Abstract
Cortical neurotransmitter availability is known to exert domain-specific effects on cognitive performance. Hence, normal variation in genes with a role in neurotransmission may also have specific effects on cognition. We tested this hypothesis by examining associations between polymorphisms in genes affecting cholinergic and noradrenergic neurotransmission and individual differences in visuospatial attention. Healthy individuals were administered a cued visual search task which varied the size of precues to the location of a target letter embedded in a 15-letter array. Cues encompassed 1, 3, 9, or 15 letters. Search speed increased linearly with precue size, indicative of a spatial attentional scaling mechanism. The strength of attentional scaling increased progressively with the number of C alleles (0, 1, or 2) of the alpha-4 nicotinic receptor gene C1545T polymorphism (n = 104). No association was found for the dopamine beta hydroxylase gene G444A polymorphism (n = 135). These findings point to the specificity of genetic neuromodulation. Whereas variation in a gene linked to cholinergic transmission systematically modulated the ability to scale the focus of visuospatial attention, variation in a gene governing dopamine availability did not. The results show that normal variation in a gene controlling a nicotinic receptor makes a selective contribution to individual differences in visuospatial attention.
INTRODUCTION
Several neurotransmitters, including acetylcholine, noradrenaline, and dopamine, have been linked to cognitive functions of the brain in a domain-specific manner (Everitt & Robbins, 1997). Therefore, variation in genes controlling aspects of neurotransmission may contribute to individual differences in cognitive functions innervated by those neurotransmitters (Fan, Wu, Fossella, & Posner, 2001), much as dopamine receptor availability modulates working memory (Sawaguchi & Goldman-Rakic, 1991) and the cholinergic agonist nicotine modulates attentional orienting (Witte, Davidson, & Marrocco, 1997). Recent efforts to identify gene–cognition links have associated cognitive performance with normal genetic variation from single nucleotide polymorphisms as well as nucleotide insertions and deletions (Goldberg et al., 2003; Fossella et al., 2002; Egan et al., 2001; Greenwood, Sunderland, Friz, & Parasuraman, 2000), reviewed in Greenwood and Parasuraman (2003). Effects of polymorphismsoncognition can be larger (Greenwood & Parasuraman, 2003) than those commonly reported for disease associations (Ioannidis, Ntzani, Trikalinos, & Contopoulos-Ioannidis, 2001). Variation in genes controlling neurotransmitter receptors or enzymes can correspondingly change protein coding. If those changes lead to altered receptor sensitivity or enzyme activity, there could be functional consequences for neurotransmission efficiency. This approach assumes that genes controlling receptors or the synthesis and degradation of neurotransmitters have selective effects on cognitive functions mediated by the associated neurotransmission system. The specificity of such effects needs to be assessed. In the present study, we tested the hypothesis that normal variation in two different neurotransmission genes exerts domain-specific effects on cognition. We show that individual differences in visuospatial attention performance are associated with variation in a cholinergic gene but not with variation in a noradrenergic gene.
Effects of genes on cognition is one source of information about the independence of cognitive processes. Orienting visuospatial attention is one of three different attentional functions—orienting, alerting, and executive control—linked to separately innervated but overlapping brain networks in an inf luential theory of attention put forward by Posner and Petersen (1990). To the extent these networks overlap, each function would be subject to the effect of more than one neuromodulator. Overlap has been postulated to exist between orienting and spatial working memory, with orienting claimed to be the rehearsal space of spatial working memory (Cowan, 2001; Awh, Jonides, & Reuter-Lorenz, 1998). Relating normal variation in neurotransmission genes to deployment of visuospatial attention can potentially determine both the role of specific genes in individual differences in specific attentional functions and the selectivity of those genetic influences.
Establishing the functional independence of different cognitive operations has been addressed in a number of different ways, including behavioral measures. With the advent of cognitive neuroscience and neuroimaging, activation of regional brain networks has been used as a distinguishing criterion (i.e., cognitive functions with different activation networks are thought to be independent). In the animal and human pharmacological literature, it is also common to argue independence or association of function based on neurotransmitter mediation or drug effects. No single method can conclusively establish whether two cognitive functions are distinct or associated, but each adds converging evidence to the overall picture. We see genetic data as yet another source of converging evidence that can help in understanding the extent to which two cognitive functions are independent.
That orienting of visuospatial attention is mediated by subcortical cholinergic innervation of the cortex suggests modulation by cholinergic genes. Deployment of visuospatial attention has been shown to depend on a cholinergically mediated network involving the prefrontal and intraparietal cortex (Nobre et al., 1997). Muscarinic cholinergic agents—specifically scopolamine— applied directly to the intraparietal sulcus modulate speed of orienting visuospatial attention (Davidson & Marrocco, 2000). Although attentional scaling has been less studied, recent work from ERP and imaging studies suggests scaling modulates brain activation in both the striate and extrastriate cortex (Muller, Bartelt, Donner, Villringer, & Brandt, 2003). Although most cholinergic receptors are muscarinic, nicotinic cholinergic receptors are important in fast synaptic transmission (Alkondon, Pereira, Eisenberg, & Albuquerque, 2000) and appear to modulate cortical functioning (Xiang, Huguenard, & Prince, 1998). Nicotine has been found to modulate the redirection of attention in rats (Phillips, McAlonan, Robb, & Brown, 2000), monkeys (Witte et al., 1997), and humans (Shirtcliff & Marrocco, 2003). The most widely distributed nicotinic acetylcholine receptor (nAChR) in the CNS is composed of alpha-4 and beta-2 nAChR subunits assembled together (Flores, Rogers, Pabreza, Wolfe, & Kellar, 1992). As each subunit type is controlled by one gene, variation in those genes could be a source of individual differences in attentional performance through effects on receptor efficiency.
Several polymorphisms have been identified in both alpha-4 and beta-2 subunit genes, although coding sequences in neuronal receptor genes are highly conserved and polymorphisms relatively rare. One polymorphism in the alpha-4 nAChR gene (CHRNA4) has been associated with autosomal dominant nocturnal frontal lobe epilepsy (Steinlein, Magnusson, et al., 1997). Another CHRNA4 polymorphism, a common C to T substitution at 1545, has been investigated for association with panic disorder (Steinlein, Deckert, et al., 1997), attention deficit/hyperactivity disorder (Kent et al., 2001), and Alzheimer’s disease (Cook et al., 2004), although linked to none of these. This polymorphism has, however, been linked in a large sample to vulnerability to nicotine addiction, both alone and within a haplotype (Feng et al., 2004).
In previous work, we examined the association between the CHRNA4 C1545T polymorphism and orienting of visuospatial attention (Parasuraman, Greenwood, Kumar, & Fossella, 2005). A spatially cued visuospatial attention task (Posner, 1980) was administered to healthy adults genotyped for this polymorphism. Increasing gene dose (0, 1, 2) of the C allele was associated with greater reaction time (RT) benefits of valid relative to neutral spatial cues and reduced RT costs of invalid relative to neutral cues. At the same time, there was no association between the CHRNA4 polymorphism and individual differences in performance on a working memory task, which was modulated by a polymorphism in a noradrenergic gene. This result suggests that (a) the neuromodulators of visuospatial attention are more cholinergic than noradrenergic and (b) that visuospatial attention and working memory are modulated by different neurotransmission systems.
A number of questions remain. It has been argued that visuospatial attention is the rehearsal process of spatial working memory (Awh et al., 1998). If so, its deployment would likely be modulated by dopamine, which has a well-documented role in working memory (Abi-Dargham et al., 2002; Sawaguchi & Goldman-Rakic, 1991). However, the orienting task used by Parasuraman et al. did not manipulate the number of items held in the attentional focus, and thus, may not activate the specific processes hypothesized to be in common with spatial working memory. Therefore, the observed lack of an effect of the dopamine beta hydroxylase (DBH) gene on attentional orienting in that study could be due either to lack of noradrenergic innervation on visuospatial attention or to the use of a task requiring attention to only one item. Therefore, a task which engages the aspect of attention claimed to be important for spatial working memory—flexible allocation of attention to variable numbers of items—would provide a better test of the hypothesis that individual differences in attention and memory are modulated by different neurotransmission genes.
In addition to being shifted in space, the focus of visuospatial attention can also be scaled in size or extent—the so-called zoom-lens property (Eriksen & St. James, 1986). We have argued that attentional shifting and scaling are (a) deployed independently and (b) guide saccadic eye movements in visual search (Greenwood & Parasuraman, 1999, 2004). Attentional scaling is measured as the benefit for target discrimination when a narrow relative to a broad attentional focus was induced by variation in the size of precues to target location. The size of location precues modulates speed of detection (Castiello & Umilta, 1990), feature and conjunction search (Greenwood & Parasuraman, 1999), and working memory (Greenwood, Lambert, Sunderland, & Parasuraman, 2005). To the extent that working memory demand varies with the number of items in the attentional focus, as claimed by Cowan (2001), the cued visual search task allows a comparison of the effects of cholinergic and dopaminergic receptor genes on that demand.
Given that the CHRNA4 polymorphism in question has been linked to attentional shifting (Parasuraman et al., 2005), and given the evidence for cholinergic involvement in attentional scaling, we hypothesized that the same polymorphism would be linked to individual differences in attentional scaling. Because working memory may be involved when a number of items have to be held in the attentional focus, and given the link between dopamine and working memory (Sawaguchi & Goldman-Rakic, 1991), a gene controlling dopamine availability was assessed as well. This also allowed for a determination of the specificity of any genetic association. The enzyme DβH is involved in the conversion of dopamine to norepinephrine. Plasma and CSF levels of the enzyme are under genetic control by the DBH gene, which is known to have several polymorphisms. One of these polymorphisms, a G-to-A substitution at position 444, exon 2 (G444A) on chromosome 9, affects plasma and CSF DβH enzyme levels, with the A allele associated with lower levels of the enzyme (Cubells, Kranzler, et al., 2000). Although the precise relationship between human DβH levels and brain dopamine and norepinephrine levels is not known, dopaminergic agents have a well-established role in the prefrontal cortex and in the mediation of working memory (Sawaguchi & Goldman-Rakic, 1991). Immunohistochemical studies have shown high concentrations of DβH-labeled fibers in several prefrontal cortical sites in both monkey (Lewis & Morrison, 1989) and postmortem human brain (Gaspar, Berger, Febvret, Vigny, & Henry, 1989). Parasuraman et al. (2005) reported that retention of memory for up to three spatial locations over a 3-sec delay decreased with the number of A alleles of the DBH G444A polymorphism. There was no association with shifts of visuospatial attention, which was linked to CHRNA4. Based on this work and on the preceding literature, we reasoned similarly that the G444A polymorphism of the DBH gene would modulate attentional scaling only if there is merit to the view that component processes of attentional orienting are important to spatial working memory.
We predicted that if the innervations of visuospatial attention and working memory are largely separate, with little overlap, then cue-induced attentional scaling would be modulated by variation in a nicotinic receptor gene, specifically the C1545T CHRNA4 polymorphism, but not by variation in a gene controlling the conversion of dopamine, specifically the G444A DBH polymorphism.
RESULTS
All significance tests were conducted at a significance level of .05.
Test–Retest Analysis
In order to assess the reliability of performance on the cued visual search task, 80 of the total sample of 105 individuals underwent retesting within a week of the initial test. Test–retest correlations of mean search RT ranged from .532 with the nine-letter cue under conjunction search to .637 with the smallest cue under feature search conditions. The average correlation was .603.
CHRNA4
Neuropsychological Performance
To determine whether CHRNA4 genotype affects standardized tests of cognitive ability, one-way analyses of variance (ANOVAs) were carried out on scores from (a) the Wechsler Memory Scale—Revised Logical Memory Delayed and (b) the Wechsler Adult Intelligence Scale—Revised Vocabulary subtest (Table 1). Because participants differed in age, age group (younger, older) was included as a factor. There were no significant differences.
Table 1.
Demographic Characteristics of Groups Genotyped for CHRNA4 C1545T Polymorphism and DBH G444A Polymorphism
| Genotype | n | Age | Sex (F/M) | WAIS Vocabulary | WMS-Da |
|---|---|---|---|---|---|
| CHRNA4 | |||||
| TT | 61 | 29.7 (21.7) | 30/16 | 51.1 (9.9) | 12.3 (4.7) |
| CT | 25 | 36.9 (24.5) | 19/5 | 52.0 (9.6) | 11.1 (4.4) |
| CC | 18 | 41.8 (25.0) | 13/6 | 53.8 (9.7) | 10.1 (3.2) |
| DBH | |||||
| AA | 22 | 36.0 (25.6) | 11/11 | 55.3 (9.62) | 11.2 (3.1) |
| GA | 49 | 35.3 (24.7) | 35/14 | 50.8 (9.9) | 11.7 (4.3) |
| GG | 64 | 32.8 (21.4) | 34/30 | 54.04 (11.0) | 11.7 (4.5) |
Wechsler Memory Scale-R, Logical Memory Delayed.
Accuracy
Accuracy ratio data were grouped according to genotype of the CHRNA4 C1545T polymorphism (TT, CT, CC) and subjected to a repeated-measures ANOVA. Ratios declined significantly with cue size, but over a narrow range from 0.959 to 0.984 [F(3,303) = 18.30]. Ratios were also lower in the harder conjunction search task [F(1,101) = 31.56]. There was a main effect of genotype [F(2,101) = 3.90], which interacted with cue size [F(6,303) = 3.58], with the effect of cue size being strongest in the CC genotype group.
Reaction Time
The measure of interest is the change in RT as a function of cue size, captured as cue size/RT slope (see below). To justify use of that measure, evidence was sought of that relationship. RT data were grouped according to genotype of the CHRNA4 C1545T polymorphism (TT, CT, CC) and subjected to a repeated-measures ANOVA. The within-subjects factor was cue size. RT was slower in conjunction than feature search [F(1,101) = 492.3] and increased with cue size [F(3,303) = 357.4]. These two effects interacted, with cue size effects greater on conjunction than feature search trials [F(3,303) = 237.2]. RT also increased with gene dose of the CHRNA4 C allele [F(2,101) = 4.6]. This effect interacted with task [F(2,101) = 6.6] and with both task and cue size [F(6,303) = 3.4]. The three-way interaction (plotted in Figure 1A and B) shows a generally linear increase in search speed with cue size that strengthened with Callele gene dose, particularly in conjunction search. This was confirmed in a follow-up ANOVA on conjunction search data alone, which showed a main effect of gene dose [F(2,101) = 5.6] and an interaction of gene dose with cue size [F(6,303) = 2.6]. Although the main effect suggests that C-allele carriers were simply slower to search, Figure 1, considered together with the significant interaction, indicates the effect of the C allele increased with cue size. Separate one-way ANOVAs comparing genotype at each level of cue size showed F values increased roughly with cue size [F(2,101) = 3.6, 4.3, 6.0, 5.4 for cue sizes 2.38, 7.56, 23.2, 40, respectively]. Significance (corrected for the number of analyses) was obtained for all but the smallest cue (3.6).
Figure 1.
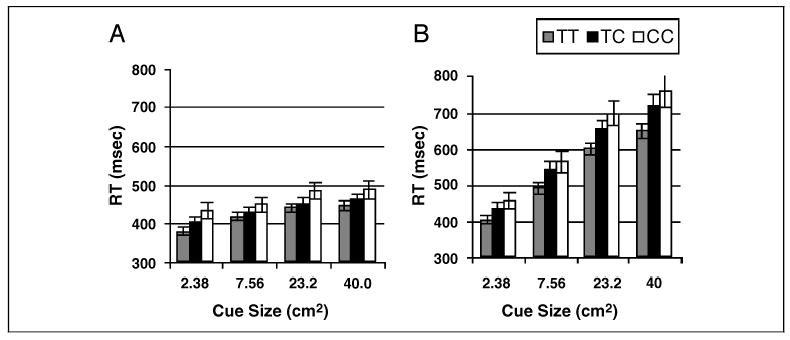
RT plotted as a function of cue size as a function of CHRNA4 genotype. (A) Feature search conditions. (B) Conjunction search conditions.
Slope
As the cued visual search task was designed to measure change in search speed as a function of cue size, the effect of cue size on RT was summarized by calculating the slope of the RT/cue size function (least squares solution). The slope index was submitted to a repeatedmeasures ANOVA with CHRNA4 genotype as the between-subjects factor and task type (feature, conjunction) as the within-subjects factor. Although there was no reason to predict that genotype would vary with age, age group was included as a between-subjects factor to eliminate age as a possible confound. Slopes were steeper on conjunction relative to feature trials, producing a main effect of task type [F(1,98) = 326.7]. There was a marginal main effect of CHRNA4 C-allele gene dose [F(2,98) = 2.22, p = .11], but a significant interaction of gene dose with task type, with slopes increasing with C-allele gene dose on conjunction but not feature search [F(2,98) = 3.22; Figure 2A]. Contrasts between genotype groups for conjunction search revealed that the TT homozygotes differed significantly from both the heterozygotes and from the CC homozygotes. The heterozygotes did not differ from the CC homozygotes (Fisher’s LSD). The effect size of CHRNA4 genotype for conjunction search (using Cohen’s 1988 d for unequal sample sizes) was .25.
Figure 2.
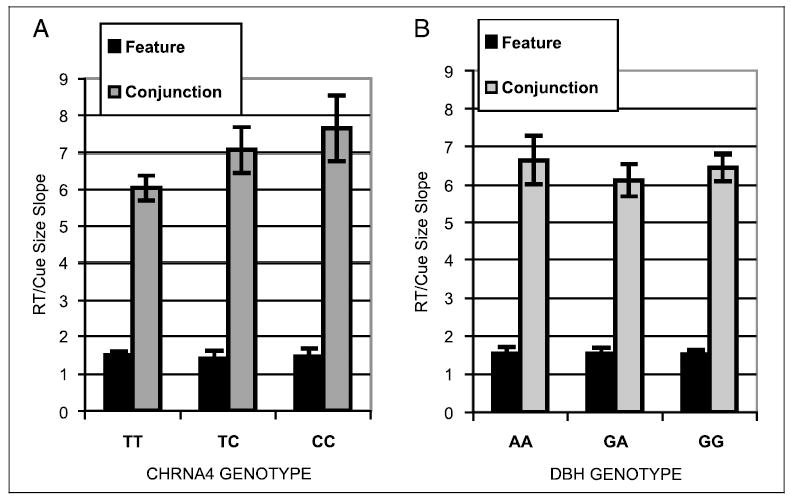
RT/cue size slope plotted as a function of CHRNA4 genotype for feature and conjunction slope conditions for (A) CHRNA4 genotype and (B) DBH genotype.
Dopamine Beta Hydroxylase
To determine the specificity of this effect of CHRNA4 genotype on attentional scaling, we also genotyped these individuals for the G444A polymorphism in the DBH gene, as described above. To determine whether DBH genotype affects standardized tests of cognitive ability, one-way ANOVAs including age as a factor were carried out on scores from (a) the Wechsler Memory Scale—Revised Logical Memory Delayed and (b) the Wechsler Adult Intelligence Scale—Revised Vocabulary subtest (Table 1). There were no significant differences.
Accuracy
Accuracy ratios were grouped according to genotype of the G444A polymorphism (AA, GA, GG) and analyzed in a repeated-measures ANOVA. Accuracy decreased with cue size over a narrow range from .967 to .980 [F(3,393) = 13.21] and was lower under conjunction compared to feature search [F(1,131) = 34.29]. There was no effect of DBH genotype.
Reaction Time
RT data were grouped according to genotype of the G444A polymorphism (AA, GA, GG) and analyzed in a repeated-measures ANOVA with cue size and task type as within-subjects variables. RT was slowed by conjunction compared to feature search [F(1,133) = 511.11] and by increases in cue size [F(3,393) = 445.46]. These effects interacted, with cue size effects greater in conjunction search [F(3,393) = 246.75]. There were no effects of DBH genotype (Figure 3).
Figure 3.

RT across feature and conjunction tasks plotted as a function of cue size for each DBH genotype.
The slope index was also submitted to a repeatedmeasures ANOVA with DBH genotype and age group as the between-subjects factors and task type (feature, conjunction) as the within-subjects factor. The only significant effects were task type, with slopes steeper in conjunction relative to feature search [F(1,132) = 372.95, Figure 2B], and Task type × Age group [F(1,132) = 8.17]. The latter interaction was due to a greater increase in slope from feature to conjunction search in the older group (1.6 to 5.9 in younger people and 1.5 to 7.6 in older people).
DISCUSSION
The present results show that investigation of the cognitive consequences of normal variation in neurotransmitter genes sheds light on the specificity of neuromodulatory influences. Whereas normal genetic variation in a nicotinic cholinergic receptor gene modulated ability to encompass variable numbers of items in the attentional focus, normal variation in a dopamine enzyme gene did not. This evidence speaks to three issues relevant to the genetics of cognition: (1) the influence of neurotransmission genes on cognition; (2) the specificity of such influences; and (3) the hypothesis that spatial working memory requires visuospatial attention (Cowan, 2001; Awh et al., 1998).
First, that a specific polymorphism in a nicotinic receptor gene affects both the ability to shift visuospatial attention in space (Parasuraman et al., 2005) and to dynamically adjust the scale of visuospatial attention (the present study) indicates that normal variation in a single gene can make a measurable contribution to individual differences in deployment of visuospatial attention. In the present study, the CHRNA4 C1545T polymorphism C allele was associated with increasingly slowed search as cue size increased. A reviewer pointed out that the selectively slowed responding following larger cues in C-allele carriers is inconsistent with our previous report that the C allele was associated with greater benefits and reduced costs of valid and invalid cues, respectively (Parasuraman et al., 2005). Results from Parasuraman et al. show that the C allele was associated with ability to selectively benefit from precise location cues, whereas the present study found no genotype effects with precise cues but slower search with imprecise cues. This suggests that C-allele carriers experience greater (Parasuraman et al., 2005) or equal (this study) benefits compared to noncarriers from a constricted attentional focus, but experience greater costs from a large attentional focus. Additional work with a larger range of tasks will be needed to fully explicate effects of the C1545T polymorphism on visuospatial attention.
The influence of a gene with a role in cholinergic neurotransmission on component processes of visuospatial attention is consistent with evidence of the cholinergic mediation of visuospatial attention (Davidson & Marrocco, 2000; Levy, Parasuraman, Greenwood, Dukoff, & Sunderland, 2000; Witte et al., 1997). Although it may not be surprising that alteration in a nicotinic receptor affects behavior innervated by cholinergic circuitry, it is noteworthy that normal variation in one gene controlling a subunit of a nicotinic receptor would lead to measurable, albeit subtle, changes in cognition. This suggests that individual differences in attention performance are partly attributable to normal variation in neurotransmission genes. This is consistent with recent studies showing that a polymorphism in the COMT gene—which controls an enzyme that breaks down dopamine in the synapse—affects executive functioning (Egan et al., 2001) and working memory (Goldberg et al., 2003).
A second issue concerns the specificity of gene–cognition links. The present finding that visuospatial attention was not modulated by variation in a gene controlling an enzyme which breaks down dopamine suggests that the observed association with a cholinergic gene is specific rather than general.
Third, the present study also speaks to the question of overlap in the cognitive processes of visuospatial attention and working memory. It has been argued that visuospatial attention and spatial working memory share a common process—that of attending to variable numbers of items. Jonides and colleagues argued that visuospatial attention is the rehearsal mechanism of spatial working memory (Awh et al., 1998), and Cowan (2001) has actually redefined working memory as the ability to control attention. There is substantial evidence that dopaminergic (Abi-Dargham et al., 2002; Sawaguchi & Goldman-Rakic, 1991) and adrenergic (Franowicz & Arnsten, 2002) receptors are important for working memory. Therefore, if control of the attentional focus is a component process of spatial working memory, then that control may be affected by the availability of dopamine. Contrary to this prediction, variations in the DBH gene, which controls conversion of dopamine to norepinephrine, did not modulate control of the attentional focus, although it has previously been shown to modulate spatial working memory (Parasuraman et al., 2005). Specifically, individuals with the GG genotype of the DBH gene, previously shown to have the best working memory performance (Parasuraman et al., 2005) and the highest CSF level of the enzyme (Cubells, van Kammen, et al., 1998), did not exhibit superior attentional scaling in the present study. Nor did our previous study find CHRNA4 genotype to modulate working memory. This work shows that visuospatial attention and working memory can be differentiated in terms of genetic inf luences. It should be noted, as pointed out by a reviewer, that visuospatial attention could have a role in working memory, which is independent of mediation by dopamine. However, given the strong evidence that dopamine modulates working memory, it is reasonable to assume that to the extent visuospatial attention is a component of working memory, it would be modulated by the efficiency of dopaminergic/noradrenergic neurotransmission.
The present findings are consistent with what is known of effects of nicotine on visuospatial attention in both humans and animals (Levin & Simon, 1998). Nicotine administration has been found to benefit attentional orienting (Witte et al., 1997), sustained attention, and divided attention (Hahn, Shoaib, & Stolerman, 2002). Of particular relevance is evidence that nicotine has selective effects on the redirection of visuospatial attention in humans. In a cued detection task, nicotine was found to speed detection RT on invalidly cued trials, but had little effect on validly cued trials (Witte et al., 1997). In contrast, alpha-2 adrenergic agonists (administered to monkeys) changed the alerting effect of neutral trials compared to no-cue trials, but did not alter the validity effect (Witte & Marrocco, 1997). This suggests that the disengagement of visuospatial attention from invalidly cued space (Posner, Walker, Friderich, & Rafal, 1984) is dependent on cholinergic circuitry. In agreement with that interpretation, the cue validity effect (invalid – valid RT) in chronic smokers was found to be inversely related to salivary levels of a metabolite of nicotine, which declined with days of abstinence (Shirtcliff & Marrocco, 2003). The present finding that a nicotinic receptor has a role in adjustment of both the size and location of the focus of visuospatial attention is consistent with that literature.
In contrast to the orderly effects of CHRNA4 genotype on the attentional scaling component of visuospatial attention, normal variation in the DBH gene did not affect attentional scaling. This is consistent with what is known of the role of the DBH enzyme in converting dopamine to norepinephrine in the brain. Catecholamine innervation, in general, is critical for normal prefrontal cortical function (Franowicz et al., 2002), upon which depends working memory and executive function. Therefore, variation in the DBH gene can be predicted to modulate efficiency of those functions. Consistent with that prediction, in a previous study we found that ability to retain up to three spatial locations over a delay—postulated to tax working memory—varied with genotype of the DBH G444A polymorphism (Parasuraman et al., 2005). It should be noted, however, that nicotinic circuitry in the hippocampus has been found to be important for working memory in rats. An antagonist specific for the alpha-4/beta-2 receptor infused into the hippocampus impaired performance on a 16-arm radial maze (Levin, Bradley, Addy, & Sigurani, 2002). This task, which requires retention of which arms of the maze the rat has previously entered, is considered to be sensitive to working memory capacity. Recent evidence implicating the nucleus basalis magnocellularis cholinergic system in both attentional and mnemonic processing (Chudasama, Dalley, Nathwani, Bouger, & Robbins, 2004) may reflect the nicotinic influences on memory. However, we did not see evidence that a CHRNA4 polymorphism modulated working memory in our previous study (Parasuraman et al., 2005). Further work will be needed to more fully determine the specificity of the effects of neurotransmission genes on cognitive performance.
What is the mechanism for the observed modulation of attentional scaling by a CHRNA4 polymorphism? There is evidence that another CHRNA4 polymorphism associated with an autosomal-dominant nocturnal epilepsy alters the affinity of its associated nicotinic receptor for acetylcholine (Steinlein, Magnusson, et al., 1997). Therefore, it can be speculated that a similar alteration in the receptor occurs with the C1545T polymorphism investigated here. Consistent with that interpretation is the finding that C1545T heterozygotes showed an intermediate effect on attention performance, indicating an additive effect of one allele. Moreover, the C1545T polymorphism has been linked to nicotine addiction in a large sample of men (Feng et al., 2004). However, the functional consequences of this polymorphism have not been extensively investigated. Therefore, the possibility must be considered that the polymorphism is not itself functional, but rather is inherited together with another polymorphism which alters receptor affinity.
The present results contribute to a small but growing literature showing effects of polymorphisms in neurotransmission genes on individual differences in cognition (Fan, Fossella, Sommer, Wu, & Posner, 2003; Goldberg et al., 2003; Egan et al., 2001). This field of cognitive neurogenetics is in its infancy and may yet be plagued by the failures to replicate which characterize SNP studies of disease (Ioannidis et al., 2001). However, work to date suggests that effect sizes can be medium to large (in the range 0.25–0.45) when cognitive measures are used as endpoints (reviewed in Greenwood & Parasuraman, 2003). This is in contrast to effect sizes of less than 1% which are commonly observed when diagnosis is used as an endpoint (Ioannidis et al., 2001). Therefore, the allelic association approach applied to investigation of the component processes of cognition may show more successful replication.
METHODS
Participants
The demographic characteristics of the healthy, screened participants are presented in Table 1 for each polymorphism. Because only some individuals were typed for both polymorphisms, the data are treated as two samples. There were 41 individuals who were typed only for the DBH polymorphism and 10 individuals who were typed only for the CHRNA4 polymorphism. They were recruited from Catholic University and the Washington, DC, community. Informed consent was obtained and vision tested to ensure at least 20/30 vision on a Rosenbaum pocket screener. Participants were cognitively screened by means of the Wechsler WAIS vocabulary subtest (Wechsler, 1981) and the Wechsler Memory Scale logical memory subtest (Wechsler, 1945). Participants over age 65 were additionally screened with the Mini-Mental State Exam. The purpose of these neuropsychological tests is to rule out possible dementia in this older group.
Stimuli and Procedures
A cued visual search task was used (Figure 4). This task required speeded search for a target letter (pink T) whose location was cued in advance. The target was embedded in a 6.3° × 4.2° array of 15 uppercase letters (3 rows, 5 letters each). The 1.5° letters were pink, blue, and green (T, N, G) and their location was randomly determined. Following a 1000-msec fixation cross, target location was precued (500 msec SOA) with a rectangle enclosing 1, 3, 9, or 15 letters. On 83% of the trials, the target appeared inside the randomly placed cue, although on 17% (catch trials) the target was absent. Both cue and target remained visible until the speeded button-press response indicating target presence/absence occurred or 2.5 sec elapsed. There were two search conditions: (a) easy or feature search in which the target letter “popped out” from the distractors by being the only pink letter; (b) hard or conjunction search in which the target properties of color and form appeared together only in the target, but separately with equal frequency among the distractors.
Figure 4.

Illustration of cued visual search task (pink is represented by gray text, green by plain text, blue by bolded text). Precues could encompass 1, 3, 9, and 15 array elements. (A) The one-element cue encompassed only one letter in the search array. (B) The nine-element cue encompassed nine letters in the search array.
Genotyping
Buccal (cheek) swabs were obtained from each participant in order to extract DNA (Richards et al., 1993) and prepared as directed by the manufacturer (MasterAMP TMBuccal Swab DNA Extraction Kit, Epicentre Technologies, Madison, WI). Yields ranged from 0.5 to 3 μg of DNA from each buccal sample. Yields were determined spectrophotometrically by absorbance at 260 nm. Taq polymerase, PCR buffer, and dNTPs were obtained from QIAGEN (Valencia, CA) and used at recommended concentrations for a 20-μl PCR reaction. PCR reactions and restriction digests (PCR-RFLP) were performed on the PTC-100 Programmable Thermal Controller (MJ Research, Watertown, MA). Gel electrophoresis in either LE or Metaphor agarose, followed by staining in ethidium bromide, was used to resolve and visualize DNA fragments. Two primers were used to identify the C-to-T polymorphism in the CHRNA4 gene (RS1044396), forward: 5′-accagggctggccaaagccagg-3′ and reverse: 5′-gtgctttggtgctgcgggtc-3′, followed by digestion with HhaI or CfoI (as described by Steinlein, Deckert, et al., 1997). The T allele results in 152- and 138-bp bands, whereas the C allele results in 152-, 105- and 33-bp bands (Figure 5). Using these methods, the sample of 106 participants was subdivided into three groups based on the number of C alleles, 0 (TT genotype, n = 61), 1 (TC genotype, n = 27), or 2 (CC genotype, n = 18). The observed frequencies in this sample were TT = 0.59, TC=0.24, and CC=0.17. A recent article with a sample of 238 reported frequencies of 0.27, 0.55, and 0.18, respectively (Cook et al., 2004).
Figure 5.

HhaI or CfoI restriction digest detects a polymorphism at position 1545 (Steinlein et al. 1997). Results of PCR-RFLP from buccal swabs of 25 participants.
The genomic PCR primers used to detect the G444A polymorphism of the DBH gene (Genbank accession X63418; Cubells, Kranzler, et al., 2000) were forward, 5′-cctggagcccagtgcttgtc-3′ and reverse, 5′-acgccctcctgggtactcgc-3′, followed by digestion with EcoNI (Cubells, van Kammen, et al., 1998). Using these methods, the sample of 138 participants was subdivided into three groups based on the number of G alleles, 0 (AA genotype, n = 22), 1 (GA genotype, n = 51), or 2 (GG genotype, n = 65). The observed frequencies in our sample were GG = 0.47, GA = 0.36, AA = 0.16. A recent article with a sample of 120 individuals (Yamamoto et al., 2003) reported frequencies of 0.25, 0.54, and 0.21, respectively.
Acknowledgments
This work was supported by NIH grant AG 19653 to RP.
Reprint requests should be sent to P. M. Greenwood, Department of Psychology, MSN 3F5, George Mason University, Fairfax, VA 22030-4444, or via e-mail:pgreenw1@gmu.edu.
References
- Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, Hwang DR, Keilp J, Kochan L, Van Heertum R, Gorman J, Laruelle M. Prefrontal dopamine D1 receptors and working memory in schizophrenia. Journal of Neuroscience. 2002;22:3708–3719. doi: 10.1523/JNEUROSCI.22-09-03708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Eisenberg HM, Albuquerque EX. Nicotinic receptor activation in human cerebral cortical interneurons: A mechanism for inhibition and disinhibition of neuronal networks. Journal of Neuroscience. 2000;20:66–75. doi: 10.1523/JNEUROSCI.20-01-00066.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awh E, Jonides J, Reuter-Lorenz PA. Rehearsal in spatial working memory. Journal of Experimental Psychology: Human Perception and Performance. 1998;24:780–790. doi: 10.1037//0096-1523.24.3.780. [DOI] [PubMed] [Google Scholar]
- Castiello U, Umilta C. Size of the attentional focus and efficiency of processing. Acta Psychologica. 1990;73:195–209. doi: 10.1016/0001-6918(90)90022-8. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Dalley JW, Nathwani F, Bouger P, Robbins TW. Cholinergic modulation of visual attention and working memory: Dissociable effects of basal forebrain 192-igg-saporin lesions and intraprefrontal infusions of scopolamine. Learning and Memory. 2004;11:78–86. doi: 10.1101/lm.70904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook LJ, Ho LW, Taylor AE, Brayne C, Evans JG, Xuereb J, Cairns NJ, Pritchard A, Lemmon H, Mann D, St Clair D, Turic D, Hollingworth P, Moore PJ, Jehu L, Archer N, Walter S, Foy C, Edmondson A, Powell J, Lovestone S, Owen MJ, Williams J, Lendon C, Rubinsztein DC. Candidate gene association studies of the alpha4 (CHRNA4) and beta2 (CHRNB2) neuronal nicotinic acetylcholine receptor subunit genes in Alzheimer’s disease. Neuroscience Letters. 2004;358:142–146. doi: 10.1016/j.neulet.2004.01.016. [DOI] [PubMed] [Google Scholar]
- Cowan N. The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behavioral and Brain Sciences. 2001;24:87–114. doi: 10.1017/s0140525x01003922. discussion 114–185. [DOI] [PubMed] [Google Scholar]
- Cubells JF, Kranzler HR, McCance-Katz E, Anderson GM, Malison RT, Price LH, Gelernter J. A haplotype at the DBH locus, associated with low plasma dopamine beta-hydroxylase activity, also associates with cocaine-induced paranoia. Molecular Psychiatry. 2000;5:56–63. doi: 10.1038/sj.mp.4000657. [DOI] [PubMed] [Google Scholar]
- Cubells JF, van Kammen DP, Kelley ME, Anderson GM, O’Connor DT, Price LH, Malison R, Rao PA, Kobayashi K, Nagatsu T, Gelernter J. Dopamine beta-hydroxylase: Two polymorphisms in linkage disequilibrium at the structural gene DBH associate with biochemical phenotypic variation. Human Genetics. 1998;102:533–540. doi: 10.1007/s004390050736. [DOI] [PubMed] [Google Scholar]
- Davidson MC, Marrocco RT. Local infusion of scopolamine into intraparietal cortex slows covert orienting in rhesus monkeys. Journal of Neurophysiology. 2000;83:1536–1549. doi: 10.1152/jn.2000.83.3.1536. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proceedings of the National Academy of Sciences, USA. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen CW, St James JD. Visual attention within and around the field of focal attention: A zoom lens model. Perception & Psychophysics. 1986;40:225–240. doi: 10.3758/bf03211502. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annual Review of Psychology. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- Fan J, Fossella J, Sommer T, Wu Y, Posner MI. Mapping the genetic variation of executive attention onto brain activity. Proceedings of the National Academy of Sciences, USA. 2003;100:7406–7411. doi: 10.1073/pnas.0732088100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Wu Y, Fossella JA, Posner MI. Assessing the heritability of attentional networks. BMC Neuroscience. 2001;2:14. doi: 10.1186/1471-2202-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Niu T, Xing H, Xu X, Chen C, Peng S, Wang L, Laird N, Xu X. A common haplotype of the nicotine acetylcholine receptor alpha 4 subunit gene is associated with vulnerability to nicotine addiction in men. American Journal of Human Genetics. 2004;75:112–121. doi: 10.1086/422194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores CM, Rogers SW, Pabreza LA, Wolfe BB, Kellar KJ. A subtype of nicotinic cholinergic receptor in rat brain is composed of alpha 4 and beta 2 subunits and is up-regulated by chronic nicotine treatment. Molecular Pharmacology. 1992;41:31–37. [PubMed] [Google Scholar]
- Fossella J, Sommer T, Fan J, Wu Y, Swanson JM, Pfaff DW, Posner MI. Assessing the molecular genetics of attention networks. BMC Neuroscience. 2002;3:14. doi: 10.1186/1471-2202-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franowicz JS, Arnsten AF. Actions of alpha-2 noradrenergic agonists on spatial working memory and blood pressure in rhesus monkeys appear to be mediated by the same receptor subtype. Psychopharmacology (Berlin) 2002;162:304–312. doi: 10.1007/s00213-002-1110-6. [DOI] [PubMed] [Google Scholar]
- Franowicz JS, Kessler LE, Borja CM, Kobilka BK, Limbird LE, Arnsten AF. Mutation of the alpha2A-adrenoceptor impairs working memory performance and annuls cognitive enhancement by guanfacine. Journal of Neuroscience. 2002;22:8771–8777. doi: 10.1523/JNEUROSCI.22-19-08771.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar P, Berger B, Febvret A, Vigny A, Henry JP. Catecholamine innervation of the human cerebral cortex as revealed by comparative immunohistochemistry of tyrosine hydroxylase and dopamine-beta-hydroxylase. Journal of Comparative Neurology. 1989;279:249–271. doi: 10.1002/cne.902790208. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS, Goldman D, Weinberger DR. Executive subprocesses in working memory: Relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Archives of General Psychiatry. 2003;60:889–896. doi: 10.1001/archpsyc.60.9.889. [DOI] [PubMed] [Google Scholar]
- Greenwood PM, Lambert JC, Sunderland T, Parasuraman R. Effects of APOE genotype on spatial attention, working memory, and their interaction in healthy, middle-aged adults: Results from the NIMH BIOCARD study. Neuropsychology. 2005;19:199–211. doi: 10.1037/0894-4105.19.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood PM, Parasuraman R. Scale of attentional focus in visual search. Perception & Psychophysics. 1999;61:837–859. doi: 10.3758/bf03206901. [DOI] [PubMed] [Google Scholar]
- Greenwood PM, Parasuraman R. Normal genetic variation, cognition, and aging. Behavioral and Cognitive Neuroscience Reviews. 2003;2:278–306. doi: 10.1177/1534582303260641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood PM, Parasuraman R. The scaling of spatial attention in visual search and its modification in healthy aging. Perception & Psychophysics. 2004;66:3–22. doi: 10.3758/bf03194857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood PM, Sunderland T, Friz JL, Parasuraman R. Genetics and visual attention: Selective deficits in healthy adult carriers of the varepsilon 4 allele of the apolipoprotein E gene. Proceedings of the National Academy of Sciences, USA. 2000;97:11661–11666. doi: 10.1073/pnas.97.21.11661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Shoaib M, Stolerman IP. Nicotine-induced enhancement of attention in the five-choice serial reaction time task: The influence of task demands. Psychopharmacology (Berlin) 2002;162:129–137. doi: 10.1007/s00213-002-1005-6. [DOI] [PubMed] [Google Scholar]
- Ioannidis JP, Ntzani EE, Trikalinos TA, Contopoulos-Ioannidis DG. Replication validity of genetic association studies. Nature Genetics. 2001;29:306–309. doi: 10.1038/ng749. [DOI] [PubMed] [Google Scholar]
- Kent L, Middle F, Hawi Z, Fitzgerald M, Gill M, Feehan C, Craddock N. Nicotinic acetylcholine receptor alpha4 subunit gene polymorphism and attention deficit hyperactivity disorder. Psychiatric Genetics. 2001;11:37–40. doi: 10.1097/00041444-200103000-00007. [DOI] [PubMed] [Google Scholar]
- Levin ED, Bradley A, Addy N, Sigurani N. Hippocampal alpha 7 and alpha 4 beta 2 nicotinic receptors and working memory. Neuroscience. 2002;109:757–765. doi: 10.1016/s0306-4522(01)00538-3. [DOI] [PubMed] [Google Scholar]
- Levin ED, Simon BB. Nicotinic acetylcholine involvement in cognitive function in animals. Psychopharmacology (Berlin) 1998;138:217–230. doi: 10.1007/s002130050667. [DOI] [PubMed] [Google Scholar]
- Levy JA, Parasuraman R, Greenwood PM, Dukoff R, Sunderland T. Acetylcholine affects the spatial scale of attention: Evidence from Alzheimer’s disease. Neuropsychology. 2000;14:288–298. doi: 10.1037//0894-4105.14.2.288. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Morrison JH. Noradrenergic innervation of monkey prefrontal cortex: A dopamine-beta-hydroxylase immunohistochemical study. Journal of Comparative Neurology. 1989;282:317–330. doi: 10.1002/cne.902820302. [DOI] [PubMed] [Google Scholar]
- Muller NG, Bartelt OA, Donner TH, Villringer A, Brandt SA. A physiological correlate of the “Zoom Lens” of visual attention. Journal of Neuroscience. 2003;23:3561–3565. doi: 10.1523/JNEUROSCI.23-09-03561.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobre AC, Sebestyen GN, Gitelman DR, Mesulam MM, Frackowiak RS, Frith CD. Functional localization of the system for visuospatial attention using positron emission tomography. Brain. 1997;120:515–533. doi: 10.1093/brain/120.3.515. [DOI] [PubMed] [Google Scholar]
- Parasuraman R, Greenwood P, Kumar R, Fossella J. Beyond heritability: Neurotransmitter genes differentially modulate visuospatial attention and working memory. Psychological Science. 2005;16:200–207. doi: 10.1111/j.0956-7976.2005.00804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JM, McAlonan K, Robb WG, Brown VJ. Cholinergic neurotransmission influences covert orientation of visuospatial attention in the rat. Psychopharmacology (Berlin) 2000;150:112–116. doi: 10.1007/s002130000437. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Posner MI, Walker JA, Friderich FJ, Rafal RD. Effects of parietal injury on covert orienting of attention. Journal of Neuroscience. 1984;4:1863–1874. doi: 10.1523/JNEUROSCI.04-07-01863.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards B, Skoletsky J, Shuber AP, Balfour R, Stern RC, Dorkin HL, Parad PB, Witt D, Klinger KW. Multiplex PCR amplification from the CFTR gene using DNA prepared from buccal brushes/swabs. Human Molecular Genetics. 1993;2:159–163. doi: 10.1093/hmg/2.2.159. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: Involvement in working memory. Science. 1991;251:947–950. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Marrocco RT. Salivary cotinine levels in human tobacco smokers predict the attentional validity effect size during smoking abstinence. Psychopharmacology (Berlin) 2003;166:11–18. doi: 10.1007/s00213-002-1293-x. [DOI] [PubMed] [Google Scholar]
- Steinlein OK, Deckert J, Nothen MM, Franke P, Maier W, Beckmann H, Propping P. Neuronal nicotinic acetylcholine receptor alpha 4 subunit (CHRNA4) and panic disorder: An association study. American Journal of Medical Genetics. 1997;74:199–201. doi: 10.1002/(sici)1096-8628(19970418)74:2<199::aid-ajmg17>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Steinlein OK, Magnusson A, Stoodt J, Bertrand S, Weiland S, Berkovic SF, Nakken KO, Propping P, Bertrand D. An insertion mutation of the CHRNA4 gene in a family with autosomal dominant nocturnal frontal lobe epilepsy. Human Molecular Genetics. 1997;6:943–947. doi: 10.1093/hmg/6.6.943. [DOI] [PubMed] [Google Scholar]
- Wechsler D. A standardized memory scale for clinical use. Journal of Psychology. 1945;19:87–95. [Google Scholar]
- Wechsler, D. (1981). Wechsler adult intelligence scale—revised. New York: Psychological Corporation.
- Witte EA, Davidson MC, Marrocco RT. Effects of altering brain cholinergic activity on covert orienting of attention: Comparison of monkey and human performance. Psychopharmacology (Berlin) 1997;132:324–334. doi: 10.1007/s002130050352. [DOI] [PubMed] [Google Scholar]
- Witte EA, Marrocco RT. Alteration of brain noradrenergic activity in rhesus monkeys affects the alerting component of covert orienting. Psychopharmacology (Berlin) 1997;132:315–323. doi: 10.1007/s002130050351. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Huguenard JR, Prince DA. Cholinergic switching within neocortical inhibitory networks. Science. 1998;281:985–988. doi: 10.1126/science.281.5379.985. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Cubells JF, Gelernter J, Benkelfat C, Lalonde P, Bloom D, Lal S, Labelle A, Turecki G, Rouleau G, Joober R. Dopamine betahydroxylase (DBH) gene and schizophrenia phenotypic variability: A genetic association study. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics. 2003;117:33–38. doi: 10.1002/ajmg.b.10011. [DOI] [PubMed] [Google Scholar]


