Abstract
Context: Adrenal androgen excess is found in ∼25-60%% of women with polycystic ovary syndrome (PCOS), but the mechanisms underlying PCOS-related adrenal androgen excess are unclear.
Objective: To determine whether adrenal androgen excess is manifest in a nonhuman primate model for PCOS, Participants: Six prenatally androgenized (PA) and 6 control female rhesus monkeys of similar age, body weight and BMI were studied during days 2-6 of two menstrual cycles or anovulatory 30-day periods.
Interventions: Pre-dexamethasone adrenal steroid levels were assessed in the first cycle (cycle 1). In a subsequent cycle (cycle 2), occurring 1-3 cycles following cycle 1, adrenal steroids were determined 14.5-16.0h after an i.m. injection of 0.5mg/kg dexamethasone [post-dexamethasone levels] and following an i.v. injection of 50μg ACTH1-39.
Results: Both before and after dexamethasone, serum levels of dehydroepiandrosterone (DHEA) in PA females exceeded those in controls. Following ACTH injection, PA females exhibited higher circulating levels of DHEA, androstenedione and corticosterone, but comparable levels of 17α-hydroxyprogesterone, cortisol, DHEAS, and testosterone, compared to controls.
Conclusion: Enhanced basal and ACTH-stimulated adrenal androgen levels in PA female monkeys may reflect up-regulation of 17,20 lyase activity in the adrenal zona reticularis, causing adrenal androgen excess comparable to that found in PCOS women with adrenal androgen excess. These findings open the possibility that PCOS adrenal hyperandrogenism may have its origins in fetal androgen excess re-programming of adrenocortical function.
Keywords: prenatally androgenized, DHEA, androstenedione, fetal programming, zona reticularis
Introduction
Polycystic ovary syndrome (PCOS) is a heterogeneous reproductive and metabolic disorder found in 6-7% of reproductive-aged women (1, 2, 3) and represents the most common human female endocrinopathy (4, 5, 6). The PCOS syndrome accounts for 82% of women presenting with hyperandrogenism (7) and 75% of women requiring treatment for anovulatory infertility (6). Characteristics of PCOS include amenorrhea/oligomenorrhea, infertility, hyperinsulinemia from insulin resistance, luteinizing hormone (LH) hypersecretion, and androgen excess (8), with many of these features of PCOS worsened by coexistent obesity (4, 5, 6). The diagnosis of PCOS requires the presence of oligo- or amenorrhea together with hyperandrogenism of ovarian origin (9), or more recently, two out of three criteria that additionally include visualization of polycystic ovaries by ultrasound (10), excluding phenotypically similar, but mechanistically different disorders, such as classical and non-classical 21-hydroxylase deficiency.
Androgen excess is the most consistent defect underlying PCOS (11, 12, 13). While the ovary is the principal source of androgen excess in PCOS women, about 25-60% of women with PCOS also demonstrate elevated levels of adrenal androgens, particularly dehydroepiandrosterone (DHEA), its sulfoconjugate (DHEAS), and androstenedione (14, 15, 16). Adrenocortico-steroidogenesis may thus provide an additional, but separate, contribution to hyperandrogenism in some PCOS women and may be an inherited, stable trait (17). Although many studies have been conducted to examine possible causes of adrenal hyperandrogenism in women with PCOS (18, 19, 20), the underlying mechanisms remain unclear.
As an established nonhuman primate model for PCOS (21, 22, 23), the prenatally androgenized (PA) female rhesus monkey provides an opportunity to examine whether exposure to androgen excess during fetal life provides a developmental origin for adrenal androgen excess in adulthood. Such female rhesus monkeys, exposed to androgen excess in utero, not only exhibit anovulation (21), enlarged multi-follicular ovaries (24), and ovarian hyperandrogenism (25), but also demonstrate elevated circulating levels of DHEAS, a conjugated androgen of adrenal origin (25). Similar to 25-60% of PCOS women (14, 15, 16), androgen biosynthesis may thus be enhanced in the adrenal cortex of PA female monkeys, in addition to the hyperandrogenism demonstrated for the PA monkey ovary. Currently, acute adrenal stimulation by parenteral adrenocorticotropic hormone (ACTH) administration is considered as the preferred method to study adrenocortical enzymatic activities in vivo (26). In this study, we employ a combined dexamethsone-ACTH test, similar to those widely used to assess adrenal steroidogenic function in humans and animal models (27), to identify profound adrenal androgen excess in PA female rhesus monkeys.
Materials and Methods
Animals
The 12 adult female rhesus monkeys (Macaca mulatta) used in this study (February 2000-June 2002) were captive-born and were housed at the National Primate Research Center, University of Wisconsin, Madison (NPRC) in accordance with routine care, management and assessment protocol (28, 29). None of the females had experienced any previous long-term, postnatal treatment (i.e., prolonged steroid therapy).
The health and general behavior of all monkeys were assessed daily and each monkey was fed once daily with a meal of 16-30 biscuits (approximately 96-180g) of Purina Monkey Chow (Ralston Purina, Inc., St. Louis, MO; product # 5038). The meal was supplemented with either 1-2 pieces of fresh fruit or bread. The number of biscuits given was varied so that at least 1-3 biscuits were found when all remaining food in an animal's cage was removed between 1700h-1800h. To provide social enrichment, eight of the 12 animals were re-united with an adult female cage-mate overnight. The remaining 4 female monkeys (all controls) were not socially housed because of incompatibility with available cage partners. The Institutional Animal Care and Use Committee of the University of Wisconsin-Madison approved all the procedures used in this study. Animal maintenance was in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals and Animal Welfare Act with its subsequent amendments.
As described previously, six PA female monkeys were exposed in utero to fetal male serum levels of testosterone, averaging 1-3 ng/ml (30). This fetal androgen excess was achieved when their dams received daily subcutaneous injections of 10 mg of testosterone propionate for 15-35 days, starting at days 40-44 of gestation. Body weights of the sires and dams of prenatally androgenized females were typical for adult rhesus monkeys (data not shown). The six control female monkeys that were unexposed to prenatal androgen excess were of similar reproductive age (control: 19.29±0.98, PA: 21.52±0.79, years; mean±SEM), body weight (control: 9.16±0.65, PA: 9.11±0.46 kg) and body mass index (BMI; control: 38.67±3.08, PA: 39.47±1.35, kg/m2) to PA female monkeys in order to prevent any confounds of BMI influencing adrenal sensitivity to ACTH (31), or of age contributing to circulating levels of cortisol (32, 33), DHEA, DHEAS or androstenedione (34, 35, 36). Since rhesus females reach menarche and menopause at about 2.5 years and 26-28 years of age, respectively (37), all female monkeys in the present study were in their mid- to late reproductive years, approximately 5-7 years before the predicted onset of menopause.
Experimental design
Pre-dexamethasone hormone level assessment during cycle 1
Initial assessment was performed on days 2-6 (all control and 3 PA female monkeys) or day 8 (1 PA female monkey with a follicular phase >20 days) of the menstrual cycle or on day 30 of an anovulatory cycle (2 PA female monkeys). A single saphenous vein blood sample was drawn to determine basal circulating adrenal steroid levels. The sample was taken at 0700h-0900h following a 14.5-16.0 h overnight fast. Blood was subsequently centrifuged at ∼2500 RPM for 10 min, and serum stored at −20 °C prior to assay.
Post-dexamethasone hormone level assessment and combined Dex-ACTH testing during cycle 2
Following the initial assessment, a combined dexamethasone-ACTH (DexACTH) test was performed during the follicular phase (days 2-6) following 1-3 menstrual cycles, or during a period of amenorrhea greater than 30 days in duration (2 PA female monkeys). Dexamethasone (0.5mg/kg body weight; American Regent Laboratories, Inc. Shirley, NY) was given as an intramuscular injection at 1600h - 1700h on the day before the ACTH infusion. At 0730h to 0800h the next day, all monkeys were anesthetized with an intramuscular injection of ketamine (ketamine HCl; 15 mg/kg body weight). A venous catheter (Polyethylene tubing, Intramedic TM, PE60, Becton Dickinson and Company, Sparks, MD) was inserted through the saphenous vein and its tip was positioned in the inferior vena cava for the entire procedure. ACTH (50μg (∼5.5μg/kg), human ACTH1-39, Organon, NJ) was infused as a bolus through the catheter at 0 min, with blood samples (4ml) withdrawn immediately before (i.e., post-dexamethasone levels), and at 15, 30, and 60 min after ACTH infusion. The ACTH dose administered to our female monkeys was greater than that reported in other nonhuman primate studies (i.e., 10ng/kg; 38), but was within a range of ACTH doses previously administered to adult humans (i.e., 0.1-16.2 μg/kg; 18, 39). Such supraphysiological amounts of infused ACTH are required to provide evidence of adrenal P450c17 enzyme dysregulation (18, 40).
Assay procedures
All hormones were assayed in NPRC Assay Services laboratories, as previously described (41, 42, 43). Assays for DHEA, androstenedione, testosterone, and corticosterone were performed following diethyl ether extraction of serum and solvent fraction separation by celite chromatography. 17α-hydroxyprogesterone (44), cortisol (44), DHEA (45), DHEAS (44), androstenedione (42) and corticosterone (46) were determined using RIAs. Testosterone (41) was assayed by EIA. Intra- and inter-assay CVs for quality control preparation (QC) values were: DHEA, QC1 – 13.4±0.8 ng/ml, 7.7% and 9.7%, respectively, QC2 – 3.4±0.2 ng/ml, 6.5% and 9.9%, respectively; androstenedione, QC1 – 98.7±4.0 pg/ml, 4.6% and 7.0%, respectively, QC2 – 14.4±1.7 pg/ml, 13.75% and 20.9%, respectively; testosterone, QC1 - 106.0±5.8 ng/ml, 1.3% and 13.5%, respectively, QC2 – 26.6±1.5 ng/ml, 2.6% and 13.6%, respectively; corticosterone, QC – 458.7±50.5 ng/ml, 3.4% and 19.1%, respectively; 17α-hydroxyprogesterone, QC1 - 3.5±0.2 ng/ml, 3.7% and 14%, respectively, QC2 – 0.6±0.1 ng/ml, 5.3% and 17.4%, respectively; cortisol: QC – 23.0±1.9 μg/dl, 7.0% and 7.3%, respectively; and DHEAS, QC – 27.3±0.8 ng/ml, 1.1% and 7.5%, respectively.
Statistical analysis
Circulating concentrations, area-under-the-curve (AUC) values, and hormone ratios were log transformed to achieve normality, homogeneity of variance, and to increase linearity (47), except for those related to Dex-ACTH test corticosterone values. Differences in circulating steroid values between 0 and 60 min following an i.v. injection of ACTH were not transformed because of negative values. Pre- and post-dexamethasone hormonal variables were compared by paired t-test, while all Dex-ACTH test hormonal variables were analyzed using two-way ANOVA, with fetal androgen exposure and time from ACTH as independent variables. When significant (P<0.05) statistical interactions were identified by ANOVA, posthoc univariate analyses were performed on the variables (Systat, Version 5.2, 1992; Macintosh, Evanston, IL). Dex-ACTH test serum corticosterone levels were analyzed using non-parametric statistical comparisons, since log transformation failed to normalize the data distribution. Log transformed data, nonlog transformed data, and Dex-ACTH test serum corticosterone levels, are expressed as back transformed means±95% confidence limits, mean±SEM, and median±interquartile interval, respectively.
Results
Pre- and post-dexamethasone circulating steroid levels
Serum DHEA levels were higher in PA than control female monkeys before (p<0.03) and after dexamethasone treatment (p<0.001), while serum 17α-hydroxyprogesterone, androstenedione, testosterone, cortisol, corticosterone and DHEAS levels were comparable between female monkey groups under similar conditions (p>0.05, Table 1). Serum levels of 17α-hydroxyprogesterone, cortisol, DHEA and testosterone were suppressed by dexamethasone therapy in both control and PA female monkeys (17α-hydroxyprogesterone, control: p<0.001, PA: p<0.002; cortisol, control: p<0.001, PA: p<0.01; DHEA, control: p<0.001, PA: p<0.006; and testosterone, control: p<0.006, PA: p<0.004). In contrast, serum corticosterone levels were significantly suppressed by dexamethasone therapy in control (p<0.007), but not in PA female monkeys. The ratio of corticosterone:cortisol following dexamethasone therapy (∼0.01; data not shown), however, was similar in both control and PA female monkeys. Serum androstenedione and DHEAS levels did not significantly decline following dexamethasone therapy in either female monkey group possibly due to sufficient ovarian contribution to the former and longer half-life of the latter.
Table 1.
Mean [95% confidence interval] circulating levels of steroid hormones and DHEAS in PA and control female rhesus monkeys before and after i.m. injection of 0.5 mg/kg dexamethasone.
| Control female monkeys | PA female monkeys | |||||
|---|---|---|---|---|---|---|
| Steroid | Before dexamethasone | After dexamethasone | P value (≤) | Before dexamethasone | After dexamethasone | P value (≤) |
| 17OHP4 | 0.57 [0.26, 1.24] | 0.07 [0.03, 0.18] | 0.001 | 0.31 [0.14, 0.68] | 0.05 [0.02, 0.14] | 0.002 |
| Cortisol | 30.41 [26.39, 35.04] | 5.21 [4, 6.78] | 0.001 | 29.17 [19.56, 43.51] | 7.31 [4.94, 10.82] | 0.01 |
| DHEA | 3.27 [1.96, 5.44] | 0.29 [0.17, 0.51] | 0.001 | 8.26 [4.96, 13.76]a | 3.07 [1.79, 5.27]b | 0.006 |
| DHEAS | 4.82 [2.79, 8.3] | 6.5 [3.84, 11.01] | NS | 4.31[2.93, 6.36] | 3.98 [2.23, 7.1] | NS |
| A | 177 [132, 239] | 107 [70, 163] | NS | 257 [190, 345] | 131 [86, 200] | NS |
| Corticosterone | 2.01 [1.24, 3.26] | 0.68 [0.54, 0.85] | 0.007 | 2.56 [1.33, 4.93] | 1.08 [0.59, 1.99] | NS |
| Testosterone | 0.21 [0.14, 0.29] | 0.08 [0.05, 0.14] | 0.006 | 0.30 [0.21, 0.43] | 0.07 [0.04, 0.12] | 0.004 |
p≤0.03, versus control females before dexamethasone;
p<0.001 versus control females after dexamethasone. Units used: 17OHP4 (17α- hydroxyprogesterone, ng/ml), cortisol (mg/dl), DHEA (ng/ml), DHEAS (μg/dl), A (Androstenedione pg/ml), Corticosterone (ng/ml), Testosterone (ng/ml). Conversion to SI units, 17α- hydroxyprogesterone*3.0257 nmol/L, Cortisol*27.59 nmol/L, DHEA*3.47 nmol/L, DHEAS*0.02714 μmol/L, Androstenedione*3.49 pmol/L, Corticosterone*2.886 nmol/L, Testosterone*3.47 nmol/L.
Circulating steroid responses to ACTH infusion after dexamethasone therapy
Adrenocortical steroidogenic responses to ACTH stimulation reflect ACTH-induced adrenal steroid biosynthesis in nonhuman primates and are illustrated in Figures 1 and 2. Serum DHEA levels were greater (p<0.001) in PA compared to control female monkeys throughout the Dex-ACTH test. Serum DHEA levels reached their post-ACTH maximum by 15 min (p<0.001) in control female monkeys, but continued to increase at least up to 60 min (p<0.05) in PA female monkeys.
Figure 1.
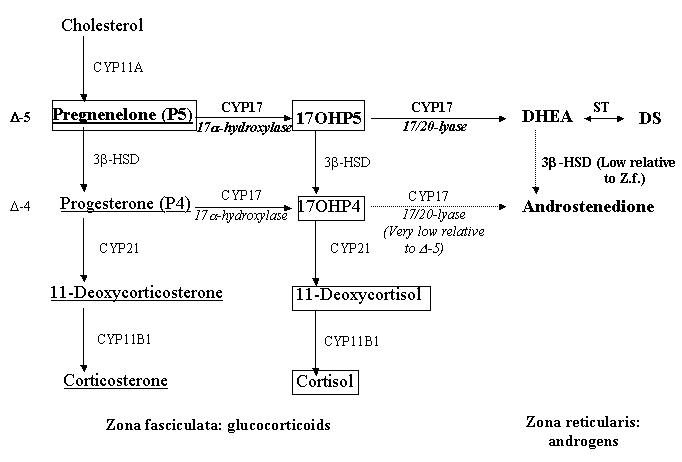
Steroid biosynthesis in the mid- to inner adrenal cortical zones, zona fasciculata (Z.f.) and zona reticularis, of Old World primates and humans (modified from Conley et al, 2004; Pattison et al, 2005). Steroids in bold represent the predominant pathway for androgen biosynthesis, steroids within boxes represent the predominant pathway for cortisol biosynthesis, and underlined steroids represent the predominant pathway for corticosterone biosynthesis. The larger arrows reflect the proposed enhanced 3β-HSD II and 17,20 lyase enzymatic function in PA female monkeys, while the lighter arrows reflect relative low enzymatic activity in both control and PA females. 17OHP5: 17α-hydroxypregnenelone; 17OHP4: 17α-hydroxyprogesterone; CYP11A: P450scc, CYP 17: P450c17, ST: sulfotransferase; 3β-HSD II: 3β-hydroxysteroid dehydrogenase II, CYP21: P450c21, CY11B1: P450c11.
While both control and PA female monkeys demonstrated increased serum androstenedione levels following ACTH stimulation (p<0.001), androstenedione levels in PA female monkeys were higher than those in control females at 15 min, 60 min (p<0.03) and at 30 min (p<0.001) post-ACTH injection. Serum androstenedione levels reached their maximum after 15 min (p<0.05) in controls, but increased until 30 min (p<0.05) in PA female monkeys. Serum DHEAS and testosterone levels were all similarly increased after ACTH injection in both control and PA female monkeys, with all values progressively increasing until 60 min (p values<0.05-0.001) following ACTH injection (Figure 2).
Figure 2.
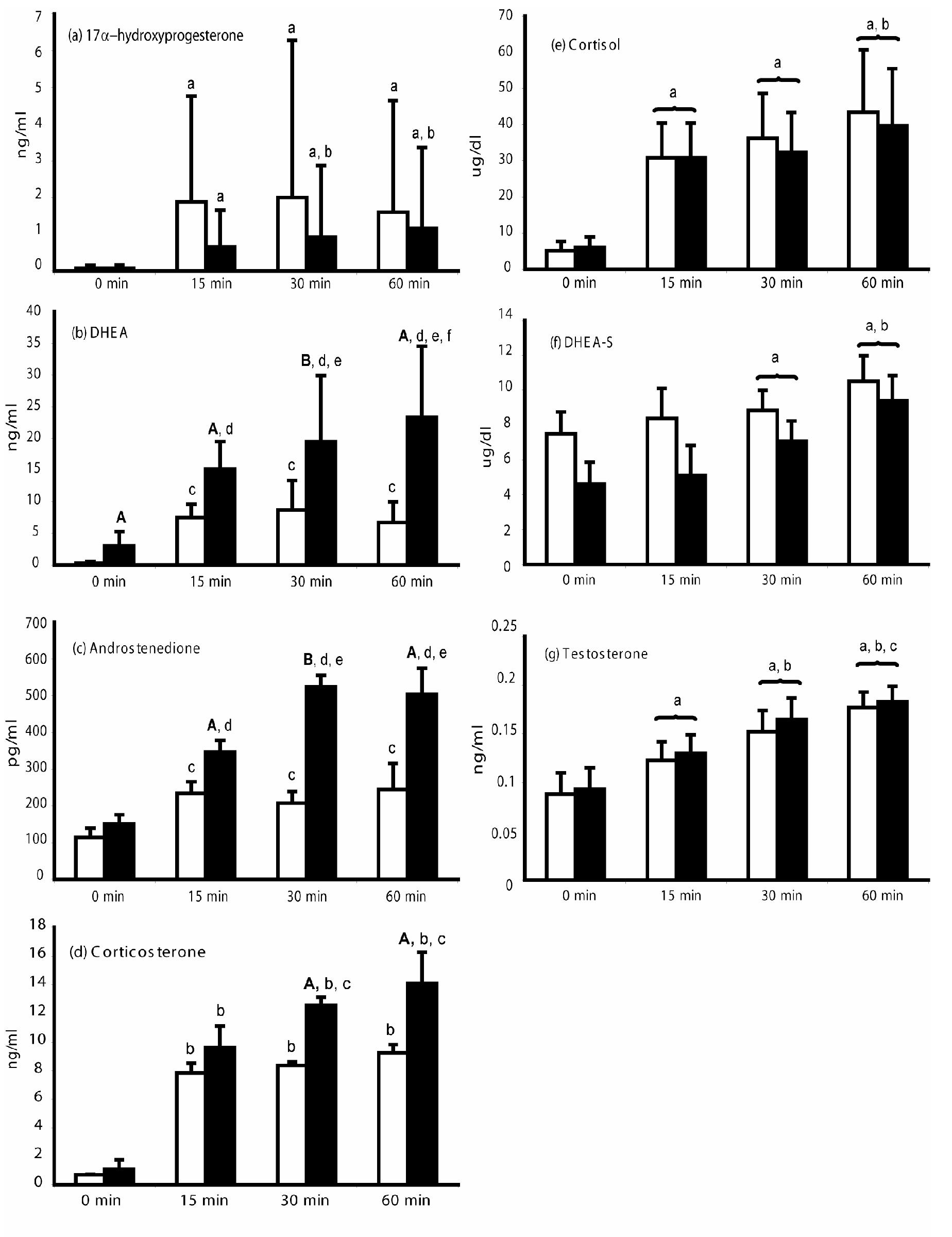
Serum steroid levels in PA (solid bars) and control (open bars) adult female rhesus monkeys following 0.5mg/kg dexamethasone i.m. at –16 to -14.5h and 50mg ACTH i.v. at 0 min (means and upper 95% confidence limits [median and upper quartile rnage for corticosterone values]). 17α-hydroxyprogesterone: a: p<0.001 vs 0 min, b: p<0.02 vs 15 min. Cortisol: a: p<0.001 vs 0 min, b: p<0.05 vs 15 or 30 min. DHEA: A: p<0.01 vs control females at respective time points; B: p<0.05 vs control females at 30 min; c: p<⇐0.001 vs 0 min; d: p<0.005 vs 0 min, e: p<0.005 vs 15 min, f: p<0.05 vs 30 min. DHEAS: a:p<0.01 vs 0 min, b: p<0.05 vs 15 or 30 min, all females combined. Androstenedione: A: p<0.03 vs control females at respective time points; B: p<0.001 vs control females at 30 min; c: p<0.05 vs 0 min; d: p<0.01 vs 0 min, e: p<0.05 vs 15 min. Testosterone: a: p<0.001 vs 0 min, b: p<0.03 vs 15 min, c: p<0.04 vs 30 min, all females combined. Corticosterone: A: p<0.05 vs control females at respective time points, b: p<0.05 vs 0 min, c: p<0.05 vs 15 min. Conversion to SI units, 17α-hydroxyprogesterone*3.0257 nmol/L, Cortisol*27.59 nmol/L, DHEA*3.47 nmol/L, DHEAS*0.02714 μmol/L, Androstenedione*3.49 pmol/L, Corticosterone*2.886 nmol/L, Testosterone*3.47 nmol/L.
Serum 17α-hydroxyprogesterone levels increased after ACTH injection in both female monkey groups (control and PA: p<0.001), with maximum elevations achieved by controls after 15 min (p<0.001), versus 30 min (p<0.05) for PA female monkeys. Serum cortisol levels increased after ACTH injection in both control and PA female monkeys, demonstrating an abrupt rise at 15 min that increased again at 60 min (p values <0.05-0.001) following ACTH (Figure 2). Serum corticosterone levels, however, were higher at both 30 min and 60 min (p values <0.05) after ACTH injection in PA compared to control female monkeys. Furthermore, serum corticosterone levels reached their maximum levels after 15 min (p<0.05) in control female monkeys, compared to 30 min (p<0.05) in PA female monkeys. Thus, in PA female monkeys, ACTH-induced increases in corticosterone, while delayed, were ultimately greater and more prolonged than those of control female monkeys.
By 60 min following ACTH injection, PA female monkeys demonstrated a greater increase from baseline in circulating levels of DHEA, androstenedione and corticosterone compared to controls (DHEA: p<0.005, androstenedione: p<0.05, corticosterone: p<0.01), but exhibited similar increases to controls for cortisol, 17α-hydroxyprogesterone, DHEAS and testosterone (Table 2).
Table 2.
Differences in circulating adrenal steroid values between 0 and 60 min following an iv injection of 50μg ACTH in control and PA female rhesus monkeys.
| Steroid | 0-60min control females | 0-60min PA females | p-value (<) |
|---|---|---|---|
| 17α-hydroxyprogesterone (ng/ml) | 3.6±1.0 | 1.2±0.9 | NS |
| Cortisol (μg/dl) | 43.6±8.0 | 33.7±8.0 | NS |
| DHEA (ng/ml) | 7.3±2.1 | 20.7±2.1 | 0.005 |
| DHEAS (μg/dl) | 3.0±1.4 | 4.8±1.4 | NS |
| Androstenedione (pg/ml) | 131±70 | 353±70 | 0.05 |
| Corticosterone (ng/ml) | 7.5±1.0 | 12.4±1.0 | 0.01 |
| Testosterone (pg/ml) | 88±24 | 90±24 | NS |
Conversion to SI units, 17α-hydroxyprogesterone*3.0257 nmol/L, Cortisol*27.59 nmol/L, DHEA*3.47 nmol/L, DHEAS*0.02714 μmol/L, Androstenedione*3.49 pmol/L, Corticosterone*2.886 nmol/L, Testosterone*3.47 nmol/L.
Ratios of adrenal steroid hormone responses during the Dex-ACTH test
After dexamethasone suppression of adrenal steroidogenesis, and following ACTH infusion, the ratio for serum androstenedione/DHEA was lower in PA compared to control female monkeys (p<0.001; Figure 3). After ACTH infusion, the serum androstenedione/DHEA ratio decreased similarly in both female monkey groups (control, p<0.005; PA, p<0.05). The serum DHEAS/DHEA ratio, in contrast, was lower in PA versus control female monkeys after dexamethasone suppression (p<0.01), and then decreased following ACTH injection in both control (p<0.005) and PA (p<0.05) female monkeys, while still remaining lower (p<0.01) in PA female monkeys throughout.
Figure 3.
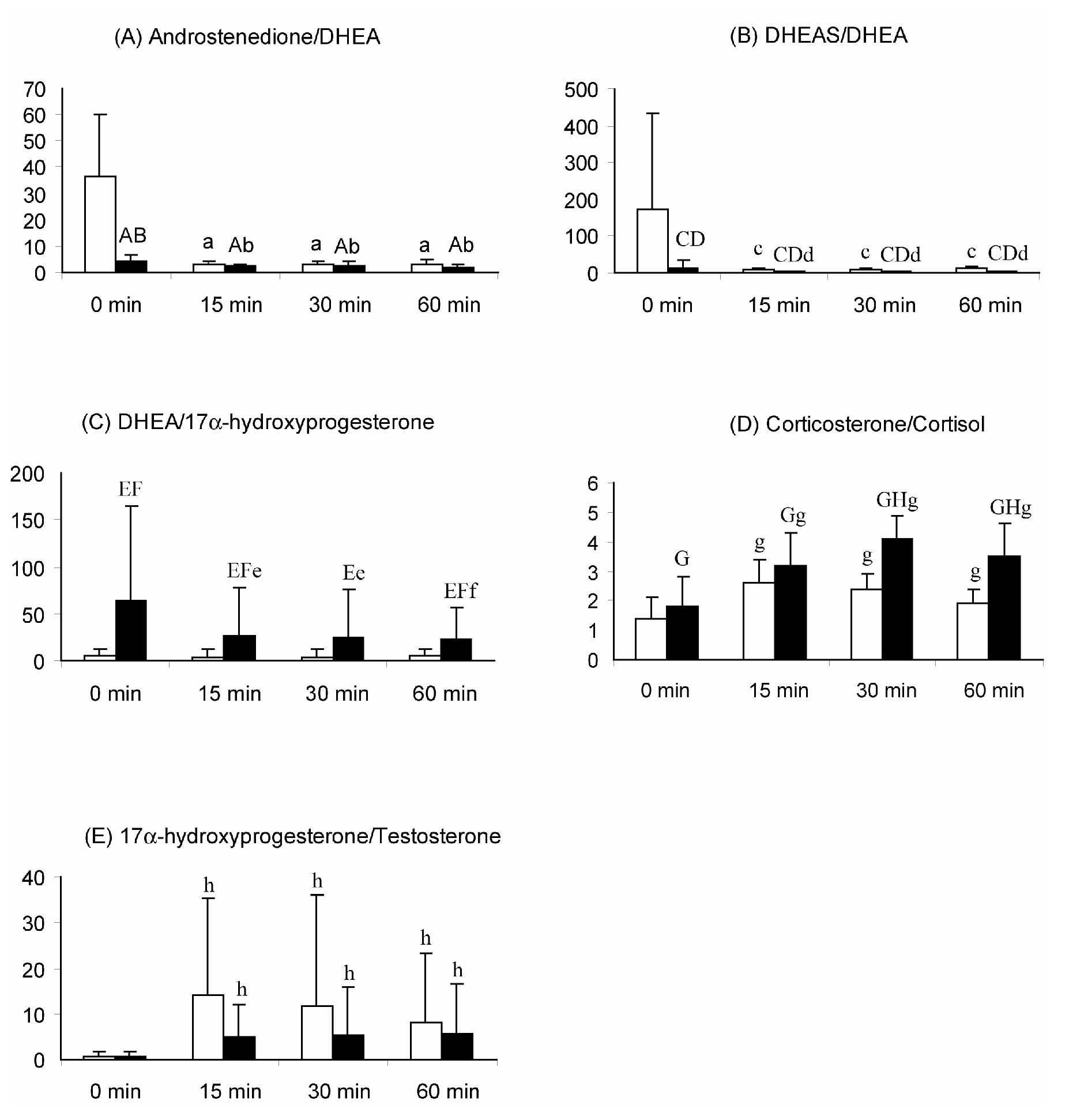
Mean (+ 95% confidence interval) ratios of serum adrenal steroid hormones following an iv injection of 50μg ACTH in control and PA female rhesus monkeys. (A): Androstenedione/DHEA (*10-2) A: p<0.012 vs control female, all time points combined; B: p<0.001 vs control female at 0 min; a: p<0.005 vs 0 min, b: p< 0.05 vs 0 min; (B): DHEAS/DHEA C: p<0.001 vs control females, all time combined; D: p< 0.01 vs control females at respective time points; c: p<0.005 vs 0 min, d: p< 0.05 vs 0 min; (C) DHEA/17α-hydroxyprogesterone; E: p<0.02 vs control females, all time points combined; F: p<0.05 vs control females at respective time points; e: p<0.03 vs 0 min; f: p<0.009 vs 0 min; (D) Corticosterone/Cortisol (*10-2) G: p<0.04 vs control females, all time points combined; H: p<0.01 vs control females at respective time points; g: p<0.005 vs 0 min; (E) 17α-hydroxyprogesterone/Testosterone h: p<0.001 vs 0 min control and PA females combined.
While serum DHEA and 17α-hydroxyprogesterone levels were elevated in both female monkey groups following ACTH stimulation, the serum DHEA/17α-hydroxyprogesterone ratio decreased in PA, but failed to change in control, female monkeys and remained higher throughout (p<0.02) in PA compared to control female monkeys (Figure 3). Serum 17α-hydroxyprogesterone/testosterone ratios, on the other hand, increased following ACTH stimulation (p<0.001) in both control and PA female monkeys, and did not differ between the two female monkey groups. Taken together, the ratios of DHEA to other steroid hormones following ACTH injection indicate relatively greater DHEA responses to ACTH in PA female monkeys than exhibited by control females.
While changes in circulating levels of corticosterone paralleled those of cortisol in controls, showing approximately an 8-fold increase in response to ACTH stimulation at 60 min, the serum corticosterone response to ACTH in PA female monkeys (∼14-fold increase) was clearly greater. Serum corticosterone/cortisol ratios were similar in PA compared to control female monkeys after dexamethasone suppression but, following ACTH stimulation, they showed a greater increase in PA female monkeys by 30 and 60 min (PA versus control female monkeys: 128% versus 71%, and 94% versus 35%, respectively; p<0.01; Figure 3).
Net increase area under curve (AUC) of adrenal steroid changes after ACTH stimulation
The net increase of AUC steroid responses to ACTH stimulation are illustrated in Table 3. The increase of AUC responses of serum corticosterone (p<0.002), DHEA (p<0.007) and androstenedione to ACTH infusion (p<0.005) were higher in PA compared to control female monkeys. The increase of AUC responses of serum 17α-hydroxyprogesterone, cortisol, DHEAS and testosterone to ACTH infusion, however, were similar in the two female monkey groups.
Table 3.
Net increase of mean ±SEM areas under the curve for serum steroid levels following an iv injection of 50μg ACTH in control and PA female rhesus monkeys.
| Steroid | Control females | PA females | P-value |
|---|---|---|---|
| 17α-hydroxyprogesterone (ng/ml*min) | 254±123 | 49±12 | NS |
| Cortisol (*103 μg/ml*min) | 1.5±0.2 | 1.8±0.4 | NS |
| DHEA ( ng/ml*min) | 378±58 | 845±129 | 0.007 |
| DHEAS (μg/ml*min) | 89±27 | 135±44 | NS |
| Androstenedione (*103 pg/ml*min) | 5.9±1.7 | 16.6±2.5 | 0.005 |
| Corticosterone ( ng/ml*min) | 396±35 | 570±27 | 0.002 |
| Testosterone (ng/ml*min) | 3.3±0.4 | 3.5±1.5 | NS |
Conversion to SI units, 17α-hydroxyprogesterone*3.0257 nmol/L, Cortisol*27.59 nmol/L, DHEA*3.47 nmol/L, DHEAS*0.02714 μmol/L, Androstenedione*3.49 pmol/L, Corticosterone*2.886 nmol/L, Testosterone*3.47 nmol/L.
Discussion
The primate adrenal cortex is unique in possessing a morphologically and functionally distinct inner zone, the zona reticularis (48). The adult zona reticularis either originates or is remodeled from another unique primate attribute, the adrenal fetal zone (49). Both the primate fetal zone and zona reticularis strongly express the androgenic biosynthetic enzyme, P450c17 (17α-hydroxylase/17,20 lyase), its catalytic accessory protein, cytochrome b5, and the relevant sulfo-conjugating enzyme, while having greatly reduced expression of 3β-hydroxysteroid dehydrogenase II (3β-HSD II) compared to the outer cortical zones, the zona fasciculata and zona glomerulosa (48, 49, 50 Figure 1). It is, therefore, not surprising that both the fetal zone and the zona reticularis are normally responsible for the relatively high circulating levels of DHEA and DHEAS typical of primates (48, 50).
As typical primates, rhesus monkeys and baboons undergo adrenarche (50), the phenomenon of increased adrenal DHEA and DHEAS secretion, similar to that manifest in humans and Great Apes (51). The developmental timing of this adrenal androgenization differs, however, with rhesus monkeys and baboons having a neonatal (50) rather than a pre-pubertal to adolescent progressive (50, 52) differentiation of the zona reticularis found in humans. Also, senescence of the zona reticularis is seen after the third month of life in rhesus monkeys and baboons, in comparison to the third decade of life in humans and, thereafter, circulating DHEAS levels progressively decline in both sexes (50). Additionally, similar to humans, 17α-hydroxyprogesterone is an inefficient substrate for 17,20 lyase in nonhuman primates (50), with negligible conversion of 17α-hydroxyprogesterone to androstenedione (53; Figure 1). Thus, rhesus monkeys provide a close approximation to human adrenal androgen physiology and in the present study provide a viable opportunity to investigate the fetal origins of pathological adrenal androgen excess in PCOS women.
Our findings demonstrate that female rhesus monkeys, exposed to experimentally-induced androgen excess during early gestation, manifest endogenous adrenal androgen excess in adulthood. In this regard, they closely resemble approximately 25-60% of PCOS women with adrenal androgen excess (7, 20). Although many studies have examined adrenal hyperandrogenism in PCOS women (11, 18, 19, 20, 26), the mechanisms underlying PCOS-related adrenal androgen excess are still unclear. Azziz and colleagues (18) demonstrated in PCOS patients with adrenal androgen excess, specific androstenedione and DHEA hyperandrogenic responses of the zona reticularis to ACTH, which appeared to be unaccompanied by other abnormalities of the adrenal (i.e., zona glomerulosa or zona fasciculata) or the hypothalamic-pituitary axis. PCOS women with adrenal androgen excess vary in their presentation of adrenocortical hyperandrogenism (54), but are most commonly identified from increased secretion of DHEA in response to ACTH (11, 20, 40) and increased basal levels of DHEAS (18, 55). In addition, PCOS women with adrenal androgen excess demonstrate enhanced ACTH-stimulated androstenedione levels (18, 20, 40). Female PA monkeys closely emulate these clinical findings since basal serum DHEA levels were increased while ACTH stimulation caused exaggerated DHEA and androstenedione elevations.
Basal serum DHEAS levels, however, were not elevated in PA female monkeys in comparison to controls, although they were increased in PA monkeys when studied 3-4 years earlier (25). These findings suggest that the absence of elevated serum DHEAS levels in the present PA female monkeys may represent an age-related decline in DHEAS levels. In this regard, if PA female rhesus monkeys are a nonhuman equivalent of PCOS women with adrenal androgen excess, then loss of elevated basal DHEAS levels with age may closely parallel that found in PCOS women over a similar time interval of 3-5 years (17). Pre-dexamethasone serum DHEAS levels in the present PA and control female rhesus monkeys are typical for their age (50), those in PA females are ∼13% less than those reported earlier (25), while control female monkeys demonstrated no such decline. Pre-dexamethasone serum 17α-hydroxyprogesterone, cortisol and testosterone levels, however, are similar to those reported previously (25). No other age comparisons are possible since additional steroid hormone measurements were not performed in the previous PA female monkey study (25).
The most likely cause of the excessive adrenal androgen secretion appears to be abnormal regulation of the 17α-hydroxylase and 17,20-lyase activities of P450c17, the rate-limiting step in androgen biosynthesis (Figure 1). Conventionally considered localized to the primate zona reticularis, promotion of 17,20 lyase activity, without enhancement of 17α-hydroxylase, requires phosphorylation of the serine residues of P450c17 (16) and the presence of cytochrome b5, which allosterically enables the interaction of P450c17 with P450 oxidoreductase, an obligate electron donor (50, 56). Adrenal androgen excess in the PA monkeys of this study, and in PCOS women, appears consistent with enhanced 17,20 lyase activity in the zona fasciculata (increased DHEA and androstenedione), in addition to 17,20 lyase activity in the zona reticularis, without enhanced 17α-hydroxylase activity (suggested by normal 17α-hydroxyprogesterone and cortisol). Increased serine phosphorylation of P450c17, increased cytochrome b5 activity, or the combination of the two, might well provide the cellular and molecular basis for the DHEA excess observed in both PA female monkeys and PCOS women with adrenal androgen excess.
To account for all three excessive steroidogenic responses (corticosterone, DHEA and androstenedione) following ACTH stimulation in PA monkeys, however, we need to propose additional enzymatic changes in adrenal steroid biosynthesis and metabolism beyond enhanced activity of 17,20-lyase in the zonae fasciculata and reticularis. Corticosterone, produced primarily in the primate zona fasciculata (50, 57; Figure 1), is elevated following ACTH stimulation in PA compared to control female monkeys, and its ratio to cortisol is also elevated in PA female monkeys. Increased 3β-HSD II activity may be present in these female monkeys, since (1) pregnenolone is the preferred substrate for cortisol biosynthesis in primates (Figure 1; 50, 53), (2) progesterone is the key precursor to corticosterone biosynthesis (Figure 1), and (3) there is a 3-4% decrease in the efficiency of cortisol biosynthesis in the zona fasciculata (derived from the increased ratio of corticosterone:cortisol at 60min following ACTH injection; 58) of PA female monkeys. Such specific enhancement of 3β-HSD II enzymatic activity in PA female monkeys would direct a small, but significant, increase in substrate metabolism towards progesterone instead of 17α-hydroxypregnenelone (Figure 1), thus providing increased substrate for corticosterone biosynthesis and other zona fasciculata products that would not normally be present. Our Dex-ACTH test most likely demonstrated such a shift in steroidogenesis via increased 3β-HSD II activity because the preferred P450c17 pathway was probably saturated following the high dose of ACTH employed. The ∼14-fold increase in corticosterone in PA female monkeys following ACTH injection compared to only an ∼8-fold increase in cortisol further supports the notion of increased 3β-HSD II activity in the zona fasciculata of these animals.
In conclusion, prenatal exposure of female rhesus monkeys to androgen excess induces irreversible physiological changes in adrenal cortex function, namely hypersecretion of adrenal DHEA, androstenedione and corticosterone. The hyperandrogenic findings closely resemble those observed in 25-60% of PCOS women who have adrenal hyperandrogenism (14, 15, 16). Fetal androgen excess may result in the elevation of circulating concentrations of adrenal androgens through a variety of defects in steroidogenic enzyme function or regulation. Since experimentally-induced fetal programming of female monkeys can so closely mimic adrenal androgen excess found in 25-60% of women with PCOS, our findings suggest that differentiation of the fetal adrenal cortex in a hyperandrogenic environment may permanently upregulate its androgenic function, probably in the zona reticularis and zona fasciculata. Such reprogramming is then retained in the development of mature, postnatal cortex zonation. Whether this adult outcome of fetal programming additionally involves altered hypothalamic-pituitary regulation of adrenal function remains to be determined.
Acknowledgements
We thank EJ Peterson, JM Turk, K Hable, S DeBruin, and RD Medley for technical assistance; F Wegner, D Wittwer, S Jacoris and Assay Services of the National Primate Research Center, University of Wisconsin-Madison (WPRC) for hormone assay expertise; D Florence DVM, I Bolton DVM, K Brunner DVM and D Welner-Kern for veterinary care; D Wade and S Maves for animal care; and JC Pattison for comments on the manuscript. This work was supported by NIH grants R01 RR013635 (to DHA) and P51 RR000167 (to WPRC). This research was conducted at a facility constructed with support from Research Facilities Improvement Program grant numbers RR15459-01 and RR020141-01.
References
- 1.Diamanti-Kandarakis E, Kouli CR, Bergiele AT, Filandra FA, Tsianateli TC, Spina GG, Zapanti ED, Bartzis MI. A survey of the polycystic ovary syndrome in the Greek island of Lesbos: hormonal and metabolic profile. J Clin Endocrinol Metab. 1999;84:4006–4011. doi: 10.1210/jcem.84.11.6148. [DOI] [PubMed] [Google Scholar]
- 2.Asuncion M, Calvo RM, San Millan JL, Sancho J, Avila S, Escobar-Morreale HF. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab. 2000;85:2434–2438. doi: 10.1210/jcem.85.7.6682. [DOI] [PubMed] [Google Scholar]
- 3.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 4.Ehrmann DA, Barnes RB, Rosenfield RL. Polycystic ovary syndrome as a form of functional ovarian hyperandrogenism due to dysregulation of androgen secretion. Endocr Rev. 1995;16:322–353. doi: 10.1210/edrv-16-3-322. [DOI] [PubMed] [Google Scholar]
- 5.Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- 6.Franks S. Polycystic ovary syndrome. N Engl J Med. 1995;333:853–861. doi: 10.1056/NEJM199509283331307. [DOI] [PubMed] [Google Scholar]
- 7.Azziz R, Sanchez LA, Knochenhauer ES, Moran C, Lazenby J, Stephens KC, Taylor K, Boots LR. Androgen excess in women: experience with over 1000 consecutive patients. J Clin Endocrinol Metab. 2004;89:453–462. doi: 10.1210/jc.2003-031122. [DOI] [PubMed] [Google Scholar]
- 8.Legro RS, Strauss JF. Molecular progress in infertility: polycystic ovary syndrome. Fertil Steril. 2002;78:569–576. doi: 10.1016/s0015-0282(02)03275-2. [DOI] [PubMed] [Google Scholar]
- 9.Zawadzki JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. Polycystic Ovary Syndrome. In: Dunaif A, Givens JR, Haseltine FP, Merriam GR, editors. Blackwell Scientific Publications; Boston, MA: 1992. pp. 377–384. [Google Scholar]
- 10.The Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group 2004 Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 19:41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 11.Rosenfield RL. Ovarian and adrenal function in polycystic ovary syndrome. Endocrinol Metab Clin North Am. 1999;28:265–293. doi: 10.1016/s0889-8529(05)70070-0. [DOI] [PubMed] [Google Scholar]
- 12.Legro RS, Driscoll D, Strauss JF, 3rd, Fox J, Dunaif A. Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proc Natl Acad Sci U S A. 1998;95:14956–14960. doi: 10.1073/pnas.95.25.14956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson-Degrave VL, Nelson-Degrave VL, Wickenheisser JK, Hendricks KL, Asano T, Fujishiro M, Legro RS, Kimball SR, Strauss JF, 3rd, McAllister JM. Alterations in mitogen-activated protein kinase kinase and extracellular regulated kinase signaling in theca cells contribute to excessive androgen production in polycystic ovary syndrome. Mol Endocrinol. 2005;19:379–390. doi: 10.1210/me.2004-0178. [DOI] [PubMed] [Google Scholar]
- 14.Wild RA, Umstot ES, Andersen RN, Ranney GB, Givens JR. Androgen parameters and their correlation with body weight in one hundred thirty-eight women thought to have hyperandrogenism. Am J Obstet Gynecol. 1983;146:602–606. doi: 10.1016/0002-9378(83)90998-5. [DOI] [PubMed] [Google Scholar]
- 15.Moran C, Knochenhauer E, Boots LR, Azziz R. Adrenal androgen excess in hyperandrogenism: relation to age and body mass. Fertil Steril. 1999;71:671–674. doi: 10.1016/s0015-0282(98)00536-6. [DOI] [PubMed] [Google Scholar]
- 16.Carmina E, Koyama T, Chang L, Stanczyk FZ, Lobo RA. Does ethnicity influence the prevalence of adrenal hyperandrogenism and insulin resistance in polycystic ovary syndrome? Am J Obstet Gynecol. 1992;167:1807–1812. doi: 10.1016/0002-9378(92)91779-a. [DOI] [PubMed] [Google Scholar]
- 17.Yildiz BO, Woods KS, Stanczyk F, Bartolucci A, Azziz R. Stability of adrenocortical steroidogenesis over time in healthy women and women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89:5558–5562. doi: 10.1210/jc.2004-0934. [DOI] [PubMed] [Google Scholar]
- 18.Azziz R, Black V, Hines GA, Fox LM, Boots LR. Adrenal androgen excess in the polycystic ovary syndrome: sensitivity and responsivity of the hypothalamic-pituitary-adrenal axis. J Clin Endocrinol Metab. 1998;83:2317–2323. doi: 10.1210/jcem.83.7.4948. [DOI] [PubMed] [Google Scholar]
- 19.Carmina E. Prevalence of adrenal androgen excess in PCOS. In: Dewailly D, editor. Androgen excess disorder in women. Lippincott-Raven; Philadelphia: 1997. pp. 385–393. [Google Scholar]
- 20.Moran C, Reyna R, Boots LS, Azziz R. Adrenocortical hyperresponsiveness to corticotropin in polycystic ovary syndrome patients with adrenal androgen excess. Fertil Steril. 2004;81:126–131. doi: 10.1016/j.fertnstert.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Abbott DH, Dumesic DA, Eisner JR, Colman RJ, Kemnitz JW. Insights into the development of PCOS from studies of prenatally androgenized female rhesus monkeys. Trends Endocrinol Metab. 1998;9:62–67. doi: 10.1016/s1043-2760(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 22.Abbott DH, Dumesic DA, Franks S. Developmental origin of polycystic ovary syndrome - a hypothesis. J Endocrinol. 2002;174:1–5. doi: 10.1677/joe.0.1740001. [DOI] [PubMed] [Google Scholar]
- 23.Abbott DH, Barnett DK, Bruns CM, Schramm RD, Dumesic DA. Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Human Reproduction Update. 2005;11:357–374. doi: 10.1093/humupd/dmi013. [DOI] [PubMed] [Google Scholar]
- 24.Abbott DH, Eisner JR, Colman RJ, Kemnitz JW, Dumesic DA. Prenatal androgen excess programs for PCOS in female rhesus monkeys. In: Chang RJ, Dunaif A, Hiendel J, editors. Polycystic Ovary Syndrome. Marcel Dekker, Inc.; New York: 2002. pp. 119–133. [Google Scholar]
- 25.Eisner JR, Barnett MA, Dumesic DA, Abbott DH. Ovarian hyperandrogenism in adult female rhesus monkeys exposed to prenatal androgen excess. Fertil Steril. 2002;77:167–172. doi: 10.1016/s0015-0282(01)02947-8. [DOI] [PubMed] [Google Scholar]
- 26.Azziz R, Ehrmann DA, Legro RS, Fereshetian AG, O'Keefe M, Ghazzi MN, PCOS/Troglitazone Study Group Troglitazone decreases adrenal androgen levels in women with polycystic ovary syndrome. Fertil Steril. 2003;79:932–937. doi: 10.1016/s0015-0282(02)04914-2. [DOI] [PubMed] [Google Scholar]
- 27.Moran C, Azziz R. The role of adrenal cortex in polycystic ovary syndrome. Obstet Gynecol Clin North Am. 2001;28:63–75. doi: 10.1016/s0889-8545(05)70185-6. [DOI] [PubMed] [Google Scholar]
- 28.Azziz R, Rafi A, Smith BR, Bradley EL, Zacur HA. On the origin of the elevated 17-hydroxyprogesterone levels after adrenal stimulation in hyperandrogenism. J Clin Endocrinol Metab. 1990;70:431–436. doi: 10.1210/jcem-70-2-431. [DOI] [PubMed] [Google Scholar]
- 29.Goy RW, Robinson JA. Prenatal exposure of rhesus monkeys to patent androgens: morphological, behavioral, and physiological consequences. Banbury Report. 1982;11:355–378. [Google Scholar]
- 30.Goy RW, Kemnitz JW. Early, persistent, and delayed effects of virilizing substances delivered transplacentally to female rhesus fetuses. In: Weiss B, editor. Application of behavioral pharmacology in toxicology. Raven Press; New York: 1983. pp. 303–314. [Google Scholar]
- 31.Resko JA, Buhl AE, Phoenix CH. Treatment of pregnant rhesus macaques with testosterone propionate: observations on its fate in the fetus. Biol Reprod. 1987;37:1185–1191. doi: 10.1095/biolreprod37.5.1185. [DOI] [PubMed] [Google Scholar]
- 32.Komindr S, Kurtz BR, Stevens MD, Karas JG, Bittle JB, Givens JR. Relative sensitivity and responsivity of serum cortisol and two adrenal androgens to alpha-adrenocorticotropin-(1-24) in normal and obese, nonhirsute, eumenorrheic women. J Clin Endocrinol Metab. 1986;63:860–864. doi: 10.1210/jcem-63-4-860. [DOI] [PubMed] [Google Scholar]
- 33.Erwin K, Blumenthal DS, Chapel T, Allwood LV. Building an academic-community partnership for increasing representation of minorities in the health professions. J Health Care Poor Underserved. 2004;15:589–602. doi: 10.1353/hpu.2004.0059. [DOI] [PubMed] [Google Scholar]
- 34.Gust DA, Wilson ME, Stocker T, Conrad S, Plotsky PM, Gordon TP. Activity of the hypothalamic-pituitary-adrenal axis is altered by aging and exposure to social stress in female rhesus monkeys. J Clin Endocrinol Metab. 2000;85:2556–2563. doi: 10.1210/jcem.85.7.6696. [DOI] [PubMed] [Google Scholar]
- 35.Colman RJ, Kemnitz JW, Lane MA, Abbott DH, Binkley N. Skeletal effects of aging and menopausal status in female rhesus macaques. J Clin Endocrinol Metab. 1999;84:4144–4148. doi: 10.1210/jcem.84.11.6151. [DOI] [PubMed] [Google Scholar]
- 36.Ibanez L, Bonnin MR, Zampolli M, Prat N, Alia PJ, Navarro MA. Usefulness of an ACTH test in the diagnosis of nonclassical 21-hydroxylase deficiency among children presenting with premature pubarche. Horm Res. 1995;44:51–56. doi: 10.1159/000184592. [DOI] [PubMed] [Google Scholar]
- 37.Urbanski HF, Downs JL, Garyfallou VT, Mattison JA, Lane MA, Roth GS, Ingram DK. Effect of caloric restriction on the 24-hour plasma DHEAS and cortisol profiles of young and old male rhesus macaques. Ann N Y Acad Sci. 2004;1019:443–447. doi: 10.1196/annals.1297.081. [DOI] [PubMed] [Google Scholar]
- 38.Kemnitz JW, Holston KA, Colman RJ. Nutrition, aging, and reproduction in rhesus monkeys. Pennington Center Nutrition Series. In: Hansel W, Bray GA, Ryan DH, editors. Part IV. Evolution of Research Methods in Nutrition and Reproduction. Louisiana State University Press; Baton Rouge: 1998. pp. 180–195. [Google Scholar]
- 39.Tiefenbacher S, Novak MA, Marinus LM, Chase WK, Miller JA, Meyer JS. Altered hypothalamic-pituitary-adrenocortical function in rhesus monkeys (Macaca mulatta) with self-injurious behavior. Psychoneuroendocrinology. 2004;29:501–515. doi: 10.1016/s0306-4530(03)00068-4. [DOI] [PubMed] [Google Scholar]
- 40.Dokmetas HS, Colak R, Kelestimur F, Selcuklu A, Unluhizarci K, Bayram F. A comparison between the 1-microg adrenocorticotropin (ACTH) test, the short ACTH (250 microg) test, and the insulin tolerance test in the assessment of hypothalamo-pituitary-adrenal axis immediately after pituitary surgery. J Clin Endocrinol Metab. 2000;85:3713–3719. doi: 10.1210/jcem.85.10.6879. [DOI] [PubMed] [Google Scholar]
- 41.Colak R, Kelestimur F, Unluhizarci K, Bayram F, Sahin Y, Tutus A. A comparison between the effects of low dose (1 microg) and standard dose (250 microg) ACTH stimulation tests on adrenal P450c17alpha enzyme activity in women with polycystic ovary syndrome. Eur J Endocrinol. 2002;147:473–477. doi: 10.1530/eje.0.1470473. [DOI] [PubMed] [Google Scholar]
- 42.Dumesic DA, Abbott DH, Eisner JR, Goy RW. Prenatal exposure of female rhesus monkeys to testosterone propionate increases serum luteinizing hormone levels in adulthood. Fertil Steril. 1997;67:155–163. doi: 10.1016/s0015-0282(97)81873-0. [DOI] [PubMed] [Google Scholar]
- 43.Dumesic DA, Schramm RD, Peterson E, Paprocki AM, Zhou R, Abbott DH. Impaired developmental competence of oocytes in adult prenatally androgenized female rhesus monkeys undergoing gonadotropin stimulation for in vitro fertilization. J Clin Endocrinol Metab. 2002;87:1111–1119. doi: 10.1210/jcem.87.3.8287. [DOI] [PubMed] [Google Scholar]
- 44.Saltzman W, Schultz-Darken NJ, Scheffler G, Wegner FH, Abbott DH. Social and reproductive influences on plasma cortisol in female marmoset monkeys. Physiol Behav. 1994;56:801–810. doi: 10.1016/0031-9384(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 45.Eisner JR, Dumesic DA, Kemnitz JW, Colman RJ, Abbott DH. Increased adiposity in female rhesus monkeys exposed to androgen excess during early gestation. Obes Res. 2003;11:279–286. doi: 10.1038/oby.2003.42. [DOI] [PubMed] [Google Scholar]
- 46.Cohen HN, Wallace AM, Beastall GH, Fogelman I, Thomson JA. Clinical value of adrenal androgen measurement in the diagnosis of delayed puberty. Lancet. 1981;1:689–692. doi: 10.1016/s0140-6736(81)91972-3. [DOI] [PubMed] [Google Scholar]
- 47.Trainor BC, Marler CA. Testosterone, paternal bahavior, and aggression in monogamous California mouse (Peromyscus californicus) Horm Behav. 2001;40:32–42. doi: 10.1006/hbeh.2001.1652. [DOI] [PubMed] [Google Scholar]
- 48.Sokal RR, Rohlf FJ. Biometry. 3 W. H. Freeman and Co.; New York: 1995. the principles and practice of statistics in biological research; pp. 413–422. [Google Scholar]
- 49.Mesiano S, Jaffe RB. Developmental and functional biology of the primate fetal adrenal cortex. Endocr Rev. 1997;18:378–403. doi: 10.1210/edrv.18.3.0304. [DOI] [PubMed] [Google Scholar]
- 50.Mapes S, Tarantal AF, Parker CR, Moran FM, Bahr JM, Pyter L, Conley AJ. Adrenocortical cytochrome b5 expression during fetal development of the rhesus macaque. Endocrinology. 2002;143:1451–1458. doi: 10.1210/endo.143.4.8718. [DOI] [PubMed] [Google Scholar]
- 51.Conley AJ, Pattison JC, Bird IM. Variations in adrenal androgen production among (nonhuman) primates. Semin Reprod Med. 2004 Nov;22:311–326. doi: 10.1055/s-2004-861548. [DOI] [PubMed] [Google Scholar]
- 52.Arlt W, Martens JW, Song M, Wang JT, Auchus RJ, Miller WL. Molecular evolution of adrenarche: structural and functional analysis of p450c17 from four primate species. Endocrinology. 2002;143:4665–4672. doi: 10.1210/en.2002-220456. [DOI] [PubMed] [Google Scholar]
- 53.Sklar CA, Kaplan SL, Grumbach MM. Evidence for dissociation between adrenarche and gonadarche: studies in patients with idiopathic precocious puberty, gonadal dysgenesis, isolated gonadotropin deficiency, and constitutionally delayed growth and adolescence. J Clin Endocrinol Metab. 1980;51:548–556. doi: 10.1210/jcem-51-3-548. [DOI] [PubMed] [Google Scholar]
- 54.Conley AJ, Bird IM. The role of cytochrome P450 17 alpha-hydroxylase and 3 beta-hydroxysteroid dehydrogenase in the integration of gonadal and adrenal steroidogenesis via the delta 5 and delta 4 pathways of steroidogenesis in mammals. Biol Reprod. 1997;56:789–799. doi: 10.1095/biolreprod56.4.789. [DOI] [PubMed] [Google Scholar]
- 55.Azziz R, Fox LM, Zacur HA, Parker CR, Boots LR. Adrenocortical sercretion of dehydroepiandrosterone in healthy women: highly variable response to adrenocorticotropin. J Clin Endocrinol Metab. 2001;86:2513–2517. doi: 10.1210/jcem.86.6.7587. [DOI] [PubMed] [Google Scholar]
- 56.Zhang LH, Rodriguez H, Ohno S, Miller WL. Serine phosphorylation of human P450c17 increases 17,20-lyase activity: implications for adrenarche and the polycystic ovary syndrome. Proc Natl Acad Sci U S A. 1995;92:10619–10623. doi: 10.1073/pnas.92.23.10619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev. 2004;25:947–970. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- 58.Pattison JC, Abbott DH, Saltzman W, Nguyen AD, Henderson G, Ju H, Pryce CR, Allen AJ, Conley AJ, Bird IM. Male Marmoset Monkeys Express an Adrenal Fetal Zone at Birth, but Not a Zona Reticularis in Adulthood. Endocrinology. 2005;146:365–374. doi: 10.1210/en.2004-0689. [DOI] [PubMed] [Google Scholar]


