Abstract
1. The electrophoretic application of γ-aminobutyrate (GABA) and glycine to septal neurones inhibited their discharge, but the currents required to cause equivalent degrees of inhibition were always smaller for GABA than for glycine.
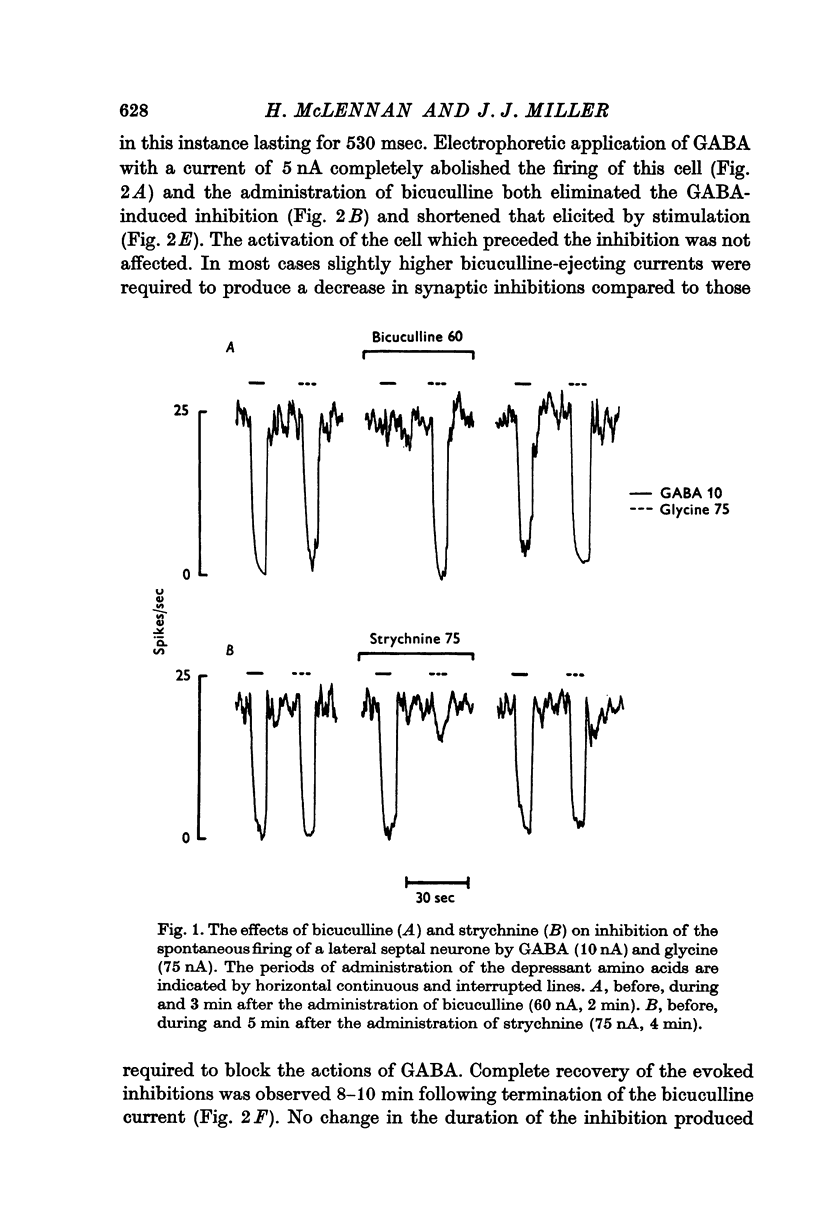
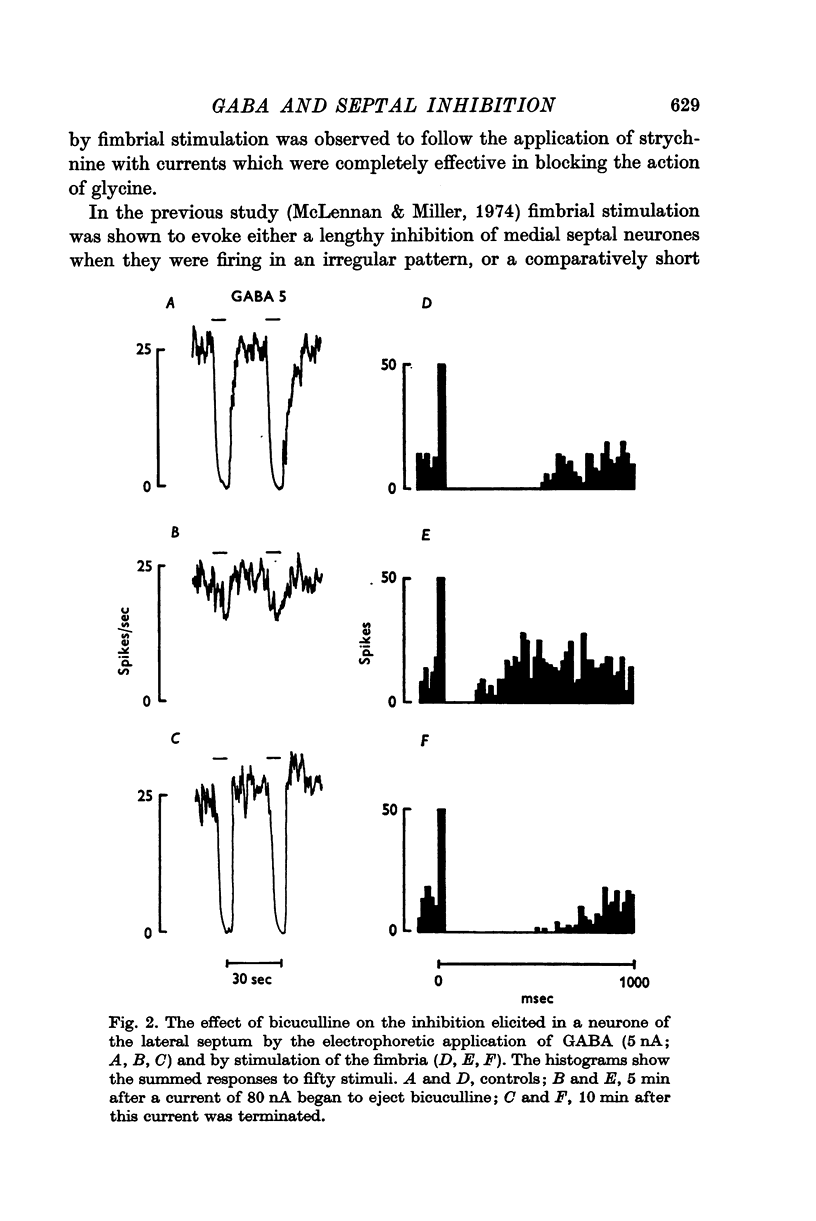
2. The actions of GABA but not those of glycine were antagonized by bicuculline and not by strychnine and vice versa.
3. The recurrent collateral inhibition of lateral septal neurones and the direct inhibition of medial cells evoked by stimulation of the fimbria were blocked by bicuculline but not by strychnine.
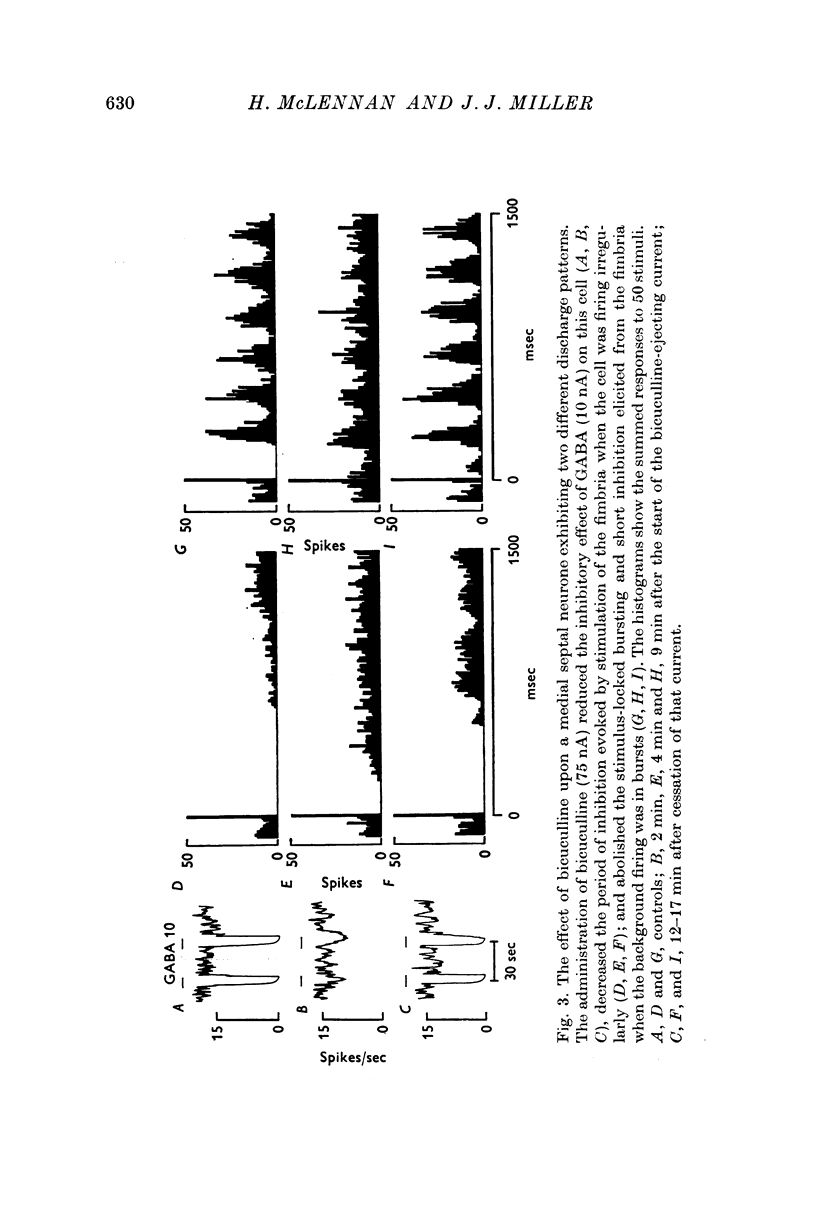
4. The bursting patterns of discharge of laterally situated medial septal neurones was disrupted by bicuculline, but required a longer application of the drug than was needed to block the direct fimbrial inhibition. It is suggested that this disruption is due to diffusion of the drug to affect the inhibitory interneurones of the lateral septum.
5. It is concluded that the inhibitory processes in the septum involve GABA as the synaptic transmitter.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Curtis D. R., Duggan A. W., Felix D., Johnston G. A. Bicuculline, an antagonist of GABA and synaptic inhibition in the spinal cord of the cat. Brain Res. 1971 Sep 10;32(1):69–96. doi: 10.1016/0006-8993(71)90156-9. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Duggan A. W., Felix D., Johnston G. A., McLennan H. Antagonism between bicuculline and GABA in the cat brain. Brain Res. 1971 Oct 8;33(1):57–73. doi: 10.1016/0006-8993(71)90305-2. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Duggan A. W., Johnston G. A. The specificity of strychnine as a glycine antagonist in the mammalian spinal cord. Exp Brain Res. 1971 Jun 29;12(5):547–565. doi: 10.1007/BF00234248. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Hösli L., Johnston G. A. A pharmacological study of the depression of spinal neurones by glycine and related amino acids. Exp Brain Res. 1968;6(1):1–18. doi: 10.1007/BF00235443. [DOI] [PubMed] [Google Scholar]
- Duggan A. W., McLennan H. Bicuculline and inhibition in the thalamus. Brain Res. 1971 Jan 8;25(1):188–191. doi: 10.1016/0006-8993(71)90579-8. [DOI] [PubMed] [Google Scholar]
- Krnjević K., Schwartz S. The action of gamma-aminobutyric acid on cortical neurones. Exp Brain Res. 1967;3(4):320–336. doi: 10.1007/BF00237558. [DOI] [PubMed] [Google Scholar]
- McLennan H., Miller J. J. The hippocampal control of neuronal discharges in the septum of the rat. J Physiol. 1974 Mar;237(3):607–624. doi: 10.1113/jphysiol.1974.sp010500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata K., Takeda K., Shinozaki H. Further study on pharmacological properties of the cerebellar-induced inhibition of deiters neurones. Exp Brain Res. 1970 Nov 26;11(4):327–342. doi: 10.1007/BF00237907. [DOI] [PubMed] [Google Scholar]
- Werman R., Davidoff R. A., Aprison M. H. Inhibitory of glycine on spinal neurons in the cat. J Neurophysiol. 1968 Jan;31(1):81–95. doi: 10.1152/jn.1968.31.1.81. [DOI] [PubMed] [Google Scholar]


