Abstract
1. Conductance changes in the acini of the cockroach salivary gland have been examined during nerve stimulation by means of two intracellular electrodes placed in the same acinus, the first electrode being used for recording membrane potential and the second for current injection.
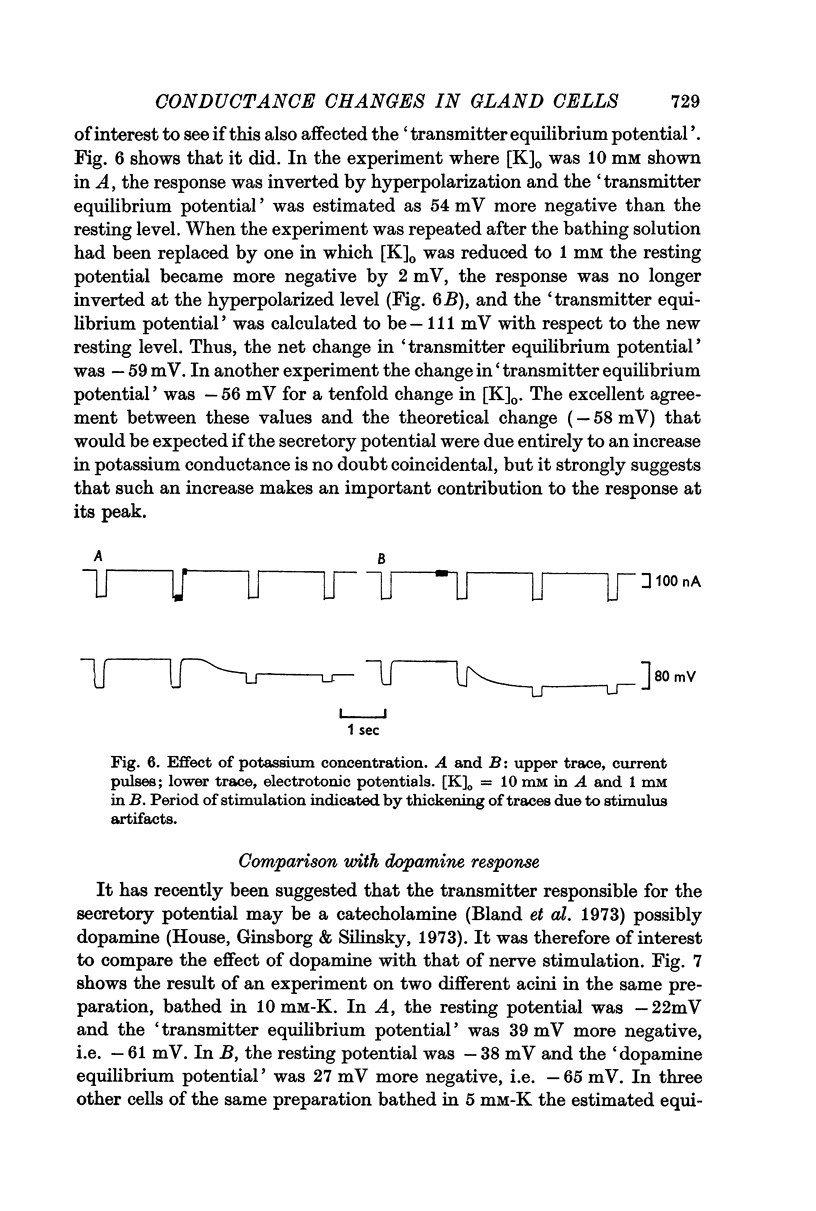
2. The transient hyperpolarization (secretory potential) in the acinus evoked by nerve stimuli is accompanied by a rise in membrane conductance. The conductance, however, remains high for a longer period than that of the response.
3. Applying the analysis of Trautwein & Dudel (1958) to the secretory potentials recorded in the acinus (assumed to behave electrically like a single cell) gives estimates of the `transmitter equilibrium potential'. The values indicate that the neurotransmitter increases the membrane potassium conductance.
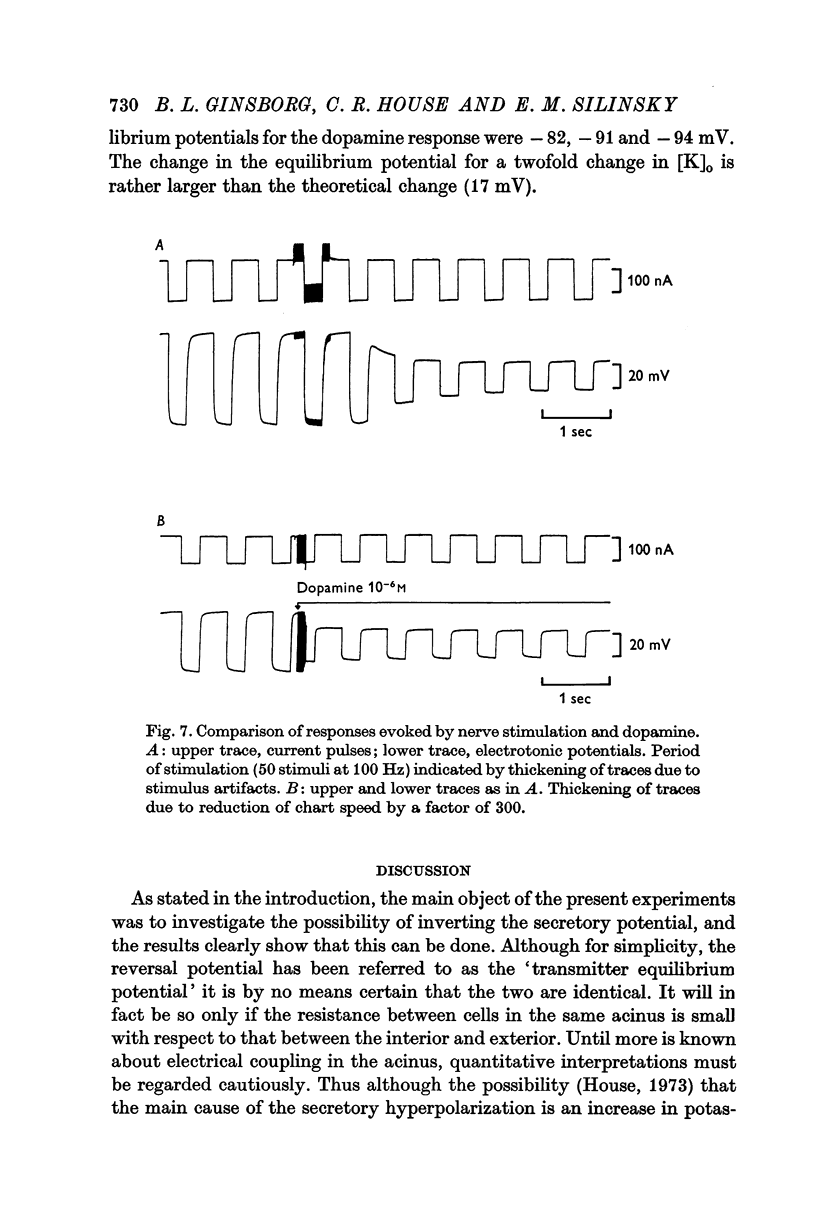
4. The hyperpolarization of the acinus evoked by 10-6 M dopamine in the bathing fluid is also associated with an increase in membrane potassium conductance.
Full text
PDF
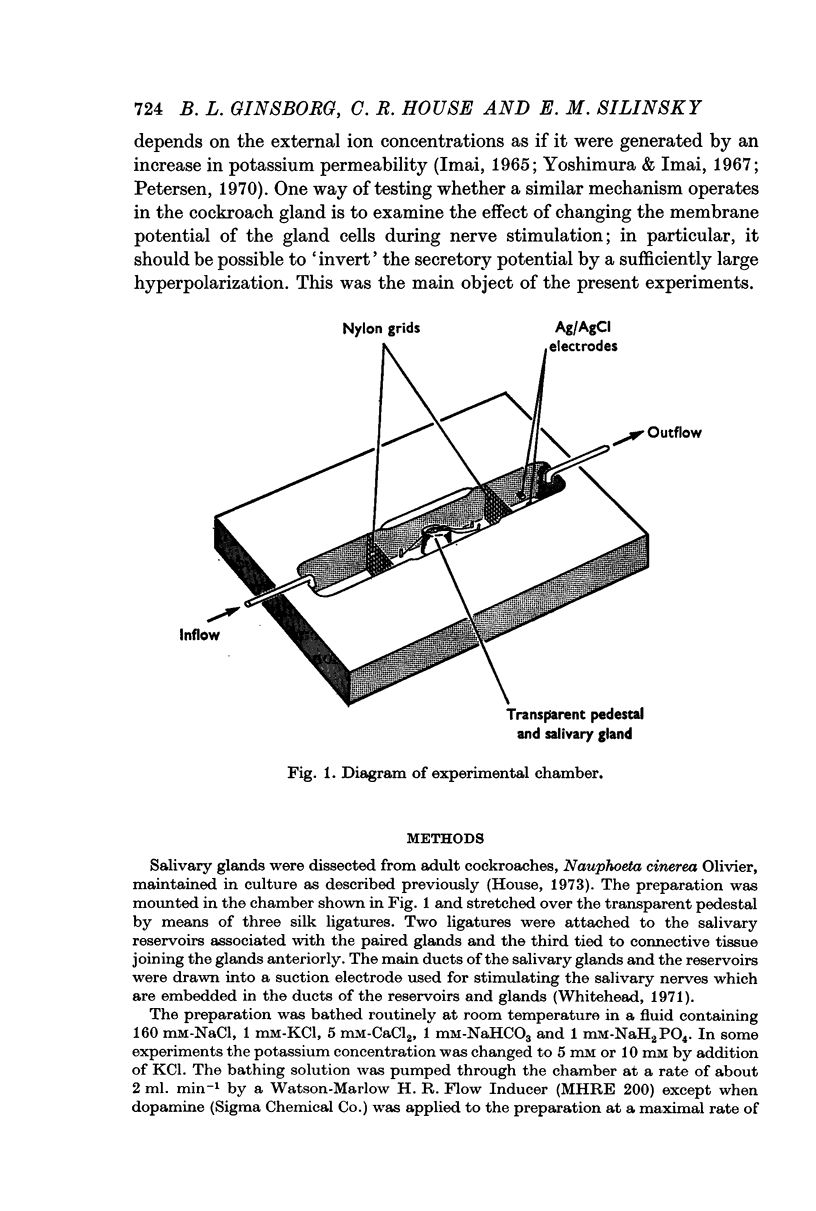
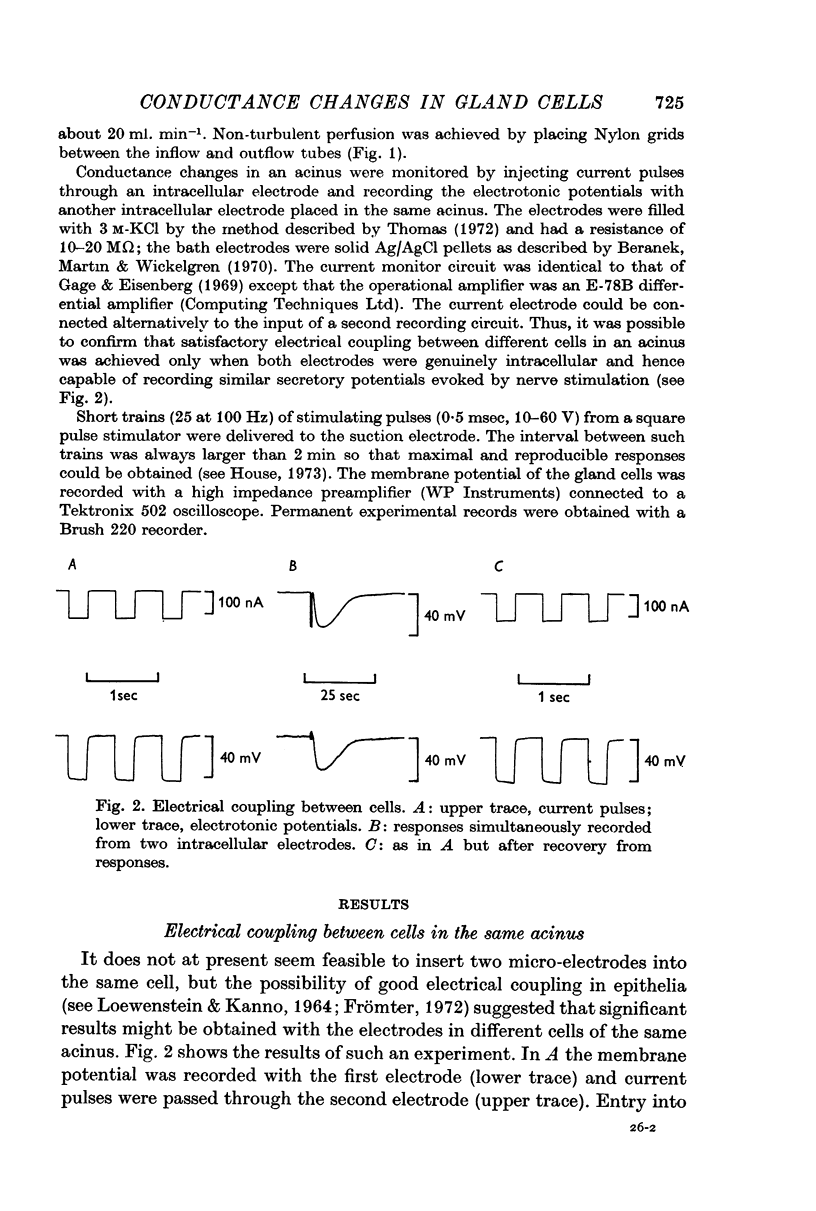
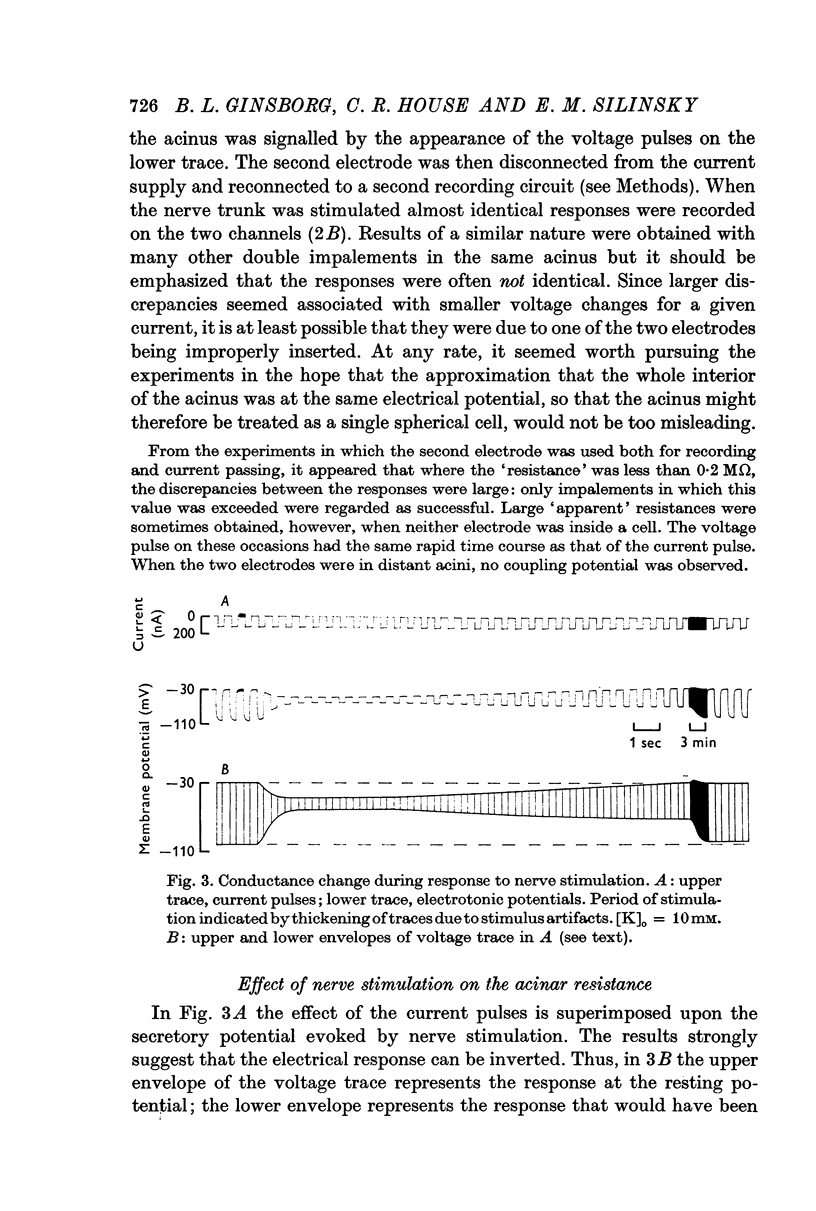

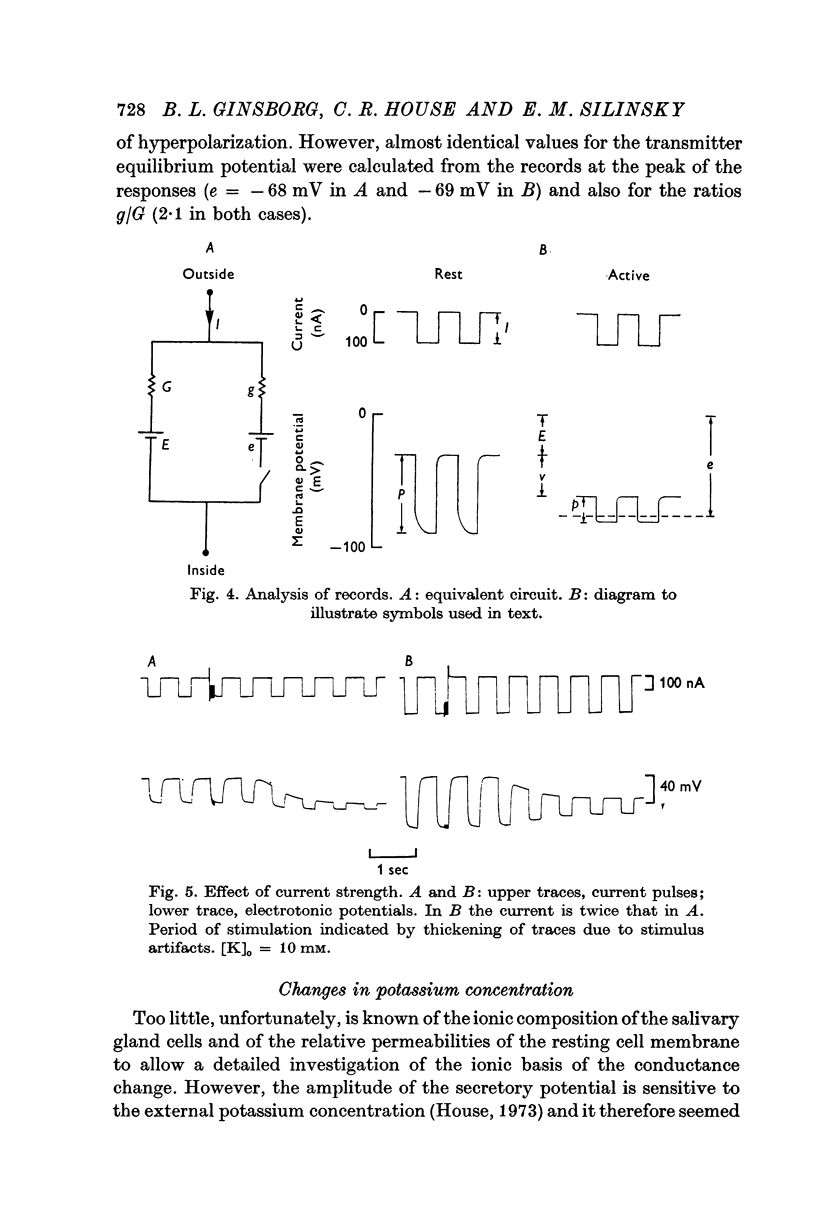

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bland K. P., House C. R., Ginsborg B. L., Laszlo I. Catecholamine transmitter for salivary secretion in the cockroach. Nat New Biol. 1973 Jul 4;244(131):26–27. doi: 10.1038/newbio244026a0. [DOI] [PubMed] [Google Scholar]
- Frömter E. The route of passive ion movement through the epithelium of Necturus gallbladder. J Membr Biol. 1972;8(3):259–301. doi: 10.1007/BF01868106. [DOI] [PubMed] [Google Scholar]
- Gage P. W., Eisenberg R. S. Capacitance of the surface and transverse tubular membrane of frog sartorius muscle fibers. J Gen Physiol. 1969 Mar;53(3):265–278. doi: 10.1085/jgp.53.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House C. R., Ginsborg B. L., Silinsky E. M. Dopamine receptors in cockroach salivary gland cells. Nat New Biol. 1973 Sep 12;245(141):63–63. doi: 10.1038/newbio245063a0. [DOI] [PubMed] [Google Scholar]
- LOEWENSTEIN W. R., KANNO Y. STUDIES ON AN EPITHELIAL (GLAND) CELL JUNCTION. I. MODIFICATIONS OF SURFACE MEMBRANE PERMEABILITY. J Cell Biol. 1964 Sep;22:565–586. doi: 10.1083/jcb.22.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. R., Wickelgren W. O., Ber1anek R. Effects of iontophoretically applied drugs on spinal interneurons of the lamprey. J Physiol. 1970 May;207(3):653–665. doi: 10.1113/jphysiol.1970.sp009086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O. H. The dependence of the transmembrane salivary secretory potential on the external potassium and sodium concentration. J Physiol. 1970 Sep;210(1):205–215. doi: 10.1113/jphysiol.1970.sp009204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRAUTWEIN W., DUDEL J. Zum Mechanismus der Membranwirkung des Acetylcholin an der Herzmuskelfaser. Pflugers Arch. 1958;266(3):324–334. doi: 10.1007/BF00416781. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Intracellular sodium activity and the sodium pump in snail neurones. J Physiol. 1972 Jan;220(1):55–71. doi: 10.1113/jphysiol.1972.sp009694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura H., Imai Y. Studies on the secretory potential of acinal cells of the dog submaxillary gland and its ionic dependency. Jpn J Physiol. 1967 Jun;17(3):280–293. doi: 10.2170/jjphysiol.17.280. [DOI] [PubMed] [Google Scholar]


