Abstract
A representative poly(β-amino ester) (PbAE) with biodegradable and pH-sensitive properties was used to formulate nanoparticle-based dosage form for tumor-targeted paclitaxel delivery. The polymer undergoes rapid dissolution when the pH of the medium is less than 6.5, and hence is expected to release its contents at once within the acidic tumor microenvironment and endo/lysosome compartments of cells. PbAE nanoparticles were prepared by solvent displacement method and characterized for particle size, charge, and surface morphology. Pluronic® F-108, a triblock copolymer of poly(ethylene oxide) (PEO) and poly(propylene oxide) (PPO) was blended with PbAE to induce surface modification of the nanoparticles. In vitro cellular uptake of tritiated [3H] paclitaxel in solution form and as nanoparticulate formulation was studied in MDA-MB-231 human breast adenocarcinoma cells grown in 12-well plates. We also examined the intracellular degradation pattern of the formulations within the cells by estimating the drug release profile. Cytotoxicity assay was performed on the formulations at different doses and time intervals. Nanoparticles prepared from poly(ε-caprolactone) (PCL) that do not display pH-sensitive release behavior were used as control. Spherical nanoparticles having positive zeta potential (~ 40 mV) were obtained in the size range of 150–200 nm with PbAE. The PEO chains of the Pluronic® were well-anchored within the nanomatrix as determined by electron spectroscopy for chemical analysis (ESCA). The intracellular accumulation of paclitaxel within tumor cells was significantly higher when administered in the nanoparticle formulations as compared to aqueous solution. Qualitative fluorescent microscopy confirmed the rapid release of the payload in case of PbAE nanoparticles into the cytosol, while the PCL nanoparticles integrity remained intact. The cytotoxicity assay results showed significantly higher tumoricidal activity of paclitaxel when administered in the nanoparticle formulations. The cell-kill effect was maximal for paclitaxel-loaded PbAE nanoparticles when normalized with respect to intracellular drug concentrations. Thus, PEO-modified PbAE nanoparticles show tremendous potential as novel carriers of cytotoxic agents for achieving improved drug disposition and enhanced efficacy.
Keywords: Biodegradable, pH-sensitive, nanoparticles, poly(β-amino ester), cytotoxicity, triggered-release, paclitaxel
INTRODUCTION
Poly (β-amino ester) (PbAE) constitutes a novel class of biodegradable cationic polymers with many desirable properties for development of site-specific drug and gene delivery systems.1,2 The synthesis of these materials is simple and straightforward and a variety of structural variants can be generated from a broad array of commercially available amine and diacrylate monomers.3 PbAE are structurally related to poly(amido amine) materials synthesized via conjugate addition of amines to bis(acrylamides).1,3,4 Given the polyamine nature of these polymers, they are particularly suited for the delivery of polynucleotides and acid-labile agents based on their ability to buffer the pH of their surroundings.3,5 Formulations prepared from PbAE may be used for delivery of low molecular weight drugs, in addition to oligonucleotides and plasmid DNA.6–9 Cytotoxicity and biocompatibility studies have shown that PbAE are significantly less toxic than currently available cationic polymers such as poly(ethyleneimine) and poly(L-lysine).1,3 In addition, PbAE degrade under physiological conditions via hydrolysis of their backbone esters to yield small molecular bis(β-amino acid) and diol products that are relatively biocompatible for systemic delivery applications.
The introduction of therapeutics that require intracellular administration and subsequent trafficking to the nucleus has created a demand for biomaterials that respond to intracellular stimuli such as pH. The PbAE possess pH-dependent solubility characteristics and are suitable for the fabrication of drug delivery systems that could be used to trigger or enhance the intracellular release of the payload upon exposure to acidic endosomal vesicles.5 Solid unprotonated samples of the PbAE are insoluble at physiological pH (7.4), but become instantly soluble in aqueous media when the pH of the solution is reduced below 6.5.5,6 As the transition from solid preparation to dissolved material occurs over the range of extracellular and endosomal/lysosomal pH (7.4 and 5.0–6.5 respectively), these polymers may be useful for the delivery of therapeutics agents in the vicinity of tumor mass and for others that must escape endosomal compartmentalization prior to fusion with lysosomes.
Biodegradable polymeric nanoparticles made from natural or synthetic polymers have drawn significant attention due to higher stability, maneuverability for industrial manufacture, and opportunity for further surface engineering.10–13 They can be tailor-made to achieve both controlled drug release and site-specificity by tuning the polymer characteristics and surface chemistry.14–16 It has been established that nanocarriers can get concentrated preferentially in the certain disease sites, such as solid tumor, by virtue of the enhanced permeation and retention (EPR) mechanism17. Once accumulated at the tumor site, they can act as local drug depot depending upon the make-up of the carrier, thus providing a source for continuous supply of encapsulated therapeutic compound into the tumor mass.10,18–21 In order to prolong the systemic circulation times of the nanoparticles and hence enhance their passive targeting efficiency, various strategies that involve creation of a hydrophilic sheath around the nanomatrices have been employed. Majority of these systems employ poly(ethylene glycol) (PEG) / poly(ethylene oxide) (PEO) chains for surface modification through physical adsorption during particle formation or by covalent linkage to the core-forming polymer (e.g., copolymer of PEG/PEO with poly(D,L-lactic acid)) prior to particle formation.22,23 More recently, the possibility of introducing them into the nanoparticles matrix through intimate blending of the PEO-containing polymer along with the core-forming polymer also has been examined.24,25
The current research project was carried out with the objective of designing a long-circulating, pH-sensitive, nanoparticulate dosage form that is capable of releasing the payload in the acidic tumor microenvironment or after internalization in tumor cells via non-specific endocytosis and consequently releasing the payload under the influence of the intracellular pH-trigger. For the proof-of-concept studies, we chose PCL as the non-pH-responsive, biodegradable polymer, fluorescein isothiocyanate (FITC) as the fluorescent label (for qualitative investigations) and paclitaxel as the cytotoxic payload (for quantitative estimations).
MATERIALS AND METHODS
Chemicals.
A hydrophobic representative PbAE (MW ~ 10,000) was synthesized by the addition reaction of 4,4’-trimethyldipiperidine with 1,4-butanediol diacrylate in dimethylformamide for 48 hours at 50°C and purified according to the synthesis scheme described earlier (Figure 1).1,9 PCL with a number average molecular weight of 14,800 Da (as verified by gel-permeation chromatography), was purchased from Polysciences Inc. (Warrington, PA). Pluronic® F-108 was kindly provided by the Performance Chemical Division of BASF Corporation (Parsinpanny, NJ). Paclitaxel was purchased from LC Laboratories (Woburn, MA) and tritiated [3H] paclitaxel with an activity of 3.2 Ci/mmol was purchased from Movravek Biochemicals (Brea, CA). Paclitaxel solution, available as commercial injection (Onxol®) was purchased from Ivax Pharmaceuticals (Miami, FL). MDA-MB-231, human breast adenocarcinoma cells, were procured from American Type Culture Collection (ATCC, Rockville, MD). The cell culture media were purchased from Fisher Scientific (Pittsburgh, PA) and all the other chemicals and reagents were of analytical grade and were used as supplied. Deionized distilled water (NanoPure II, Dubuque, IA) was used for all aqueous preparations.
Figure 1.
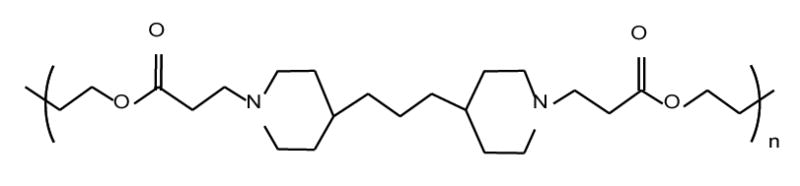
The chemical structure of a representative hydrophobic poly(β-amino ester) (PbAE) used for the preparation of poly(ethylene oxide)-modified nanoparticles. The PbAE was synthesized by addition reaction of 4,4’-trimethyldipiperidine with 1,4-butanediol diacrylate in dimethylformamide for 48 hours at 50°C.
Preparation of PEO-modified Polymeric Nanoparticles.
The PEO-modified PbAE nanoparticles were prepared by solvent displacement method as reported earlier.9,26 Briefly, a solution of PbAE was prepared in absolute ethanol and was introduced slowly into a pre-cooled (temperature ~ 15°C) aqueous solution containing known final concentration of Pluronic® F-108, ranging from 0.1% w/v to 1.0% w/v, under constant magnetic stirring. The rate of addition of organic phase to the aqueous phase, volume ratios, and the stirring speed were optimized to ensure batch-to-batch reproducibility. In another set of experiments, both PbAE and the Pluronic® were co-dissolved in ethanol to form a blend and introduced into water (without stabilizer). The final concentration of Pluronic® F-108 in the blend varied from 5% w/w to 25% w/w of the polymer. In both cases, the pH of water used was adjusted to 7.0 and magnetic stirring was continued for about 5 hours to evaporate the majority of organic solvent from the bulk. The nanoparticles were collected by centrifugation (10,000 rpm for 20 minutes), washing with buffered solution, and were freeze dried. An identical protocol was followed for preparation of the control (PEO-modified PCL) nanoparticles by replacing PbAE with PCL and acetone was used as solvent for the polymer instead of ethanol.
For drug-loaded nanoparticles, paclitaxel was dissolved along with the polymer in organic phase before introduction into aqueous medium. For quantitative experiments, a suitable quantity of [3H]-paclitaxel (supplied as methanolic solution) was added to the organic phase (0.23 μCi of radiolabeled was used for each mg of unlabeled paclitaxel).
Characterization of Nanoparticles.
The lyophilized nanoparticles were re-suspended and diluted suitably in deionized distilled water (pH ~ 7.0) and particle size was determined by Coulter® N4-Plus submicron particle sizer (Coulter Corporation, FL) at multiple scattering angle detection. Surface morphology of the freeze-dried sample was observed with a Hitachi S-4800 field emission scanning electron microscope (Hitachi Instruments, San Jose, CA). For zeta potential measurements, a diluted aqueous suspension of nanoparticles was mounted in a 90-Plus particle sizer / zetasizer (Brookhaven Instruments, NY) and mean zeta potential was computed using the Smoluchowski equation. For ESCA studies, the spectra of the freeze-dried samples were recorded on Surface Sciences Instrument X-probe spectrophotometer with a monochromatized A1 X-ray source. A 5.0 eV flood gun was used to neutralize the surface charge. The surface elemental composition was determined using standard Scofield photoemission cross sections. Identification of chemical functional groups was obtained from the high-resolution peak analysis of the carbon 1s (C1s) envelopes.
The loading capacity (μg of drug per mg of nanoparticles) and efficiency (% loaded as a function of amount of drug added) of paclitaxel in PbAE and PCL nanoparticles was determined by dissolving a known amount of the drug-loaded nanoparticles either in absolute ethanol (for PbAE) or acetone (for PCL) and the amount of paclitaxel in solution was determined by a UV assay. For [3H]-labeled paclitaxel, the loading capacity and efficiency was determined from radioactivity measurements.
Nanoparticle Uptake and Distribution in Tumor Cells.
The human breast adenocarcinoma cells (MDA-MB-231) were grown in Dulbecco’s minimum essential medium (DMEM) supplemented with 10% (v/v) fetal bovine serum, penicillin-streptomycin, HEPES buffer and sodium pyruvate. The incubation conditions were maintained at 5% CO2 levels.
For studying nanoparticles uptake and distribution by fluorescent microscopy, MDA-MB-231 cells were grown on circular glass cover-slips placed in six-well plates. Fluorescently-labeled PEO-modified PbAE or PCL nanoparticles (fluorescein isothiocyanate was incorporated into organic phase along with polymer during preparation step) were added at a concentration of 200 μg/ml per well containing 2 ml of complete serum-free medium (SFM). The cover-slips were collected at periodic time intervals after washing the cells with sterile phosphate buffered saline pH 7.4 (PBS) and mounted on glass slides using Fluoromount G®.
Measurement of Intracellular Drug Concentrations.
For studying quantitative uptake of nanoparticles containing [3H]-paclitaxel, the cells were seeded into 12-well plates (approx. 10,000 cells per well) and allowed to adhere by overnight incubation. The growth medium was replaced with serum free media (SFM) and the formulations were added as solution (in case of plain solution) or suspensions in sterile phosphate buffered saline (PBS, pH 7.4). For continuous exposure studies, the plates were incubated and at periodic time intervals, the cells were washed with sterile PBS and finally digested with 1% w/v Triton® X-100 solution (1 ml per well). For acute exposure studies, the SFM containing the formulations was replaced with the growth medium (DMEM) with a brief washing step with PBS between media change. The incubation was continued and once again, the cells were washed and digested at periodic time intervals. All the cell lysates were collected in pre-labeled scintillation vials. To each ml of the cell lysates, 10 ml of the ScintiSafe Econo® (scintillation cocktail) was added and the samples were allowed to quench for 2 hours in dark before measuring in a liquid scintillation analyzer (TriCarb 1600TR, Packard Instrument Co., CT). The counts-per-minute were converted into mCi using appropriate calibration curves. The values were converted to molar concentrations of paclitaxel and normalized with respect to protein content per well.
Cytotoxicity Studies.
The cells (approx. 5,000 per well) were seeded into 96-well plates and allowed to adhere overnight. The growth medium was replaced with SFM and the formulations containing graded concentrations of paclitaxel were added to the wells. Eight wells were used for each experimental condition by varying the drug concentrations and the incubation period was for 6 hours. At the end of which, the SFM was replaced with a mixture of DMEM and (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS – commercially available as CellTiter 96® AQueous Non-Radioactive Cell Proliferation Assay kit from Promega Corporation, Madison, WI) incubated for 4 hours. Absorbance of the controls and the treated cells were read at 492 nm using a microplate reader (Synergy HT, Bio-Tek Instruments, VT) and percentage viability values were calculated relative to untreated cells as a control.
RESULTS
Nanoparticles Preparation and Characterization.
Spherical nanoparticles having a smooth surface and distinct boundary were obtained by solvent diffusion method (Figure 2). Heating of the organic phase was necessary to get a homogenous solution of PbAE and during solvent displacement this hot solution (temperature of about 50°C) was introduced slowly into a pre-cooled aqueous phase. Nanoparticles were formed instantaneously with the newly formed nanosurface being sterically stabilized through the PEO chains of the polymeric stabilizer (Pluronic®). Earlier we have reported PEO coating of the nanoparticles via physical adsorption from the bulk solution for which we used 0.1% w/v aqueous solution (which amounts to 80 mg of Pluronic® F-108 per 100 mg of PbAE). In the current study, we standardized incorporation of the PEO chains through blending with the core-forming polymer and found 20% w/w of Pluronic® (by weight, which amounts to 25 mg and 100 mg PbAE) to be ideal to achieve steric stabilization of the nanoparticle formulation. This optimum concentration of Pluronic® F-108 was determined based on the quantitative and qualitative results from ESCA studies (measurement of -C-O- signal with repeated washing), changes in particle size determination after washing steps and after freeze drying, ease of re-dispersion of the freeze-dried nanoparticles, and ease of intravenous administration through a 27-gauge needle in mice by tail vein injection.
Figure 2.
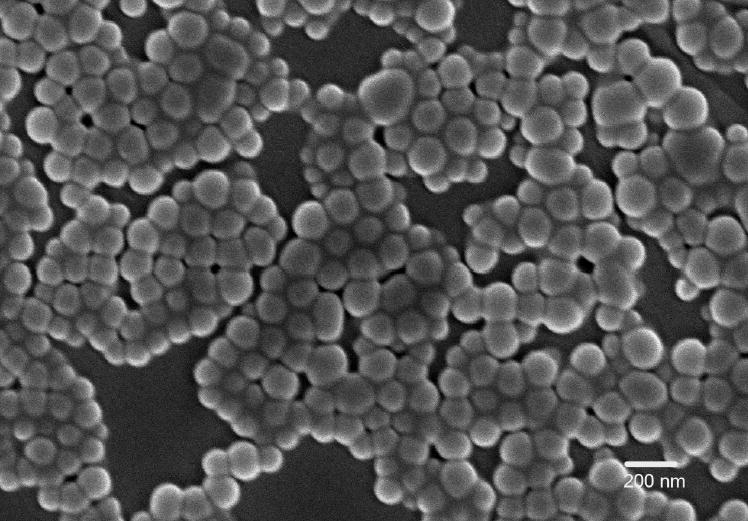
Scanning electron microscopy image of freeze-dried poly(ethylene oxide)-modified poly(β-amino ester) nanoparticles.
The particles obtained were in the range of 100 to 150 nm with a mean diameter of 113 nm. The blank PbAE nanoparticles had a surface charge of 46.8 mV (in purified water) and upon drug loading the value was slightly reduced to 39.4 mV. We optimized the formulations at a drug loading of 20% by weight and achieved an entrapment efficiency of 95% through regulation of the phase ratios and polymer concentrations. The PCL nanoparticles had a mean diameter of about 200 nm and the surface charge was 30.8 mV (with 20% by weight drug loading).
The degree of PEO surface modification of PbAE nanoparticles was estimated by ESCA for both methods (adsorption and blending). The results are summarized in Table 1 showing high-resolution -C-H- (hydrocarbon), -C-O- (ether) and -C=O- (carbonyl) peaks in the C1s envelope of the control and PEO-modified nanoparticles at their characteristic binding energies. The surface presence of the PEO chains was confirmed by an increase in the ether (-C-O-) signature of the spectra, which is indicative of the presence of ethylene oxide residues. After evaporating the organic solvent by stirring at room temperature for about 4 hours, majority of the excess (un-adsorbed) Pluronic® from the bulk was eliminated by a single centrifugation-washing step. At each processing step, we see significantly higher amounts of PEO chains being associated with the nanoparticles when incorporated through blending rather than adsorption. Each washing step strips-off certain amount of PEO chains from the nanoparticles surface, thus exposing the underlying polymeric substrate. The two methods of surface modification employ different concentrations of Pluronic® - 80 mg and 25 mg for adsorption and blending, respectively, per 100 mg of PbAE. For the experimental purpose, we used nanoparticles after the first washing step and it is evident that there was a 69% increase in (-C-O-) amount in case of blending against 10% and 37% (for adsorption from 0.1% 0.5% w/v Pluronic® solutions respectively) for passive coating. PCL nanoparticles exhibited a similar trend (results not shown) and indicated significant amounts of PEO chains present on the nanoparticles surface. The binding was stronger for PCL nanoparticles as the percentage of PEO chains stripped-of with washing steps was significantly less compared to PbAE nanoparticles. This maybe due to higher hydrophobicity of PCL as compared to PbAE.
Table 1.
High resolution C1s peak analysis from ESCA for comparison of surface elemental composition between the control and poly(ethylene oxide)-modified poly(β-amino ester) (PbAE) nanoparticlesa.
|
Mean Percentage |
||||
|---|---|---|---|---|
| CC/CH | CO | OC=O | Percentage increase in CO | |
| Surface Modification by Pluronic® F-108 Blending | ||||
| PbAE NP prepared without F-108 (control) | 73 | 20 | 7 | 0 |
| PbAE-F108 Blend NP prepared with 20% w/v F-108, centrifuged | 48 | 48 | 4 | 144 |
| PbAE-F108 Blend NP prepared with 20% w/v F-108, 1 wash | 62 | 33 | 5 | 69 |
| PbAE-F108 Blend NP prepared with 20% w/v F-108, 2 washes | 72 | 22 | 6 | 14 |
| Surface Modification by Pluronic® F-108 Adsorption | ||||
| PbAE NP prepared with 0.1% w/v F-108, centrifuged | 58 | 38 | 4 | 92 |
| PbAE NP prepared with 0.1% w/v F-108, 1 wash | 70 | 22 | 8 | 10 |
| PbAE NP prepared with 0.1% w/v F-108, 2 washes | 72 | 20 | 8 | 1 |
| PbAE NP prepared with 0.5% w/v F-108, centrifuged | 36 | 61 | 3 | 209 |
| PbAE NP prepared with 0.5% w/v F-108, 1 wash | 66 | 27 | 7 | 37 |
| PbAE NP prepared with 0.5% w/v F-108, 2 washes | 72 | 21 | 7 | 7 |
ESCA studies were performed at the NESAC/BIO, University of Washington, Seattle, WA. The spectra of the freeze-dried nanoparticle samples were recorded on Surface Sciences Instrument X-probe spectrophotometer with a monochromatized Al X-ray source. A 5.0 eV flood gun was used to neutralize the surface charge. The surface elemental composition was determined using standard Scofield photoemission cross sections. Identification of chemical functional groups was obtained from the high-resolution peak analysis of the carbon 1s (C1s) envelopes.
Nanoparticle Uptake and Distribution in Tumor Cells.
To demonstrate the rapid intracellular disintegration of the PbAE nanoparticles, we encapsulated FITC within polymeric (PbAE and PCL) nanoparticles and incubated with the tumor cells for different time periods. In Figure 3, we represent the images of the cells obtained after 1 hour of incubation with formulations. The PCL nanoparticles maintained structural integrity and the fluorescence was limited to the nanoparticles entity only. However, in case of cells exposed to PbAE nanoparticles, we observed diffused fluorescence that was present in the entire cell. There were only a small number of intact PbAE nanoparticles present within the cellular components after 1 hour.
Figure 3.
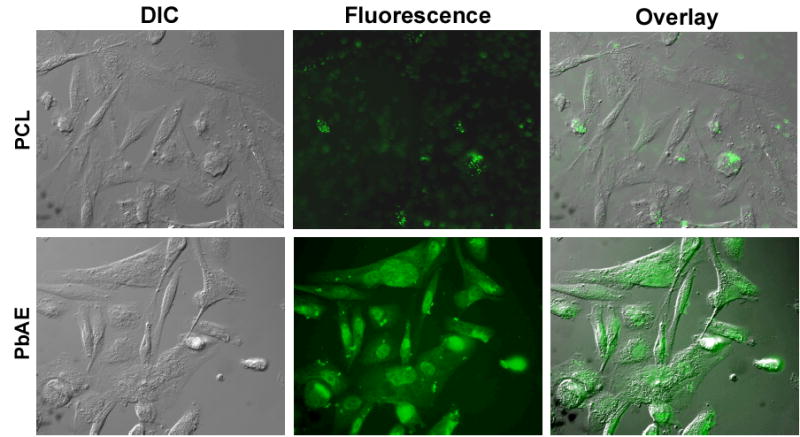
Fluorescence microscopy evidence for pH-sensitive triggered intracellular release of the payload from poly(ethylene oxide) (PEO)-modified poly(β-amino ester) (PbAE) nanoparticles. PEO-modified poly(ɛ-caprolactone) (PCL) nanoparticles served as a non-pH responsive control. Fluorescently-labeled PEO-modified PbAE or PCL nanoparticles were added to MDA-MB-231 human breast adenocarcinoma cells growing in culture at 37°C at a concentration of 200 μg/ml in 2 ml of serum-free medium. Periodically, the cover-slips were collected, washed with sterile phosphate buffered saline (pH 7.4) to remove any surface-adherent nanoparticles, and mounted on a glass slide for observation with a microscope.
Measurement of Intracellular Drug Concentrations.
We carried out two sets of studies for quantitative estimation of the total drug delivered to the intracellular matrix. In the first design, a fixed population of the cells (MDA-MB-231) was incubated with paclitaxel-containing formulations (namely paclitaxel in solution, paclitaxel in PbAE nanoparticles and paclitaxel in PCL nanoparticles at a dose of 1 mM) continuously. At periodic time intervals, the cells were washed with PBS to strip the drug that is present outside the cells and the drug that is captured within the intracellular matrix as measured after lysing and digesting the cells. The results are depicted in Figure 4. The intracellular concentrations achieved were significantly higher for paclitaxel formulated as nanoparticles compared to the solution form. At any given time interval, the total paclitaxel concentration within the cells was significantly higher when administered in PbAE nanoparticles as opposed to PCL nanoparticles. The intracellular drug concentration did not drop with time indicating a balance of flux being established and maintained during the study period of 6 hours. The pattern of cellular concentration was similar for paclitaxel in solution and paclitaxel in PbAE nanoparticles showing a steady build-up and retention. However, for paclitaxel in PCL nanoparticles, the intracellular concentration was fluctuating with time due to slower release.
Figure 4.
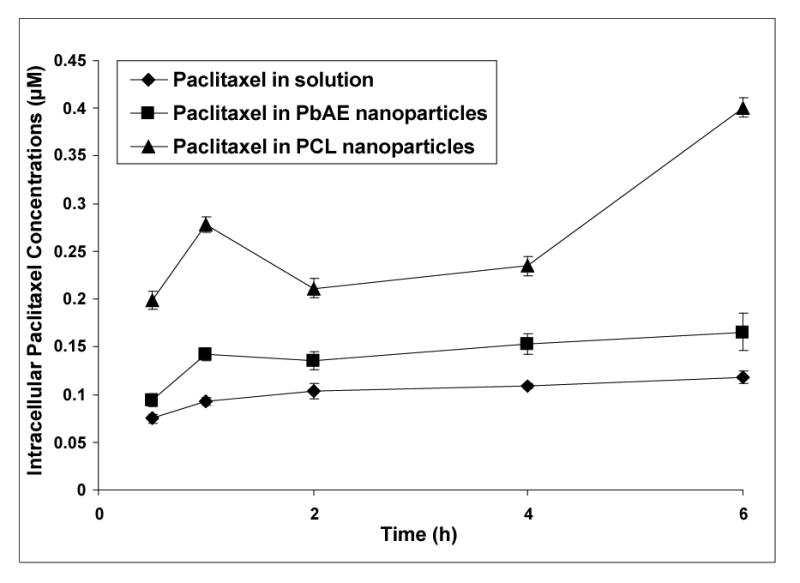
Intracellular paclitaxel concentrations upon continuous exposure of the drug in different formulations. Comparison between aqueous solution formulation, poly(ethylene oxide) (PEO)-modified poly(β-amino ester) (PbAE) nanoparticles, and PEO-modified poly(ɛ-caprolactone) (PCL) nanoparticles. For studying quantitative uptake of nanoparticles containing [3H]-paclitaxel, MDA-MB-231 human breast adenocarcinoma cells were seeded into 12-well plates (approx. 10,000 cells per well) and allowed to adhere by overnight incubation at 37°C. The growth medium was replaced with serum-free media and the formulations were added as an aqueous solution or as nanoparticle suspensions in sterile phosphate buffered saline (PBS, pH 7.4). For continuous exposure studies, the plates were incubated and at periodic time intervals, the cells were washed with sterile PBS and digested with 1% w/v Triton® X-100 solution. Radioactivity levels in the cell lysates was determined using a liquid scintillation counter and the values were converted into micromolar concentrations of paclitaxel normalized to protein content per well.
To examine the role of dose-dependency on the cellular uptake of formulations, we exposed the cells to graded concentration of paclitaxel (1, 10 and 100 mM) for two hours and measured the intracellular concentrations. In all cases, the intracellular concentration increased with dose, though the extent of increase was not linear. The PbAE nanoparticles accumulated 4.7, 2.1 and 1.3 times higher concentrations of paclitaxel as compared to the control (paclitaxel in solution) at 1, 10 and 100 mM dose levels respectively. For PCL nanoparticles, the corresponding values were 3.6, 5.1 and 1.9 respectively.
For the second design, we incubated the paclitaxel containing formulations with the cells for a period of two hours continuously in SFM. The cells were gently washed with PBS twice and the incubation was continued in the growth medium (DMEM) for additional six hours. The paclitaxel dose selected was 10 mM for the study to maintain higher sensitivity of detection. The results are represented in Figure 5. For paclitaxel in solution, a steady drop in intracellular concentration was observed with time. For paclitaxel in PCL nanoparticles, the intracellular concentration was maintained at a steady level that was significantly higher than the solution form. For paclitaxel in PbAE nanoparticles however, the cellular concentration rapidly dropped within one hour to levels similar to that of solution and maintained at the same thereon.
Figure 5.
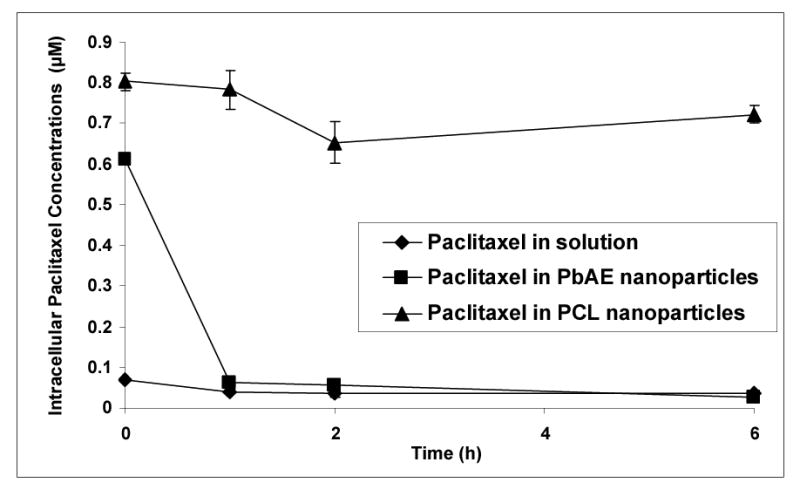
Intracellular paclitaxel concentrations upon acute exposure of different formulations. Comparison between aqueous solution formulation, poly(ethylene oxide) (PEO)-modified poly(β-amino ester) (PbAE) nanoparticles, and PEO-modified poly(ɛ-caprolactone) (PCL) nanoparticles. For studying quantitative uptake of nanoparticles containing [3H]-paclitaxel, MDA-MB-231 human breast adenocarcinoma cells were seeded into 12-well plates (approx. 10,000 cells per well) and allowed to adhere by overnight incubation at 37°C. For acute exposure studies, the serum-free media containing the formulations was replaced with the growth medium (DMEM) followed by a brief washing step with sterile phosphate buffered saline (PBS, pH 7.4) between media change. At periodic time intervals, the cells were washed with sterile PBS and digested with 1% w/v Triton® X-100 solution. Radioactivity levels in the cell lysates was determined using a liquid scintillation counter and the values were converted into micromolar concentrations of paclitaxel normalized to protein content per well.
Cytotoxicity Studies.
The cells were incubated with the paclitaxel containing formulation for a period of six hours (last time point in continuous exposure studies) at graded doses of the drug (from 1 nm to 10 mM) and the cell viability was checked using a commercially available kit. The results are represented in Figure 6 as percentage viability against log (dose). Cells exposed to the drug in the solution form remained nearly 100% viable, but the ones exposed to the drug-loaded nanoparticles showed significant cytotoxicity. A dose-dependent toxicity profile was observed for nanoparticle formulations, especially from 0.1 μM and higher. There was no statistically significant difference between the two nanoparticulate formulations except at the highest dose studied at which the paclitaxel-loaded PbAE nanoparticles showed significant cytotoxicity to tumor cells. Cells exposed to the respective controls (i.e., PbAE and PCL nanoparticles without any drug) showed 100% viability.
Figure 6.
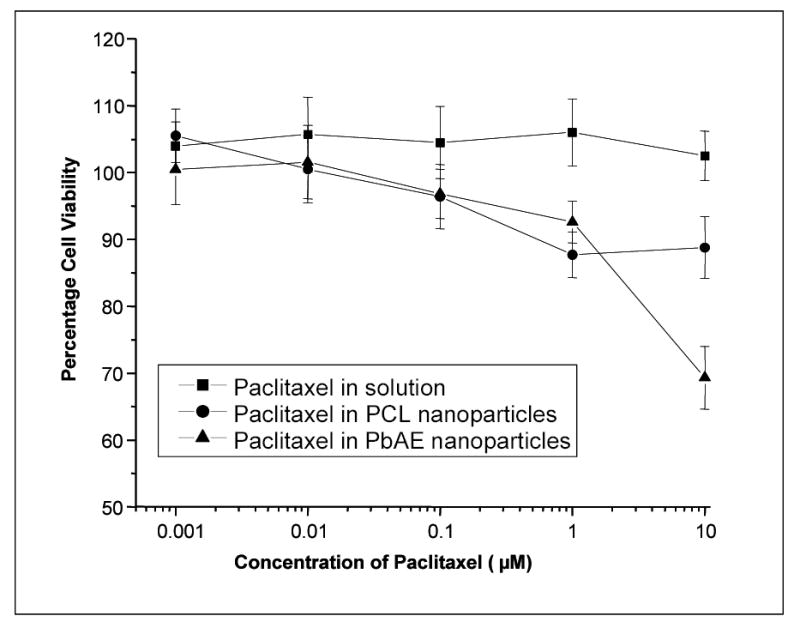
Percentage cell viability as a function of paclitaxel concentrations delivered in various formulations. Comparison between aqueous solution formulation, poly(ethylene oxide) (PEO)-modified poly(β-amino ester) (PbAE) nanoparticles, and PEO-modified poly(ɛ-caprolactone) (PCL) nanoparticles. MDA-MB-231 human breast adenocarcinoma cells, seeded in 96-well plates, at a density of approximately 5,000 cells per well, were allowed to adhere overnight at 37°C. The growth medium was replaced with serum-free media (SFM) and the graded concentrations of paclitaxel, either as an aqueous solution or as nanoparticle suspension, were added to the wells. After 6 hours of incubation, SFM was replaced with a mixture of growth media (DMEM) and (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS), and incubated for an additional 4 hours. Absorbance of the control and the treated cells were read at 492 nm using a microplate reader and percentage cell viability values were calculated relative to the untreated control.
Combining the results obtained from quantification and cytotoxicity studies indicate that the paclitaxel in PbAE nanoparticles demonstrate high cell-kill effect at comparative or lower doses. For example, at 10 μM dose level, the amount of paclitaxel accumulated within the cells were 0.06, 0.6 and 0.8 μM for paclitaxel in solution, in PbAE nanoparticles and in PCL nanoparticles respectively. The corresponding percentage cell viabilities were 100, 69 and 88 indicating a highly efficient tumoricidal activity for paclitaxel upon encapsulation within PbAE nanoparticles.
DISCUSSIONS
In our earlier report, we have described the standardization of preparation procedure for paclitaxel-loaded PbAE nanoparticles, PEO-surface modification by adsorption of Pluronic® during synthesis of nanoparticles, morphological analysis by scanning electron microscopy, in vitro nanoparticle degradation, paclitaxel loading and release study and nanoparticle trafficking into the BT-20 cells in vitro. In yet another investigation, we reported further insights into effects of PEO modification of the nanoparticles and intracellular release of rhodamine-123 in tumor cells in vitro. In the present investigation, we are following a different approach for PEO-surface-modification that is more efficient and dependable and we have quantified the cellular uptake and subsequent fate of nanoparticles within the cells. In addition, we have correlated the cellular concentrations to the cytotoxicity and provided evidence to prove the rapid intracellular release of the payload. We have used a well-characterized, biodegradable polymer as the standard (PCL) for comparison in all experiments.
Nanoparticles Preparation and Characterization.
The solvent displacement technique is well suited for preparation of nanoparticles using a hydrophobic polymer and for encapsulation of hydrophobic compounds. By tuning a limited number of variables (like polymer concentration, volume of organic phase, concentration and type of stabilizer etc.), one can vary the particle size and the drug loading efficiency. In general, a very high encapsulation efficiency (>90%) is achieved for hydrophobic drugs due to greater solubility of the drug in the nanomatrix containing the hydrophobic polymer solubilized in the organic phase rather than the aqueous phase. For the PbAE nanoparticles, we used absolute ethanol as the water-miscible organic phase, and for PCL we used acetone. The method was optimized to afford high drug loading (about 20% by weight) and to yield sub-200 nm particles. The PbAE nanoparticles maintained a cationic charge due to protonation of the tertiary amines of the polymer backbone at the measuring conditions (water used to suspend nanoparticles had pH of about 6.5). Cationic charge is essential for effective intracellular delivery as it is one of determinants for initial interaction between the delivery system and the cell surface (negatively charged) triggering the process of endocytosis. PCL nanoparticles have slightly lower cationic charge as there was no contribution from the polymeric core to the overall charge of the nanoparticle system.
PEO and its derivatives (available as Pluronics®, Poloxamers®, Poloxamines® and other block polymers of biodegradable polyesters) have been extensively used as biomaterials due to excellent biocompatibility and low toxicity. The majority of these polymers are not biodegradable and hence their application in systemic drug delivery is limited by their molecular weight. High molecular weight Pluronics® are acceptable and popular for oral delivery applications, while only those with a relatively low molecular weight (generally less than 30 kDa, but preferably between 5 to 15 kDa) have been proposed for parenteral administration. The lack of clearance of high molecular weight PEO derivatives is apparently due to the poor filtration properties of high molecular weight polymers difficulties through the kidneys after systemic administration. PEO-surface-modification is a simple, logical and popular means to improve the stability and performance of the nanoparticles and to avoid the interaction of the hydrophobic core particles with proteins and enzymes by providing them with a protective hydrophilic sheath.
Passive coating via physical adsorption with the Pluronic® F-108 (PEO122-PPO56-PEO122) has been reported earlier by us and others.9,26–30 Pluronic® F-108 has an average molecular weight of 14,000 daltons. Simple physical adsorption from an aqueous solution of the PEO-containing polymer is a straightforward surface modification methodology.17,23 This is further simplified when the PEO-containing polymer has an adjacent hydrophobic block (like PPO). As we see in Pluronics®, the hydrophobic center block (PPO) has high affinity for a surface of similar nature (like the surface of the PbAE/PCL nanoparticles) and can get easily anchored onto nanoparticles surface leaving the hydrophilic PEO side chains protrude into the surrounding aqueous medium.17,26,27 This kind of orientation of the long PEO chains offers hydrophilization of the nanoparticles surface and also a strong steric hindrance preventing particle aggregation and thus increasing the stability. For Pluronic® F-108, the PEO chain length of 122 ethylene oxide residues would provide extension and flexibility in the aqueous medium for effective steric repulsion of proteins and cells.
It must be remembered that the coating efficiency depends on extent and strength of PPO bonding with the nanoparticles surface. It has been observed by us and others that there is a decrease in the amount of ethylene oxide groups bound to the nanoparticles surface with washing and there is a possibility of their displacement with plasma proteins when administered into the systemic circulation.17,26,27,31 One of the recent strategy to limit the desorption process has been to introduce the Pluronic® as a blend with the core-forming polymer itself.24,25 In this case, the Pluronic® is co-dissolved with the PbAE/PCL in the water-miscible organic solvent and then introduced into the aqueous phase to induce precipitation of the nanocore. It is obvious that a certain population of the Pluronic® will be partitioned in favor of the aqueous phase in which it has greater solubility. However, the remaining part of the Pluronic® gets trapped within the nanomatrix with the central PPO block buried in the hydrophobic interior of the particle with the PEO chains protruding into the hydrophilic surrounding medium. The incorporation efficiency of the surface-modifying agent was much higher through the blending process as compared to the physical adsorption. The qualitative evidence for the incorporation has been provided with 1H-NMR earlier25. In the current investigation, we have provided evidence for the surface presence of PEO groups through ESCA.
We have limited the comparison of the two surface-modifying strategies for PbAE and PCL nanoparticle formulations. From Table 1, it is evident that there is greater retention of the PEO groups on the nanoparticles surface with blending process confirming a stronger anchoring of the central PPO blocks on the hydrophobic nanoparticle matrix. The percentage increase in PEO surface groups was significantly higher for the polymer blended nanoparticles at comparatively low Pluronic® concentrations. Though we observed the stripping of the PEO chains with washing steps, the extent of PEO chains retained on the nanoparticles surface were significantly higher for the blend nanoparticles than for the passively adsorbed systems. The Pluronic®-PbAE blend nanoparticles, prepared with 20% w/w Pluronic® F-108, showed superior flow properties and stability and did not aggregate even after repeated washing and freeze-drying steps.
Nanoparticle Uptake and Distribution in Tumor Cells.
It has been proven beyond doubt that the PbAE nanoparticles shows pronounced pH sensitivity.2,5,6 In one of the earlier investigations, it was shown that very low levels (~ 15%) of encapsulated rhodamine-dextran were released after 48 hours at pH 7.4, but lowering the pH to about 5.1 resulted in rapid and complete release of the encapsulated material.6 Intracellular pH measurements via fluorescently labeled DNA showed proton sponge effect for the PbAE through the buffering of the imidazole group.5 Although it is believed that the nanoparticles made from PbAE release encapsulated material in the endosomal pH range through a dissolution mechanism that involves protonation of amines in the polymer matrix, the current study provides comparative and supportive evidence relative to PCL, a non-pH-sensitive polymer. From Figure 2, it can be seen that the FITC-containing PCL nanoparticles get accumulated within the tumor cells and remain intact during the incubation period (one hour). However, the FITC-containing PbAE nanoparticles tend to lose their structural integrity and release the fluorescent dye into the cytosol, resulting in a diffused (not limiting to the nanomatrix alone) fluorescence staining not only the entire intracellular matrix but also the background.
Measurement of Intracellular Drug Concentrations.
The quantitative investigation was carried with dual objectives: (1) to learn about the rate and extent of accumulation of drug in intracellular matrix when exposed as different formulations; (2) to learn about the pattern of intracellular degradation and release from different formulation once taken up by the cells. We followed the continuous exposure protocol to achieve the first objective and an acute exposure protocol for the second.
The amount of paclitaxel that is concentrated within the cells varied according to the dose exposed. Almost a linear trend was followed for control (paclitaxel in solution) wherein the drug enters the cell through multiple pathways such as membrane diffusion, pinocytosis etc. The nanoparticle uptake is predominantly governed by (and limited to) non-specific endocytosis.32 This is probably why we see an initial increase in the intracellular concentration of paclitaxel when the dose is increase from 1 to 10 μM and then a decrease when normalized with the control.
The continuous exposure studies were carried out a concentration of 1mM of paclitaxel in all formulations (solution and nanoparticles). At all time intervals, paclitaxel in nanoparticles was concentrated at a higher extent than the solution form. Due to high drug loading capacity, the amount of drug that is trafficked into the cell for every nanoparticle is much higher compared to the solution form. Among the two polymeric nanoparticles studies, the PCL nanoparticles resulted in significantly higher drug concentration that the PbAE, though the intracellular concentrations were fluctuating and erratic with time of incubation. This can be explained based on polymer characteristics. PbAE is a pH-sensitive polymer which dissolves in the intracellular pH range (5.0–6.5) while PCL is expected to remain as is. The influx process reaches equilibrium for each formulation depending upon the pathway and the amount available extracellularly. The simplest and fastest is for the drug in solution (case 1) where the first limiting factor is the diffusion step across the cell membrane and the second is the concentration of the free drug within the intracellular matrix. That is why we observe a steady state reached quickly and maintained so throughout the study period. There is also a possibility of efflux of the drug if concentration gradient across the cell membrane were to prevail. For the polymeric nanoparticles, the first limiting step is the endocytosis-mediated uptake, while the second (concentration of free drug within the cells) is governed by the fate of the nanoparticles within the intracellular environment. In case of PCL nanoparticles (case 2), the polymer being highly stable at the conditions prevailing within the cells, the process of particle influx will continue to the rate and extent as governed by the endocytosis process. This process may reach saturation at times and hence will follow an unpredictable pattern. However, for PbAE (case 3), the scenario could be different due to the pH-triggered dissolution behavior. Once within the cells, the nanoparticles may quickly disintegrate / dissolve (within acidic endolysosomal compartments) releasing the free drug within the intracellular matrix. With progress of these two events, equilibrium would be reached between influx process, the particle dissolution process and the eventual efflux process of the free drug. The situation is comparable to that of drug in solution (case 1) except for the fact the drug is present in the nanoparticulate form extracellularly. We observe a small increase in cellular concentration (first two time-points in Figure 3 for paclitaxel in PbAE nanoparticles) followed by a parallel intracellular concentration to paclitaxel in solution form.
In the second protocol followed (acute exposure), the pattern observed in Figure 5 can be explained on two bases – polymer characteristics and contribution from the method used for estimating the drug. The PCL nanoparticles, once accumulated within the cells during the incubation period (2 hours with the formulations), undergo slow degradation – showing a near-steady concentration. The PbAE nanoparticles are rapidly dissoluted within the cells, free drug gets released intracellularly, and with the extracellular medium being clear of any free drug, there is diffusion of the drug in the opposite direction alongside the concentration gradient. Hence we see a rapid fall in the intracellular concentration (between first two time-points). Once the intracellular concentration reaches a certain value (comparable to that of the drug in solution form), a baseline concentration is maintained within cells (almost overlapping pattern from first hour onwards for paclitaxel in solution and in PbAE nanoparticles).
Cytotoxicity Studies.
This is a direct function of the effect of intracellular drug accumulation and is the terminal indicator of the efficiency of the formulations. Drug when exposed in the solution form did not cause any cytotoxicity at all the dose levels studied. We learn from the quantitative estimations that the intracellular drug levels reached were very low. Within the polymeric nanoparticles, the difference in cell-kill effect is obvious only at the highest dose studied (10 μM) though from 0.1 μM onwards, a significantly improved cytotoxicity was observed compared to control. The intracellular concentration achieved for the paclitaxel in solution, in PbAE nanoparticles and in PCL nanoparticles were 0.07, 0.6 and 0.8 μM respectively at 10 μM dose. The corresponding cell viability values were 102, 69 and 88%. This can be explained on polymer characteristics and the method used for estimating the intracellular drug concentration. The drug encapsulated in PCL nanoparticles is retained within the particles themselves upon reaching the site of action (intracellular matrix). While for the drug within PbAE nanoparticles, the entire payload is dumped into the cytosol rapidly upon ingestion of the particles resulting in efficient cell kill. There is also contribution from the method used for estimating the intracellular drug concentration. It is understood that we are estimating the total drug within the cells – drug in solution plus drug held within the intact nanoparticles. For the PCL nanoparticles, the contribution from the first component is minimal while for the PbAE nanoparticles, it’s the maximal. This means, though the PCL nanoparticles show higher intracellular concentration, the free drug that is available for pharmacological action is limited.
CONCLUSIONS
PEO-modified PbAE nanoparticles can be prepared successfully by blending the polymer with Pluronic® F-108. The PPO central block gets anchored onto nanoparticle surface more strongly than mere physical adsorption leaving the hydrophilic PEO chains mobile on surface leading to steric stabilization of the nanocarrier. High concentrations of paclitaxel can be loaded into the nanoparticles which can be tuned to achieve better intracellular delivery of drugs at high concentrations. PbAE nanoparticles, being pH sensitive, deliver the entire payload more rapidly compared to PCL-based system. Besides, the PCL nanoparticles showed a slow intracellular degradation pattern while the PbAE-based system shows rapid dissolution and elimination. PbAE-based nanoparticles can be highly efficient carrier systems for cytotoxic agents to achieve rapid tumoricidal action.
Acknowledgments
This study was supported by the National Cancer Institute’s grant R01-CA095522 from the National Institutes of Health. The authors would like to thank Professor Robert Campbell for the particles size and zeta potential measurements and Professor Richard Deth for the liquid scintillation counter. Additionally, Dr. Lara Gamble help with the ESCA investigations at the NESAC/BIO, University of Washington, Seattle, WA is gratefully acknowledged. NESAC/BIO is supported by the National Institutes of Health grant EB-002027. Ms. Sushma Kommareddy is thanked for the SEM imaging and Ms. Wei Fu is thanked for her assistance in fluorescent microscopy.
References
- 1.Lynn DM, Anderson DG, Putnam D, Langer R. Accelerated discovery of synthetic transfection vectors: parallel synthesis and screening of a degradable polymer library. J Am Chem Soc. 2001;123:8155–8156. doi: 10.1021/ja016288p. [DOI] [PubMed] [Google Scholar]
- 2.Lynn, D. M.; Anderson, D. G.; Akinc, A.; Langer, R. Deagradable poly (β-amino ester)s for gene delivery. Polymeric gene delivery: Principles and applications; CRC Press LLC: Boca Raton, 2005; pp 227–241.
- 3.Akinc A, Anderson DG, Lynn DM, Langer R. Synthesis of poly(beta-amino ester)s optimized for highly effective gene delivery. Bioconjug Chem. 2003;14:979–988. doi: 10.1021/bc034067y. [DOI] [PubMed] [Google Scholar]
- 4.Akinc A, Lynn DM, Anderson DG, Langer R. Parallel synthesis and biophysical characterization of a degradable polymer library for gene delivery. J Am Chem Soc. 2003;125:5316–5323. doi: 10.1021/ja034429c. [DOI] [PubMed] [Google Scholar]
- 5.Akinc A, Langer R. Measuring the pH environment of DNA delivered using nonviral vectors: implications for lysosomal trafficking. Biotechnol Bioeng. 2002;78:503–508. doi: 10.1002/bit.20215. [DOI] [PubMed] [Google Scholar]
- 6.Lynn DM, Amiji MM, Langer R. pH-Responsive Polymer microspheres: rapid release of encapsulated material within the range of intracellular pH. Angew Chem Int Ed Engl. 2001;40:1707–1710. [PubMed] [Google Scholar]
- 7.Berry D, Lynn DM, Sasisekharan R, Langer R. Poly(beta-amino ester)s promote cellular uptake of heparin and cancer cell death. Chem Biol. 2004;11:487–498. doi: 10.1016/j.chembiol.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 8.Little SR, Lynn DM, Ge Q, Anderson DG, Puram SV, et al. Poly-beta amino ester-containing microparticles enhance the activity of nonviral genetic vaccines. Proc Natl Acad Sci U S A. 2004;101:9534–9539. doi: 10.1073/pnas.0403549101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Potineni A, Lynn DM, Langer R, Amiji MM. Poly(ethylene oxide)-modified poly(beta-amino ester) nanoparticles as a pH-sensitive biodegradable system for paclitaxel delivery. J Control Rel. 2003;86:223–234. doi: 10.1016/s0168-3659(02)00374-7. [DOI] [PubMed] [Google Scholar]
- 10.Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003;55:329–347. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 11.Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. J Control Rel. 2001;70:1–20. doi: 10.1016/s0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- 12.Speiser PP. Nanoparticles and liposomes: a state of the art. Methods Find Exp Clin Pharmacol. 1991;13:337–342. [PubMed] [Google Scholar]
- 13.Douglas SJ, Davis SS, Illum L. Nanoparticles in drug delivery. Crit Rev Ther Drug Carrier Syst. 1987;3:233–261. [PubMed] [Google Scholar]
- 14.Gref R, Luck M, Quellec P, Marchand M, Dellacherie E, et al. ‘Stealth’ corona-core nanoparticles surface modified by polyethylene glycol (PEG): influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surf B: Biointerfaces. 2000;18:301–313. doi: 10.1016/s0927-7765(99)00156-3. [DOI] [PubMed] [Google Scholar]
- 15.Labhasetwar V, Song C, Humphrey W, Shebuski R, Levy RJ. Arterial uptake of biodegradable nanoparticles: effect of surface modifications. J Pharm Sci. 1998;87:1229–1234. doi: 10.1021/js980021f. [DOI] [PubMed] [Google Scholar]
- 16.Tan JS, Butterfield DE, Voycheck CL, Caldwell KD, Li JT. Surface modification of nanoparticles by PEO/PPO block copolymers to minimize interactions with blood components and prolong blood circulation in rats. Biomaterials. 1993;14:823–833. doi: 10.1016/0142-9612(93)90004-l. [DOI] [PubMed] [Google Scholar]
- 17.Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol Rev. 2001;53:283–318. [PubMed] [Google Scholar]
- 18.Emerich DF, Thanos CG. Nanotechnology and medicine. Expert Opin Biol Ther. 2003;3:655–663. doi: 10.1517/14712598.3.4.655. [DOI] [PubMed] [Google Scholar]
- 19.Barratt G. Colloidal drug carriers: achievements and perspectives. Cell Mol Life Sci. 2003;60:21–37. doi: 10.1007/s000180300002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Otsuka H, Nagasaki Y, Kataoka K. PEGylated nanoparticles for biological and pharmaceutical applications. Adv Drug Deliv Rev. 2003;55:403–419. doi: 10.1016/s0169-409x(02)00226-0. [DOI] [PubMed] [Google Scholar]
- 21.Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev. 2002;54:631–651. doi: 10.1016/s0169-409x(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 22.Bhadra D, Bhadra S, Jain P, Jain NK. Pegnology: a review of PEG-ylated systems. Pharmazie. 2002;57:5–29. [PubMed] [Google Scholar]
- 23.Moghimi SM, Hunter AC. Poloxamers and poloxamines in nanoparticle engineering and experimental medicine. Trends Biotechnol. 2000;18:412–420. doi: 10.1016/s0167-7799(00)01485-2. [DOI] [PubMed] [Google Scholar]
- 24.Csaba N, Caamano P, Sanchez A, Dominguez F, Alonso MJ. PLGA:poloxamer and PLGA:poloxamine blend nanoparticles: new carriers for gene delivery. Biomacromolecules. 2005;6:271–278. doi: 10.1021/bm049577p. [DOI] [PubMed] [Google Scholar]
- 25.Csaba N, Gonzalez L, Sanchez A, Alonso MJ. Design and characterisation of new nanoparticulate polymer blends for drug delivery. J Biomater Sci Polym Ed. 2004;15:1137–1151. doi: 10.1163/1568562041753098. [DOI] [PubMed] [Google Scholar]
- 26.Shenoy DB, Amiji MM. Poly(ethylene oxide)-modified poly(epsilon-caprolactone) nanoparticles for targeted delivery of tamoxifen in breast cancer. Int J Pharm. 2005;293:261–270. doi: 10.1016/j.ijpharm.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 27.Chawla JS, Amiji MM. Biodegradable poly(epsilon -caprolactone) nanoparticles for tumor-targeted delivery of tamoxifen. Int J Pharm. 2002;249:127–138. doi: 10.1016/s0378-5173(02)00483-0. [DOI] [PubMed] [Google Scholar]
- 28.Amiji M, Park K. Surface modification of polymeric biomaterials with poly(ethylene oxide), albumin, and heparin for reduced thrombogenicity. J Biomater Sci Polym Ed. 1993;4:217–234. doi: 10.1163/156856293x00537. [DOI] [PubMed] [Google Scholar]
- 29.Moghimi SM, Muir IS, Illum L, Davis SS, Kolb-Bachofen V. Coating particles with a block co-polymer (poloxamine-908) suppresses opsonization but permits the activity of dysopsonins in the serum. Biochim Biophys Acta. 1993;1179:157–165. doi: 10.1016/0167-4889(93)90137-e. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Caldwell K, Rapoport N. Surface properties of Pluronic-coated polymeric colloids. Langmuir. 1994;10:4475–4482. [Google Scholar]
- 31.Moghimi SM. Prolonging the circulation time and modifying the body distribution of intravenously injected polystyrene nanospheres by prior intravenous administration of poloxamine-908. A ‘hepatic-blockade’ event or manipulation of nanosphere surface in vivo? Biochim Biophys Acta. 1997;1336:1–6. doi: 10.1016/s0304-4165(97)00060-3. [DOI] [PubMed] [Google Scholar]
- 32.Panyam J, Labhasetwar V. Sustained cytoplasmic delivery of drugs with intracellular receptors using biodegradable nanoparticles. Mol Pharm. 2004;1:77–84. doi: 10.1021/mp034002c. [DOI] [PubMed] [Google Scholar]


