Abstract
MntH, a bacterial homolog of mammalian natural resistance associated macrophage protein 1 (Nramp1), is a primary transporter for Mn2+ influx in Salmonella enterica serovar Typhimurium and Escherichia coli. S. enterica serovar Typhimurium MntH contributes to H2O2 resistance and is important for full virulence. Consistent with its phenotype and function, mntH is regulated at the transcriptional level by both H2O2 and substrate cation. We have now identified three trans-acting regulatory factors and the three corresponding cis-acting mntH promoter motifs that mediate this regulation. In the presence of hydrogen peroxide, mntH is activated by OxyR, acting through an OxyR-binding motif centered just upstream of the likely −35 RNA polymerase-binding site. In the presence of Fe2+, mntH is repressed primarily by Fur, acting through a Fur-binding motif overlapping the −35 region. In the presence of Mn2+, mntH is repressed primarily by the Salmonella equivalent of E. coli b0817, a distant homolog of the Bacillus subtilis manganese transport repressor, MntR, acting through an inverted-repeat motif located between the likely −10 polymerase binding site and the ribosome binding site. E. coli b0817 was recently shown to bind the identical inverted-repeat motif in the E. coli mntH promoter and hence has been renamed MntR (S. I. Patzer and K. Hantke, J. Bacteriol. 183:4806-4813, 2001). Using Δfur, ΔmntR, and Δfur ΔmntR mutant strains as well as mutations in the Fur- and MntR-binding motif elements, we found that Fe2+ can also mediate repression through the Mn2+ repressor MntR.
Nramp1, the product of the ity/bcg/lsh locus in the mouse, is the type member of a protein family now found throughout the domains Eucarya and Bacteria (7), with putative homologs recently identified in some members of the domain Archaea (23). Nramp1 proteins confer “natural resistance” to phylogenetically and antigenically diverse microbial pathogens, exemplified in the mouse resistance to Salmonella enterica serovar Typhimurium, Mycobacterium bovis, and Leishmania donovani. Nramp1 is expressed strongly in macrophages and neutrophils and is recruited to the phagosomal membrane following phagocytosis. It is an electrogenic divalent cation transporter like its biochemically characterized homolog Nramp2. Its substrate specificity has not yet been fully detailed, but direct evidence that it can transport Fe2+ has recently been published (15) to complement previous indirect data that it can transport both Fe2+ and Mn2+ (4, 22, 25). Like Nramp2, it is energized by the proton gradient (15, 18).
The few bacterial Nramp homologs biochemically characterized all appear to transport Mn2+ and have been named MntH. Derepression of Bacillus subtilis MntH increases sensitivity to growth inhibition by Mn2+ (35). Mn2+ inhibits Ni2+ uptake in Xenopus laevis oocytes transfected with the Mycobacterium tuberculosis MntH homolog, Mramp (1). Direct 54Mn2+ and 55Fe2+ uptake studies have shown that Escherichia coli (24, 27) and S. enterica serovar Typhimurium (24) MntH proteins transport both metals. However, kinetic analysis further showed that MntH is highly selective for Mn2+ under physiological conditions and contributes little or nothing to Fe2+ uptake (24). Collectively these findings suggest that Mn2+ is of general importance in both free-living and pathogenic bacteria. This hypothesis is supported by the findings that MntH contributes to H2O2 resistance in both E. coli and S. enterica serovar Typhimurium, that S. enterica serovar Typhimurium mntH is induced upon entry into murine macrophages, and that S. enterica serovar Typhimurium MntH function is required for full virulence even in BALB/c mice which are themselves functionally Nramp1−/− (24). Certain metal-dependent growth phenotypes are also affected by mutation of the E. coli mntH locus (27).
Clues to the physiological importance of Mn2+ in S. enterica serovar Typhimurium can be found by examining how its uptake and homeostasis are regulated. In our initial mntH characterization (24) we noted that addition of peroxide or starvation for a divalent cation (but not generation of an intracellular superoxide radical) regulated mntH expression. Consistent with these phenotypic data, we noted strong candidate OxyR- and Fur-binding sequence motifs in the mntH promoter region, as well as a striking inverted repeat between the putative −10 and ribosome binding sites. We have now confirmed the identities of three trans-acting factors and three corresponding cis-acting DNA motifs which mediate H2O2 and metal responses. While additional regulators of S. enterica serovar Typhimurium Mn2+ transport likely remain to be discovered, these results provide an initial baseline for understanding mntH expression.
MATERIALS AND METHODS
Construction of cis-acting DNA mutations in the mntH promoter.
Four mntH::lacZ reporter plasmids were used in this study. pMLZ104 contains the wild-type mntH promoter. pDGK261, pDGK262, and pDGK263 are identical to pMLZ104 except for block substitution mutations in the OxyR-, Fur-, and MntR-binding motifs, respectively. pMLZ104 (24) is derived from mini-F′ plasmid pFZY1 and places transcription of lacZYA under the control of an EcoRI-HindIII fragment encompassing the 120 bp of nupC encoding the N terminus of the product, the 342-bp nupC-mntH intragenic region, and the 308 bp of mntH encoding the N terminus of the product. Block substitutions into putative cis-acting elements were derived from the bacteriophage P22 Pant promoter by PCR as described below. All primer sequences are available from the authors upon request.
Initially, a high-copy-number plasmid pDGK247, in which three novel restriction sites were created between suspected mntH promoter motifs, was constructed. An upstream KpnI-SalI piece of the insert was amplified from pMLZ104's high-copy-number precursor, pDGK227 (24), with sense primer DK133 and antisense mutagenic primer DK134 and ligated into pBluescript II SK(+) to yield pDGK246. Then a downstream SalI-SacI piece of the insert was amplified from pDGK227 with sense mutagenic primer DK135 and antisense primer DK136 and ligated into the corresponding sites of pDGK246 to create pDGK247 containing a full-length EcoRI-HindIII insert bearing novel SphI, SalI, and BamHI sites. Responses of this modified promoter to all trans-acting factors were identical to that of the wild-type promoter. Motif mutants were made by PCR mutagenesis of pDGK247 by block substitutions of the three desired motifs. Since the Pant promoter of bacteriophage P22 (GenBank accession no. X01916) has similarly spaced −35, −10, ribosome binding, and translation start sites and since no endogenous S. enterica serovar Typhimurium transcription factors are known or suspected to bind in the region (16), motifs in the mntH promoter were replaced with equal lengths of isopositional sequence from Pant with two modifications. First, in changing the Fur-binding motif, which overlaps the −35 RNA polymerase binding region, the native mntH −35 hexamer was conserved. Second, to facilitate identification of the mutations once they were back in a single-copy vector, a 1- or 2-bp change was introduced in the Pant sequence to incorporate a distinct novel restriction enzyme site in each mutant. Basal activities of all three cis-acting motif mutants were somewhat lower than that of wild type as measured by mntH::lacZ activity following overnight growth in M9-glucose with no added H2O2 or inhibitory cation. These basal activities were, however, independent of strain background (trans-acting factor mutations); thus it was possible to normalize activity in any given assay to the relevant basal activity in the absence of any additives to the culture.
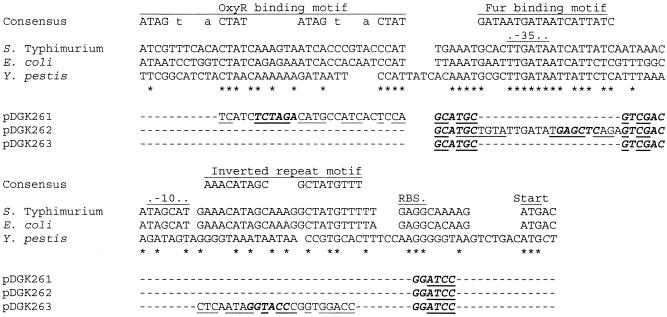
Upstream modified T7 primer DK146 and mutagenic antisense primer DK143 yielded an EcoRI-SphI fragment upon digestion, which was substituted back into pDGK247 to yield pDGK251, in which the suspected OxyR-binding motif of the mntH promoter was replaced an by equal-length isopositional Pant sequence containing a novel diagnostic XbaI site. Mutagenic sense primer DK144 and downstream modified T3 primer DK147 yielded an SphI-HindIII fragment upon digestion, which was substituted back into pDGK247 to yield pDGK252, in which the suspected Fur-binding motif of the mntH promoter was replaced by an equal-length isopositional Pant sequence containing a diagnostic SacI site. Upstream modified T7 primer DK146 and mutagenic antisense primer DK150 yielded an EcoRI-BamHI fragment upon digestion, which was substituted back into pDGK247 to yield pDGK253 to replace the suspected MntR-binding motif by an equal-length isopositional Pant sequence containing a novel diagnostic KpnI site. To make the final block substitution reporter constructs, the EcoRI-HindIII insert in pMLZ104 was replaced by the EcoRI-HindIII inserts from pDGK251, pDGK252, and pDGK253 to yield pDGK261, pDGK262, and pDGK263, respectively. Mutations were confirmed on the basis of XbaI, SacI, and KpnI digestion patterns, respectively, followed by the sequencing of the entire promoter region. The specific mutant sequences obtained after the Pant substitutions are shown in Fig. 1.
FIG. 1.
Wild-type and mutant candidate cis-acting motifs in the mntH promoter. Proximal regions of the mntH promoters from S. enterica serovar Typhimurium (24), E. coli (24, 27), and Y. pestis (5) were aligned and compared to consensus motifs for OxyR (40) and Fur (2, 9). The S. enterica serovar Typhimurium and E. coli mntH promoters both have a candidate OxyR-binding site upstream of the predicted −35 region and a candidate Fur-binding site overlapping and extending downstream of the −35 region. Also highlighted is an inverted-repeat motif between the predicted −10 region and the ribosome binding site (RBS) of the mntH coding region. Of these three motifs, only a putative Fur-binding site is readily discernible in the Y. pestis mntH promoter. Below these motifs are lines indicating the substitutions made in the three cis-acting S. enterica serovar Typhimurium promoter mutants used in this study, pDGK261, pDGK262, and pDGK263. Identical nucleotides in the three promoter regions (asterisks), novel restriction sites introduced between motifs or within block substitutions (described in Materials and Methods) (bases in bold italic type), and isopositional sequences from the bacteriophage P22 Pant promoter (bases in lightface roman type) are indicated. As some substituted bases fortuitously matched those found in the wild-type S. enterica serovar Typhimurium mntH promoter, the bases actually changed in pDGK261, pDGK262, and pDGK263 are underlined. In the consensus OxyR-binding motif the bases shown in lowercase type are common but not completely conserved bases at these sites (38).
Construction of trans-acting element mutations and double-mutant strains.
Strains MM2211 (JA4023), bearing an oxyR::Tn10 insertion mutation, and MM2212 (JA3199), bearing the zbf-5126::Tn10 fur-9 mutations, were obtained from J. Foster, while strains MM2625 (JS219), bearing the fur41::kan deletion mutation, and MM2626 (JS220), bearing the mntR51::cam deletion mutation were made by the method of Datsenko and Wanner (11). Mutant alleles were transferred into the SL1344 strain background by P22 transduction by standard methods. Subsequently, P22 transduction was also used to construct strains carrying mutations in the various combinations of cis- and trans-acting factors (Table 1).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype and/or construction | Source or reference |
|---|---|---|
| Strains | ||
| MM2089 | SL1344 wild type | 24 |
| MM2211 | UK1 oxyR::Tn10 (JA4023) | J. Fostera |
| MM2212 | SF1 iroA::MudJ zbf-5126::Tn10 fur-9 (JA3199) | J. Foster |
| MM2625 | 14028 Δfur41::kan (JS219) | This study |
| MM2626 | 14028 ΔmntR51::cam (JS220) | This study |
| MM2525 | SL1344 oxyR::Tn10; P22 from MM2211 into SL1344 | This study |
| MM2526 | SL1344 zbf-5126::Tn10 fur-9; P22 from MM2212 into SL1344 | This study |
| MM2635 | SL1344 Δfur41::kan; P22 from MM2625 into SL1344 | This study |
| MM2634 | SL1344 ΔmntR51::cam; P22 from MM2626 into SL1344 | This study |
| MM2656 | SL1344 Δfur41::kan ΔmntR51::cam; P22 from MM2625 into MM2634 | This study |
| MM2507 | MM2089/pMLZ104 | This study |
| MM2529 | MM2525/pMLZ104 | This study |
| MM2530 | MM2526/pMLZ104 | This study |
| MM2646 | MM2635/pMLZ104 | This study |
| MM2645 | MM2634/pMLZ104 | This study |
| MM2654 | MM2656/pMLZ104 | This study |
| MM2616 | MM2089/pDGK261 | This study |
| MM2617 | MM2089/pDGK262 | This study |
| MM2618 | MM2089/pDGK263 | This study |
| MM2762 | MM2646/pDGK262 | This study |
| MM2763 | MM2646/pDGK263 | This study |
| MM2759 | MM2645/pDGK262 | This study |
| MM2760 | MM2645/pDGK263 | This study |
| Plasmids | ||
| pMLZ104 | pFZY1 mini-F′ plasmid derivative, with an mntH::lacZYA fusion controlled by wild-type mntH promoter on an EcoRI-HindIII insert | 24 |
| pDGK227 | pBluescript II SK(+) containing EcoRI-HindIII mntH promoter insert from pMLZ104 | 24 |
| pDGK246 | Intermediate in construction of pDGK247 (see Materials and Methods) | This study |
| pDGK247 | pBluescript II SK (+) containing EcoRI-HindIII mntH promoter insert containing SphI, SalI, and BamHI sites between putative cis-acting elements in mntH promoter | This study |
| pDGK251 | pDGK247 with P22 Pant sequence and XbaI site in place of PmntH OxyR-binding motif | This study |
| pDGK252 | pDGK247 with P22 Pant sequence and SacI site in place of PmntH Fur-binding motif | This study |
| pDGK253 | pDGK247 with P22 Pant sequence and KpnI site in place of PmntH MntR-binding motif | This study |
| pDGK261 | pMLZ104 with P22 Pant sequence and XbaI site in place of PmntH OxyR-binding motif | This study |
| pDGK262 | pMLZ104 with P22 Pant sequence and SacI site in place of PmntH Fur-binding motif | This study |
| pDGK263 | pMLZ104 with P22 Pant sequence and KpnI site in place of PmntH MntR-binding motif | This study |
Department of Microbiology, University of South Alabama, Mobile.
Growth and treatment of cells for transcription reporter assays.
S. enterica serovar Typhimurium SL1344 strains bearing the desired chromosomal mutation and reporter plasmid were grown aerobically overnight at 37°C from 7% glycerol frozen stocks in Luria-Bertani medium. The medium contained 50 μg of ampicillin/ml to maintain the reporter plasmid and 50 μg of kanamycin/ml or 25 μg of chloramphenicol/ml depending on the marker carried by the chromosomal insertion. Overnight cultures were washed twice and resuspended at an optical density at 600 nm (OD600) of 0.5 to 1.5 in M9 salts (6.4 g of Na2HPO4 · 7H2O, 15 g of anhydrous KH2PO4, 2.5 g of NaCl, and 5 g of NH4Cl per liter) supplemented with 1 mM MgSO4 and 0.0021% (wt/vol) histidine (since SL1344 is a histidine auxotroph). Unless otherwise specified, the term M9 refers to this Mg2+- and histidine-supplemented medium. For H2O2 challenge experiments, M9-washed cells were diluted to an OD600 of 0.020 in the same medium supplemented with 0.2% (wt/vol) glucose and 50 μg of ampicillin/ml, grown at 37°C to an OD600 of 0.2 to 0.3 either aerobically (with shaking at 225 rpm) or “microaerobically” (full, tightly capped tubes without agitation), and then treated with H2O2 that was freshly diluted into sterile H2O from 3 or 30% stocks. For metal repression experiments, M9-washed cells were diluted to an OD600 of 0.020 in M9 medium supplemented with 0.2% (wt/vol) glucose, 50 μg of ampicillin/ml, and the desired concentration of metal and grown for 18 to 24 h overnight at 37°C to an OD600 of 0.9 to 1.5.
β-Galactosidase assays.
Aliquots of cells were subjected to colorimetric β-galactosidase activity assays using o-nitrophenyl-β-d-galactopyranoside as the substrate in accordance with standard methods. Activity is expressed in Miller units, defined as 1,000 times the scattering-corrected OD420 per OD600 of cells per minute.
RESULTS
Sequence of the mntH promoter.
Genomic sequence inspection suggests that mntH is a stand-alone transcriptional unit in many γ-proteobacteria. S. enterica serovar Typhimurium, E. coli, and Yersinia pestis share a gene arrangement in which mntH and the nucleoside permease gene, nupC, are transcribed divergently with approximately 300 bp separating the two open reading frames (25). The available partial genomic sequence indicates, by contrast, that the mntH and nupC genes of Klebsiella pneumoniae (Klebsiella pneumoniae Genome Server, http://genome.wustl.edu/gsc/Projects/K.pneumoniae, 2002) are unlinked. When the nupC-mntH intragenic sequences from the first three organisms are aligned and regions corresponding to documented nupC cis-acting elements are excluded from consideration (10), about 100 to 150 bp remains. This presumably contains the entire mntH promoter, as experiments with the wild-type gene and a gene controlled only by 150 bp 5′ to the coding sequence show the same activity (data not shown). Inspection and computer predictions identify likely −35 and −10 RNA polymerase binding sites and a reasonable Shine-Dalgarno ribosome binding site (Fig. 1). With the knowledge that H2O2 and EDTA each induce E. coli and S. enterica serovar Typhimurium mntH, plausible candidate OxyR- and Fur-binding motifs can readily be discerned in these two promoters (Fig. 1). Also evident in these promoters (but not in that of Y. pestis) is a perfect 9-bp inverted repeat separated by a 4-bp spacer, located between the −10 and Shine-Dalgarno sites.
Behavior of the wild-type mntH promoter.
To define wild-type expression, we used a previously constructed transcriptional fusion of a truncated mntH gene to a promoterless lacZYA operon on a nominally single-copy plasmid (mini-F replication origin) transformed into wild-type S. enterica serovar Typhimurium SL1344 (24). Since the chromosomal mntH locus remains intact in this system, reporter transcription should reflect wild-type physiology. There is no evidence for posttranscriptional regulation of mntH, and MntH (like LacZ) appears stable because bacteria stored at 4°C retain their initial 54Mn2+ transport capacity for several days (data not shown). Consequently, LacZ protein content and activity should faithfully reflect MntH content and activity.
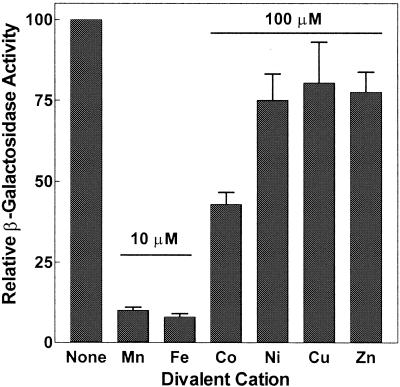
The wild-type reporter expressed significantly more LacZ in minimal medium (M9-glucose) than in complex medium (Luria-Bertani medium), and activity was greatest during late log phase and the transition to stationary phase. Whether this involves more than the interplay of the peroxide and metal regulators described below remains to be determined although, since identical responses were obtained in S. enterica serovar Typhimurium LT2, which lacks a functional rpoS, RpoS regulation is unlikely to be significant (data not shown). For the purposes of this study, however, it had two practical consequences. First, since there was negligible expression in early-log-phase cells, we used early-log-phase cultures growing in M9-glucose to evaluate activation by H2O2 or EDTA. Second, since both growth and LacZ activity were reproducible in wild-type cultures grown to saturation, we grew cells overnight in M9-glucose to evaluate repression by cations. Baseline metal repression behavior for the wild-type mntH::lacZ fusion shown in Fig. 2 and the dose-response curves of Fig. 4 indicate that Mn2+ and Fe2+ are the most potent repressors of mntH transcription. Co2+ can also repress but only at higher concentrations, while significant repression by 100 μM Ni2+, Cu2+, or Zn2+ was not observed (Fig. 2). Concentrations of divalent metals Mg2+ and Ca2+, monovalent metals Na+ and K+, and the osmolite choline chloride could be varied between 0 and 100 mM with no effect on mntH::lacZ expression (data not shown).
FIG. 2.
Repression of mntH transcription by transition metals. Wild-type reporter strain MM2507 (S. enterica serovar Typhimurium SL1344 bearing plasmid pMLZ104) was grown aerobically overnight at 37°C in M9-0.2% glucose-50 μg of ampicillin/ml containing the sulfate or chloride salts of various divalent first-row transition metals at a concentration of 10 or 100 μM as indicated. The β-galactosidase activity of saturated cultures (OD600 of 1.0 to 1.5 ) was normalized to that of a culture containing no added metal (about 300 Miller units). Results represent the means and standard errors of two to five independent experiments in each case.
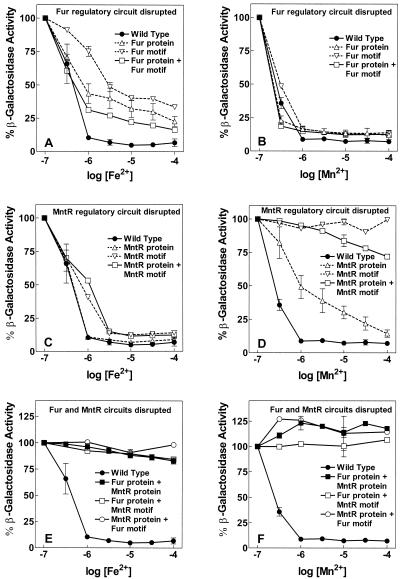
FIG. 4.
Control of iron and manganese repression by Fur and MntR. Cultures were grown overnight in M9-0.2% glucose-50 μg of ampicillin/ml with no added metal, with 10 μM MnSO4, or with 10 μM FeSO4. For each strain the β-galactosidase activity of metal-challenged cultures was normalized to that of the same strain grown in the absence of added metal. Results are the means and standard errors of two to five independent experiments. (A) Fe2+ repression dose-response curves comparing the wild-type strain/promoter combination (MM2507) to strains bearing a mutated Fur protein (MM2646), a mutated Fur-binding motif (MM2617), or both (MM2762). (B) Mn2+ repression dose-response curves for the same four strains as in panel A. (C) Fe2+ repression dose-response curves comparing the wild-type strain/promoter combination (MM2507) to strains bearing a mutated MntR protein (MM2645), a mutated MntR-binding motif (MM2618), or both (MM2760). (D) Mn2+ repression dose-response curves for the same four strains as in panel C. (E) Fe2+ repression dose-response curves comparing the wild-type strain/promoter combination (MM2507) to strains bearing a mutated Fur protein and a mutated MntR protein (MM2654), a mutated Fur protein and a mutated MntR-binding motif (MM2763), or a mutated MntR protein and a mutated Fur-binding motif (MM2759). (F) Mn2+ repression dose-response curves for the same four strains as in panel E.
To evaluate cis-acting promoter elements, we constructed block substitution mutants lacking individual putative binding motifs. The replacement sequence (Fig. 1) was derived from the Pant promoter of bacteriophage P22 since its −35, −10, and Shine-Dalgarno elements are spaced similarly to those of mntH and since no endogenous S. enterica serovar Typhimurium transcription factors appear to influence ant transcriptional activity (15). To evaluate trans-acting regulatory factors, we obtained or constructed S. enterica serovar Typhimurium SL1344 derivatives (Table 1) bearing defined mutations in OxyR, Fur, and MntR. MntR is b0817 in Blattner E. coli notation (6).
OxyR mediates H2O2 activation of mntH.
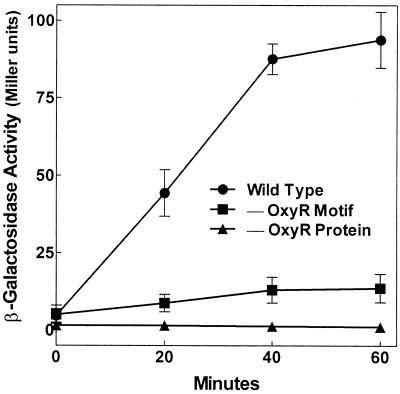
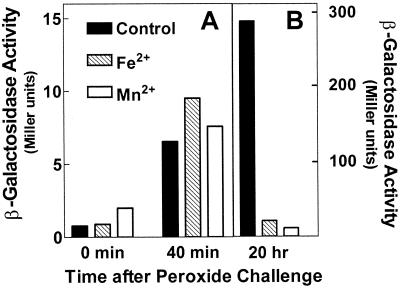
The wild-type promoter can be induced during log-phase growth by addition of H2O2. The time course for activation by 100 μM H2O2 in an early-log-phase microaerobic culture (see Materials and Methods) is shown in Fig. 3. The response began within 5 min (data not shown) and was transient, consistent with expectations that H2O2 is rapidly degraded. Activation was greatly muted by mutation of the OxyR-binding motif and was eliminated entirely in an oxyR mutant strain. Thus the mntH response to peroxide is almost completely mediated by OxyR. The slight residual activation may be a response to some downstream metabolite or an alternative but less efficacious response to peroxide itself. Further, mntH expression in the absence of exogenous peroxide was equivalently low and at the limit of detection during either microaerobic or aerobic growth (data not shown). In contrast to peroxide activation, superoxide has no effect on mntH expression as shown previously (24).
FIG. 3.
Activation of mntH transcription by H2O2. Cultures were grown at 37°C in M9 with 0.2% glucose and 50 μg of ampicillin/ml to an OD600 of 0.3, when they were supplemented with 100 μM H2O2. β-Galactosidase activity was determined at different times following H2O2 challenge. The response of the wild-type strain/promoter combination (MM2507) is compared to that of a strain in which the OxyR-binding motif of the mntH::lacZYA reporter is mutated (MM2616) and to that of a strain in which the OxyR protein is mutated (MM2529). Data shown here are for cells grown microaerobically (see Materials and Methods). A similar pattern was seen in cells grown aerobically, except that maximal induced β-galactosidase activity was three- to fivefold lower for each strain. Results are the means and standard errors of three independent experiments.
Fur mediates Fe2+ repression of mntH.
EDTA (100 μM) induced mntH when added to rapidly growing cells but not when added to less rapidly growing cells, suggesting that mntH is repressed by one or more divalent cations (24). As no chelator is sufficiently selective to deplete a single transition metal from a solution, we identified specific regulatory cations and specific cellular response pathways in this study by means of “add-back” experiments. Expression of mntH in overnight M9-glucose cultures was substantial and was not affected by pretreatment of the medium with a chelator such as Chelex (data not shown) but could be potently inhibited by addition of Mn2+ and Fe2+. The most prominent Fe2+- and Mn2+-dependent regulator in the enterobacteria is Fur. Since a plausible Fur-binding motif is apparent in the mntH promoter, cis- and trans-acting Fur mutants were evaluated in add-back experiments with each metal. While the wild-type mntH::lacZ reporter was strongly repressed by addition of Fe2+ at concentrations of 1 μM or greater, this repression was diminished but not eliminated when either the Fur protein or the Fur-binding motif in the promoter or both were mutated (Fig. 4A). The fur-9 allele expresses a Fur containing a point mutation that eliminates the response to Fe2+ or Mn2+, but the fur-9 allele still regulates some promoters in response to acid (13). Overnight expression of the wild-type mntH promoter showed less than a twofold dependence on the pH of the growth medium between pH 6.4 and 8.0; moreover, the pattern was identical in the wild-type, fur-9, and Fur null strain backgrounds (data not shown). Thus both the Fur protein and the Fur-binding motif are involved in Fe2+-dependent, but not in acid-dependent, regulation of mntH. In contrast, addition of Mn2+ to these same strains elicited essentially the same response, i.e., full repression, from the wild-type system, the Fur protein mutant, the Fur-binding motif mutant, and the double mutant (Fig. 4B). Therefore, the primary effect of Mn2+ on mntH is not mediated through Fur. The weaker inhibition of mntH expression by Co2+, by contrast, appears to be mediated entirely via Fur (data not shown).
The inverted-repeat motif and MntR mediate Mn2+ repression of mntH.
We noted that a distant homolog of the B. subtilis Mn2+ transport repressor, MntR, was present in S. enterica serovar Typhimurium and was homologous to the protein encoded by the E. coli b0817 locus. We hypothesized that S. enterica serovar Typhimurium “MntR” was likely the trans-acting factor that bound at the inverted-repeat motif in the mntH promoter that we had previously noted (24). Patzer and Hantke recently showed that the E. coli b0817 gene product bound to the identical inverted-repeat motif in the E. coli mntH promoter (34). Consequently, this locus has been named mntR. Experiments equivalent to those with the Fur protein and Fur-binding motif mutants were performed with an MntR protein mutant and an inverted-repeat motif (MntR-binding motif) mutant, respectively. The results (Fig. 4D) indicate that the MntR regulatory circuit is clearly the principal mediator of Mn2+ repression. While the wild-type mntH::lacZ reporter was strongly repressed by addition of Mn2+ at concentrations of 0.3 μM or greater, the dose-response curve for Mn2+ was shifted to the right when the MntR protein was mutated. Repression by Mn2+ was completely eliminated when the MntR-binding motif or both the MntR protein and the MntR-binding motif were mutated. Fe2+ repression was not altered by elimination of the MntR protein and was only minimally shifted to the right by elimination of the MntR-binding motif or both the MntR protein and MntR-binding motif (Fig. 4C).
Fur and MntR account for all cation repression.
The data shown in Fig. 4A to D indicate that Fe2+ repression is not completely abolished by any mutation in the Fur regulatory circuit. In contrast, Mn2+ repression is completely abolished by mutation of the MntR-binding motif but only partially abolished by mutation of the MntR protein. This suggests that S. enterica serovar Typhimurium can compensate for the loss of either individual regulatory circuit. We therefore examined Fe2+ and Mn2+ repression in strains where both regulatory circuits were disrupted (Fig. 4E and F). Three combinations were tested: a mutant Fur protein combined with a mutant MntR protein, a mutant Fur protein combined with a mutant MntR-binding motif, and a mutant Fur-binding motif combined with a mutant MntR protein. Repression by Fe2+ or Mn2+ was completely abolished in all three cases even at concentrations on the threshold of growth inhibition (100 μM). Thus, Fur and MntR regulation is sufficient to account for all observed cation regulatory effects.
Hydrogen peroxide activation is independent of metal repression.
We observed that Fe2+ and Mn2+ still repressed mntH::lacZ when OxyR regulation was inactivated by a cis- or trans-acting mutation. Likewise, H2O2 still activated mntH::lacZ when either the Fur or MntR systems had been inactivated by a cis- or trans-acting mutation (data not shown). This suggested that metal repression and peroxide activation are substantially independent responses. To determine whether Fe2+ and Mn2+ repression and H2O2 activation of mntH are coupled, the wild-type strain and promoter system were subjected to both metal and H2O2 challenge in the same culture. Fe2+ and Mn2+ repressed overnight accumulation of LacZ to comparable extents in wild-type cells whether or not they had been challenged with 100 μM H2O2 in early log phase (Fig. 5B), while in the complementary experiment H2O2 activated mntH in wild-type cells in early log phase even if 10 μM Fe2+ or Mn2+ had been included in the medium to repress mntH (Fig. 5A).
FIG. 5.
Occurrence of H2O2 activation and metal repression in the same culture. Three cultures of MM2507, containing wild-type forms of all three trans-acting factors and cis-acting motifs, were grown microaerobically at 37°C in M9-0.2% glucose-50 μg of ampicillin/ml with no added metal, with 10 μM MnSO4, or with 10 μM FeSO4. At an OD600 of 0.3 each culture was supplemented with 100 μM H2O2 and β-galactosidase activity was determined at different times following H2O2 challenge. The cultures were then allowed to grow overnight to saturation (OD600 of 1.0 to 1.5 ), and β-galactosidase activity was determined again. The data show that H2O2 activation still occurs even if 10 μM MnSO4 or 10 μM FeSO4 was present (A) and that manganese and iron repression still occurs even if the cells had been previously challenged with H2O2 (B). Note the different scales for β-galactosidase activity in the two panels.
DISCUSSION
S. enterica serovar Typhimurium mntH is controlled by at least three transcription factors with distinguishable binding sites and roles: OxyR, Fur, and MntR. In agreement with the data of Patzer and Hantke (34), there is no evidence for metal regulation other than by Mn2+ and Fe2+ acting through MntR and Fur. As the data in this report demonstrate, however, regulation is more than the sum of three factors functioning independently. In terms of metal repression, our results differ somewhat from those of Patzer and Hantke regarding the E. coli mntH gene (34). These authors interpreted their data to indicate that Fur and MntR are strictly specific for Fe2+ and Mn2+, respectively, in E. coli. In contrast, in S. enterica serovar Typhimurium, we see significant Fe2+ repression when either component of the Fur regulatory circuit has been disrupted (Fig. 4A), but only when the MntR circuit is intact. This residual repression is completely inhibited by disruption of the MntR regulatory circuit (Fig. 4F). The simplest interpretation is that Fe2+ also stimulates repressive binding of MntR to the MntR-binding motif, albeit with a poorer overall affinity than Mn2+. While the difference between the results for E. coli and S. enterica serovar Typhimurium could simply be due to a species difference, there are at least two reasons why Fe2+ regulation through the MntR circuit might not have been observed in the E. coli studies. First, Fe2+ repression via the MntR circuit alone produces somewhat different dose-response curves than Fe2+ repression through the Fur circuit alone (Fig. 4A and C); the E. coli studies examined only a single cation concentration. Second and more importantly, the reporter systems used in these two studies differ significantly. In E. coli the mntH::lacZ reporter was a chromosomal insertion which inactivated the mntH gene. Altered Mn2+ homeostasis resulting from loss of a primary uptake transporter could alter regulatory patterns. In contrast, our S. enterica serovar Typhimurium results were obtained by using a nominally single-copy or low-copy-number plasmid reporter in an otherwise wild-type strain, thus maintaining normal Mn2+ and Fe2+ homeostasis and preserving normal regulatory conditions as much as possible.
Except for slight differences in dose response among the three Fur circuit mutants in Fig. 4A, our data are consistent with the hypothesis that residual Fe2+ repression of mntH in Fur protein and/or Fur-binding motif mutants is straightforward regulation by MntR, acting through the MntR-binding motif using Fe2+ as a cofactor. Attributing residual Mn2+ repression to the normal Fur regulatory circuit is more problematic. There are extensive previous data that Fur can repress via the Fur-binding motif with Mn2+ as a cofactor (8, 19, 20, 31, 38). The Mn2+ repression seen in the MntR protein mutant (Fig. 4D) is consistent with these examples. However, mutation of the MntR-binding motif alone abolishes Mn2+ repression (Fig. 4D); hence the possible interaction of a Mn2+-liganded Fur protein with the Fur-binding motif does not appear to be sufficient to mediate Mn2+ repression. Further, mutation of the MntR protein and Fur-binding motif also abolishes Mn2+ repression (Fig. 4F); hence the Fur protein does not appear to mediate repression by binding to the MntR-binding motif. Yet, finally, mutation of both the MntR and Fur proteins also abolishes Mn2+ repression (Fig. 4F), which implies that Fur is the only other trans-acting factor involved. The most direct conclusion based on the present data is that residual Mn2+ repression is mediated through the Fur protein, but the cis-acting sequence required for Mn2+-cofactored Fur to be effective contains elements from both the Fur- and MntR-binding motifs. There is the formal possibility that repression involves some other protein which is expressed only in the presence of Fur; however the same uncertainties as to the cis-acting binding motif apply to it as well. Until additional cis-acting mntH promoter mutants are tested, the mechanistic details of Fur-mediated residual Mn2+ repression of mntH will remain unclear.
What is clear is that a transporter exclusively dedicated to the uptake of Mn2+ is repressed strongly, via normal Fur regulation, by the minor and physiologically unimportant substrate Fe2+ (23a). What might be the physiological relevance of this regulation? It may be related to the fact that MntH is extraordinarily active when expressed, capable of concentrating submicromolar amounts of extracellular Mn2+ to over 10 mM in the cytoplasm (24). The iron content of enterobacteria, by contrast, is typically far lower, in the range of a few hundred micromolar (21, 26, 33), essentially all of it bound to protein. Either the [Mn2+]/[Fe2+] ratio or the total content of these two redox-active metals is likely to be under strong homeostatic control. It could also be argued that, under some environmental conditions, MntH can mediate physiologically significant amounts of iron uptake. However, transport data (23a) suggest that this is highly unlikely in S. enterica serovar Typhimurium.
It is not surprising that Mn2+ and Fe2+ still repress overnight expression of mntH in cultures that were exposed to H2O2 challenge earlier in their growth. Exogenous H2O2 is degraded rapidly after its introduction into culture medium. Moreover, the amount of MntH protein (as well as of catalase and peroxidase proteins) induced by this transient exposure would be diluted to insignificance by subsequent growth. Finally, no mechanism by which cells might be predisposed to resist metal repression many generations after exposure to H2O2 is apparent. It is less self-evident, and therefore more interesting, that H2O2 can induce mntH expression when Mn2+ or Fe2+ is present at 10 μM in the medium, 10 to 30 times their half-maximal repressive concentrations. The possibility that metal repression is somehow inoperative in early-log-phase cultures seems unlikely. A more plausible explanation is that OxyR-mediated activation is configured spatially on the mntH promoter such that it can override both Fur-mediated and MntR-mediated metal repression. How this occurs mechanistically, particularly with the MntR-binding motif relatively distant from the OxyR binding site, is an intriguing question.
Since the 22-bp inverted repeat has recently been documented as the binding site for the novel MntR regulator (34) and since we had previously noted a similar motif (19 of 22 bp identical) in the control region of the S. enterica serovar Typhimurium sitABCD operon (24), the prevalence of MntR and MntR-regulated genes throughout Bacteria was of interest. Extensive queries using BLAST (3), including searches for degenerate matches to the inverted-repeat motif, suggest first that close homologs to MntR are restricted to the E. coli and S. enterica lineages with only one ortholog per species and second that MntR regulates only a few genes in any given organism unless its binding site is very degenerate. Besides E. coli and S. enterica serovar Typhimurium mntH and S. enterica serovar Typhimurium sitABCD, we have identified only two other strong candidate MntR-regulated transcriptional units. One is the mntR locus itself, which has a single copy of the inverted repeat motif in both species (18 of 22 bp identical in E. coli, 19 of 22 bp identical in S. enterica serovar Typhimurium). The second locus is in the presumed promoter region of yebN in each species, whose product is b1821 in Blattner E. coli notation (6), which contains two tandem inverted-repeat motifs exhibiting 15 to 18 of 22 bp identical to those of the MntH motif separated by a spacer of 18 or 21 bp. The promoter at the mntR locus has not been characterized, but the locus has two intriguing properties. First, the MntR-binding motif at the mntR locus lies upstream of a plausible −35 site, such that MntR could either repress or activate transcription. Second, the MntR gene stop codon overlaps the start codon of the gene encoding putative membrane protein YbiR, b0818 in Blattner E. coli notation (6), whose BLAST hits are annotated variously as carboxylate or arsenate transporters. We are investigating the possibility that YbiR mediates the Mn2+ efflux activity that we observed during our characterization of MntH as an Mn2+ uptake protein (24). If so, this would suggest a model whereby MntR represses two Mn2+ uptake systems and simultaneously activates both itself and the YbiR Mn2+ efflux transporter in the presence of sufficient Mn2+.
Most of the bacterial Mn2+ enzymes identified to date function either in stress response or in central energy metabolism. Stress Mn2+ enzymes include superoxide dismutase SodA (32, 41, 42), the newly discovered nonheme catalase KatN of S. enterica serovar Typhimurium (36), and the orthologous protein phosphatases PrpA and PrpB (28, 29, 39), which modulate repair of envelope damage in E. coli. Metabolic Mn2+ enzymes include ppGpp synthase/hydrolase SpoT, which modulates the bacterial response to amino acid starvation (12, 30, 37) and phosphoglyceromutase YibO, b3612 in Blattner E. coli notation (6), which is regulated distinctly from the 2,3-bisphosphoglycerate-cofactored GpmA (14, 17) and for which a distinctive role is not yet known.
The overall role of Mn2+ in Salmonella, including its role in both Nramp1-dependent and Nramp1-independent aspects of virulence, probably involves the interplay of both stress responses and metabolic adaptability. The regulation of mntH, the regulation of a second Mn2+ transporter of S. enterica serovar Typhimurium, encoded by sitABCD (23a), and the involvement of possible Mn2+ efflux systems will provide clues to the scope and the ultimate significance of Mn2+ in bacterial physiology. Understanding OxyR, Fur, and MntR regulation of mntH should enable further regulatory questions to be addressed, particularly questions regarding the sensitivity of mntH expression to growth medium and growth state.
Acknowledgments
We thank J. Foster for strains, M. Zaharik and B. Finlay for plasmid pMLZ104, and Erica Giavasis for performing many β-galactosidase assays.
This work was supported by NIH GM61748 to M.E.M. and grant 00-25 from the Roy J. Carver Charitable Trust to J.M.S.
REFERENCES
- 1.Agranoff, D., I. M. Monahan, J. A. Mangan, P. D. Butcher, and S. Krishna. 1999. Mycobacterium tuberculosis expresses a novel pH-dependent divalent cation transporter belonging to the Nramp family. J. Exp. Med. 190:717-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Althaus, E. W., C. E. Outten, K. E. Olson, H. Cao, and T. V. O'Halloran. 1999. The ferric uptake regulation (Fur) repressor is a zinc metalloprotein. Biochemistry 38:6559-6569. [DOI] [PubMed] [Google Scholar]
- 3.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 4.Atkinson, P. G., and C. H. Barton. 1998. Ectopic expression of Nramp1 in COS-1 cells modulates iron accumulation. FEBS Lett. 425:239-242. [DOI] [PubMed] [Google Scholar]
- 5.Bearden, S. W., and R. D. Perry. 1999. The Yfe system of Yersinia pestis transports iron and manganese and is required for full virulence of plague. Mol. Microbiol. 32:403-414. [DOI] [PubMed] [Google Scholar]
- 6.Blattner, F. R., G. Plunkett, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 7.Cellier, M. F., I. Bergevin, E. Boyer, and E. Richer. 2001. Polyphyletic origins of bacterial Nramp transporters. Trends Genet. 17:365-370. [DOI] [PubMed] [Google Scholar]
- 8.Compan, I., and D. Touati. 1993. Interaction of six global transcription regulators in expression of manganese superoxide dismutase in Escherichia coli K-12. J. Bacteriol. 175:1687-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coy, M., and J. B. Neilands. 1991. Structural dynamics and functional domains of the fur protein. Biochemistry 30:8201-8210. [DOI] [PubMed] [Google Scholar]
- 10.Craig, J. E., Y. Zhang, and M. P. Gallagher. 1994. Cloning of the nupC gene of Escherichia coli encoding a nucleoside transport system, and identification of an adjacent insertion element, IS186. Mol. Microbiol. 11:1159-1168. [DOI] [PubMed] [Google Scholar]
- 11.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Boer, H. A., A. J. Bakker, and M. Gruber. 1977. Breakdown of ppGpp in spoT and spoT− cells of Escherichia coli. Manganese and energy requirement and tetracycline inhibition. FEBS Lett. 79:19-24. [DOI] [PubMed] [Google Scholar]
- 13.Foster, J. W., and H. K. Hall. 1992. Effect of Salmonella typhimurium ferric uptake regulator (fur) mutations on iron- and pH-regulated protein synthesis. J. Bacteriol. 174:4317-4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fraser, H. I., M. Kvaratskhelia, and M. F. White. 1999. The two analogous phosphoglycerate mutases of Escherichia coli. FEBS Lett. 455:344-348. [DOI] [PubMed] [Google Scholar]
- 15.Goswami, T., A. Bhattacharjee, P. Babal, S. Searle, E. Moore, M. Li, and J. M. Blackwell. 2001. Natural-resistance-associated macrophage protein 1 is an H+/bivalent cation antiporter. Biochem. J. 354:511-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grana, D., P. Youderian, and M. M. Susskind. 1985. Mutations that improve the ant promoter of Salmonella phage P22. Genetics 110:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grana, X., P. Perez de la Ossa, C. Broceno, M. Stocker, J. Garriga, P. Puigdomenech, and F. Climent. 1995. 2,3-Bisphosphoglycerate-independent phosphoglycerate mutase is conserved among different phylogenic kingdoms. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 112:287-293. [DOI] [PubMed] [Google Scholar]
- 18.Gunshin, H., B. Mackenzie, U. V. Berger, Y. Gunshin, M. F. Romero, W. F. Boron, S. Nussberger, J. L. Gollan, and M. A. Hediger. 1997. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 388:482-488. [DOI] [PubMed] [Google Scholar]
- 19.Hamed, M. Y. 1993. Binding of the ferric uptake regulation repressor protein (Fur) to Mn(II), Fe(II), Co(II), and Cu(II) ions as co-repressors: electronic absorption, equilibrium, and 57Fe Mossbauer studies. J. Inorg. Biochem. 50:193-210. [DOI] [PubMed] [Google Scholar]
- 20.Hassan, H. M., and H. C. Sun. 1992. Regulatory roles of Fnr, Fur, and Arc in expression of manganese-containing superoxide dismutase in Escherichia coli. Proc. Natl. Acad. Sci. USA 89:3217-3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hudson, A. J., S. C. Andrews, C. Hawkins, J. M. Williams, M. Izuhara, F. C. Meldrum, S. Mann, P. M. Harrison, and J. R. Guest. 1993. Overproduction, purification and characterization of the Escherichia coli ferritin. Eur. J. Biochem. 218:985-995. [DOI] [PubMed] [Google Scholar]
- 22.Jabado, N., A. Jankowski, S. Dougaparsad, V. Picard, S. Grinstein, and P. Gros. 2000. Natural resistance to intracellular infections. Natural resistance-associated macrophage protein 1 (NRAMP1) functions as a pH-dependent manganese transporter at the phagosomal membrane. J. Exp. Med. 192:1237-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawarabayasi, Y., Y. Hino, H. Horikawa, K. Jin-no, M. Takahashi, M. Sekine, S. Baba, A. Ankai, H. Kosugi, A. Hosoyama, S. Fukui, Y. Nagai, K. Nishijima, R. Otsuka, H. Nakazawa, M. Takamiya, Y. Kato, T. Yoshizawa, T. Tanaka, Y. Kudoh, J. Yamazaki, N. Kushida, A. Oguchi, K. Aoki, S. Masuda, M. Yanagii, M. Nishimura, A. Yamagishi, T. Oshima, and H. Kikuchi. 2001. Complete genome sequence of an aerobic thermoacidophilic crenarchaeon, Sulfolobus tokodaii strain7. DNA Res. 8:123-140. [DOI] [PubMed] [Google Scholar]
- 23a.Kehres, D. G., A. Janakiraman, J. M. Slauch, and M. E. Maguire. 2002. SitABCD is the alkaline Mn2+ transporter of Salmonella enterica serovar Typhimurium. J. Bacteriol. 184:3159-3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kehres, D. G., M. L. Zaharik, B. B. Finlay, and M. E. Maguire. 2000. The NRAMP proteins of Salmonella typhimurium and Escherichia coli are selective manganese transporters involved in the response to reactive oxygen. Mol. Microbiol. 36:1085-1100. [DOI] [PubMed] [Google Scholar]
- 25.Kuhn, D. E., B. D. Baker, W. P. Lafuse, and B. S. Zwilling. 1999. Differential iron transport into phagosomes isolated from the RAW264.7 macrophage cell lines transfected with Nramp1Gly169 or Nramp1Asp169. J. Leukoc. Biol. 66:113-119. [DOI] [PubMed] [Google Scholar]
- 26.Li, D. S., K. Ohshima, S. Jiralerspong, M. W. Bojanowski, and M. Pandolfo. 1999. Knock-out of the cyaY gene in Escherichia coli does not affect cellular iron content and sensitivity to oxidants. FEBS Lett. 456:13-16. [DOI] [PubMed] [Google Scholar]
- 27.Makui, H., E. Roig, S. T. Cole, J. D. Helmann, P. Gros, and M. F. Cellier. 2000. Identification of the Escherichia coli K-12 NRAMP orthologue (MntH) as a selective divalent metal ion transporter. Mol. Microbiol. 35:1065-1078. [DOI] [PubMed] [Google Scholar]
- 28.Missiakas, D., and S. Raina. 1997. Protein misfolding in the cell envelope of Escherichia coli: New signaling pathways. Trends Biochem. Sci. 22:59-63. [DOI] [PubMed] [Google Scholar]
- 29.Missiakas, D., and S. Raina. 1997. Signal transduction pathways in response to protein misfolding in the extracytoplasmic compartments of E. coli: role of two new phosphoprotein phosphatases PrpA and PrpB. EMBO J. 16:1670-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murray, K. D., and H. Bremer. 1996. Control of spoT-dependent ppGpp synthesis and degradation in Escherichia coli. J. Mol. Biol. 259:41-57. [DOI] [PubMed] [Google Scholar]
- 31.Nealson, K. H., and D. Saffarini. 1994. Iron and manganese in anaerobic respiration: environmental significance, physiology, and regulation. Annu. Rev. Microbiol. 48:311-343. [DOI] [PubMed] [Google Scholar]
- 32.Niederhoffer, E. C., C. M. Naranjo, K. L. Bradley, and J. A. Fee. 1990. Control of Escherichia coli superoxide dismutase (sodA and sodB) genes by the ferric uptake regulation (fur) locus. J. Bacteriol. 172:1930-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niehaus, F., K. Hantke, and G. Unden. 1991. Iron content and FNR-dependent gene regulation in Escherichia coli. FEMS Microbiol. Lett. 84:319-324. [DOI] [PubMed] [Google Scholar]
- 34.Patzer, S. I., and K. Hantke. 2001. Dual repression by Fe2+-Fur and Mn2+-MntR of the mntH gene, encoding an NRAMP-like Mn2+ transporter in Escherichia coli. J. Bacteriol. 183:4806-4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Que, Q., and J. D. Helmann. 2000. Manganese homeostasis in Bacillus subtilis is regulated by MntR, a bifunctional regulator related to the diphtheria toxin repressor family of proteins. Mol. Microbiol. 35:1454-1468. [DOI] [PubMed] [Google Scholar]
- 36.Robbe-Saule, V., C. Coynault, M. Ibanez-Ruiz, D. Hermant, and F. Norel. 2001. Identification of a non-haem catalase in Salmonella and its regulation by RpoS (sigmaS). Mol. Microbiol. 39:1533-1545. [DOI] [PubMed] [Google Scholar]
- 37.Schreiber, G., E. Z. Ron, and G. Glaser. 1995. ppGpp-mediated regulation of DNA replication and cell division in Escherichia coli. Curr. Microbiol. 30:27-32. [DOI] [PubMed] [Google Scholar]
- 38.Schrum, L. W., and H. M. Hassan. 1993. Transcriptional activation of Mn-superoxide dismutase gene (sodA) of Escherichia coli by MnCl2. Biochim. Biophys. Acta 1216:186-190. [DOI] [PubMed] [Google Scholar]
- 39.Shi, L., D. G. Kehres, and M. E. Maguire. 2001. The PPP-family protein phosphatases PrpA and PrpB of Salmonella enterica serovar Typhimurium possess distinct biochemical properties. J. Bacteriol. 183:7053-7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toledano, M. B., I. Kullik, F. Trinh, P. T. Baird, T. D. Schneider, and G. Storz. 1994. Redox-dependent shift of OxyR-DNA contacts along an extended DNA-binding site: a mechanism for differential promoter selection. Cell 78:897-909. [DOI] [PubMed] [Google Scholar]
- 41.Touati, D. 1988. Transcriptional and posttranscriptional regulation of manganese superoxide dismutase biosynthesis in Escherichia coli, studied with operon and protein fusions. J. Bacteriol. 170:2511-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsolis, R. M., A. J. Baumler, and F. Heffron. 1995. Role of Salmonella typhimurium Mn-superoxide dismutase (SodA) in protection against early killing by J774 macrophages. Infect. Immun. 63:1739-1744. [DOI] [PMC free article] [PubMed] [Google Scholar]