Abstract
1. Cyclic adenosine 3′,5′-monophosphate (cyclic AMP) levels and catecholamine release were measured in cat adrenal glands perfused in situ with Locke solution.
2. Cyclic AMP was present in the medulla in an amount which represented approximately one fifth of that present in the cortex.
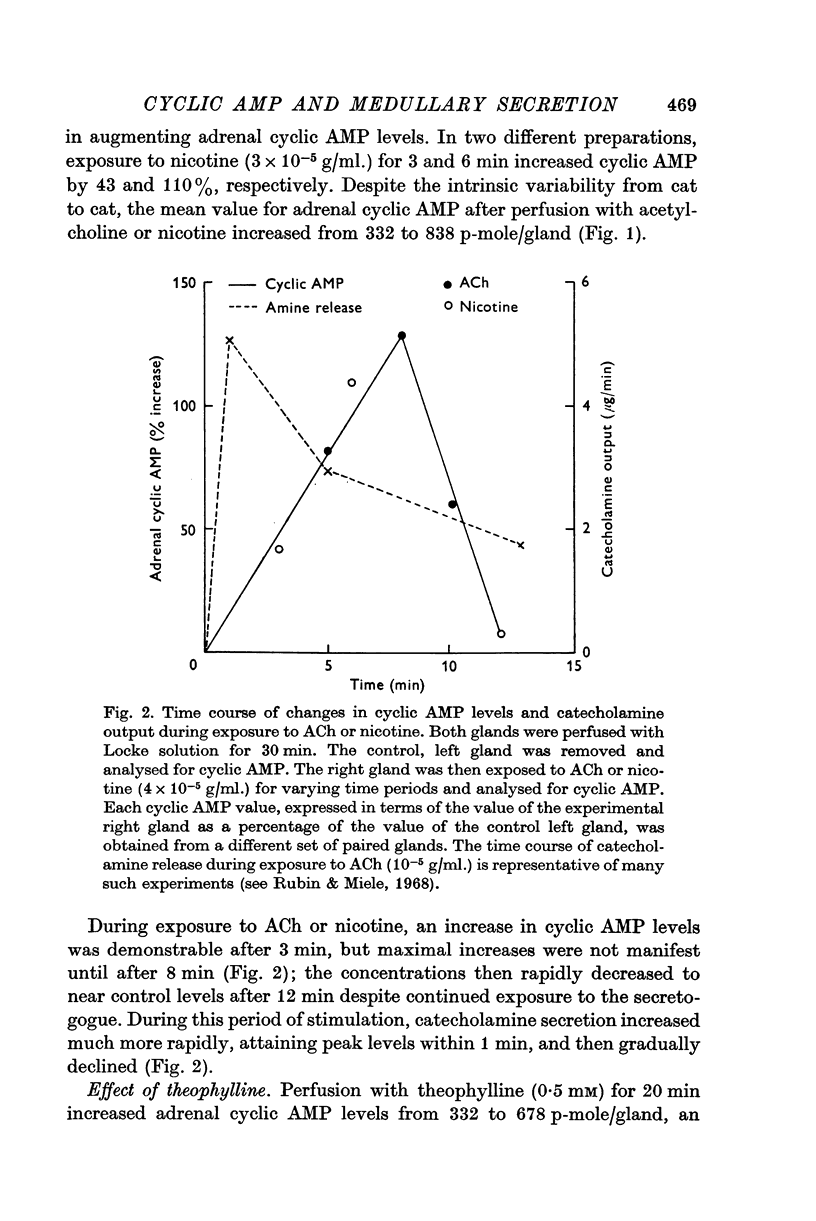
3. Perfusion with acetylcholine (ACh) or nicotine increased cyclic AMP both in the intact adrenal and in the perfusate. The time course of the changes in tissue cyclic AMP during stimulation was out of phase with the time course of catecholamine release. Maximal increases in cyclic AMP were not manifest until after 8 min of exposure to the secretogogue, whereas maximal rates of secretion occurred during the first minute.
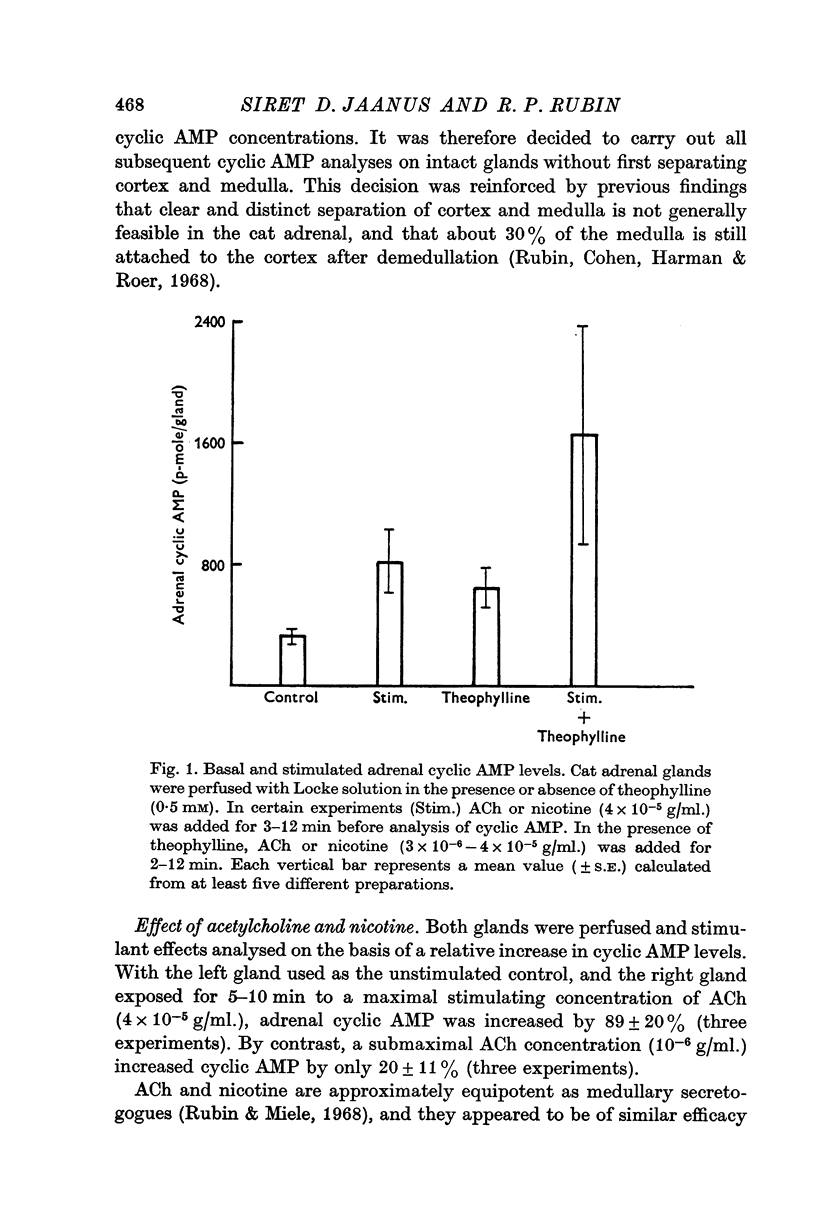
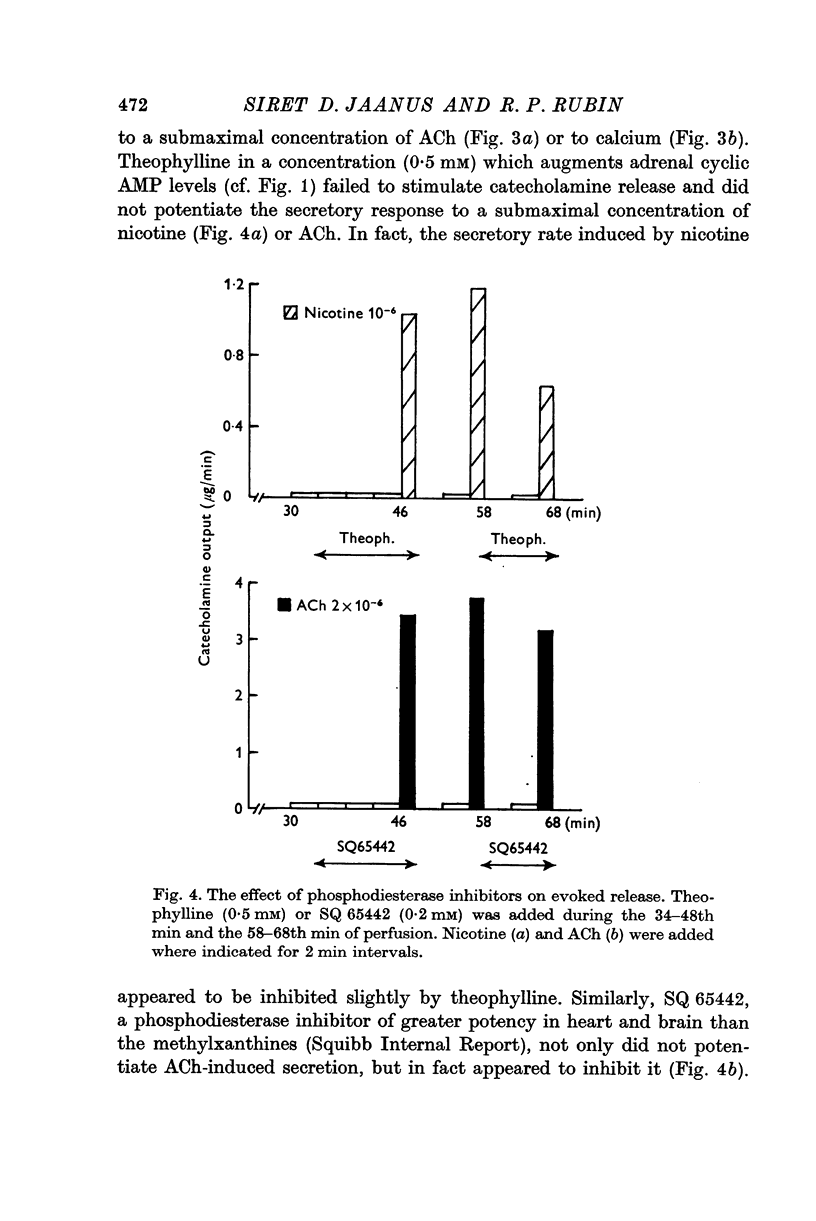
4. Theophylline (0·5 mM) increased basal and stimulated adrenal cyclic AMP levels, but did not potentiate the secretory response to ACh or nicotine.
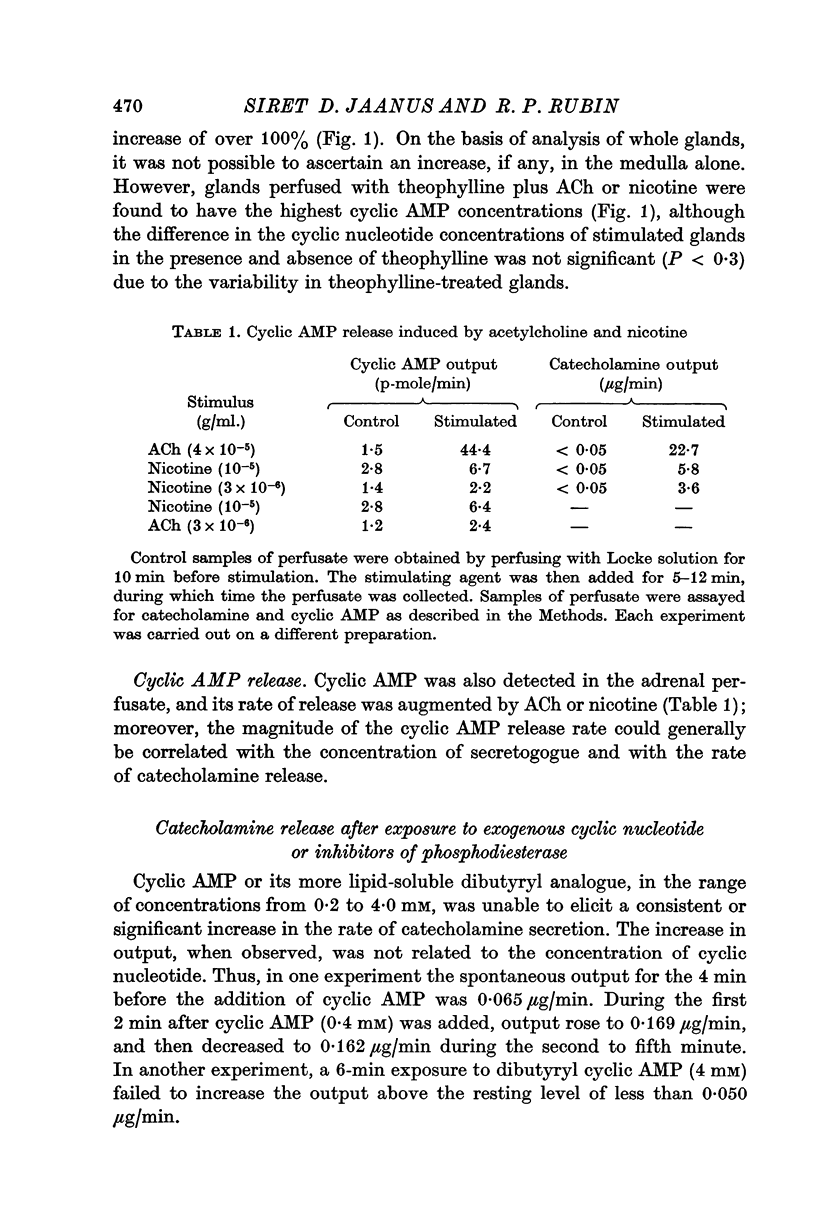
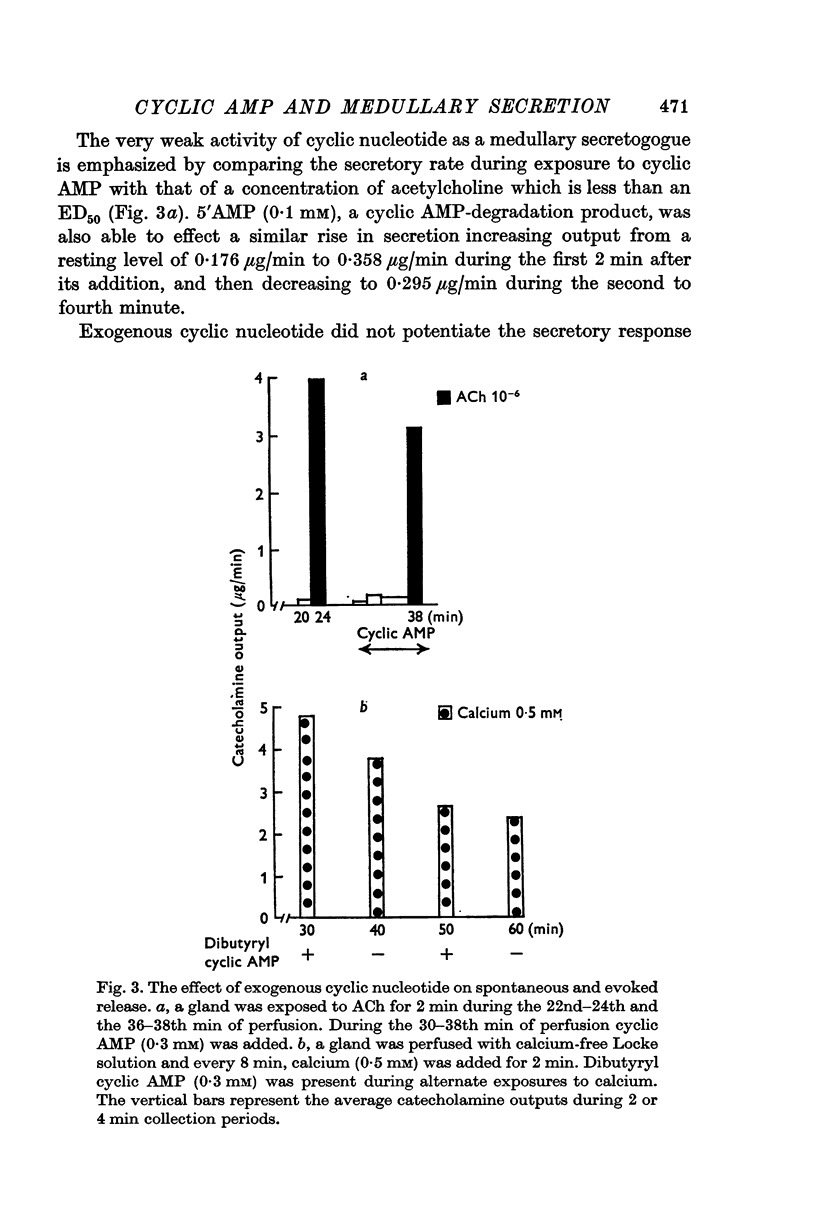
5. Perfusion with cyclic AMP or its dibutyryl derivative (0·2-4 mM) failed to effect a consistent or significant increase in the rate of catecholamine release and was unable to potentiate the secretory response to a submaximal concentration of ACh or calcium.
6. The results suggest that, unlike calcium, cyclic AMP is not a direct mediator of medullary secretion.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTON A. H., SAYRE D. F. A study of the factors affecting the aluminum oxide-trihydroxyindole procedure for the analysis of catecholamines. J Pharmacol Exp Ther. 1962 Dec;138:360–375. [PubMed] [Google Scholar]
- Beall R. J., Sayers G. Isolated adrenal cells: steroidogenesis and cyclic AMP accumulation in response to ACTH. Arch Biochem Biophys. 1972 Jan;148(1):70–76. doi: 10.1016/0003-9861(72)90116-6. [DOI] [PubMed] [Google Scholar]
- Benz L., Eckstein B., Matthews E. K., Williams J. A. Control of pancreatic amylase release in vitro: effects of ions, cyclic AMP, and colchicine. Br J Pharmacol. 1972 Sep;46(1):66–67. doi: 10.1111/j.1476-5381.1972.tb06849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carchman R. A., Jaanus S. D., Rubin R. P. The role of adrenocorticotropin and calcium in adenosine cyclic 3', 5'-phosphate production and steroid release from the isolated, perfused cat adrenal gland. Mol Pharmacol. 1971 Sep;7(5):491–499. [PubMed] [Google Scholar]
- Case R. M., Johnson M., Scratcherd T., Sherratt H. S. Cyclic adenosine 3',5'-monophosphate concentration in the pancreas following stimulation by secretin, cholecystokinin-pancreozymin and acetylcholine. J Physiol. 1972 Jun;223(3):669–684. doi: 10.1113/jphysiol.1972.sp009868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. The role of calcium in the secretory response of the adrenal medulla to acetylcholine. J Physiol. 1961 Nov;159:40–57. doi: 10.1113/jphysiol.1961.sp006791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A., Costa E. Involvement of adenosine 3',5'-monophosphate in the activation of tyrosine hydroxylase elicited by drugs. Science. 1973 Mar 2;179(4076):902–904. doi: 10.1126/science.179.4076.902. [DOI] [PubMed] [Google Scholar]
- Jaanus S. D., Carchman R. A., Rubin R. P. Further studies on the relationship between cycl AMP levels and adrenocortical activity. Endocrinology. 1972 Oct;91(4):887–895. doi: 10.1210/endo-91-4-887. [DOI] [PubMed] [Google Scholar]
- Montague W., Cook J. R. The role of adenosine 3':5'-cyclic monophosphate in the regulation of insulin release by isolated rat islets of Langerhans. Biochem J. 1971 Mar;122(1):115–120. doi: 10.1042/bj1220115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul M. I., Kvetnanský R., Cramer H., Silbergeld S., Kopin I. J. Immobilization stress induced changes in adrenocortical and medullary cyclic AMP content in the rat. Endocrinology. 1971 Feb;88(2):338–344. doi: 10.1210/endo-88-2-338. [DOI] [PubMed] [Google Scholar]
- Peach M. J. Stimulation of release of adrenal catecholamine by adenosine 3':5'-cyclic monophosphate and theophylline in the absence of extracellular Ca 2+ . Proc Natl Acad Sci U S A. 1972 Apr;69(4):834–836. doi: 10.1073/pnas.69.4.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poisner A. M. Caffeine-induced catecholamine secretion: similarity to caffeine-induced muscle contraction. Proc Soc Exp Biol Med. 1973 Jan;142(1):103–105. doi: 10.3181/00379727-142-36967. [DOI] [PubMed] [Google Scholar]
- Poisner A. M. Direct stimulant effect of aminophylline on catecholamine release from the adrenal medulla. Biochem Pharmacol. 1973 Feb 15;22(4):469–476. doi: 10.1016/0006-2952(73)90288-8. [DOI] [PubMed] [Google Scholar]
- Rasmussen H. Cell communication, calcium ion, and cyclic adenosine monophosphate. Science. 1970 Oct 23;170(3956):404–412. doi: 10.1126/science.170.3956.404. [DOI] [PubMed] [Google Scholar]
- Rubin R. P., Cohen M. S., Harman S. M., Roer E. M. The localization of adrenaline-rich medullary chromaffin cells adjacent to the adrenal cortex. J Endocrinol. 1968 Aug;41(4):541–545. doi: 10.1677/joe.0.0410541. [DOI] [PubMed] [Google Scholar]
- Rubin R. P., Jaanus S. D. A study of the release of catecholamines from the adrenal medulla by indirectly acting sympathomimetic amines. Naunyn Schmiedebergs Arch Pharmakol Exp Pathol. 1966;254(2):125–137. doi: 10.1007/BF00535900. [DOI] [PubMed] [Google Scholar]
- Rubin R. P., Miele E. A study of the differential secretion of epinephrine and norepinephrine from the perfused cat adrenal gland. J Pharmacol Exp Ther. 1968 Nov;164(1):115–121. [PubMed] [Google Scholar]
- Rubin R. P. The role of calcium in the release of neurotransmitter substances and hormones. Pharmacol Rev. 1970 Sep;22(3):389–428. [PubMed] [Google Scholar]
- Rubin R. P. The role of energy metabolism in calcium-evoked secretion from the adrenal medulla. J Physiol. 1970 Jan;206(1):181–192. doi: 10.1113/jphysiol.1970.sp009005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serck-Hanssen G., Christoffersen T., Morland J., Osnes J. B. Adenyl cyclase activity in bovine adrenal medulla. Eur J Pharmacol. 1972 Aug;19(2):297–300. doi: 10.1016/0014-2999(72)90026-x. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Kipnis D. M., Utiger R., Parker C. Radioimmunoassay for the measurement of adenosine 3',5'-cyclic phosphate. Proc Natl Acad Sci U S A. 1969 Sep;64(1):367–373. doi: 10.1073/pnas.64.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]


