Abstract
1. Records of field potentials and of the firing patterns of single neurones evoked by stimulation of the fimbria and fornix have been obtained from the septal nuclei of the rat.
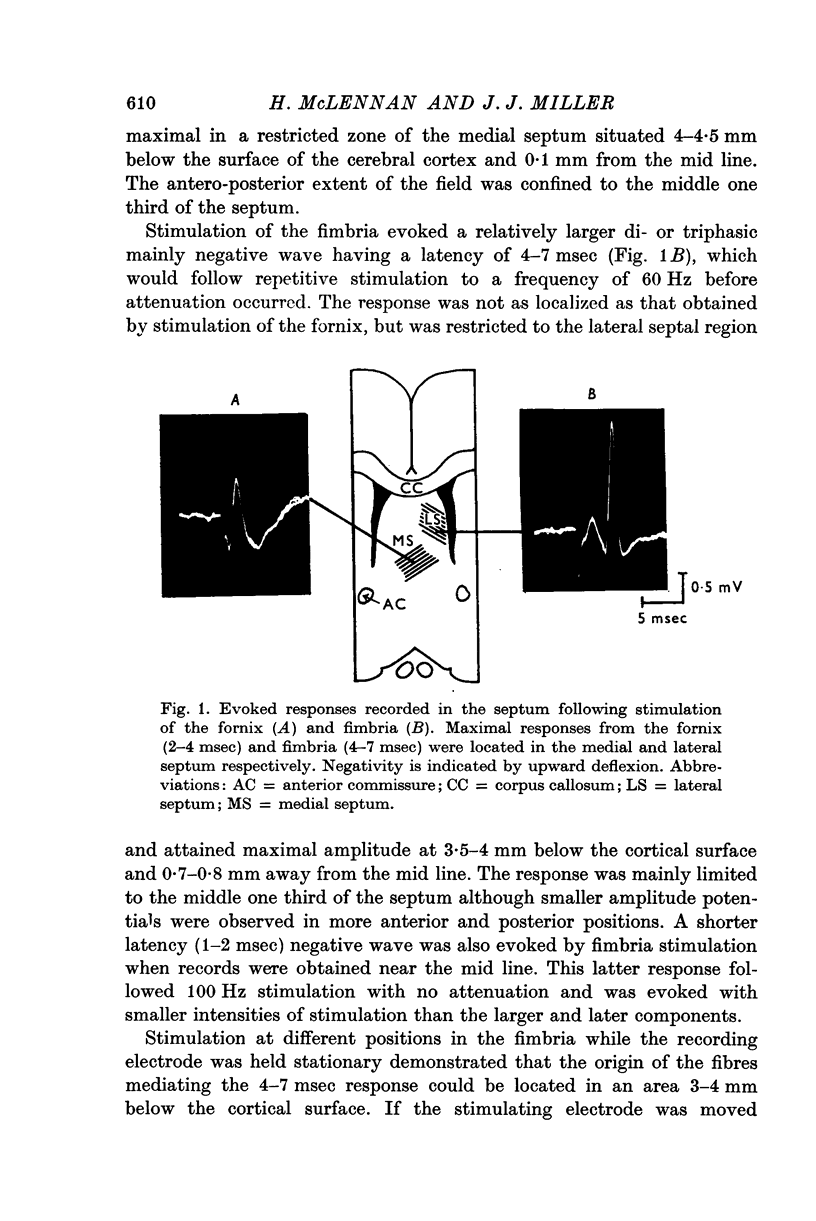
2. Fimbrial stimulation caused orthodromic activation of lateral septal neurones which was followed by a lengthy inhibition. In the medial septum such stimulation could elicit an antidromic response; but inhibition whose duration depended upon whether the neurones were firing irregularly or in synchronized bursts was obtained without prior activation.
3. Stimulation of the medial septum evoked an antidromic response in the lateral septal neurones, which was followed by inhibition.
4. For all of these inhibitory phenomena, bursts of action potentials of small amplitude and correlated with the start of the inhibitory periods were observed, and are believed to indicate the firing of inhibitory interneurones.
5. Stimulation of the fornix caused excitation of medial septal neurones but was without effect on those in the lateral septum.
6. A scheme is proposed in which the direct inhibition of medial septal neurones from the fimbria is suggested to act as a `reset' mechanism, while the phasic input from lateral septum resulting from the recurrent inhibitory pathways regulates the frequency of bursting in the medial septum.
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P., ECCLES J. C., LOYNING Y. PATHWAY OF POSTSYNAPTIC INHIBITION IN THE HIPPOCAMPUS. J Neurophysiol. 1964 Jul;27:608–619. doi: 10.1152/jn.1964.27.4.608. [DOI] [PubMed] [Google Scholar]
- ANDERSEN P., ECCLES J. C., SEARS T. A. THE VENTRO-BASAL COMPLEX OF THE THALAMUS: TYPES OF CELLS, THEIR RESPONSES AND THEIR FUNCTIONAL ORGANIZATION. J Physiol. 1964 Nov;174:370–399. doi: 10.1113/jphysiol.1964.sp007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P., Bland B. H., Dudar J. D. Organization of the hippocampal output. Exp Brain Res. 1973 Apr 30;17(2):152–168. doi: 10.1007/BF00235025. [DOI] [PubMed] [Google Scholar]
- BRUECKE F., PETSCHE H., PILLAT B., DEISENHAMMER E. [The influencing of the "hippocampus arousal reaction" in the rabbit by electrical stimulation in the septum]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1959;269:319–338. [PubMed] [Google Scholar]
- CRAWFORD J. M., CURTIS D. R., VOORHOEVE P. E., WILSON V. J. STRYCHNINE AND CORTICAL INHIBITION. Nature. 1963 Nov 30;200:845–846. doi: 10.1038/200845a0. [DOI] [PubMed] [Google Scholar]
- Dreifuss J. J., Murphy J. T., Gloor P. Contrasting effects of two identified amygdaloid efferent pathways on single hypothalamic neurons. J Neurophysiol. 1968 Mar;31(2):237–248. doi: 10.1152/jn.1968.31.2.237. [DOI] [PubMed] [Google Scholar]
- GREEN J. D., ARDUINI A. A. Hippocampal electrical activity in arousal. J Neurophysiol. 1954 Nov;17(6):533–557. doi: 10.1152/jn.1954.17.6.533. [DOI] [PubMed] [Google Scholar]
- Gogolák G., Petsche H., Sterc J., Stumpf C. Septum cell activity in the rabbit under reticular stimulation. Brain Res. 1967 Aug;5(4):508–510. doi: 10.1016/0006-8993(67)90022-4. [DOI] [PubMed] [Google Scholar]
- Murphy J. T., Renaud L. P. Mechanisms of inhibition in the ventromedial nucleus of the hypothalamus. J Neurophysiol. 1969 Jan;32(1):85–102. doi: 10.1152/jn.1969.32.1.85. [DOI] [PubMed] [Google Scholar]
- PETSCHE H., GOGOLAK G., VANZWIETEN P. A. RHYTHMICITY OF SEPTAL CELL DISCHARGES AT VARIOUS LEVELS OF RETICULAR EXCITATION. Electroencephalogr Clin Neurophysiol. 1965 Jul;19:25–33. doi: 10.1016/0013-4694(65)90004-0. [DOI] [PubMed] [Google Scholar]
- PETSCHE H., STUMPF C., GOGOLAK G. [The significance of the rabbit's septum as a relay station between the midbrain and the hippocampus. I. The control of hippocampus arousal activity by the septum cells]. Electroencephalogr Clin Neurophysiol. 1962 Apr;14:202–211. doi: 10.1016/0013-4694(62)90030-5. [DOI] [PubMed] [Google Scholar]
- STUMPF C., PETSCHE H., GOGOLAK G. The significance of the rabbit's septum as a relay station between the midbrain and the hippocampus. II. The differential influence of drugs upon both the septal cell firing pattern and the hippocampus theta activity. Electroencephalogr Clin Neurophysiol. 1962 Apr;14:212–219. doi: 10.1016/0013-4694(62)90031-7. [DOI] [PubMed] [Google Scholar]
- STUMPF C. THE FAST COMPONENT IN THE ELECTRICAL ACTIVITY OF RABBIT'S HIPPOCAMPUS. Electroencephalogr Clin Neurophysiol. 1965 Apr;18:477–486. doi: 10.1016/0013-4694(65)90128-8. [DOI] [PubMed] [Google Scholar]
- Siegel A., Tassoni J. P. Differential efferent projections from the ventral and dorsal hippocampus of the cat. Brain Behav Evol. 1971;4(3):185–200. doi: 10.1159/000125433. [DOI] [PubMed] [Google Scholar]
- Stumpf C. Drug action on the electrical activity of the hippocampus. Int Rev Neurobiol. 1965;8:77–138. doi: 10.1016/s0074-7742(08)60756-4. [DOI] [PubMed] [Google Scholar]
- Tömböl T., Petsche H. The histological organizarion of the pacemaker for the hippocampal theta rhythm in the rabbit. Brain Res. 1969 Feb;12(2):414–426. doi: 10.1016/0006-8993(69)90009-2. [DOI] [PubMed] [Google Scholar]
- YOKOTA T., FUJIMORI B. EFFECTS OF BRAIN-STEM STIMULATION UPON HIPPOCAMPAL ELECTRICAL ACTIVITY, SOMATOMOTOR REFLEXES AND AUTONOMIC FUNCTIONS. Electroencephalogr Clin Neurophysiol. 1964 Apr;16:375–382. doi: 10.1016/0013-4694(64)90071-9. [DOI] [PubMed] [Google Scholar]


