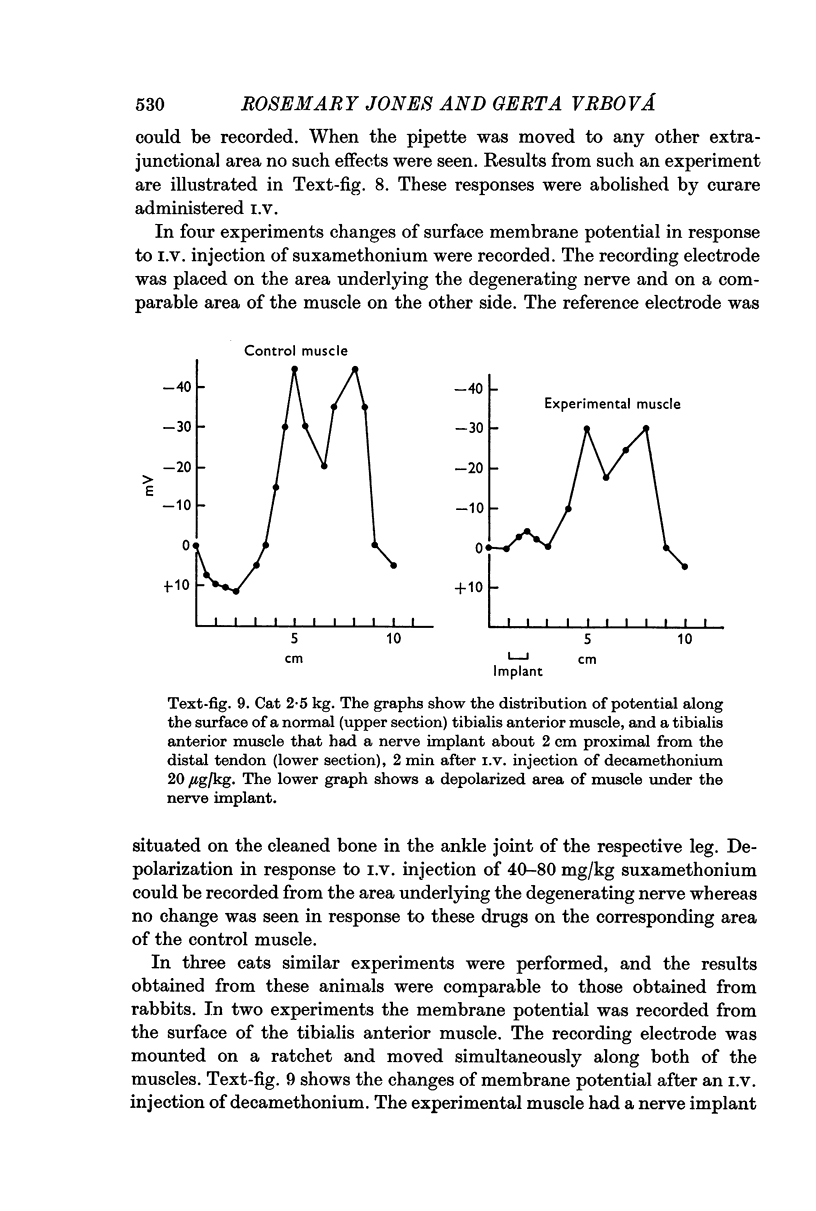
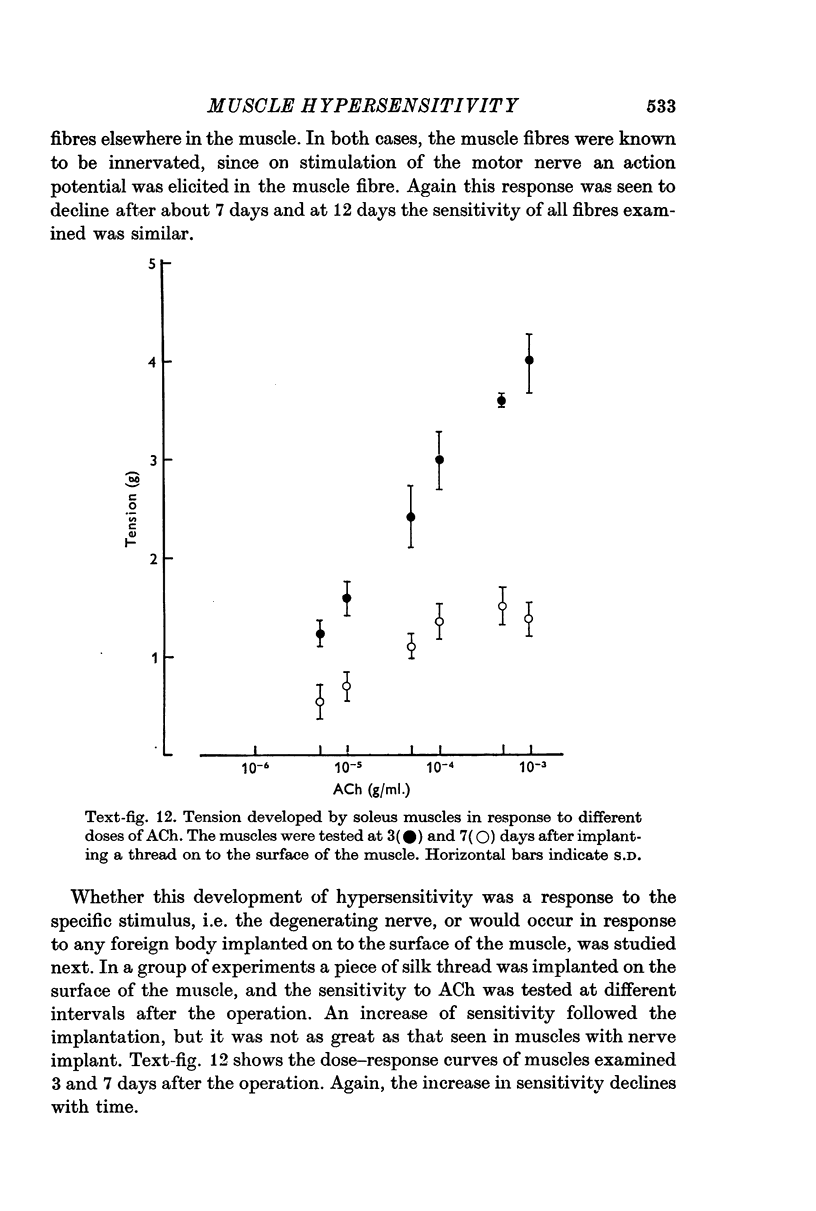
4. Hypersensitivity is also seen in muscles on which a small piece of thread or degenerating nerve has been placed. Hypersensitivity following these procedures declines within a few days, unlike denervation hypersensitivity which persists until innervation is restored.
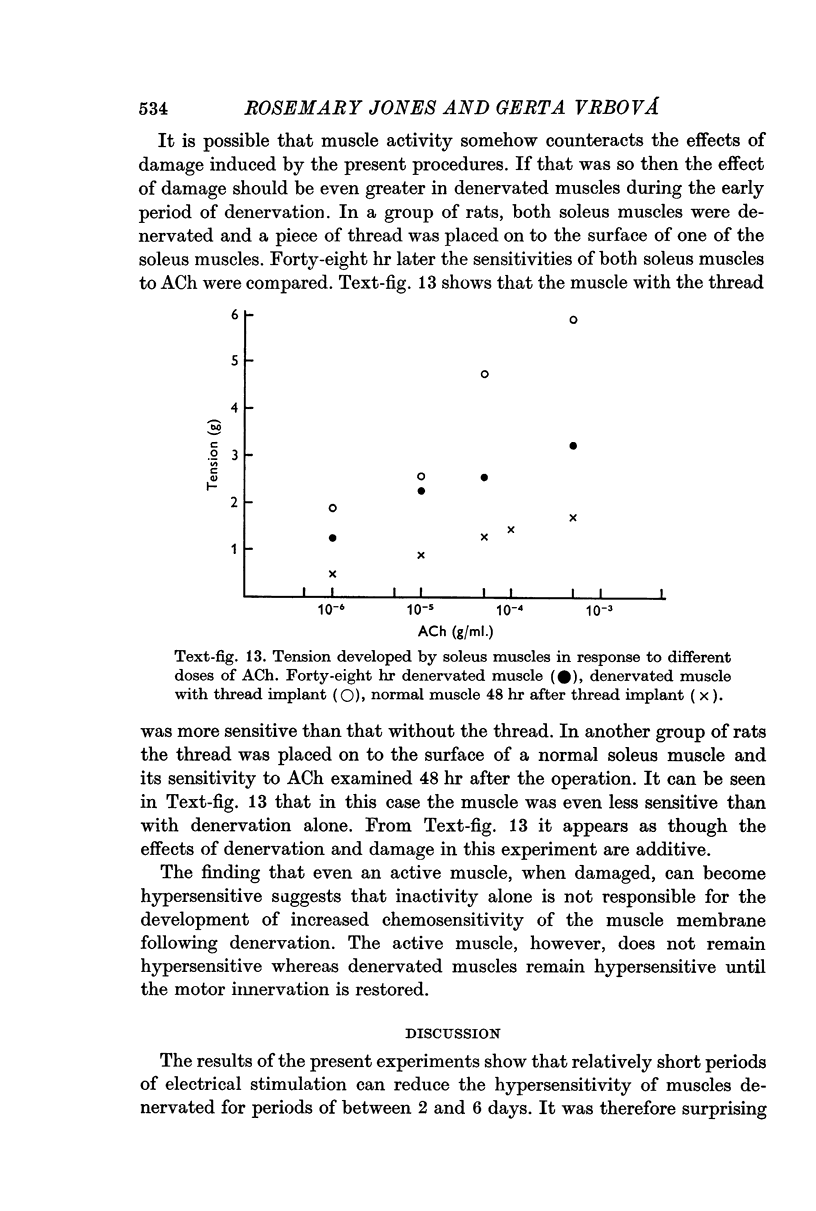
5. The present results suggest that activity alone cannot prevent the development of hypersensitivity in the presence of degenerating nerve fibres, or muscle damage. Activity does however counteract increased sensitivity. It is suggested that two factors interact to produce denervation hypersensitivity; the presence of degenerating nerve tissue and concomitant cellular changes bring about changes in the muscle fibre membrane causing it to become hypersensitive; and the loss of muscle activity, resulting in the persistence of hypersensitivity until innervation is restored.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELSSON J., THESLEFF S. A study of supersensitivity in denervated mammalian skeletal muscle. J Physiol. 1959 Jun 23;147(1):178–193. doi: 10.1113/jphysiol.1959.sp006233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque E. X., Thesleff S. Influence of phospholipase C on some electrical properties of the skeletal muscle membrane. J Physiol. 1967 May;190(1):123–137. doi: 10.1113/jphysiol.1967.sp008197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. L. The actions of acetylcholine on denervated mammalian and frog's muscle. J Physiol. 1937 Jun 3;89(4):438–461. doi: 10.1113/jphysiol.1937.sp003491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAMOND J., MILEDI R. A study of foetal and new-born rat muscle fibres. J Physiol. 1962 Aug;162:393–408. doi: 10.1113/jphysiol.1962.sp006941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fambrough D. M. Acetylcholine sensitivity of muscle fiber membranes: mechanism of regulation by motoneurons. Science. 1970 Apr 17;168(3929):372–373. doi: 10.1126/science.168.3929.372. [DOI] [PubMed] [Google Scholar]
- Grampp W., Harris J. B., Thesleff S. Inhibition of denervation changes in skeletal muscle by blockers of protein synthesis. J Physiol. 1972 Mar;221(3):743–754. doi: 10.1113/jphysiol.1972.sp009780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann E., Young J. Z. The re-innervation of muscle after various periods of atrophy. J Anat. 1944 Jan;78(Pt 1-2):15–43. [PMC free article] [PubMed] [Google Scholar]
- HAJEK I., GUTMANN E., SYROVY I. PROTEOLYTIC ACTIVITY IN DENERVATED AND REINNERVATED MUSCLE. Physiol Bohemoslov. 1964;13:32–38. [PubMed] [Google Scholar]
- Harris J. B., Thesleff S. Nerve stump length and membrane changes in denervated skeletal muscle. Nat New Biol. 1972 Mar 15;236(63):60–61. doi: 10.1038/newbio236060a0. [DOI] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE DEVELOPMENT OF ACETYLCHOLINE SENSITIVITY IN NERVE-FREE SEGMENTS OF SKELETAL MUSCLE. J Physiol. 1964 Mar;170:389–396. doi: 10.1113/jphysiol.1964.sp007339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. An investigation of spontaneous activity at the neuromuscular junction of the rat. J Physiol. 1956 Jun 28;132(3):650–666. doi: 10.1113/jphysiol.1956.sp005555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUCO J. V., EYZAGUIRRE C. Fibrillation and hypersensitivity to ACh in denervated muscle: effect of length of degenerating nerve fibers. J Neurophysiol. 1955 Jan;18(1):65–73. doi: 10.1152/jn.1955.18.1.65. [DOI] [PubMed] [Google Scholar]
- Lomo T., Rosenthal J. Control of ACh sensitivity by muscle activity in the rat. J Physiol. 1972 Mar;221(2):493–513. doi: 10.1113/jphysiol.1972.sp009764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunt G. G., Stefani E., De Robertis E. [Increased incorporation of [G-3H]leucine into a possible "receptor" proteolipid in denervated muscle in vivo. J Neurochem. 1971 Aug;18(8):1545–1553. doi: 10.1111/j.1471-4159.1971.tb00016.x. [DOI] [PubMed] [Google Scholar]
- MILEDI R. Junctional and extra-junctional acetylcholine receptors in skeletal muscle fibres. J Physiol. 1960 Apr;151:24–30. [PMC free article] [PubMed] [Google Scholar]
- MILEDI R. Properties of regenerating neuromuscular synapses in the frog. J Physiol. 1960 Nov;154:190–205. doi: 10.1113/jphysiol.1960.sp006573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILEDI R. The acetylcholine sensitivity of frog muscle fibres after complete or partial devervation. J Physiol. 1960 Apr;151:1–23. [PMC free article] [PubMed] [Google Scholar]
- Maclagan J., Vrbová G. A study of the increased sensitivity of denervated and re-innervated muscle to depolarizing drugs. J Physiol. 1966 Jan;182(1):131–143. doi: 10.1113/jphysiol.1966.sp007813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Slater C. R. On the degeneration of rat neuromuscular junctions after nerve section. J Physiol. 1970 Apr;207(2):507–528. doi: 10.1113/jphysiol.1970.sp009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert E. D., Oester Y. T. Nerve impulses and trophic effect. Absence of fibrillation after prolonged and reversible conduction block. Arch Neurol. 1970 Jan;22(1):57–63. doi: 10.1001/archneur.1970.00480190061010. [DOI] [PubMed] [Google Scholar]
- THESLEFF S. Supersensitivity of skeletal muscle produced by botulinum toxin. J Physiol. 1960 Jun;151:598–607. doi: 10.1113/jphysiol.1960.sp006463. [DOI] [PMC free article] [PubMed] [Google Scholar]