Abstract
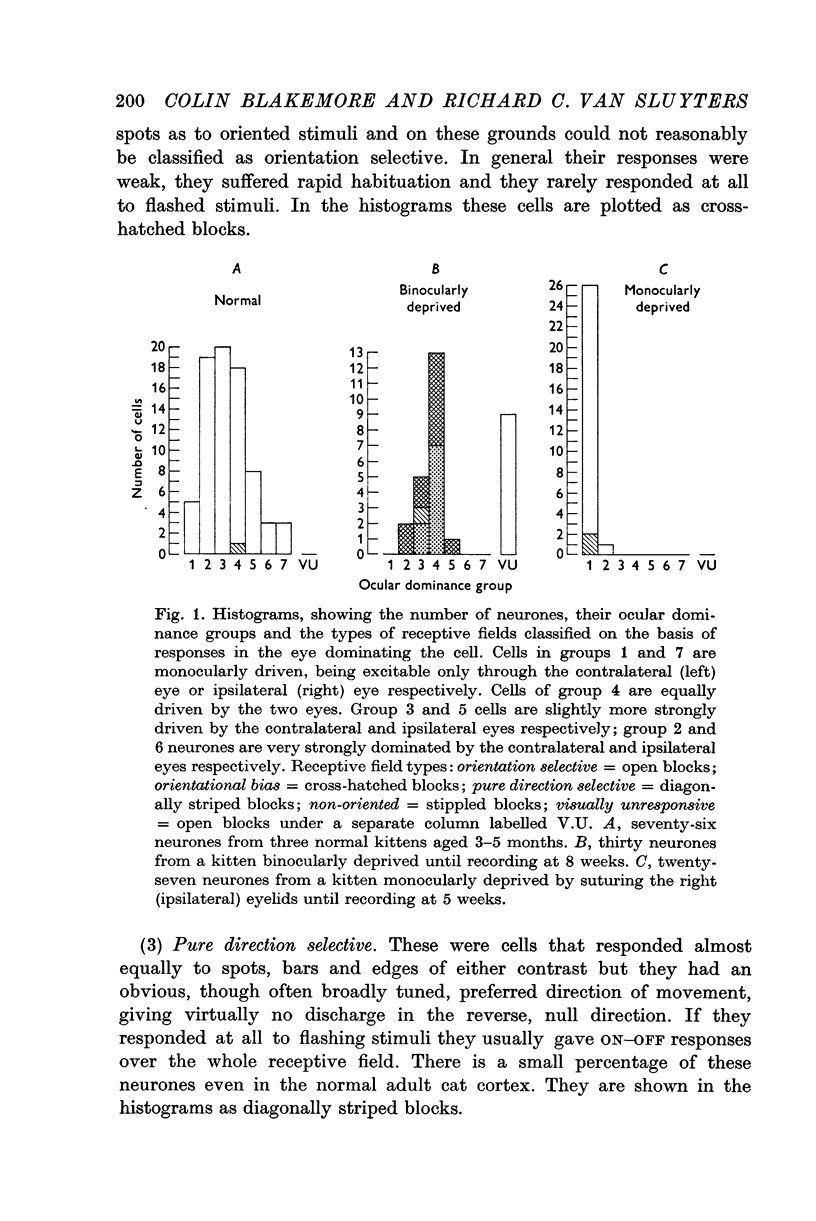
1. It was confirmed that suturing the lids of one eye (monocular deprivation), until only 5 weeks of age, leaves virtually every neurone in the kitten's visual cortex entirely dominated by the other eye. On the other hand, deprivation of both eyes causes no change in the normal ocular dominance of cortical neurones, most cells being clearly binocularly driven.
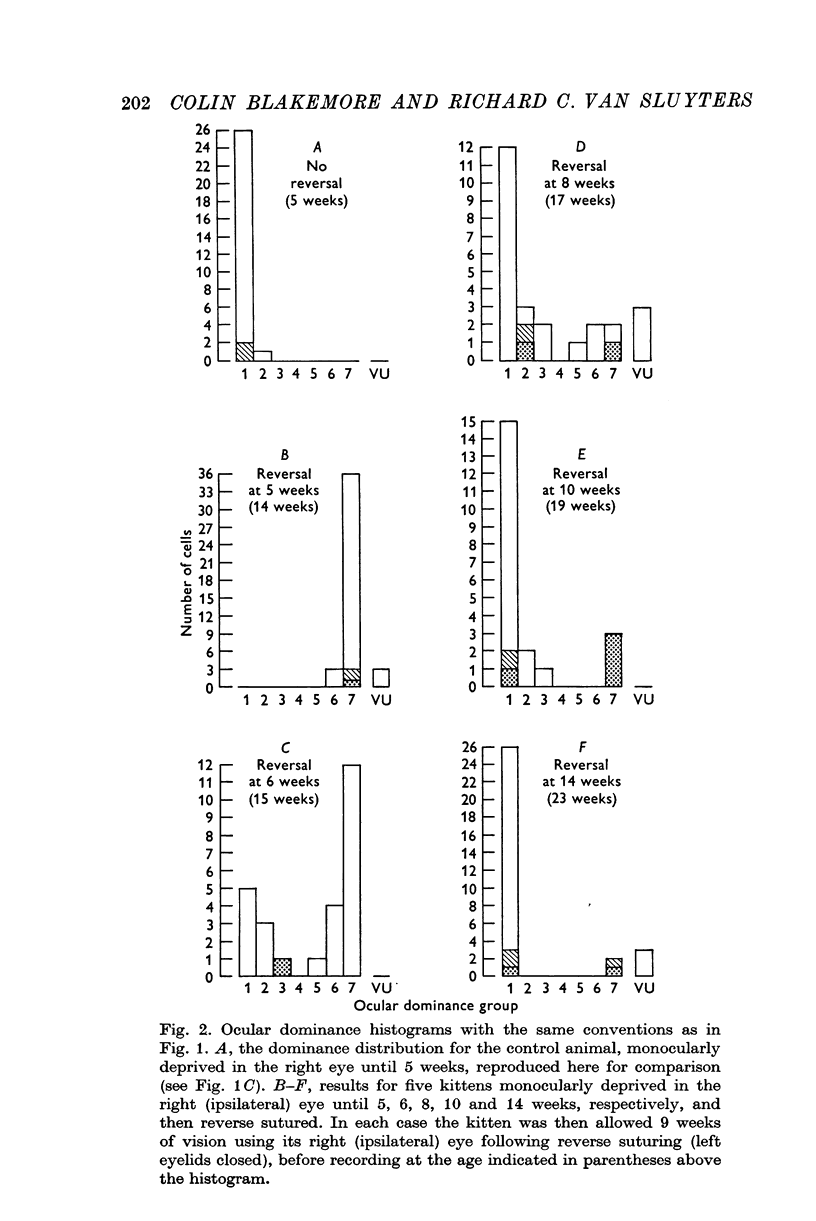
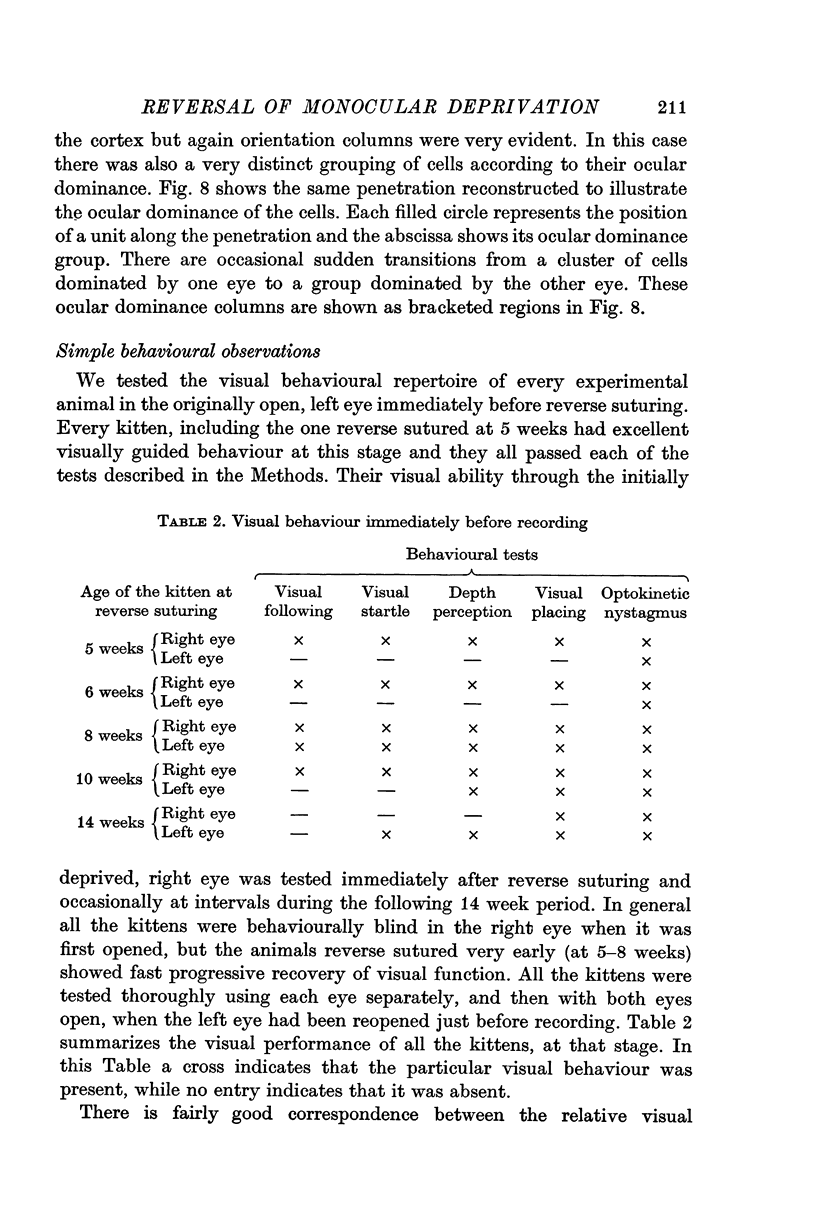
2. Kittens were monocularly deprived until various ages, from 5 to 14 weeks, at which time reverse suturing was performed: the initially deprived right eye was opened and the left eye closed for a further 9 weeks before recording from the visual cortex.
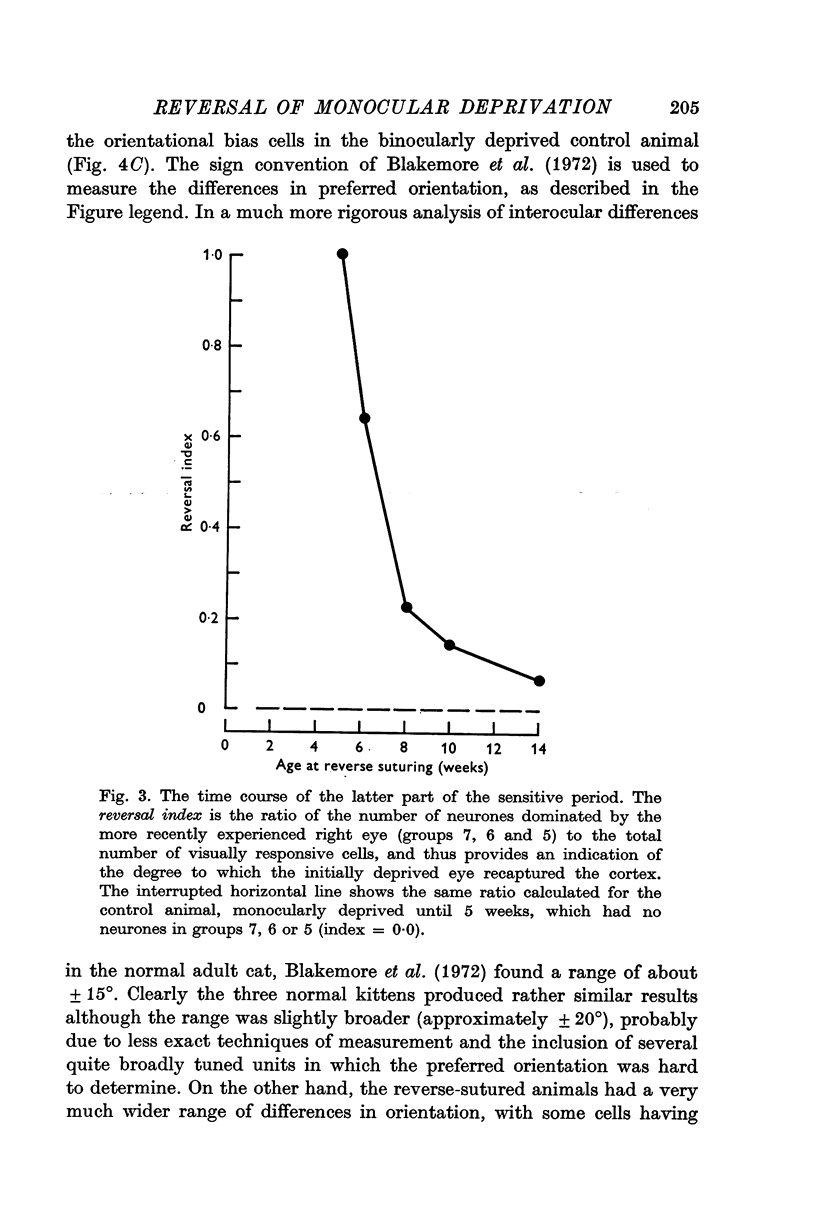
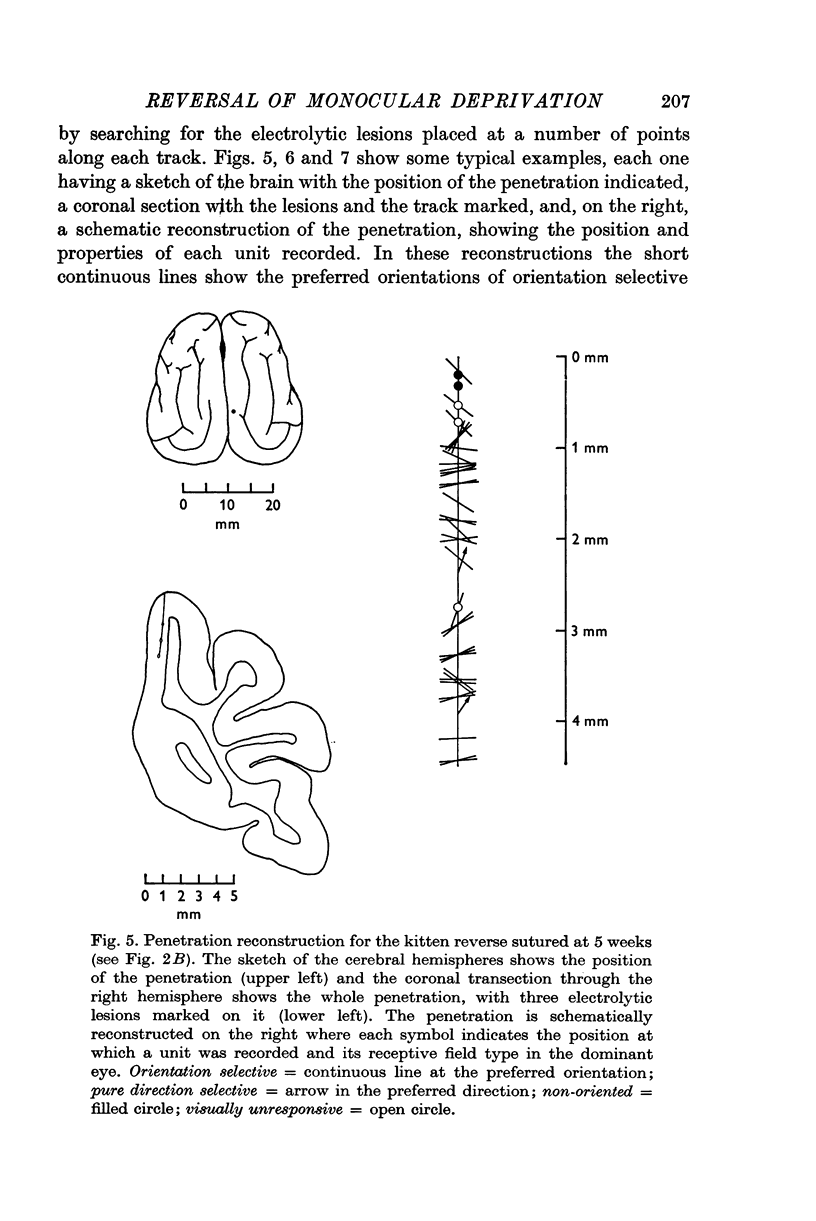
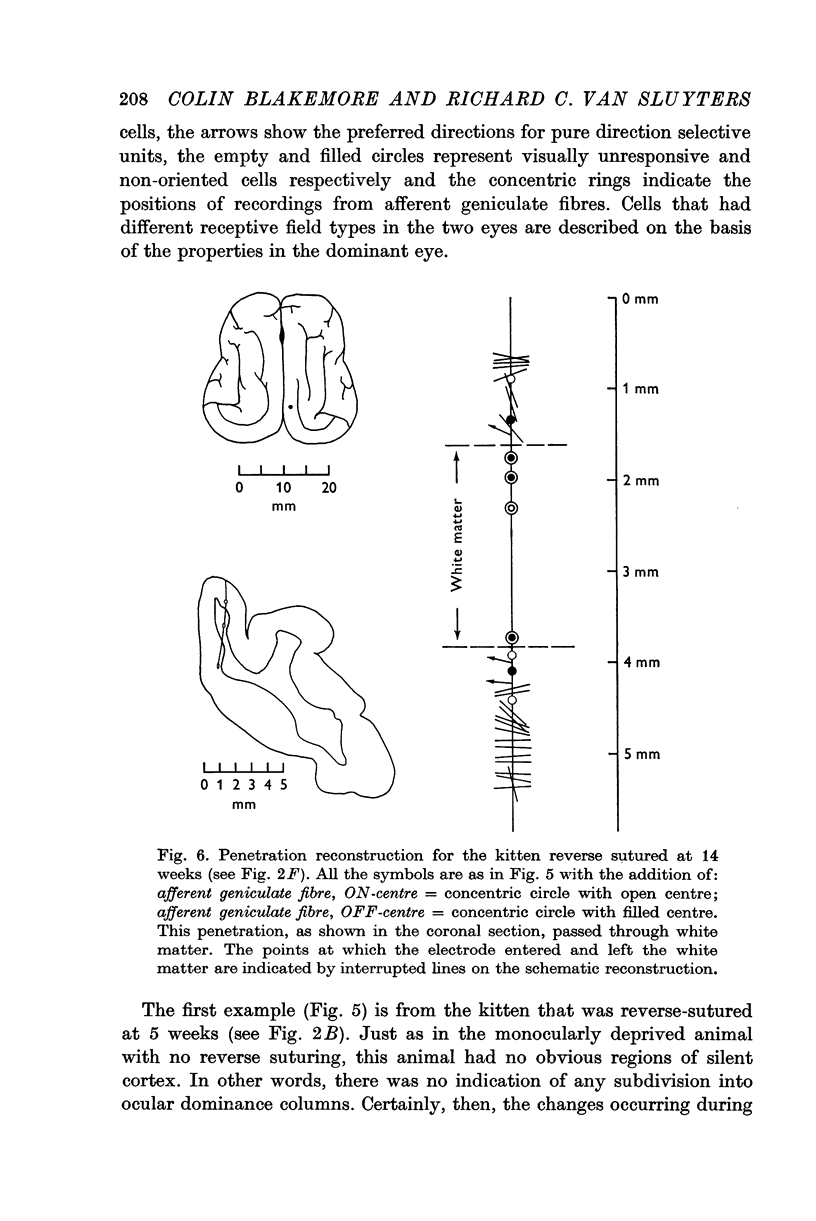
3. Reverse suturing at 5 weeks caused a complete switch in ocular dominance: every cell was dominated by the initially deprived right eye. Reverse suturing at 14 weeks, however, had almost no further effect on ocular dominance: most cells were still driven solely by the left eye. Animals reverse sutured at intermediate ages had cortical neurones strongly dominated by one eye or the other, and they were organized into clear columnar groups according to ocular dominance.
4. Thus, between 5 weeks and 4 months of age, there is a period of declining sensitivity to both the effects of an initial period of monocular deprivation and the reversal of those effects by reverse suturing.
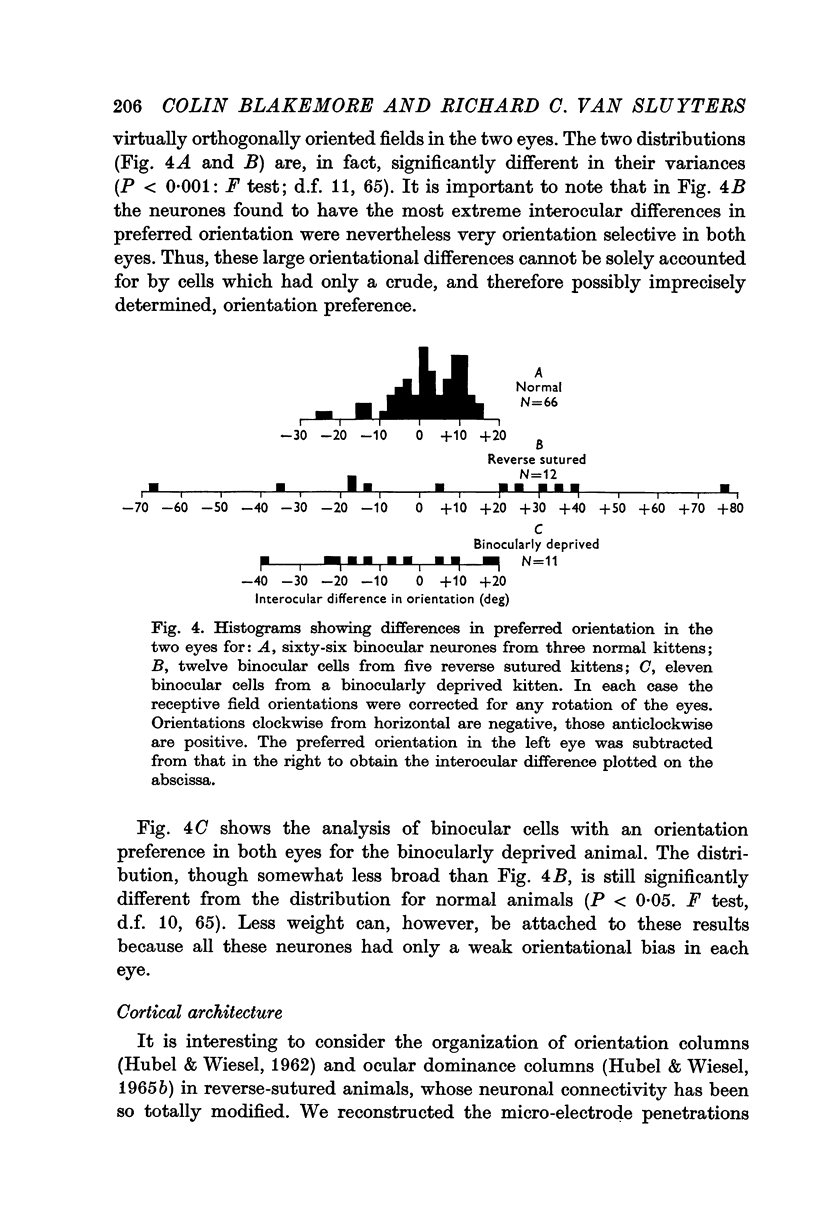
5. The small proportion of binocular cells in reverse sutured kittens (which have never had simultaneous binocular vision) often differed considerably in their receptive field properties in the two eyes. In particular, if the cells were orientation selective in both eyes the two preferred orientations could differ by up to 70°.
6. The relative importance of innate and environmental contributions to the properties of cortical cells is discussed.
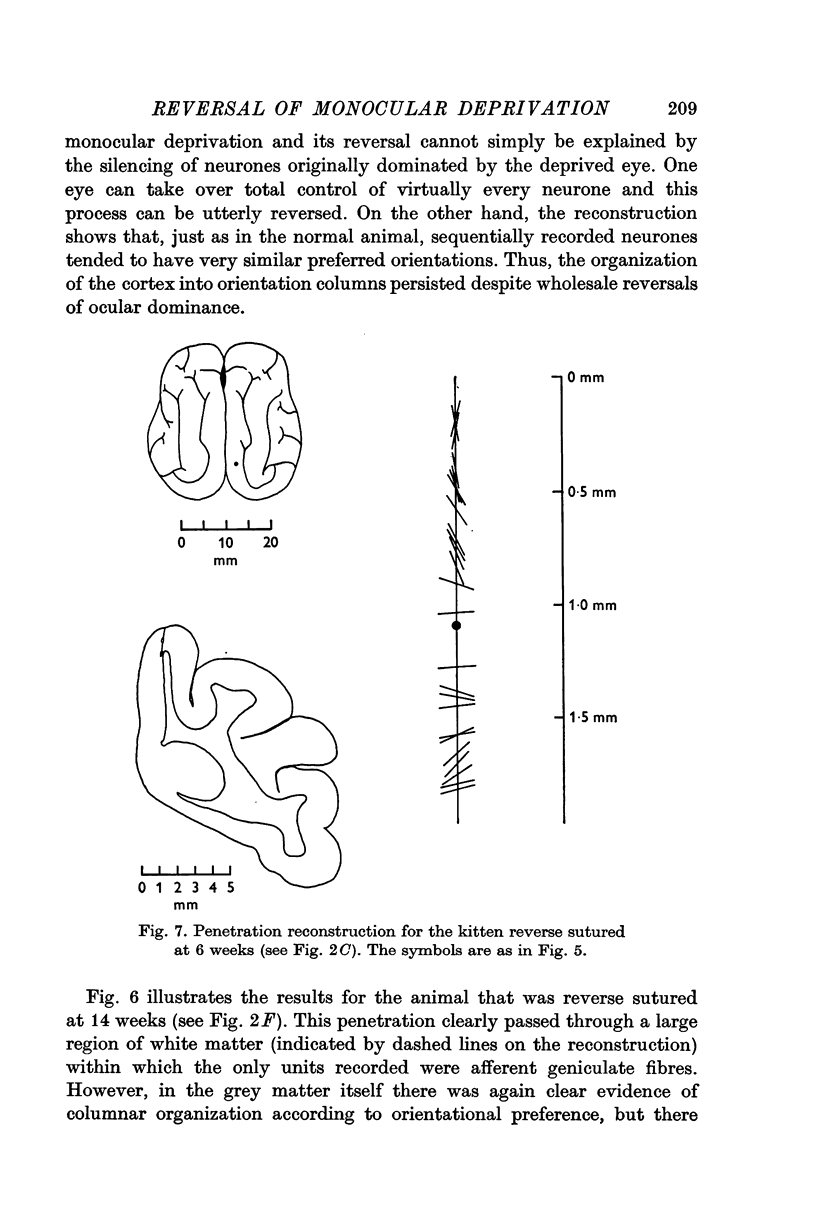
Full text
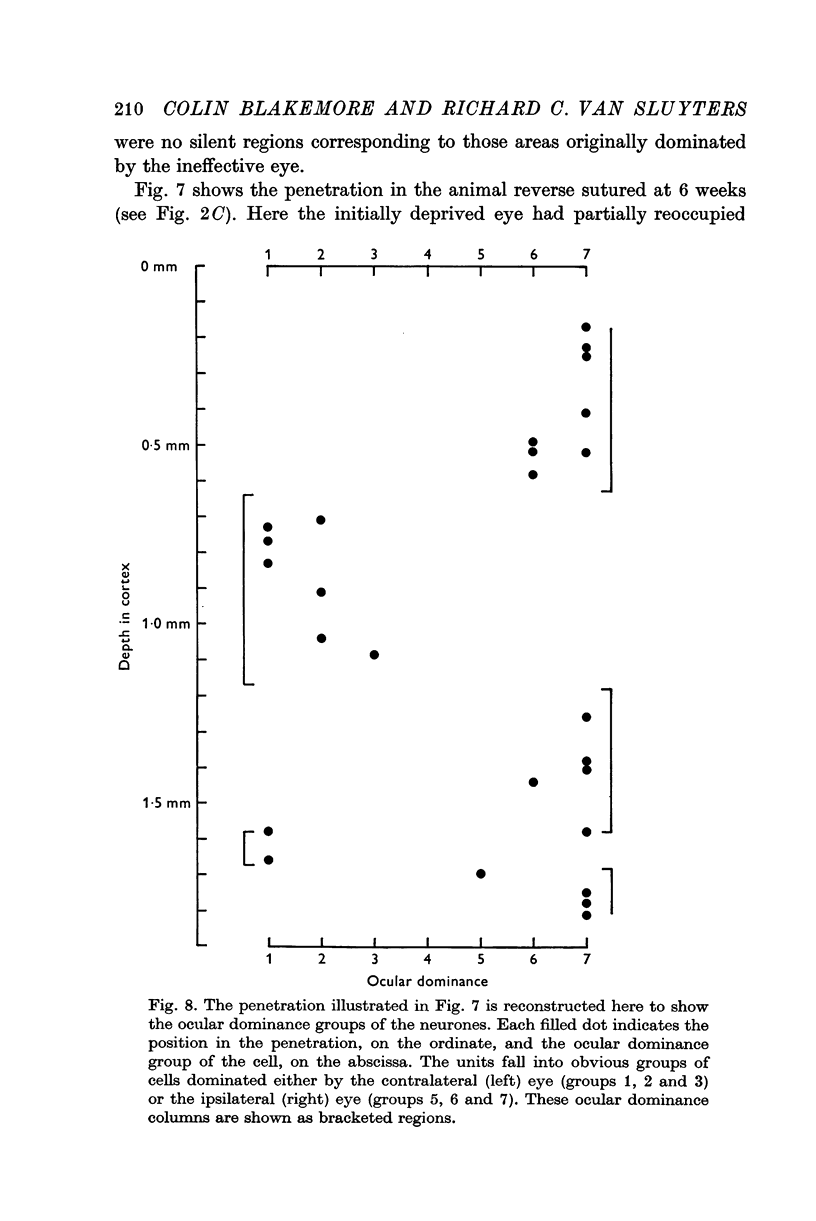
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlow H. B., Blakemore C., Pettigrew J. D. The neural mechanism of binocular depth discrimination. J Physiol. 1967 Nov;193(2):327–342. doi: 10.1113/jphysiol.1967.sp008360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Pettigrew J. D. Lack of specificity of neurones in the visual cortex of young kittens. J Physiol. 1971 Oct;218 (Suppl):98P–100P. [PubMed] [Google Scholar]
- Blakemore C., Cooper G. F. Development of the brain depends on the visual environment. Nature. 1970 Oct 31;228(5270):477–478. doi: 10.1038/228477a0. [DOI] [PubMed] [Google Scholar]
- Blakemore C., Fiorentini A., Maffei L. A second neural mechanism of binocular depth discrimination. J Physiol. 1972 Nov;226(3):725–749. doi: 10.1113/jphysiol.1972.sp010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Mitchell D. E. Environmental modification of the visual cortex and the neural basis of learning and memory. Nature. 1973 Feb 16;241(5390):467–468. doi: 10.1038/241467a0. [DOI] [PubMed] [Google Scholar]
- Blakemore C., Pettigrew J. D. Eye dominance in the visual cortex. Nature. 1970 Jan 31;225(5231):426–429. doi: 10.1038/225426a0. [DOI] [PubMed] [Google Scholar]
- COLONNIER M. THE TANGENTIAL ORGANIZATION OF THE VISUAL CORTEX. J Anat. 1964 Jul;98:327–344. [PMC free article] [PubMed] [Google Scholar]
- Chow K. L., Stewart D. L. Reversal of structural and functional effects of long-term visual deprivation in cats. Exp Neurol. 1972 Mar;34(3):409–433. doi: 10.1016/0014-4886(72)90038-6. [DOI] [PubMed] [Google Scholar]
- Dews P. B., Wiesel T. N. Consequences of monocular deprivation on visual behaviour in kittens. J Physiol. 1970 Feb;206(2):437–455. doi: 10.1113/jphysiol.1970.sp009023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON E. J., WALK R. D. The "visual cliff". Sci Am. 1960 Apr;202:64–71. [PubMed] [Google Scholar]
- Ganz L., Fitch M., Satterberg J. A. The selective effect of visual deprivation on receptive field shape determined neurophysiologically. Exp Neurol. 1968 Dec;22(4):614–637. doi: 10.1016/0014-4886(68)90153-2. [DOI] [PubMed] [Google Scholar]
- Ganz L., Fitch M. The effect of visual deprivation on perceptual behavior. Exp Neurol. 1968 Dec;22(4):638–660. doi: 10.1016/0014-4886(68)90154-4. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. RECEPTIVE FIELDS AND FUNCTIONAL ARCHITECTURE IN TWO NONSTRIATE VISUAL AREAS (18 AND 19) OF THE CAT. J Neurophysiol. 1965 Mar;28:229–289. doi: 10.1152/jn.1965.28.2.229. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. RECEPTIVE FIELDS OF CELLS IN STRIATE CORTEX OF VERY YOUNG, VISUALLY INEXPERIENCED KITTENS. J Neurophysiol. 1963 Nov;26:994–1002. doi: 10.1152/jn.1963.26.6.994. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein A., Held R. Dissociation of the visual placing response into elicited and guided components. Science. 1967 Oct 20;158(3799):390–392. doi: 10.1126/science.158.3799.390. [DOI] [PubMed] [Google Scholar]
- Hirsch H. V., Spinelli D. N. Modification of the distribution of receptive field orientation in cats by selective visual exposure during development. Exp Brain Res. 1971 Jun 29;12(5):509–527. doi: 10.1007/BF00234246. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Binocular interaction in striate cortex of kittens reared with artificial squint. J Neurophysiol. 1965 Nov;28(6):1041–1059. doi: 10.1152/jn.1965.28.6.1041. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970 Feb;206(2):419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick W. R. Another tungsten microelectrode. Med Biol Eng. 1972 Jul;10(4):510–515. doi: 10.1007/BF02474199. [DOI] [PubMed] [Google Scholar]
- Pettigrew J., Olson C., Barlow H. B. Kitten visual cortex: short-term, stimulus-induced changes in connectivity. Science. 1973 Jun 15;180(4091):1202–1203. doi: 10.1126/science.180.4091.1202. [DOI] [PubMed] [Google Scholar]
- Sherman S. M. Development of interocular alignment in cats. Brain Res. 1972 Feb 25;37(2):187–203. doi: 10.1016/0006-8993(72)90666-x. [DOI] [PubMed] [Google Scholar]
- Van Essen D., Kelly J. Correlation of cell shape and function in the visual cortex of the cat. Nature. 1973 Feb 9;241(5389):403–405. doi: 10.1038/241403a0. [DOI] [PubMed] [Google Scholar]
- WIESEL T. N., HUBEL D. H. EFFECTS OF VISUAL DEPRIVATION ON MORPHOLOGY AND PHYSIOLOGY OF CELLS IN THE CATS LATERAL GENICULATE BODY. J Neurophysiol. 1963 Nov;26:978–993. doi: 10.1152/jn.1963.26.6.978. [DOI] [PubMed] [Google Scholar]
- WIESEL T. N., HUBEL D. H. SINGLE-CELL RESPONSES IN STRIATE CORTEX OF KITTENS DEPRIVED OF VISION IN ONE EYE. J Neurophysiol. 1963 Nov;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. J Neurophysiol. 1965 Nov;28(6):1029–1040. doi: 10.1152/jn.1965.28.6.1029. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H. Extent of recovery from the effects of visual deprivation in kittens. J Neurophysiol. 1965 Nov;28(6):1060–1072. doi: 10.1152/jn.1965.28.6.1060. [DOI] [PubMed] [Google Scholar]
- Young J. Z. Receptive fields of the visual system of the squid. Nature. 1973 Feb 16;241(5390):469–471. doi: 10.1038/241469a0. [DOI] [PubMed] [Google Scholar]



