Abstract
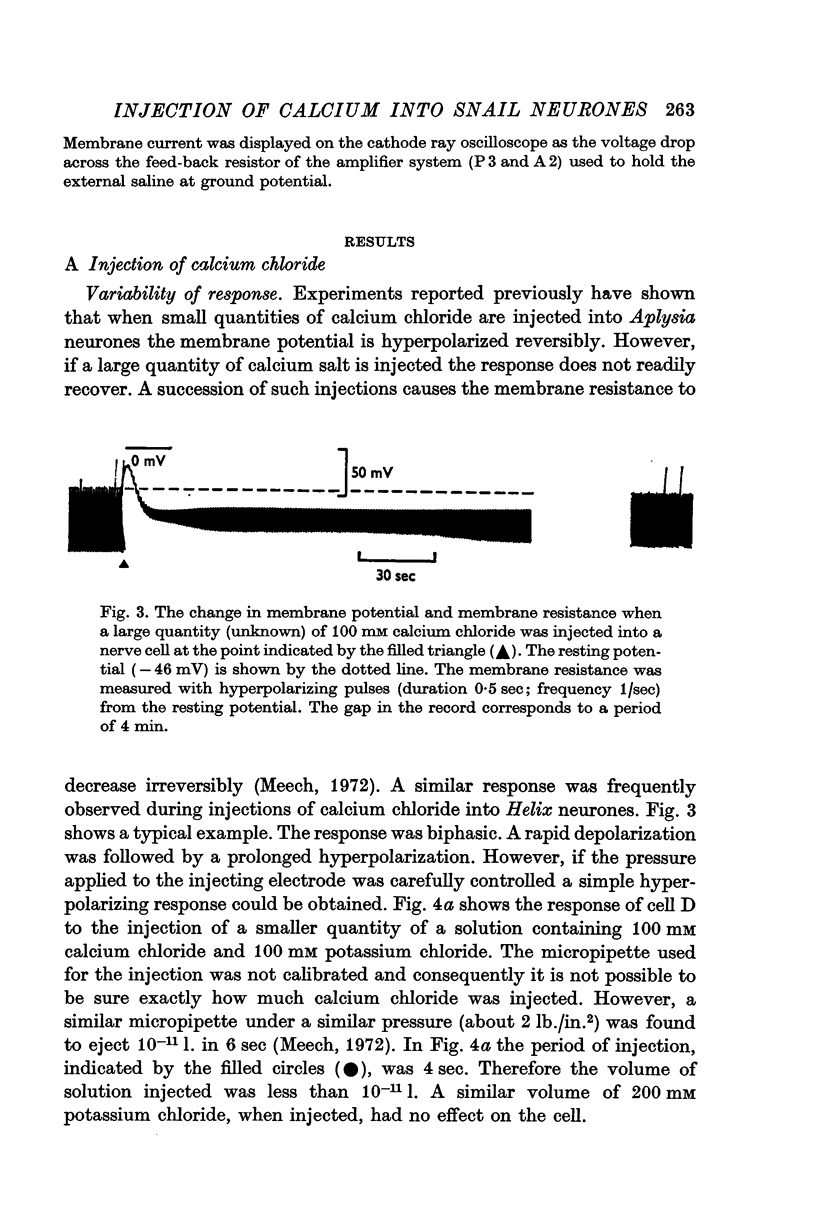
1. When calcium chloride was injected into Helix aspersa neurones there was a fall in membrane resistance and the membrane potential became hyperpolarized.
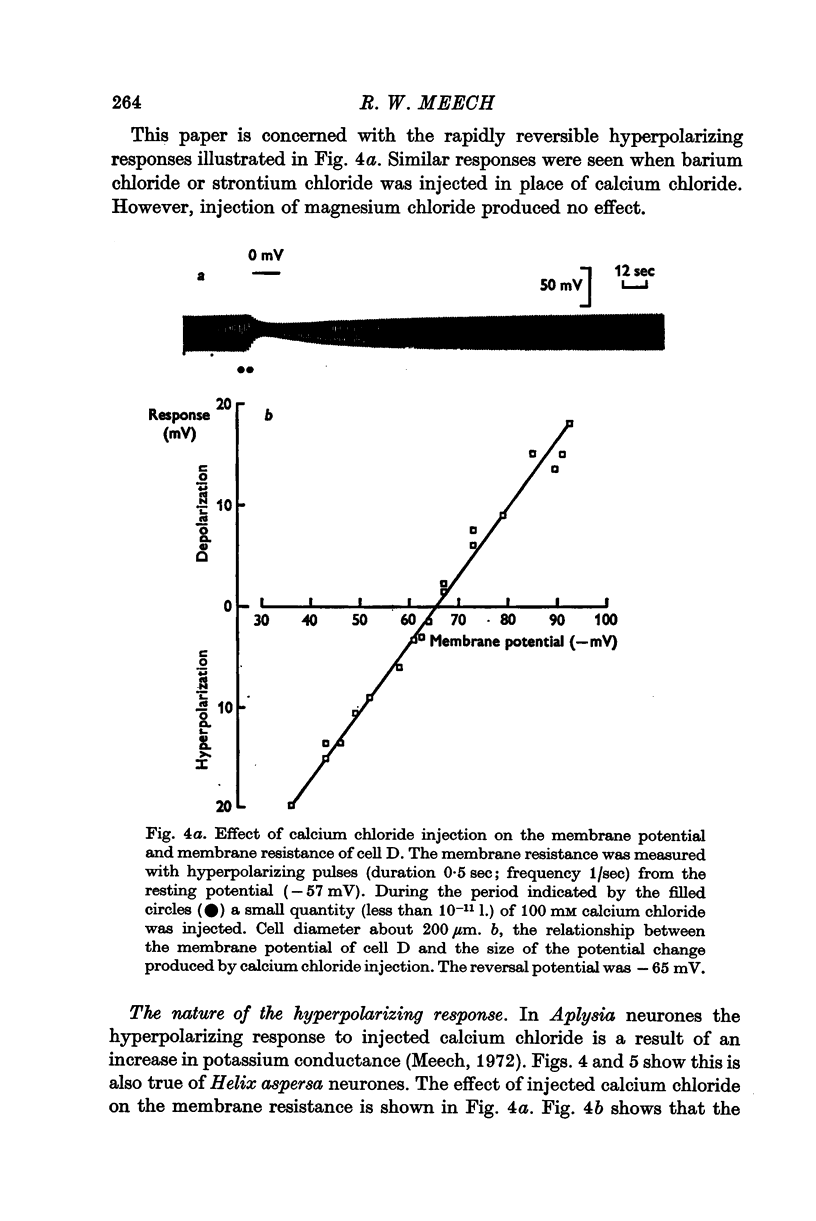
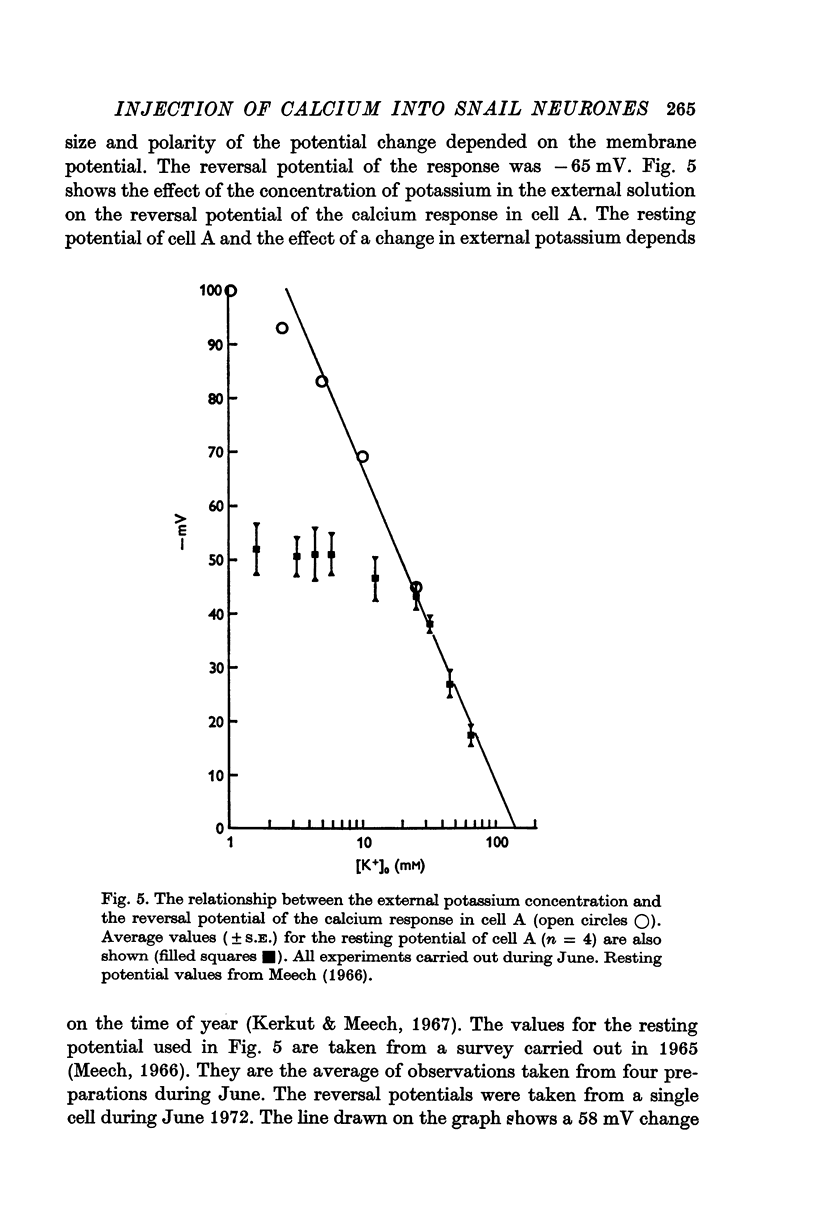
2. The reversal potential of the response was dependent on the concentration of potassium in the external solution.
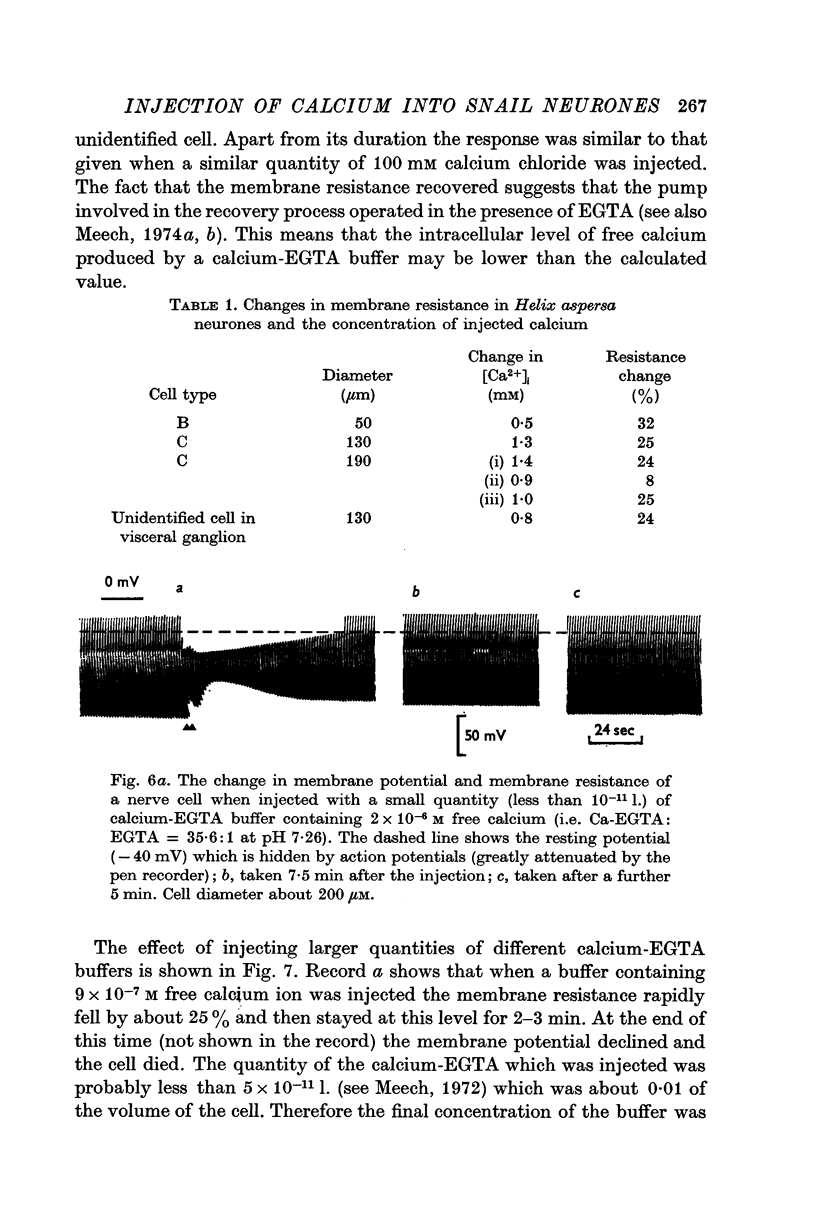
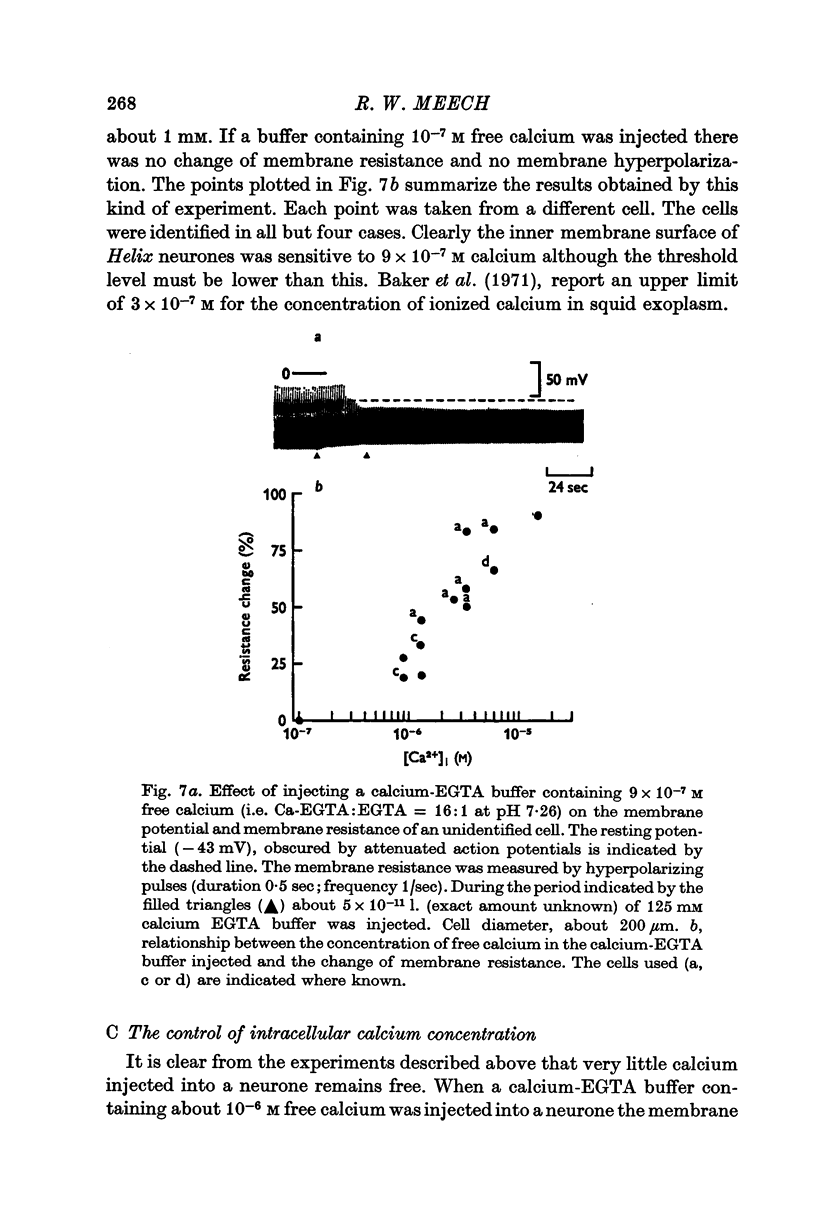
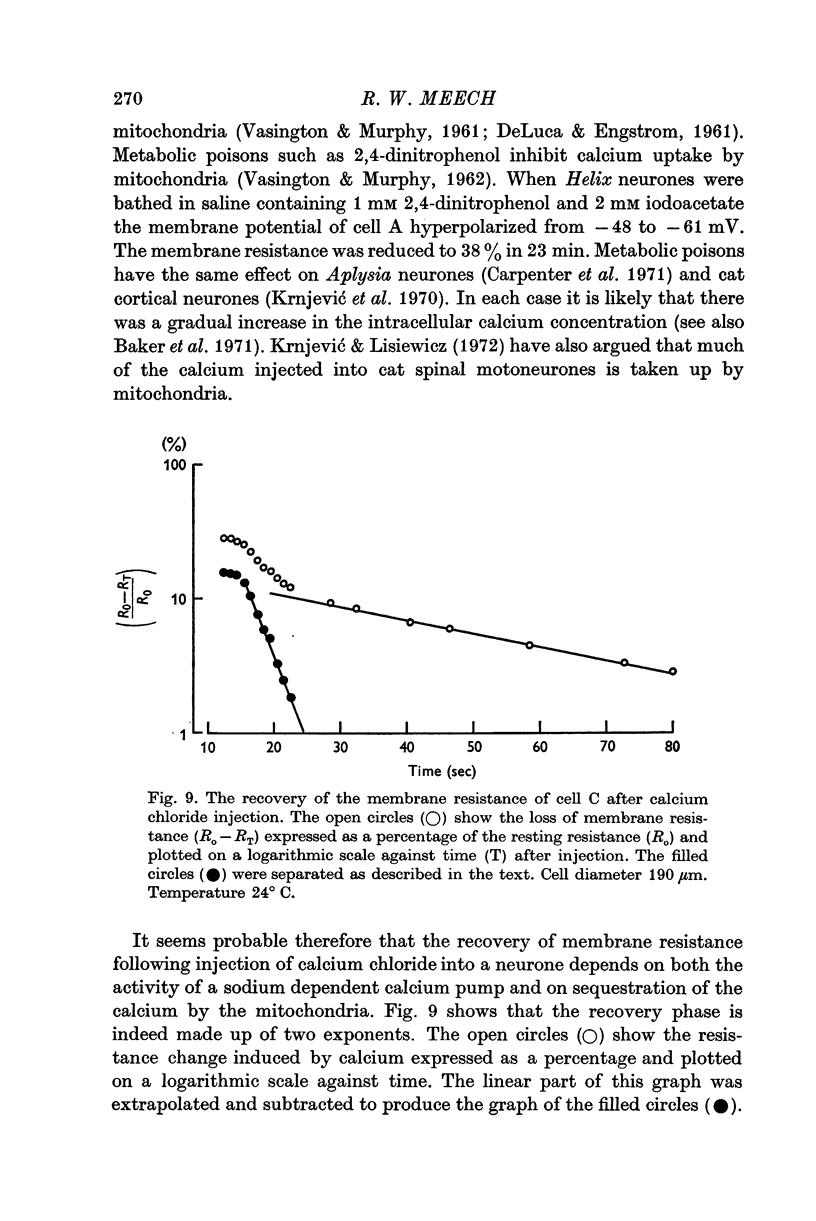
3. Injection of a calcium—EGTA buffer containing 9 × 10-7 M free calcium reduced the membrane resistance by 25%. When calcium chloride was injected it was necessary to increase the total intracellular calcium concentration by about 10-3 M to produce similar change of resistance.
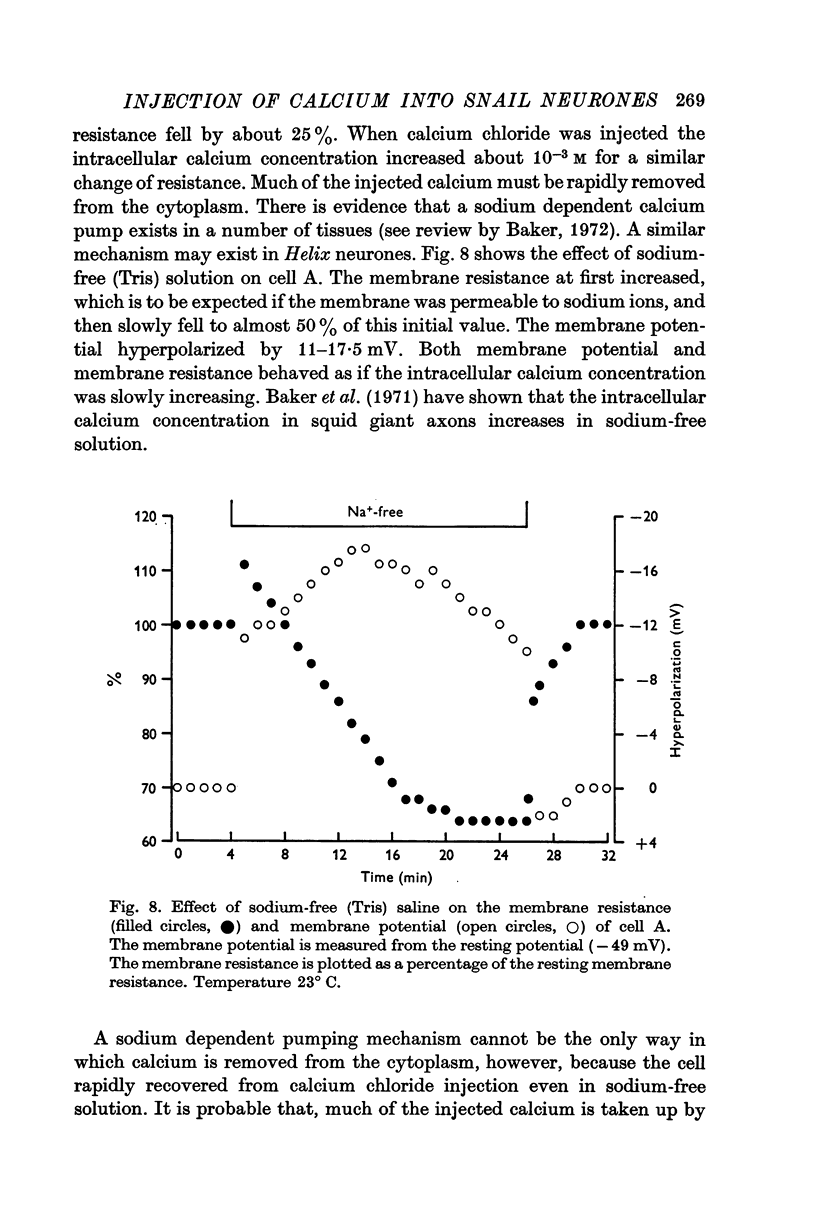
4. In sodium-free (Tris) solution there was a slow fall of membrane resistance as if the intracellular calcium concentration had increased. There was a similar resistance change in the presence of 2,4-dinitrophenol and iodoacetate.
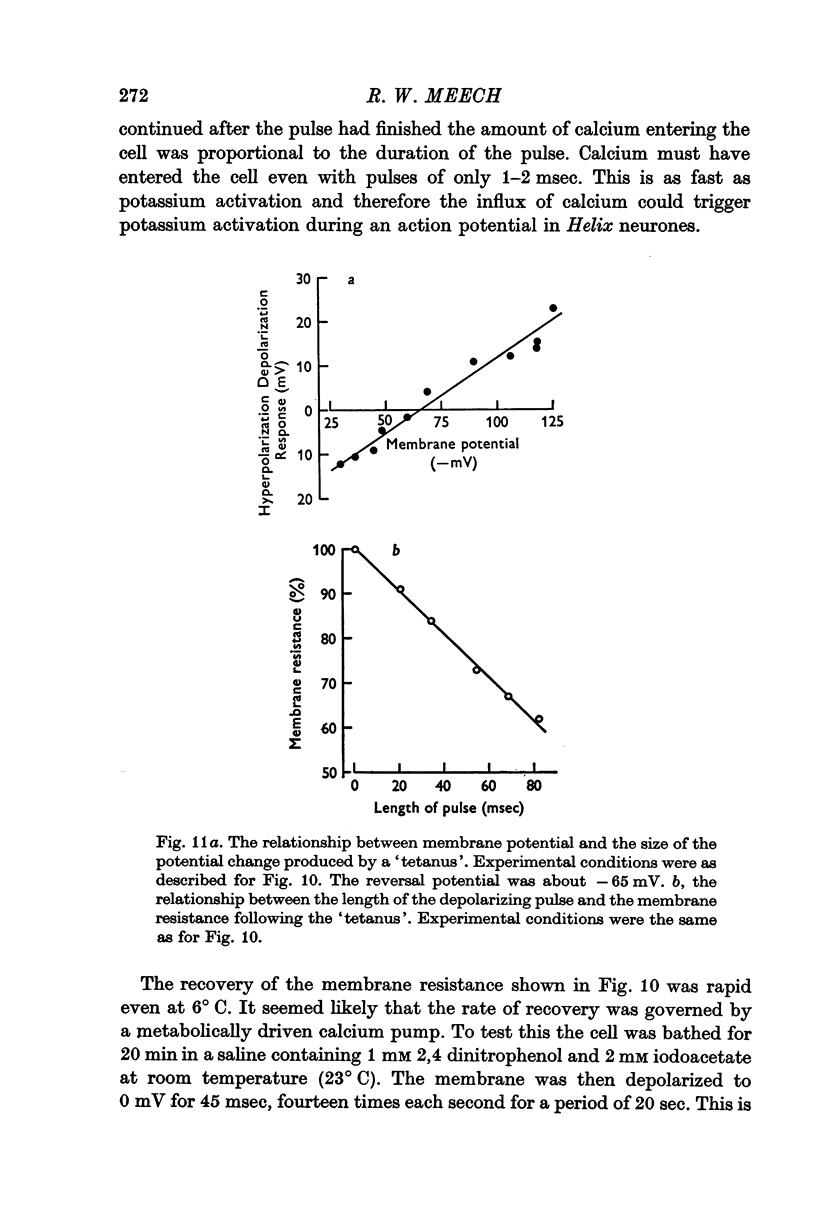
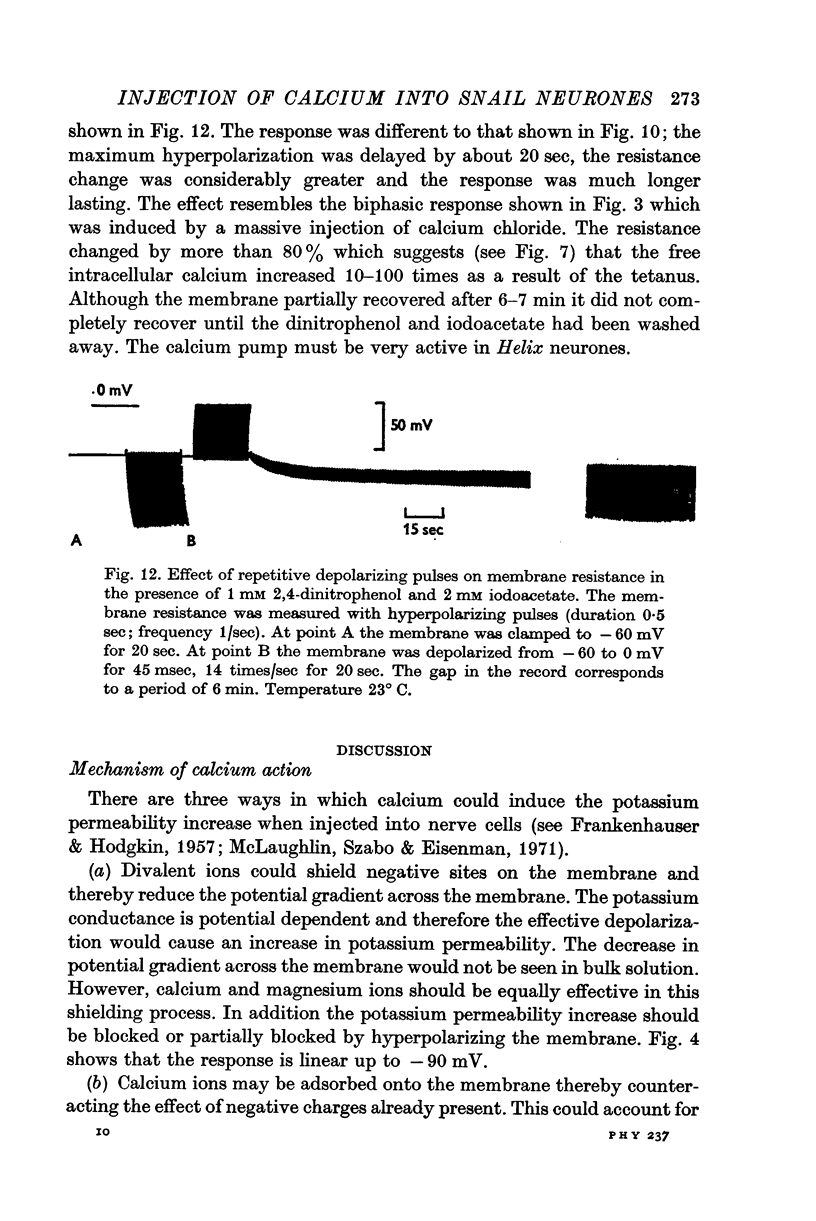
5. A series of repetitive depolarizing pulses produced a long lasting reduction in membrane resistance which was enhanced by 2,4-dinitrophenol and iodoacetate.
6. It is concluded that (a) injection of calcium causes an increase in potassium permeability, (b) the injected calcium is rapidly pumped from the cytoplasm by a sodium-dependent mechanism and by mitochondria, and (c) 1-2 msec depolarizing pulses stimulate an influx of calcium. This influx is rapid enough to trigger potassium activation during an action potential.
Full text
PDF

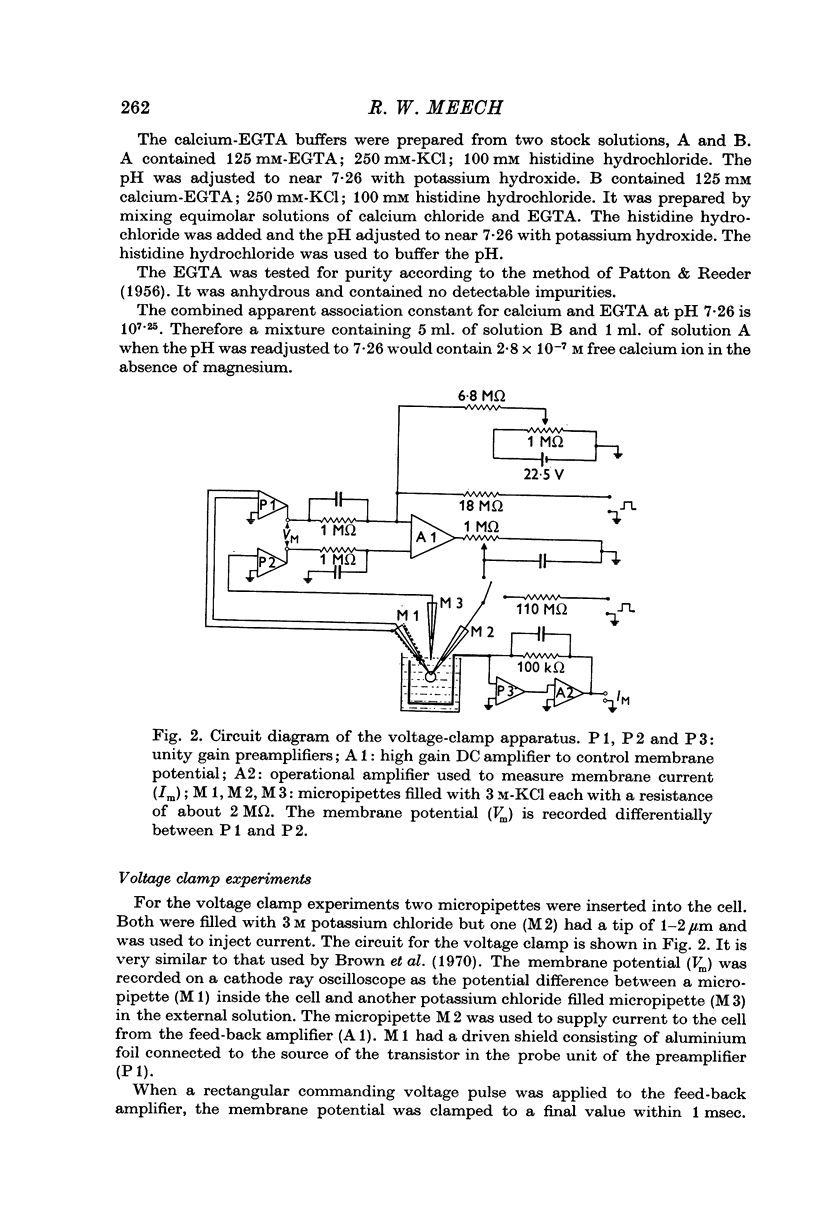






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRINK F. The role of calcium ions in neural processes. Pharmacol Rev. 1954 Sep;6(3):243–298. [PubMed] [Google Scholar]
- Baker P. F., Crawford A. C. Mobility and transport of magnesium in squid giant axons. J Physiol. 1972 Dec;227(3):855–874. doi: 10.1113/jphysiol.1972.sp010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Hodgkin A. L., Ridgway E. B. Depolarization and calcium entry in squid giant axons. J Physiol. 1971 Nov;218(3):709–755. doi: 10.1113/jphysiol.1971.sp009641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Meves H., Ridgway E. B. Effects of manganese and other agents on the calcium uptake that follows depolarization of squid axons. J Physiol. 1973 Jun;231(3):511–526. doi: 10.1113/jphysiol.1973.sp010246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodwick M. S., Junge D. Post-stimulus hyperpolarization and slow potassium conductance increase in Aplysia giant neurone. J Physiol. 1972 Jun;223(2):549–570. doi: 10.1113/jphysiol.1972.sp009862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Brown H. M. Light response of a giant Aplysia neuron. J Gen Physiol. 1973 Sep;62(3):239–254. doi: 10.1085/jgp.62.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. M., Hagiwara S., Koike H., Meech R. M. Membrane properties of a barnacle photoreceptor examined by the voltage clamp technique. J Physiol. 1970 Jun;208(2):385–413. doi: 10.1113/jphysiol.1970.sp009127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarandini D. J., Gerschenfeld H. M. Ionic mechanism of cholinergic inhibition in molluscan neurons. Science. 1967 Jun 23;156(3782):1595–1596. doi: 10.1126/science.156.3782.1595. [DOI] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Prediction of repetitive firing behaviour from voltage clamp data on an isolated neurone soma. J Physiol. 1971 Feb;213(1):31–53. doi: 10.1113/jphysiol.1971.sp009366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELUCA H. F., ENGSTROM G. W. Calcium uptake by rat kidney mitochondria. Proc Natl Acad Sci U S A. 1961 Nov 15;47:1744–1750. doi: 10.1073/pnas.47.11.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfraind J. M., Krnjević K., Pumain R. Unexpected features of the action of dinitrophenol on cortical neurones. Nature. 1970 Nov 7;228(5271):562–564. doi: 10.1038/228562a0. [DOI] [PubMed] [Google Scholar]
- KERKUT G. A., THOMAS R. C. ACETYLCHOLINE AND THE SPONTANEOUS INHIBITORY POST SYNAPTIC POTENTIALS IN THE SNAIL NEURONE. Comp Biochem Physiol. 1963 Jan;8(1):39–45. doi: 10.1016/0010-406x(63)90067-7. [DOI] [PubMed] [Google Scholar]
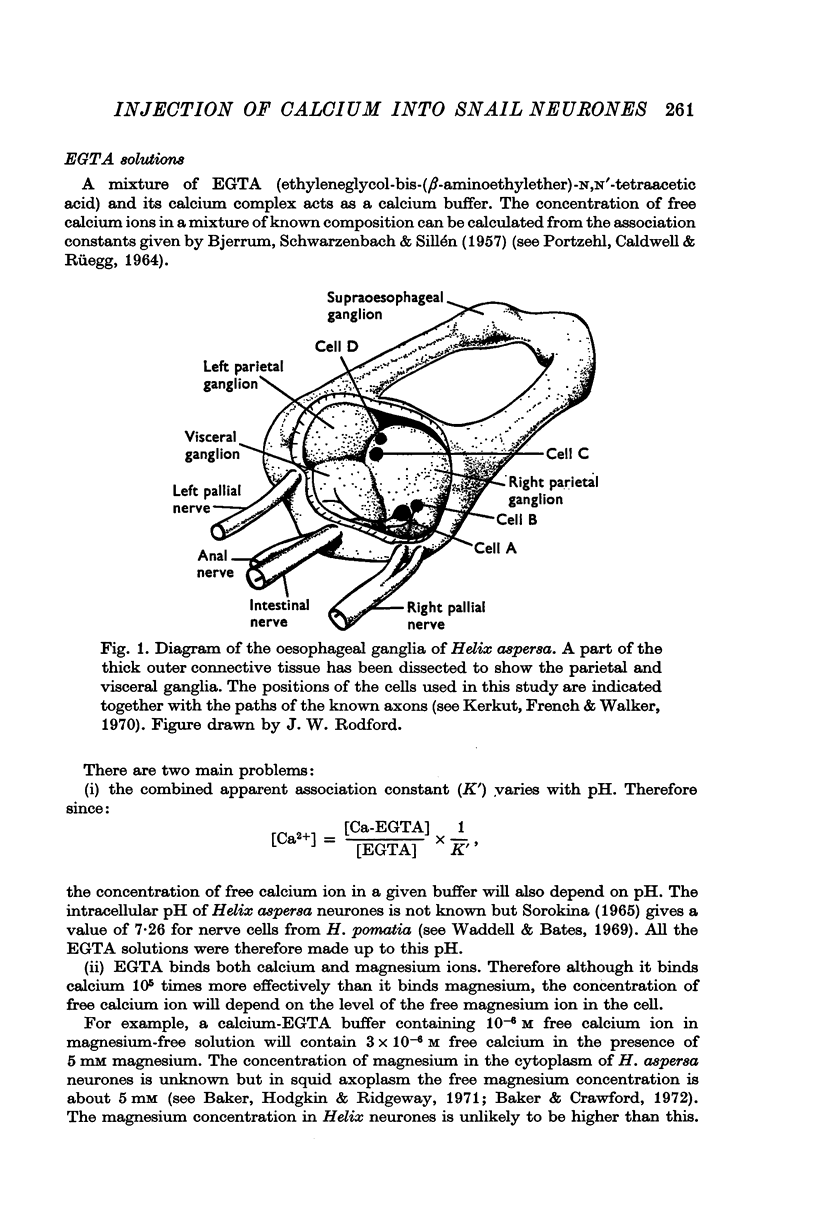
- Kerkut G. A., French M. C., Walker R. J. The location of axonal pathways of identifiable neurones of Helix aspersa using the dye Procion yellow M-4R. Comp Biochem Physiol. 1970 Feb 15;32(4):681–690. doi: 10.1016/0010-406x(70)90820-0. [DOI] [PubMed] [Google Scholar]
- Kerkut G. A., Meech R. W. The effect of ions on the membrane potential of snail neurones. Comp Biochem Physiol. 1967 Feb;20(2):411–429. doi: 10.1016/0010-406x(67)90257-5. [DOI] [PubMed] [Google Scholar]
- McLaughlin S. G., Szabo G., Eisenman G. Divalent ions and the surface potential of charged phospholipid membranes. J Gen Physiol. 1971 Dec;58(6):667–687. doi: 10.1085/jgp.58.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W. Intracellular calcium injection causes increased potassium conductance in Aplysia nerve cells. Comp Biochem Physiol A Comp Physiol. 1972 Jun 1;42(2):493–499. doi: 10.1016/0300-9629(72)90128-4. [DOI] [PubMed] [Google Scholar]
- Moreton R. B. Electrophysiology and ionic movements in the central nervous system of the snail, Helix aspersa. J Exp Biol. 1972 Oct;57(2):513–541. doi: 10.1242/jeb.57.2.513. [DOI] [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- SHANES A. M. Electrochemical aspects of physiological and pharmacological action in excitable cells. I. The resting cell and its alteration by extrinsic factors. Pharmacol Rev. 1958 Mar;10(1):59–164. [PubMed] [Google Scholar]
- TOBIAS J. M. Experimentally altered structure related to function in the lobster axon with an extrapolation to molecular mechanisms in excitation. J Cell Physiol. 1958 Aug;52(1):89–125. doi: 10.1002/jcp.1030520107. [DOI] [PubMed] [Google Scholar]
- Treherne J. E., Moreton R. B. The environment and function of invertebrate nerve cells. Int Rev Cytol. 1970;28:45–88. doi: 10.1016/s0074-7696(08)62540-1. [DOI] [PubMed] [Google Scholar]
- VASINGTON F. D., MURPHY J. V. Ca ion uptake by rat kidney mitochondria and its dependence on respiration and phosphorylation. J Biol Chem. 1962 Aug;237:2670–2677. [PubMed] [Google Scholar]
- Waddell W. J., Bates R. G. Intracellular pH. Physiol Rev. 1969 Apr;49(2):285–329. doi: 10.1152/physrev.1969.49.2.285. [DOI] [PubMed] [Google Scholar]
- Whittam R. Control of membrane permeability to potassium in red blood cells. Nature. 1968 Aug 10;219(5154):610–610. doi: 10.1038/219610a0. [DOI] [PubMed] [Google Scholar]



