Abstract
A cued, visuospatial attention task and a working memory task were administered to 89 healthy adults genotyped for a T-to-C polymorphism in CHRNA4, a nicotinic receptor subunit gene. Increasing gene dose of the C allele of the CHRNA4 gene (i.e., no C alleles, one C allele, two C alleles) was associated with increased reaction time (RT) benefits of valid attentional cuing and reduced RT costs of invalid cues, but was not associated with working memory performance. In a second experiment, 103 healthy persons were genotyped for a G-to-A polymorphism of the dopamine beta-hydroxylase (DBH) gene. Increasing gene dose of the G allele of the DBH gene was associated with increased working memory accuracy at a high memory load. However, there was no consistent association between the DBH gene and visuospatial attention. Thus, a double dissociation was observed, with visuospatial attention associated with CHRNA4 but not the DBH gene and, conversely, working memory associated with the DBH gene but not CHRNA4. The results show that normal allelic variations in single neurotransmitter genes modulate individual differences in processing components of cognitive functions in healthy individuals.
Twin studies show that genetic factors contribute substantially to individual differences in normal cognition, but the specific genes involved are unknown (Plomin & Crabbe, 2000). Recent advances in molecular genetics now provide a complementary approach to the twin method, allelic association. Rather than only demonstrating and quantifying heritability, this method enables particular genes to be associated with individual differences in cognition in healthy, unrelated persons.
Allelic association requires identification of candidate genes that might influence a given cognitive process because of the functional role of each gene’s protein product in the brain. Allelic variation results from slight differences in the chain of nucleic acids making up a gene. Typically, one nucleotide is substituted for another, resulting in what is termed a single-nucleotide polymorphism. The protein whose production is directed by the gene with the changed sequence is correspondingly altered. Such polymorphisms, which are found about once in every 1,000 DNA base pairs in unrelated individuals, can influence brain function and cognition in many ways (Craig & McClay, 2003).
Determining the role of a specific gene in a given cognitive ability poses a challenge. Single-nucleotide polymorphisms with functional significance for cognition and behavior constitute only a small part of the human genome, but there are still so many of them that forging valid links to cognition is difficult. One solution is to capitalize on the breakthroughs in neuroscience that have improved understanding of the neural bases of cognition (Plomin & Kosslyn, 2001). By identifying the regional brain networks involved in a particular cognitive function, and by tracing the neurochemical innervation of those networks, researchers can in principle link variation in genes that influence neurotransmitter function to individual differences in that cognitive function. Recent studies using this approach with healthy persons have shown that individual differences in performance of cognitive tasks can be linked to polymorphisms in single genes (Diamond, Briand, Fossella, & Gehlbach, 2004; Egan et al., 2001; Fan, Fossella, Sommer, Wu, & Posner, 2003; Fan, Wu, Fossella, & Posner, 2001; Fossella et al., 2002; Greenwood, Sunderland, Friz, & Parasuraman, 2000; Parasuraman, Greenwood, & Sunderland, 2002; see Greenwood & Parasuraman, 2003, for a review).
Attention provides a useful model system for investigating the genetics of cognition because the associated brain networks and their neurochemical innervation are known (Everitt & Robbins, 1997; Posner & Petersen, 1990), and some attentional functions are heritable (Fan et al., 2001). There is strong evidence linking visuospatial attention to a distributed network of cortical regions that center on the intraparietal sulcus (Corbetta, Kincade, Ollinger, McAvoy, & Schulman, 2000; Posner & Peterson, 1990). This system is innervated by the ascending basal forebrain cholinergic pathway (Everitt & Robbins, 1997), as shown by lesion (Voytko, 1996), pharmacological (Davidson & Marrocco, 2000), and neurodegenerative disease studies (Parasuraman, Greenwood, Haxby, & Grady, 1992).
Cholinergic receptors influence neuronal function in parietal cortex (Xiang, Huguenard, & Prince, 1998), where they regulate fast synaptic transmission (Alkondon, Pereira, Eisenberg, & Albuquerque, 2000). Although most cholinergic receptors are muscarinic, nicotinic receptors are particularly important for attention (Phillips, McAlonan, Robb, & Brown, 2000). Human cholinergic innervation in parietal cortex appears to be nicotinic, not muscarinic (Mentis et al., 2001). We therefore hypothesized that polymorphisms of nicotinic acetylcholine receptors (nAChRs) might be candidates for modulation of visuospatial attention.
EXPERIMENT 1
Several subunits assemble together to form an nAChR. The most widely distributed nAChR in the brain is the alpha4/beta2 receptor, which is composed of alpha4 and beta2 subunits assembled together (Flores, DeCamp, Kilo, Rogers, & Hargreaves, 1996). Several polymorphisms in the gene controlling the alpha4 subunit, CHRNA4, have been identified. We examined the association between a common polymorphism in CHRNA4 (Steinlein, Deckert, et al., 1997) and individual differences in a visuospatial attention task that previous neuroimaging (Corbetta et al., 2000) and pharmacological (Davidson & Marrocco, 2000) studies have shown to be mediated by intraparietal cortex. We hypothesized that normal allelic variation in a gene that controls cholinergic neurotransmission is likely to be associated with component processes of visuospatial attention in healthy individuals. To examine the specificity of the association, we predicted that the same individuals who would show an association between the CHRNA4 gene and individual differences in the visuospatial attention task would not show an association between this gene and a working memory task.
Method
Participants
Complete behavioral and genomic data were obtained for a sample of 89 healthy adults (61 females, 28 males) with a mean age of 35.2 (± 2.5) years. All had normal vision and no neuropsychological deficits.
Tasks
Each trial of the visuospatial attention task began with a fixation cross (0.95° × 0.95°) that was presented centrally on the display for 1 s and followed by an arrow cue that pointed to a location to the left, to the right, or to both the left and the right. A variable time later (500- or 2,000-ms stimulus onset asynchrony, SOA), the cue was followed by a letter target (0.95° × 1.14°) presented 6.7° to the left or right of fixation. The letter remained on the display until the participant responded, indicating whether it was a consonant or vowel. The arrow was a valid cue pointing to the target location on 62.5% of the trials; it was invalid (18.75%) or neutral (18.75%) on the remaining trials. Participants were required to maintain fixation on the central cross for the duration of each trial. The intertrial interval varied randomly (2,200, 2,500, or 2,800 ms). Reaction time (RT) and accuracy were measured.
Each trial of the spatial working memory task began with a fixation cross (0.95° × 0.95°) presented centrally on the display for 1 s. After 1 s, one, two, or three black dots (0.67°) appeared at randomly chosen screen locations for 500 ms. Simultaneously with the offset of the dot display, the fixation cross reappeared for a 3-s delay, at the end of which a single red test dot (0.67°) appeared alone, either at the same location as a target dot (match) or at a different location (nonmatch). Participants had 2 s to decide whether the location of the test dot matched the location of one of the target dots. RT and accuracy were measured.
Genotyping
All participants were genotyped for the C1545T polymorphism of the CHRNA4 gene. Genomic material was obtained via buccal cell brush and prepared (MasterAMP TMBuccal Swab DNA Extraction Kit, Epicentre Technologies, Madison, WI). Yields ranged from 0.5 to 3 μg of DNA from each buccal sample, as determined spectrophotometrically by absorbance at 260 nm. Taq polymerase, polymer chain reaction (PCR) buffer, and deoxyribonucleotide triphosphates were obtained from QIAGEN (Valencia, CA) and used at recommended concentrations for a 20-ul PCR reaction. PCR reactions and restriction fragment-length polymorphism digests were performed on a PTC-100 Programmable Thermal Controller (MJ Research, Waltham, MA). Gel electrophoresis in either LE or Metaphor agarose followed by staining in ethidium bromide was used to resolve and visualize DNA fragments. Two primers were used to identify the T-to-C polymorphism in the alpha4 nicotinic receptor subunit. Administration of forward (5′-accagggctggccaaagccagg-3′) and reverse (5′-gtgctttggtgctgcgggtc-3′) primers was followed by digestion with the HhaI or CfoI enzymes (as described by Steinlein, Deckert, et al., 1997). The T allele results in 152 and 138 base-pair bands, whereas the C allele results in 152, 105, and 33 base-pair bands. Using these methods, we subdivided the sample of 89 participants into three groups based on the number of C alleles, none (TT genotype, n = 46), one (TC genotype, n = 24), or two (CC genotype, n = 19).
Procedure
The attention (two blocks of 80 trials) and working memory (three blocks of 84 trials) tasks were presented in counterbalanced order with brief intervening breaks.
Results
Visuospatial Attention
Because accuracy in the cued letter-discrimination task exceeded 91%, no further analysis of accuracy was conducted. The means of the median RTs were computed separately for each condition and for the three CHRNA4 genotype subgroups (see Table 1). A 3 (genotype) × 3 (cue validity) × 2 (SOA) analysis of variance (ANOVA) showed a significant effect for cue validity, F(2, 172) = 48.7, p < .0001. RT was higher for invalidly cued targets (cost) than for validly cued targets (benefit), with RT following neutral cues having an intermediate value. The main effect of SOA was also significant, F(2, 86) = 16.6, p < .0001. The main effect of CHRNA4 genotype was not significant (F < 1), but interacted with cue validity, F(4, 172) = 2.9, p < .05.
TABLE 1.
Mean of Median Reaction Times (in Milliseconds) on the Visuospatial Attention Task for the Three CHRNA4 Genotypes
| Genotype
|
|||
|---|---|---|---|
| Cue validity | TT | TC | CC |
| 500-ms SOA | |||
| Valid | 592.3 (15.1) | 596.4 (25.4) | 608.9 (22.8) |
| Neutral | 597.5 (14.4) | 634.3 (29.0) | 633.1 (23.9) |
| Invalid | 625.3 (15.4) | 648.0 (32.6) | 647.4 (25.7) |
| 2,000-ms SOA | |||
| Valid | 610.5 (16.4) | 611.7 (25.9) | 618.6 (20.8) |
| Neutral | 610.2 (16.0) | 632.7 (26.3) | 652.2 (22.5) |
| Invalid | 657.3 (18.6) | 659.2 (30.7) | 654.7 (23.4) |
Note. The three genotypes TT, TC, and CC correspond to increasing gene dose (0, 1, and 2, respectively) of the C allele of the CHRNA4 gene. Standard errors are in parentheses. SOA = stimulus-onset asynchrony.
The interaction justified separate analyses of RT benefits (neutral RT – valid RT) and costs (invalid RT – neutral RT). We restricted this analysis to the SOA for which total RT costs and benefits were maximal (2,000 ms). The effect of CHRNA4 genotype was significant for RT benefits, F(2, 86) = 7.5, p < .001; the effect-size d corrected for the unequal sample sizes of the genotype subgroups (Cohen, 1988) was 0.45. The effect of CHRNA4 on RT costs was also significant, F(2, 86) = 3.5, p < .05, d = 0.3. RT benefits increased (see Fig. 1a) with increasing gene dose of the C allele (0, 1, 2). RT benefits were significantly greater for the TC and CC genotypes (one and two C alleles, respectively) than for the TT (no C alleles) genotype (p < .05, Fisher’s test). In contrast, RT costs decreased with increased C allele dosage (see Fig. 1b). RT cost was significantly (p < .05) greater for the lowest C allele dosage (TT) than for the highest C allele dosage (CC).
Fig. 1.
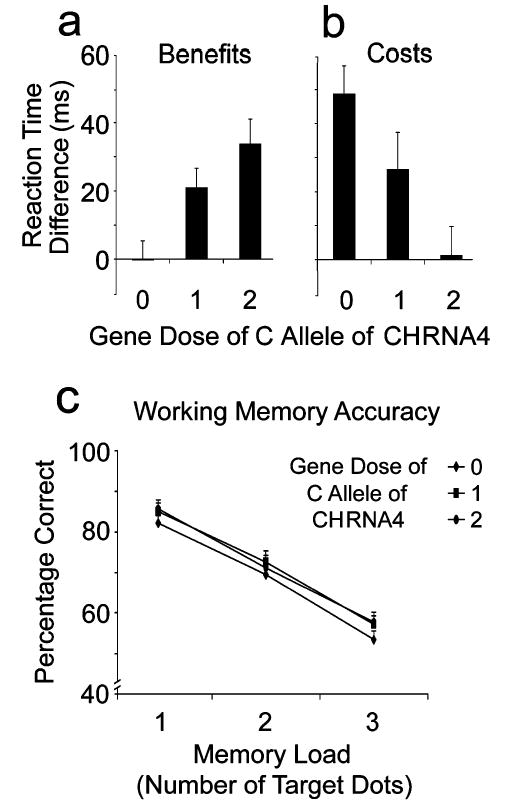
Effects of allelic variation in the CHRNA4 gene on visuospatial attention in the 2,000-ms stimulus-onset asynchrony condition and on working memory: (a) reaction time (RT) benefits of valid location cues in the visuospatial attention task (neutral RT – valid RT), (b) RT costs of invalid location cues in the same task (invalid RT – neutral RT), and (c) match accuracy in the working memory task as a function of number of spatial locations to be maintained in working memory. The three genotypes TT, TC, and CC correspond to increasing gene dose (0, 1, 2) of the C allele of the CHRNA4 gene.
Working Memory
Accuracy for match trials on the memory task was computed for each of the three memory loads (one, two, or three target locations). A 3 (CHRNA4 genotype) × 3 (memory load) ANOVA showed a significant effect for memory load, F(2, 172) = 189.2, p < .0001. Accuracy declined as the number of locations to be remembered increased from one to two to three (see Fig. 1c). However, neither the main effect of CHRNA4 genotype, F(2, 86) < 1, nor its interaction with memory load, F < 1, was significant.
Intertask Correlations
Intertask correlations were computed for the conditions of the attention task in which genetic association was observed. Memory accuracy at the highest memory load correlated .11 with RT benefits and −.17 with RT costs at the 2,000-ms SOA of the visuospatial attention task. Neither correlation was significant (p > .05). However, there was a significant (p < .01) correlation between overall memory accuracy and overall RT, −.33.
Discussion
Normal allelic variation in the CHRNA4 gene was associated with individual differences in the efficiency of component processes of a visuospatial attention task. Although overall accuracy or speed of performance on this task was unrelated to CHRNA4 genotype, component processes—the endogenous orienting and reorienting of visuospatial attention as elicited by valid and invalid location cues—were reliably and systematically related to variants of the CHRNA4 gene in a sample of healthy individuals. At the same time, variation in the CHRNA4 gene did not modulate either overall performance or component processes in a working memory task.
The CHRNA4 gene was chosen as a candidate gene for its putative role in the modulation of cholinergic activity in the posterior parietal cortex, which is known to mediate spatial attention. This brain region is involved in both endogenous and exogenous shifts of attention. We did not examine the latter in this study, but predict the same association with CHRNA4. The pattern of association with spatial attention and dissociation with working memory provides preliminary evidence for the role of the CHRNA4 gene in the control of cholinergically related functions in the brain. Thus, the results add to the large body of evidence linking the forebrain ascending cholinergic system and spatial attention (Everitt & Robbins, 1997).
An association between the CHRNA4 gene and components of spatial attention is reasonable given that this gene is known to control production of the alpha4 nAChRs and that these receptors are thought to play an important role in attentional functions (Phillips et al., 2000). However, the precise functional role of the protein products of the different variants of the CHRNA4 gene and their effects on cortical cholinergic activity are not fully understood, so it is difficult to interpret the direction of the effects of allelic variation in the CHRNA4 gene on component processes in the spatial attention task. Whereas increased gene dosage of the C allele was associated with an increase in RT benefits of valid location cuing, it was associated with a decrease in RT costs of invalid location cuing. Although this differential pattern of results cannot be easily interpreted given current knowledge, the orderly nature and moderate to large size of the effects is encouraging. Receptor polymorphisms are known to exert their effects by influencing the affinity of a receptor for an ion. For example, a different polymorphism in the CHRNA4 gene (involving an insertion of three nucleotides) that has been linked to autosomal dominant, nocturnal frontal lobe epilepsy affects the size of the acetylcholine-evoked current in vitro (Steinlein, Magnusson, et al., 1997). In addition, the increase in RT following invalid cues—RTcosts—is greater in Alzheimer’s disease patients (Parasuraman et al., 1992) than in age-matched control subjects. Therefore, the association of the C allele with lower costs and the T allele with higher costs may reflect differences in acetylcholine neurotransmitter activity (Steinlein, Deckert, et al., 1997), although no biochemical evidence has yet linked this polymorphism to changes in regulation in messenger RNA or protein structure.
No association was found between the CHRNA4 gene and working memory. The effects of CHRNA4 on visuospatial attention were moderate to large. With the sample size used (N = 89), power to detect a moderate-size (0.25) effect of CHRNA4 on working memory was greater than 88%. Given the lack of association between CHRNA4 and working memory, we examined whether another gene would be associated with working memory, which has been linked to the brain dopaminergic system and to prefrontal cortical activation. We hypothesized that a gene playing a role in a dopaminergic function—mediated in prefrontal cortex—might modulate working memory. Accordingly, we examined the effects of variation in the dopamine beta-hydroxylase (DBH) gene on performance on the same spatial attention and working memory tasks studied in Experiment 1.
EXPERIMENT 2
Dopaminergic agents mediate working memory (Muller, von Cramon, & Pollmann, 1998) and influence prefrontal cortex activity (Ranganath, Johnson, & D’Esposito, 2003). For example, the binding potential of D1 but not D2 dopamine receptors modulates working memory (Abi-Dargham et al., 2002). Thus, genes with a role in the prefrontal dopaminergic system are good candidates for association with working memory. We chose as a candidate the DBH gene. The DBH enzyme is involved in the conversion of dopamine to norepinephrine. Plasma levels of this enzyme are under genetic control by the DBH gene, which is known to have several polymorphisms. One of these polymorphisms, a G-to-A substitution at position 444, exon 2 (G444A) on chromosome 9, affects DBH levels in plasma and cerebrospinal fluid (CSF), with the A allele associated with lower levels of the enzyme (Cubells et al., 2000). We predicted that variation in the DBH gene would be related to individual differences in the working memory task, but not the visuospatial attention task.
Method
Participants
Complete behavioral and genomic data were obtained for a sample of 103 healthy adults (69 females, 34 males) with a mean age of 31.5 (± 2.1) years.
Tasks
The same spatial attention and working memory tasks from Experiment 1 were used.
Genotyping
Genomic material was obtained via buccal cell brush and prepared as described for Experiment 1. To detect the G444A polymorphism, we administered forward (5′-cctggagcccagtgcttgtc-3′) and reverse (5′-acgccctcctgggtactcgc-3′) genomic PCR primers, followed by digestion with EcoNI (Cubells et al., 1998). Using these methods, we subdivided the sample of 103 participants into three groups based on the number of G alleles, none (AA genotype, n = 17), one (AG genotype, n = 39), or two (GG genotype, n = 47).
Results
Visuospatial Attention
Accuracy on this task was above 92% and so was not analyzed further. The means of the median RTs (see Table 2) were submitted to ANOVA, with factors of genotype (the three DBH subgroups), cue validity, and SOA. Effects were obtained for cue validity, F(2, 200) = 53.6, p < .00001; SOA, F(1, 100) = 23.2, p < .0001; and the Validity × SOA interaction, F(2, 200) = 4.8, p < .01, reflecting the expected effects of location cuing in the spatial attention task. Neither the main effect of DBH genotype, F < 1, nor its interaction with cue validity, F(4, 200) = 1.8, p = .13, was significant. However, the Genotype × SOA × Cue Validity interaction was significant, F(4, 200) = 3.9, p < .01, which probably reflects the somewhat anomalous trend for the AA genotype to have low RT for invalid cues in the 500-ms SOA (resulting in negative cost; see Table 2). Because we tested for genotype effects of CHRNA4 at the 2,000-ms SOA in Experiment 1, we repeated those analyses for DBH genotype. Neither RT benefits (Fig. 2a), F(2, 100) = 1.9, p = .15, nor RT costs (Fig. 2b), F(2, 100) = 1.7, p = .19, were significantly associated with DBH genotype.
TABLE 2.
Mean of Median Reaction Times (in Milliseconds) on the Visuospatial Attention Task for the Three Dopamine Beta-Hydroxylase (DBH) Genotypes
| Genotype
|
|||
|---|---|---|---|
| Cue validity | AA | AG | GG |
| 500-ms SOA | |||
| Valid | 555.4 (24.7) | 577.6 (14.7) | 567.5 (15.8) |
| Neutral | 590.8 (24.9) | 593.4 (13.3) | 582.8 (16.7) |
| Invalid | 573.5 (23.9) | 622.2 (15.4) | 615.9 (19.7) |
| 2,000-ms SOA | |||
| Valid | 585.5 (27.5) | 596.1 (14.1) | 584.1 (15.3) |
| Neutral | 579.3 (26.3) | 602.6 (14.3) | 599.7 (16.5) |
| Invalid | 615.1 (29.8) | 636.5 (18.2) | 633.3 (18.5) |
Note. The three genotypes AA, AG, and GG correspond to increasing gene dose (0, 1, and 2, respectively) of the G allele of the DBH gene. Standard errors are in parentheses. SOA = stimulus-onset asynchrony.
Fig. 2.
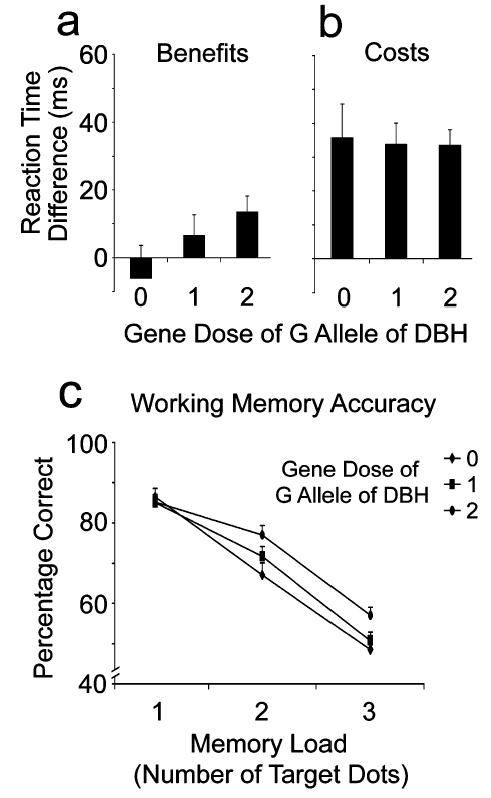
Effects of allelic variation in the dopamine beta-hydroxylase (DBH) gene on visuospatial attention in the 2,000-ms stimulus-onset asynchrony condition and on working memory: (a) reaction time (RT) benefits of valid location cues in the visuospatial attention task (neutral RT – valid RT), (b) RT costs of invalid location cues in the same task (invalid RT – neutral RT), and (c) match accuracy in the working memory task as a function of number of spatial locations to be maintained in working memory. The three genotypes AA, AG, and GG correspond to increasing gene dose (0, 1, 2) of the G allele of the DBH gene.
Working Memory
The effect of memory load was significant, F(2, 200) = 242.1, p < .0001; accuracy declined with the number of target locations to be remembered. The main effect of DBH genotype was not significant, F(2, 100) = 2.1, p = .13, but the Genotype × Memory Load interaction was, F(4, 200) = 2.7, p < .05. As Figure 2c shows, although accuracy was equivalent for all three genotypes at the lowest memory load, it was higher with higher gene doses of the G allele at the other two memory loads, and particularly the highest (three targets), as confirmed by a simple effects analysis, F(2, 100) = 3.1, p < .05, d = 0.25. Memory accuracy was significantly (p < .05) greater for the GG genotype (G gene dose = 2) than for both the AG genotype (G gene dose = 1) and the AA genotype (G gene dose = 0).
Intertask Correlations
Working memory accuracy at the highest load correlated .07 with RT benefits and −.16 with RT costs at the 2,000-ms SOA. The correlation between overall working memory accuracy and average RT on the visuospatial attention task was −.11. All correlations were nonsignificant (p > .05).
Discussion
The ability to maintain memory for location over a delay was significantly reduced as the number of locations increased, confirming the sensitivity of the working memory task to increased memory load. Increasing gene dose of the G allele of the DBH gene was associated with better working memory performance as memory load increased. The effect of genotype was most apparent at the highest memory load tested (three target locations). Thus, the association between the DBH gene and working memory was particularly marked under conditions that most taxed the working memory system.
Cubells et al. (1998) reported that the G444A polymorphism of the DBH gene influences levels of the DBH enzyme in CSF. Immunohistochemical studies have shown high concentrations of DBH-labeled fibers in several prefrontal cortical sites in postmortem human brain (Gaspar, Berger, Febvret, Vigny, & Henry, 1989). Although the precise relationship between the enzymatic activity of DBH and human brain dopamine levels is not known, the association we found between DBH genotype and working memory is consistent with the well-known role of dopaminergic agents in prefrontal cortex and its dopaminergic mediation of working memory (Abi-Dargham et al., 2002). At the same time, performance on the visuospatial attention task was not consistently associated with the DBH gene. With the sample size of 103, power to detect a moderate (0.25) effect of DBH on visuospatial attention exceeded 94%.
That retention in working memory decreased with the number of A alleles of the DBH gene can be considered in the context of evidence of the role of the A allele in mental disorders. A low plasma level of the DBH enzyme—associated with the A allele (Cubells et al., 1998)—is a risk factor for depression (Meyers et al., 1999). Wood, Joyce, Miller, Mulder, and Kennedy (2002) found that among depressed individuals, inheritance of an A allele is associated with higher levels of other psychiatric symptoms, suggesting that the GG genotype may protect against development of more severe symptoms. Our results indicate that the GG genotype may also allow for more efficient working memory within the normal range. Given evidence that working memory is a core deficit of schizophrenia (Silver, Feldman, Bilker, & Gur, 2003), the effect of DBH genotype on brain dopamine and norepinephrine levels may be common to both normal individual variation in working memory and abnormal changes characteristic of schizophrenia.
GENERAL DISCUSSION
In Experiment 1, we investigated a polymorphism in the CHRNA4 gene, which regulates binding of nAChR alpha4 subunits in parietal cortex (Marutle, Warpman, Bogdanovic, Lannfelt, & Nordberg, 1999), a critical brain region for visuospatial attention (Corbetta et al., 2000). Increasing gene dose of the C allele of CHRNA4 was systematically associated with the costs and benefits of location cuing in a spatial attention task, but not with performance on a working memory task.
In Experiment 2, we investigated a polymorphism in the DBH gene, the A allele of which is associated with lower plasma and CSF levels of the DBH enzyme (Cubells et al., 2000), which is involved in the conversion of dopamine into norepinephrine. Given prefrontal dopaminergic mediation of working memory (Muller et al., 1998), we predicted an association between DBH genotype and individual differences in working memory, but not visuospatial attention. The results supported this prediction.
Together, Experiments 1 and 2 provided complementary results. In a sample of 89 healthy, unrelated persons, visuospatial attention was associated with CHRNA4, but not with the DBH gene. In another sample of 103 healthy adults, working memory was associated with the DBH gene, but not with CHRNA4. These findings are consistent with a double dissociation between the effects of the CHRNA4 and DBH genes on attention and working memory.
That genetic modulation of visuospatial attention and working memory can be differentiated also has implications for understanding the links between these two cognitive functions. Notwithstanding the substantial converging evidence for cholinergic involvement in parietally mediated spatial attention, and for dopaminergic control of prefrontally mediated working memory, these neurocognitive systems may also overlap. Awh, Anllo-Vento, and Hillyard (2000) proposed that visuospatial attention is the rehearsal mechanism for working memory, given neuroimaging evidence that the networks mediating visuospatial attention and working memory overlap at frontal and parietal sites. Working memory tasks also activate parietal regions (Jonides et al., 1993). Bleckley, Durso, Crutchfield, Engle, and Khanna (2003) showed that individuals with high working memory capacity are better able to flexibly allocate visuospatial attention than individuals with low capacity. Finally, there is physiological evidence of cholinergic modulation of dopaminergic neurons (Gurden, Takita, & Jay, 2000).
Despite these indicators of anatomical, neurochemical, and functional overlap between visuospatial attention and working memory, our results indicate that examining polymorphisms in neurotransmitter genes can differentiate these cognitive systems. The double dissociation we found, and the moderate to large effect sizes, are particularly striking indicators of the strength of the associations. Moreover, the task components of attention and working memory for which genetic associations were found were not significantly intercorrelated.
Notwithstanding our finding that CHRNA4 and the DBH gene modulate individual differences in components of visuospatial attention and working memory with moderate to large effect sizes (.25 to .45), other genes and environmental factors undoubtedly also play a role. Furthermore, only particular processing components of these tasks were differentially related to CHRNA4 and DBH genotype: Overall RT in the visuospatial attention task and average retention accuracy in the working memory task were unaffected. The use of these processing components as phenotypes to test for genetic associations rests on the assumption that they are reliable. We confirmed that they are by examining test-retest correlations in a subset of the participants (n = 73) who performed the attention and working memory tasks twice. Reliability coefficients were .67 for RT benefits and .72 for RT costs at the 2,000-ms SOA and .64 for working memory accuracy in the three-location condition.
These results add to the growing body of evidence that normal variation in single genes can be reliably associated with individual differences in components of cognition (Fossella et al., 2002; Greenwood et al., 2000). It must be emphasized that we obtained relatively large effects of normal genetic variation on cognitive performance in healthy individuals. We also calculated an effect size of 0.41 for the association between the COMT (catechol-O-methyltransferase) gene and executive function in healthy adults reported by Egan et al. (2001). These findings contrast with the low (~ 0.01) effect sizes often found in genetic association studies of disease (Ioannidis, Ntzani, Trikalinos, & Contopoulos-Ioannidis, 2001). The present results show that it is possible to detect genotype-cognition associations in healthy individuals with moderate-size samples, given that candidate genes are chosen on the basis of theories of brain function, and that appropriate cognitive task components are chosen as phenotypes.
Acknowledgments
This work was supported by National Institutes of Health Grant AG 19653 to R.P.
References
- Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, Hwang DR, Keilp J, Kochan L, Van Heertum R, Gorman JM, Laruelle M. Prefrontal dopamine D1 receptors and working memory in schizophrenia. Journal of Neuroscience. 2002;22:3708–3719. doi: 10.1523/JNEUROSCI.22-09-03708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Eisenberg HM, Albuquerque EX. Nicotinic receptor activation in human cerebral cortical interneurons: A mechanism for inhibition and disinhibition of neuronal networks. Journal of Neuroscience. 2000;20:66–75. doi: 10.1523/JNEUROSCI.20-01-00066.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awh E, Anllo-Vento L, Hillyard SA. The role of spatial selective attention in working memory for locations: Evidence from event-related potentials. Journal of Cognitive Neuroscience. 2000;12:840–847. doi: 10.1162/089892900562444. [DOI] [PubMed] [Google Scholar]
- Bleckley MK, Durso FT, Crutchfield JM, Engle RW, Khanna MM. Individual differences in working memory capacity predict visual attention allocation. Psychonomic Bulletin & Review. 2003;10:884–889. doi: 10.3758/bf03196548. [DOI] [PubMed] [Google Scholar]
- Cohen, J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale, NJ: Erlbaum.
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nature Neuroscience. 2000;3:292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Craig, I.W., & McClay, J. (2003). The role of molecular genetics in the postgenomic era. In R. Plomin, J.C. DeFries, I.W. Craig, & P. McGuffin (Eds.), Behavioral genetics in the postgenomic era (pp. 19–40). Washington, DC: American Psychological Association.
- Cubells JF, Kranzler HR, McCance-Katz E, Anderson GM, Malison RT, Price LH, Gelernter J. A haplotype at the DBH locus, associated with low plasma dopamine beta-hydroxylase activity, also associates with cocaine-induced paranoia. Molecular Psychiatry. 2000;5:56–63. doi: 10.1038/sj.mp.4000657. [DOI] [PubMed] [Google Scholar]
- Cubells JF, van Kammen DP, Kelley ME, Anderson GM, O’Connor DT, Price LH, Malison R, Rao PA, Kobayashi K, Nagatsu T, Gelernter J. Dopamine beta-hydroxylase: Two polymorphisms in linkage disequilibrium at the structural gene DBH associate with biochemical phenotypic variation. Human Genetics. 1998;102:533–540. doi: 10.1007/s004390050736. [DOI] [PubMed] [Google Scholar]
- Davidson MC, Marrocco RT. Local infusion of scopolamine into intraparietal cortex slows covert orienting in rhesus monkeys. Journal of Neurophysiology. 2000;83:1536–1549. doi: 10.1152/jn.2000.83.3.1536. [DOI] [PubMed] [Google Scholar]
- Diamond A, Briand L, Fossella J, Gehlbach L. Genetic and neurochemical modulation of prefrontal cognitive functions in children. American Journal of Psychiatry. 2004;161:125–132. doi: 10.1176/appi.ajp.161.1.125. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proceedings of the National Academy of Sciences, USA. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annual Review of Psychology. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- Fan J, Fossella JA, Sommer T, Wu Y, Posner MI. Mapping the genetic variation of attention onto brain activity. Proceedings of the National Academy of Sciences, USA. 2003;100:7406–7411. doi: 10.1073/pnas.0732088100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Wu Y, Fossella JA, Posner MI. Assessing the heritability of attentional networks. BMC Neuroscience. 2001;2(1):14–18. doi: 10.1186/1471-2202-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores CM, DeCamp RM, Kilo S, Rogers SW, Hargreaves KM. Neuronal nicotinic receptor expression in sensory neurons of the rat trigeminal ganglion: Demonstration of alpha3beta4, a novel subtype in the mammalian nervous system. Journal of Neuroscience. 1996;16:7892–7901. doi: 10.1523/JNEUROSCI.16-24-07892.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossella J, Sommer T, Fan J, Wu Y, Swanson JM, Pfaff DW, Posner MI. Assessing the molecular genetics of attention networks. BMC Neuroscience. 2002;3(1):14–19. doi: 10.1186/1471-2202-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar P, Berger B, Febvret A, Vigny A, Henry JP. Catecholamine innervation of the human cerebral cortex as revealed by comparative immunohistochemistry of tyrosine hydroxylase and dopamine-beta-hydroxylase. Journal of Comparative Neurology. 1989;279:249–271. doi: 10.1002/cne.902790208. [DOI] [PubMed] [Google Scholar]
- Greenwood P, Parasuraman R. Normal genetic variation, cognition, and aging. Behavioral and Cognitive Neuroscience Reviews. 2003;2:278–306. doi: 10.1177/1534582303260641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood PM, Sunderland T, Friz JL, Parasuraman R. Genetics and visual attention: Selective deficits in healthy adult carriers of the varepsilon 4 allele of the apolipoprotein E gene. Proceedings of the National Academy of Sciences, USA. 2000;97:11661–11666. doi: 10.1073/pnas.97.21.11661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurden H, Takita M, Jay TM. Essential role of D1 but not D2 receptors in the NMDA receptor-dependent long-term potentiation at hippocampal-prefrontal cortex synapses in vivo. Journal of Neuroscience. 2000;20:RC106. doi: 10.1523/JNEUROSCI.20-22-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis JP, Ntzani EE, Trikalinos TA, Contopoulos-Ioannidis DG. Replication validity of genetic association studies. Nature Genetics. 2001;29:306–309. doi: 10.1038/ng749. [DOI] [PubMed] [Google Scholar]
- Jonides J, Smith EE, Koeppe RA, Awh E, Minoshima S, Mintun MA. Spatial working memory in humans as revealed by PET. Nature. 1993;363:623–625. doi: 10.1038/363623a0. [DOI] [PubMed] [Google Scholar]
- Marutle A, Warpman U, Bogdanovic N, Lannfelt L, Nordberg A. Neuronal nicotinic receptor deficits in Alzheimer patients with the Swedish amyloid precursor protein 670/671 mutation. Journal of Neurochemistry. 1999;72:1161–1169. doi: 10.1046/j.1471-4159.2000.0721161.x. [DOI] [PubMed] [Google Scholar]
- Mentis MJ, Sunderland T, Lai J, Connolly C, Krasuski J, Levine B, Friz J, Sobti S, Schapiro M, Rapoport SI. Muscarinic versus nicotinic modulation of a visual task: A PET study using drug probes. Neuropsychopharmacology. 2001;25:555–564. doi: 10.1016/S0893-133X(01)00264-0. [DOI] [PubMed] [Google Scholar]
- Meyers BS, Alexopoulos GS, Kakuma T, Tirumalasetti F, Gabriele M, Alpert S, Bowden C, Meltzer HY. Decreased dopamine beta-hydroxylase activity in unipolar geriatric delusional depression. Biological Psychiatry. 1999;45:448–452. doi: 10.1016/s0006-3223(98)00085-7. [DOI] [PubMed] [Google Scholar]
- Muller U, von Cramon DY, Pollmann S. D1- versus D2-receptor modulation of visuospatial working memory in humans. Journal of Neuroscience. 1998;18:2720–2728. doi: 10.1523/JNEUROSCI.18-07-02720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasuraman R, Greenwood PM, Haxby JV, Grady CL. Visuospatial attention in dementia of the Alzheimer type. Brain. 1992;115:711–733. doi: 10.1093/brain/115.3.711. [DOI] [PubMed] [Google Scholar]
- Parasuraman R, Greenwood PM, Sunderland T. The apolipoprotein E gene, attention, and brain function. Neuropsychology. 2002;16:254–274. doi: 10.1037//0894-4105.16.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JM, McAlonan K, Robb WG, Brown VJ. Cholinergic neurotransmission influences covert orientation of visuospatial attention in the rat. Psychopharmacology (Berlin) 2000;150:112–116. doi: 10.1007/s002130000437. [DOI] [PubMed] [Google Scholar]
- Plomin R, Crabbe J. DNA Psychological Bulletin. 2000;126:806–828. doi: 10.1037/0033-2909.126.6.806. [DOI] [PubMed] [Google Scholar]
- Plomin R, Kosslyn S. Genes, brain, and cognition. Nature Neuroscience. 2001;4:1153–1155. doi: 10.1038/nn1201-1153. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Johnson MK, D’Esposito M. Prefrontal activity associated with working memory and episodic long-term memory. Neuropsychologia. 2003;41:378–389. doi: 10.1016/s0028-3932(02)00169-0. [DOI] [PubMed] [Google Scholar]
- Silver H, Feldman P, Bilker W, Gur RC. Working memory deficit as a core neuropsychological dysfunction in schizophrenia. American Journal of Psychiatry. 2003;160:1809–1816. doi: 10.1176/appi.ajp.160.10.1809. [DOI] [PubMed] [Google Scholar]
- Steinlein OK, Deckert J, Nothen MM, Franke P, Maier W, Beckmann H, Propping P. Neuronal nicotinic acetylcholine receptor alpha 4 subunit (CHRNA4) and panic disorder: An association study. American Journal of Medical Genetics. 1997;74(2):199–201. doi: 10.1002/(sici)1096-8628(19970418)74:2<199::aid-ajmg17>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Steinlein OK, Magnusson A, Stoodt J, Bertrand S, Weiland S, Berkovic SF, Nakken KO, Propping P, Bertrand D. An insertion mutation of the CHRNA4 gene in a family with autosomal dominant nocturnal frontal lobe epilepsy. Human Molecular Genetics. 1997;6:943–947. doi: 10.1093/hmg/6.6.943. [DOI] [PubMed] [Google Scholar]
- Voytko ML. Cognitive functions of the basal forebrain cholinergic system in monkeys: Memory or attention? Behavioural Brain Research. 1996;75:13–25. doi: 10.1016/0166-4328(95)00143-3. [DOI] [PubMed] [Google Scholar]
- Wood JG, Joyce PR, Miller AL, Mulder RT, Kennedy MA. A polymorphism in the dopamine beta-hydroxylase gene is associated with “paranoid ideation” in patients with major depression. Biological Psychiatry. 2002;51:365–369. doi: 10.1016/s0006-3223(01)01367-1. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Huguenard JR, Prince DA. Cholinergic switching within neocortical inhibitory networks. Science. 1998;281:985–988. doi: 10.1126/science.281.5379.985. [DOI] [PubMed] [Google Scholar]


