Abstract
1. The impulse discharge of ganglion cells and the proximal negative response were recorded with extracellular micro-electrodes in Necturus retina and spatial influences of background illumination studied.
2. When a steady background of constant illuminance was extended in diameter from 0·25 to 1 mm, the response to a small concentrically placed test flash was enhanced (sensitization).
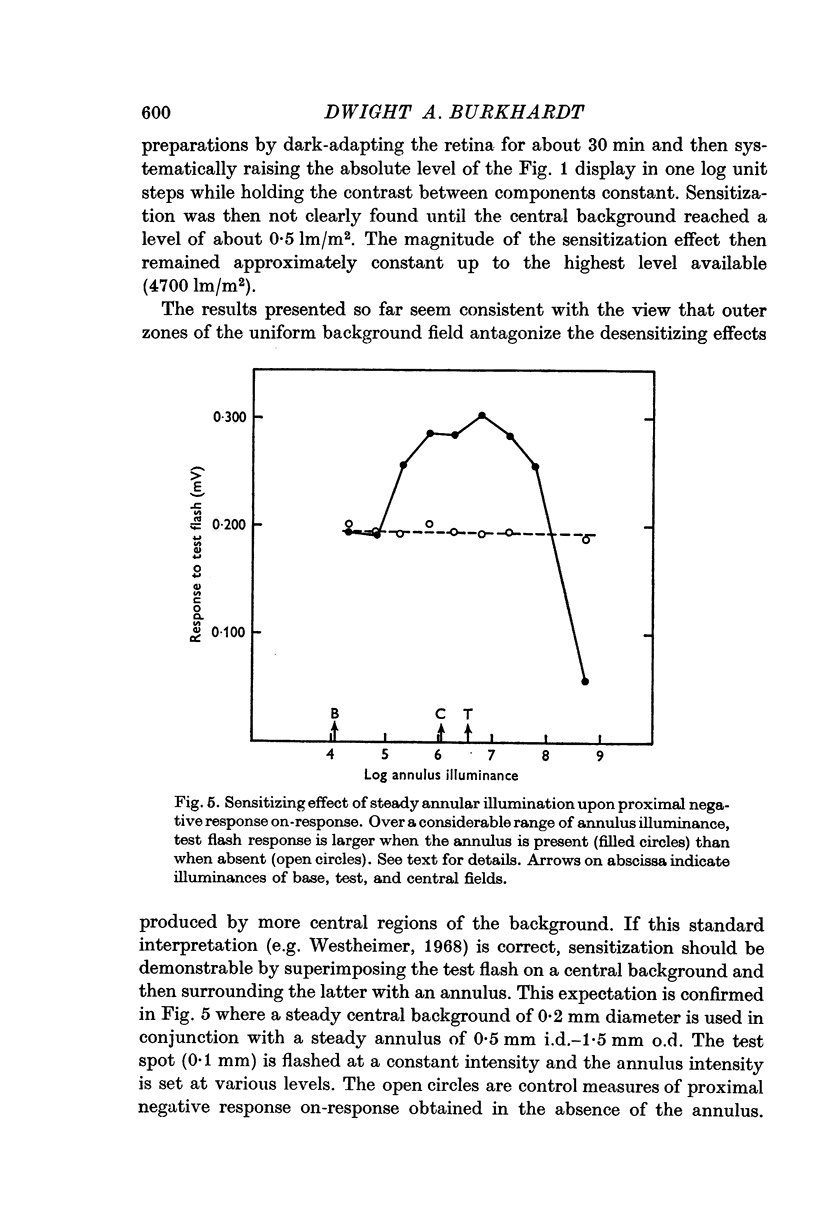
3. This sensitization was tonic, showed marked spatial summation, was also found by surrounding a central background with an annulus, and when quantified by threshold measurements, closely resembled the sensitization effect found psychophysically in human vision.
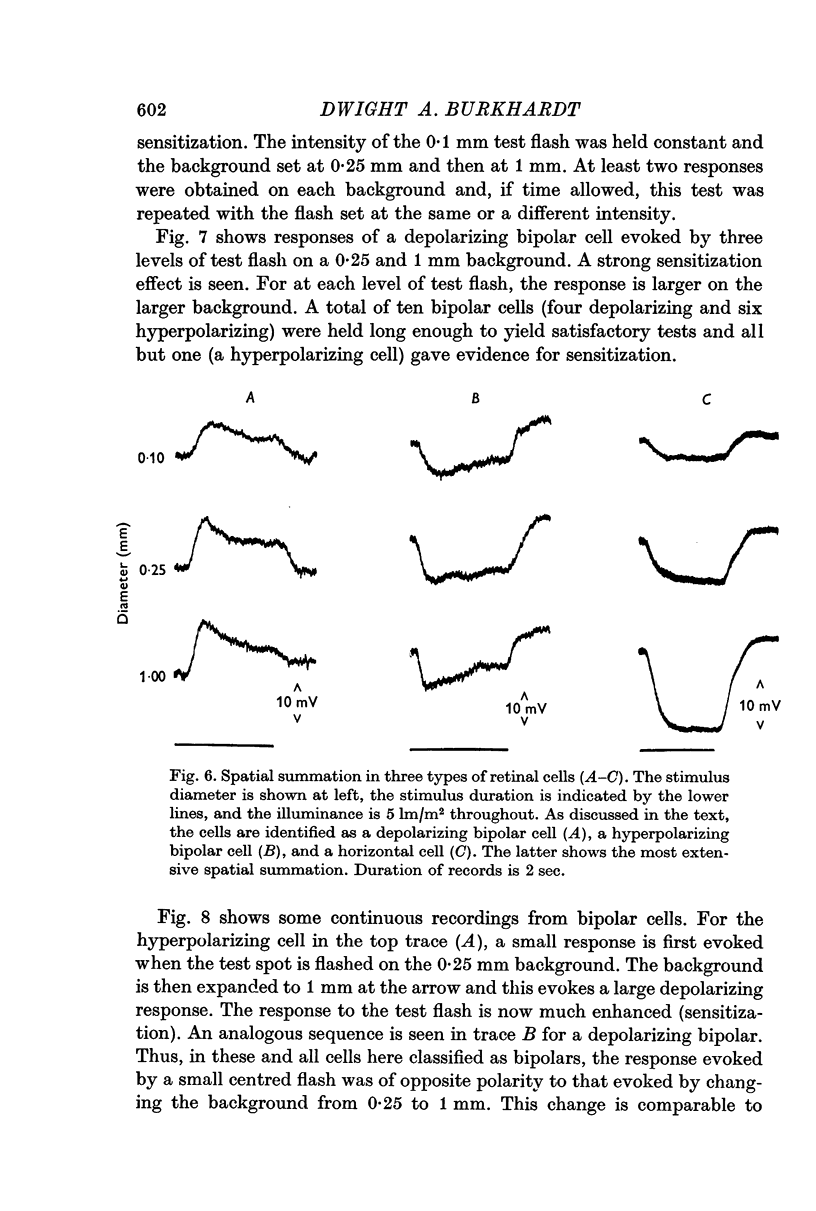
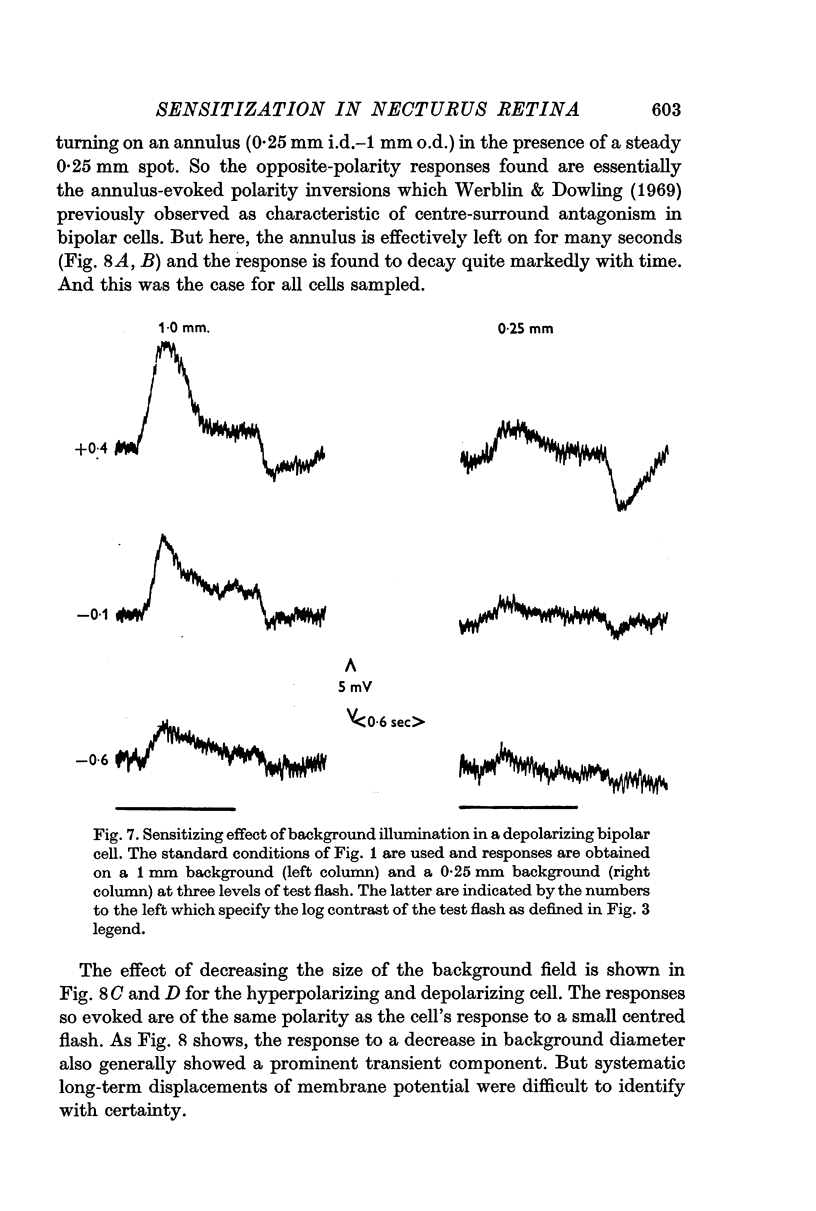
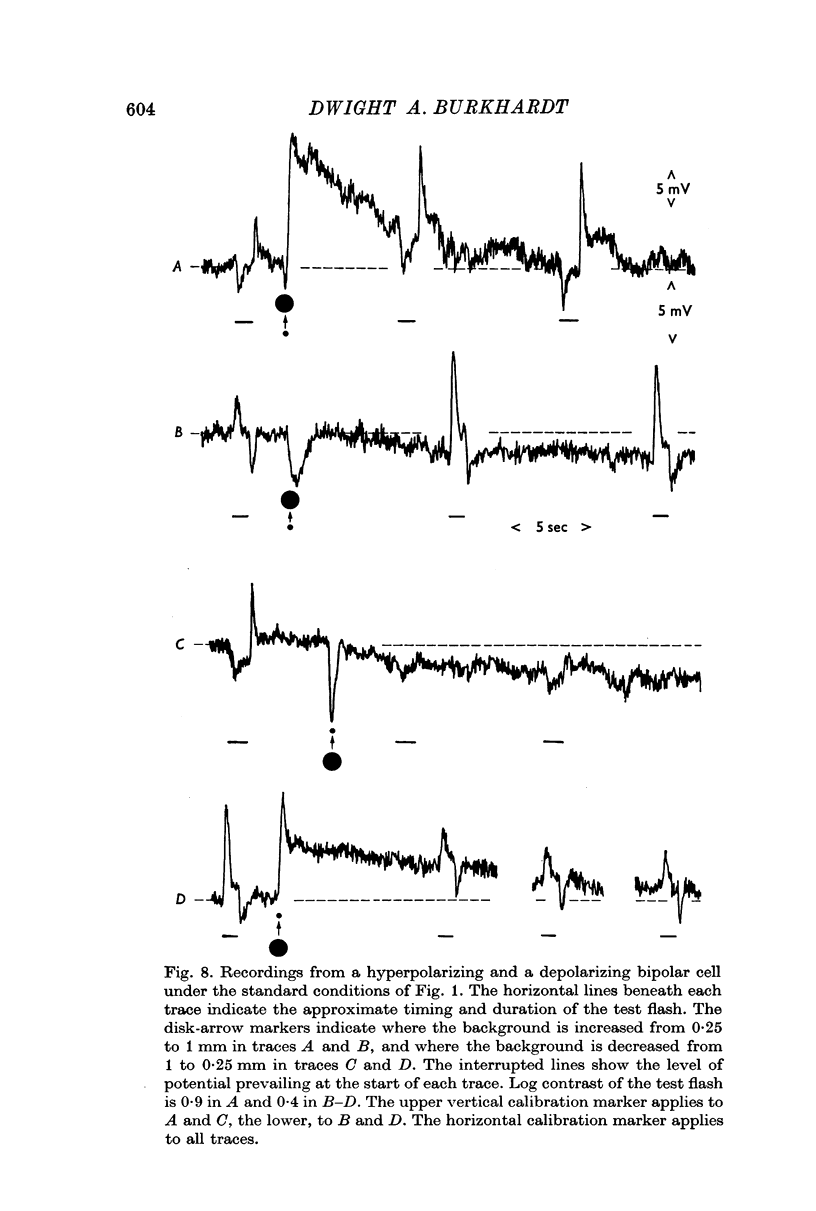
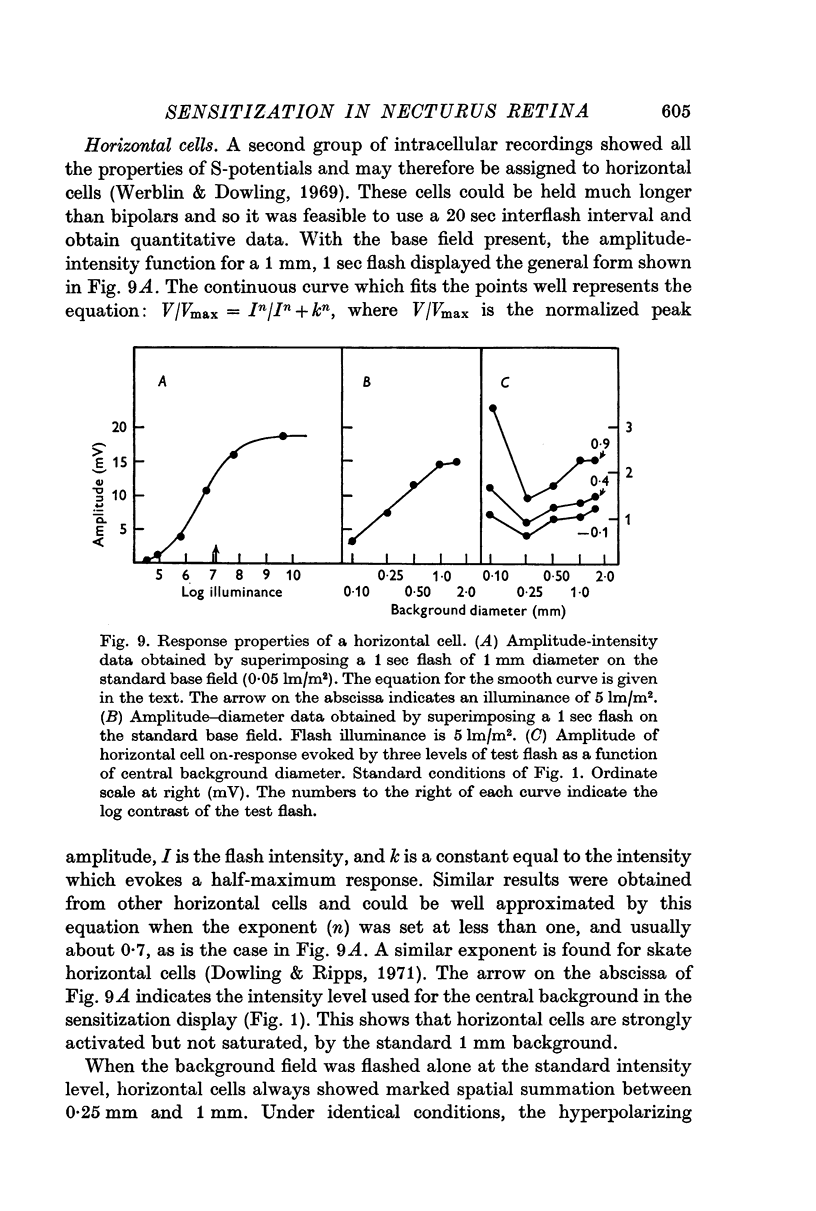
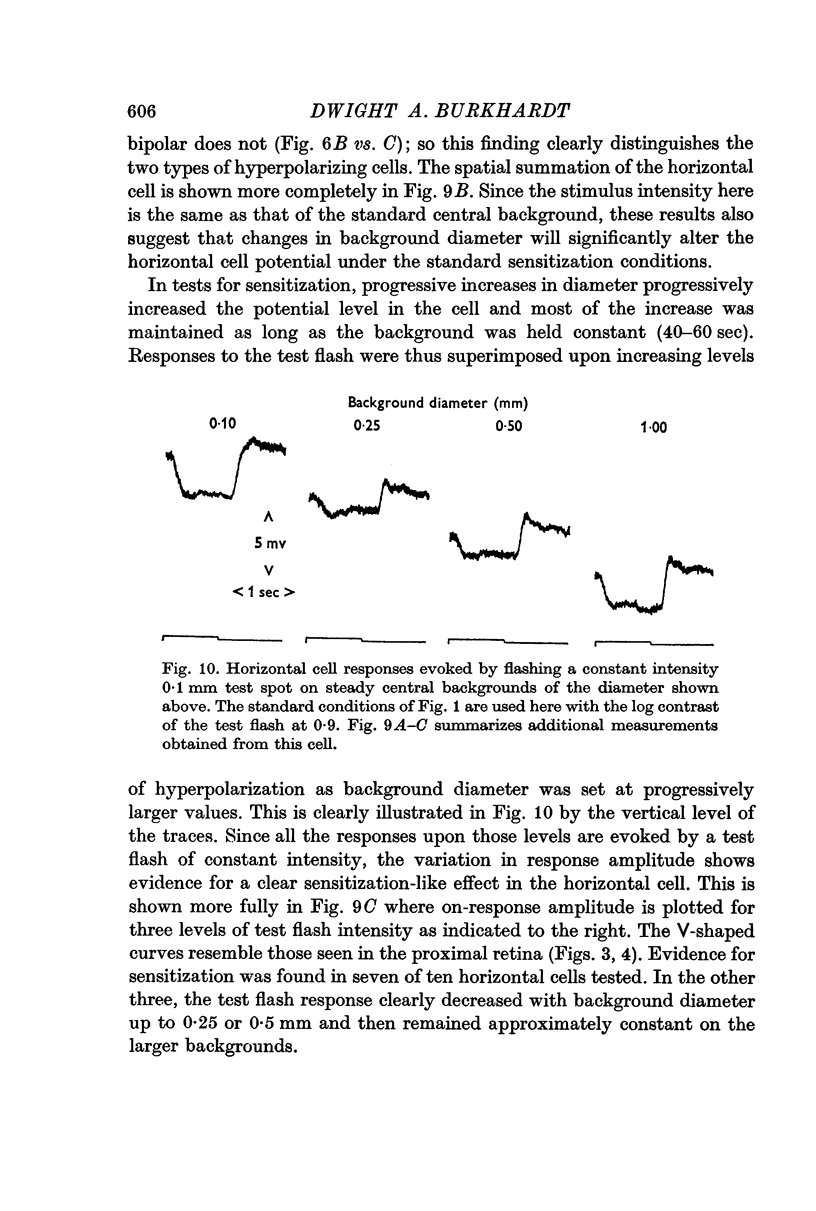
4. Sensitization was prominent in intracellular recordings from bipolar cells and usually apparent in horizontal cells. In bipolar cells, a background change which produced sensitization evoked a large but predominantly transient potential whose polarity was opposite to that evoked by a central flash.
5. The tonicity and large spatial extent of the sensitization effect strongly suggest that it is mediated by horizontal cells. They might do this by either acting upon receptors, bipolar cells, or both.
Full text
PDF

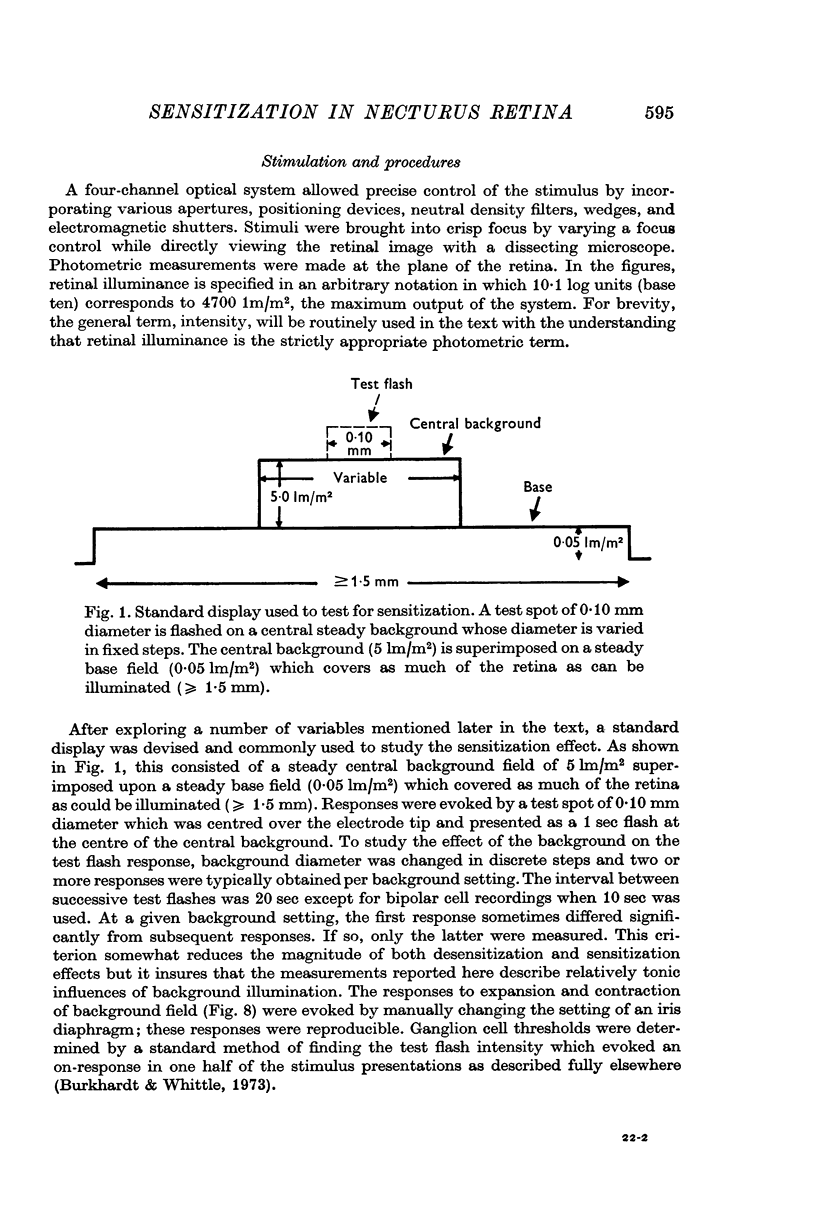
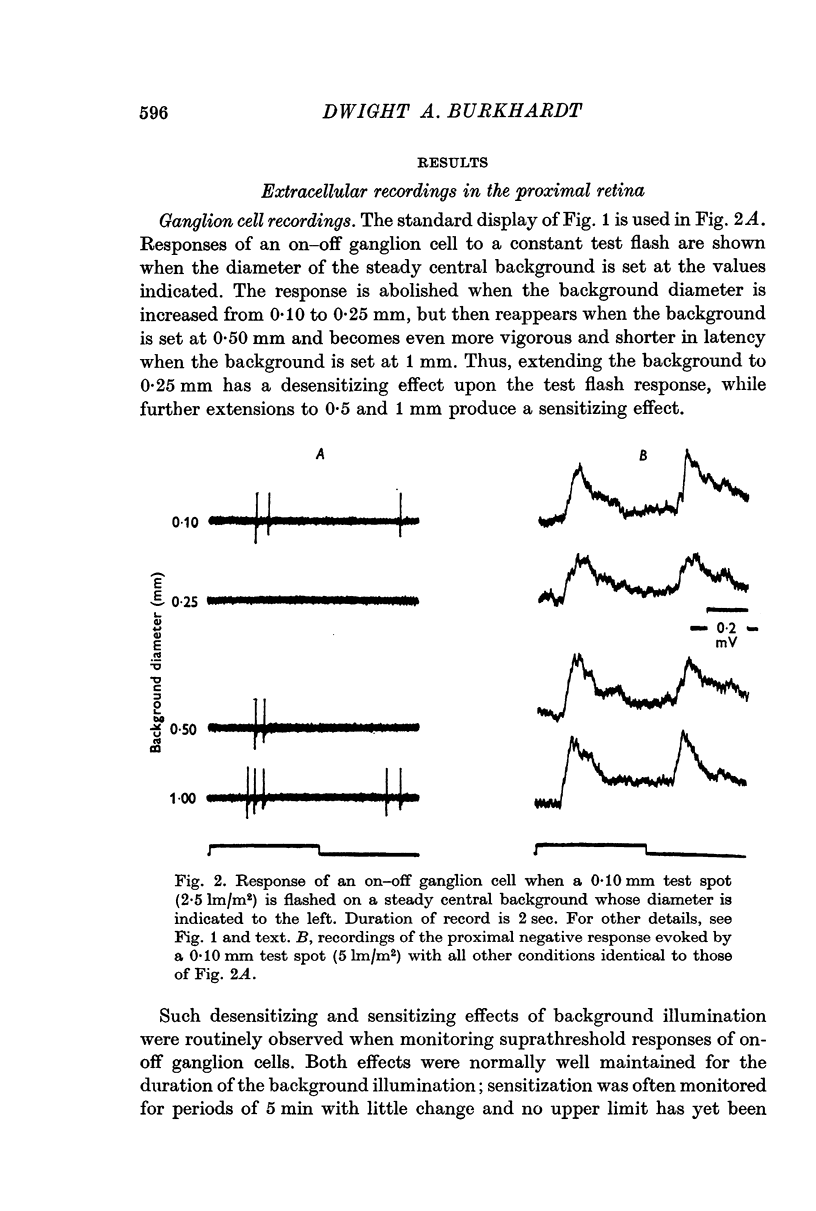
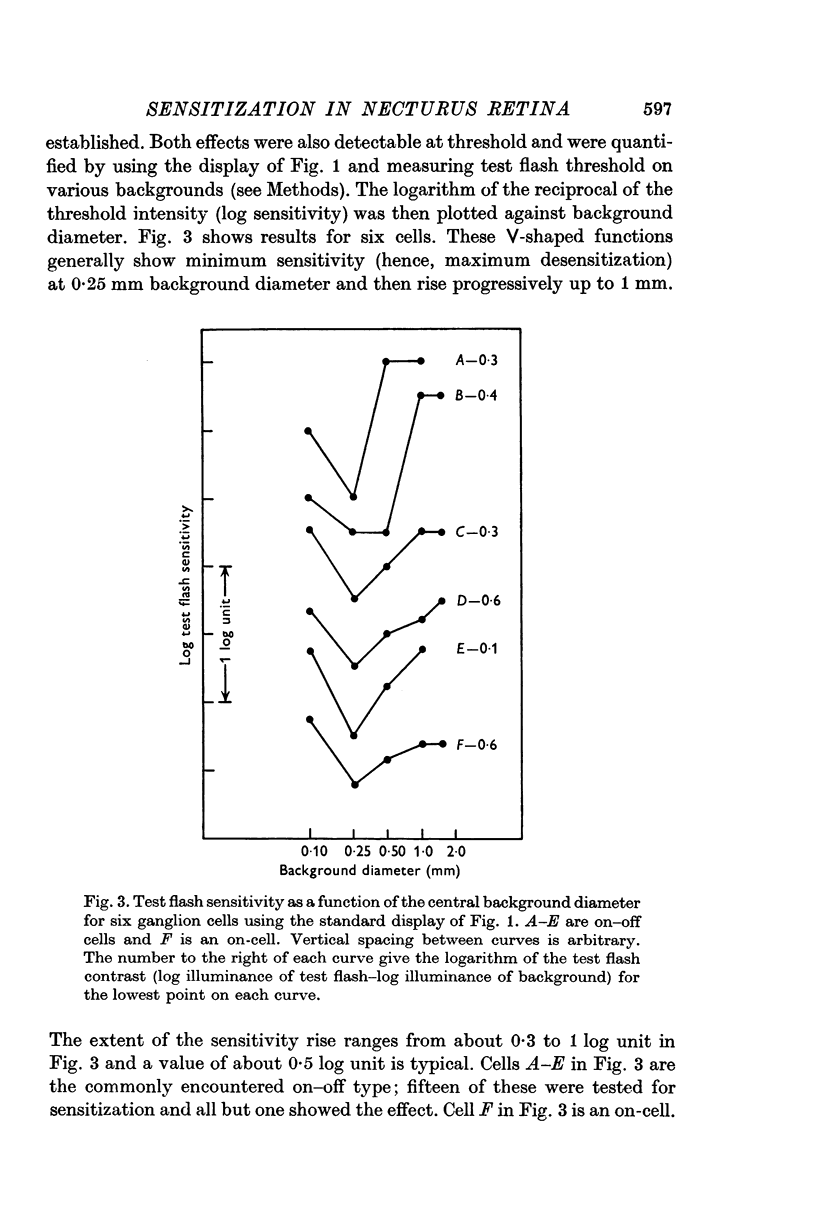

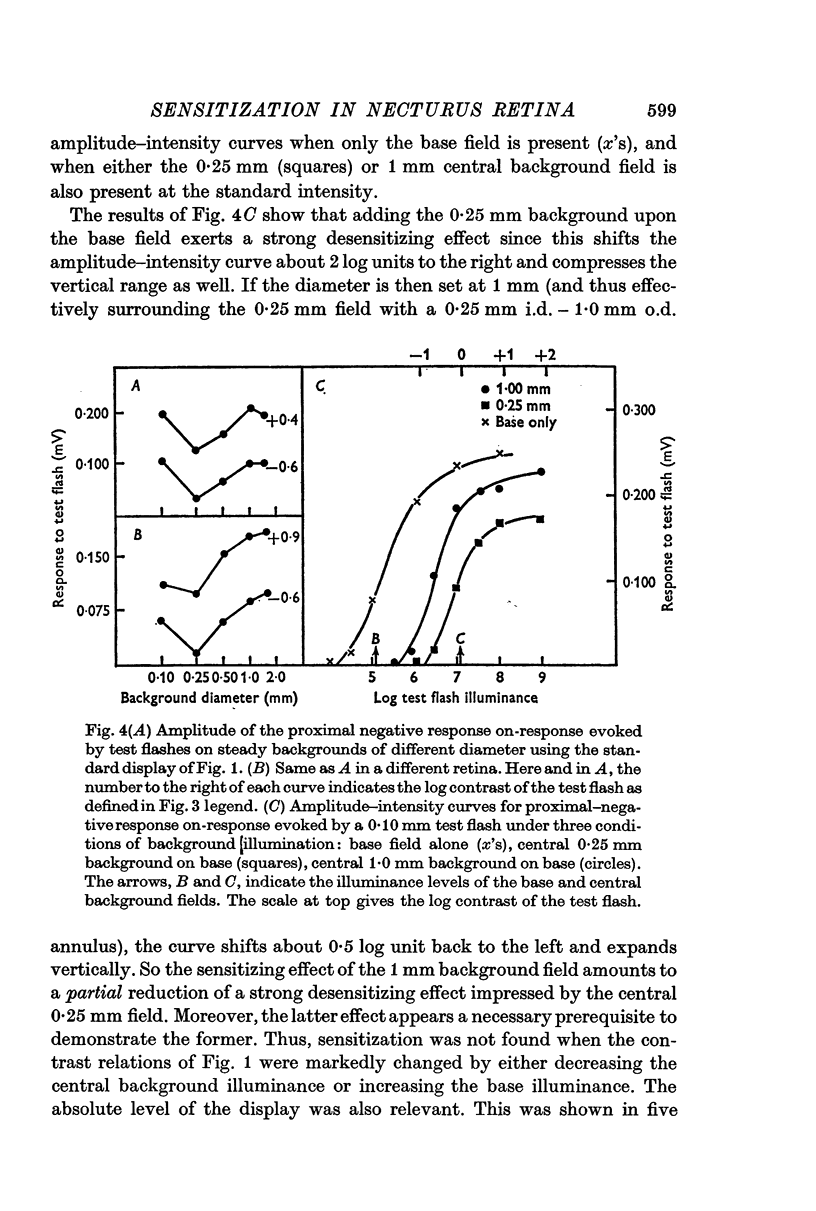





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARLOW H. B. Summation and inhibition in the frog's retina. J Physiol. 1953 Jan;119(1):69–88. doi: 10.1113/jphysiol.1953.sp004829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G., O'Bryan P. M. Receptive fields of cones in the retina of the turtle. J Physiol. 1971 Apr;214(2):265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt D. A., Berntson G. G. Light adaptation and excitation: lateral spread of signals within the frog retina. Vision Res. 1972 Jun;12(6):1095–1111. doi: 10.1016/0042-6989(72)90100-9. [DOI] [PubMed] [Google Scholar]
- Burkhardt D. A., Whittle P. Intensity coding in the frog retina. Quantitative relations between impulse and graded activity. J Gen Physiol. 1973 Mar;61(3):305–322. doi: 10.1085/jgp.61.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervetto L., MacNichol E. F., Jr Inactivation of horizontal cells in turtle retina by glutamate and aspartate. Science. 1972 Nov 17;178(4062):767–768. doi: 10.1126/science.178.4062.767. [DOI] [PubMed] [Google Scholar]
- Dowling J. E., Ripps H. S-potentials in the skate retina. Intracellular recordings during light and dark adaptation. J Gen Physiol. 1971 Aug;58(2):163–189. doi: 10.1085/jgp.58.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J. E., Werblin F. S. Organization of retina of the mudpuppy, Necturus maculosus. I. Synaptic structure. J Neurophysiol. 1969 May;32(3):315–338. doi: 10.1152/jn.1969.32.3.315. [DOI] [PubMed] [Google Scholar]
- Maffei L., Cervetto L., Fiorentini A. Transfer characteristics of excitation and inhibition in cat retinal ganglion cells. J Neurophysiol. 1970 Mar;33(2):276–284. doi: 10.1152/jn.1970.33.2.276. [DOI] [PubMed] [Google Scholar]
- Marmarelis P. Z., Naka K. I. Spatial distribution of potential in a flat cell. Application to the catfish horizontal cell layers. Biophys J. 1972 Nov;12(11):1515–1532. doi: 10.1016/S0006-3495(72)86179-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee S. P., Westheimer G. Specificity of cone mechanisms in lateral interaction. J Physiol. 1970 Jan;206(1):117–128. doi: 10.1113/jphysiol.1970.sp009001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K. Local adaptation in cat LGN cells: evidence for a surround antagonism. Vision Res. 1971 Jun;11(6):501–509. doi: 10.1016/0042-6989(71)90073-3. [DOI] [PubMed] [Google Scholar]
- Negishi K. Reduction and enhancement of S-potential observed with two simultaneous light stimuli in the isolated fish retina. Vision Res. 1971;Suppl 3:65–76. doi: 10.1016/0042-6989(71)90031-9. [DOI] [PubMed] [Google Scholar]
- Nelson R. A comparison of electrical properties of neurons in Necturus retina. J Neurophysiol. 1973 May;36(3):519–535. doi: 10.1152/jn.1973.36.3.519. [DOI] [PubMed] [Google Scholar]
- Proenza L. M., Burkhardt D. A. Proximal negative response and retinal sensitivity in the mudpuppy, Necturus maculosus. J Neurophysiol. 1973 May;36(3):502–518. doi: 10.1152/jn.1973.36.3.502. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Creutzfeldt O., Scheich H. An experimental comparison between the ganglion cell receptive field and the receptive field of the adaptation pool in the cat retina. Pflugers Arch. 1969;307(3):133–137. doi: 10.1007/BF00592079. [DOI] [PubMed] [Google Scholar]
- Sturr J. F., Teller D. Y. Sensitization by annular surrounds: dichoptic properties. Vision Res. 1973 May;13(5):909–918. doi: 10.1016/0042-6989(73)90071-0. [DOI] [PubMed] [Google Scholar]
- Sunga R. N., Enoch J. M. A static perimetric technique believed to test receptive field properties. 3. Clinical trials. Am J Ophthalmol. 1970 Aug;70(2):244–272. doi: 10.1016/0002-9394(70)90014-0. [DOI] [PubMed] [Google Scholar]
- Teller D. Y., Andrews D. P., Barlow H. B. Local adaptation in stabilized vision. Vision Res. 1966 Dec;6(12):701–705. doi: 10.1016/0042-6989(66)90081-2. [DOI] [PubMed] [Google Scholar]
- Teller D. Y., Matter C., Phillips W. D., Alexander K. Sensitization by annular surrounds: sensitization and masking. Vision Res. 1971 Dec;11(12):1445–1458. doi: 10.1016/0042-6989(71)90065-4. [DOI] [PubMed] [Google Scholar]
- Toyoda J. Membrane resistance changes underlying the bipolar cell response in the carp retina. Vision Res. 1973 Feb;13(2):283–294. doi: 10.1016/0042-6989(73)90107-7. [DOI] [PubMed] [Google Scholar]
- Werblin F. S. Adaptation in a vertebrate retina: intracellular recording in Necturus. J Neurophysiol. 1971 Mar;34(2):228–241. doi: 10.1152/jn.1971.34.2.228. [DOI] [PubMed] [Google Scholar]
- Werblin F. S., Dowling J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969 May;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]
- Westheimer G. Bleached rhodopsin and retinal interaction. J Physiol. 1968 Mar;195(1):97–105. doi: 10.1113/jphysiol.1968.sp008448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westheimer G. Rod-cone independence for sensitizing interaction in the human retina. J Physiol. 1970 Jan;206(1):109–116. doi: 10.1113/jphysiol.1970.sp009000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westheimer G. Spatial interaction in human cone vision. J Physiol. 1967 May;190(1):139–154. doi: 10.1113/jphysiol.1967.sp008198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westheimer G. Spatial interaction in the human retina during scotopic vision. J Physiol. 1965 Dec;181(4):881–894. doi: 10.1113/jphysiol.1965.sp007803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkovsky P., Nelson J., Ripps H. Action spectra and adaptation properties of carp photoreceptors. J Gen Physiol. 1973 Apr;61(4):401–423. doi: 10.1085/jgp.61.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]


