Abstract
In vitro cell culture system is a useful model for aging-related changes in a wide spectrum of biomedical research. In this study, we explored the passage and donor age-dependent changes in baboon macrovascular endothelial cells that are relevant to both in vitro cell culture aging models and experiments using cell culture techniques. We collected baboon femoral arterial samples from nine baboons ranging in age from 6 months to 30 years (equivalent to humans approximately 18 months to 90 years of age). We then cultured baboon femoral artery endothelial cells (BFAECs) in standard DMEM medium with 20% fetal calf serum with 1:3 split for subculture. Endothelial functions were documented by morphology, Dil-LDL uptake and expression of eNOS, MCP-1, vWF, VCAM-1, ICAM-1, and E-Selectin with or without cytokine stimulation. Most of the cells became nonmitotic after 30 population doublings, or 10 passages, when they became flattened, enlarged, and senescent. While it took approximately 3 days to reach confluence from three-dilution seeding at early passages (< 6), confluence was not achieved even after 7 days of culture for cells after the 9th or 10th passage. There was a linear decline in eNOS expression with passage. However, this decline was significantly less in endothelial cells from a young baboon (6 months) than those from an old baboon (30 years). While basal expression of adhesion molecules was not changed with passaging, responses to cytokine stimulation appeared to be increased in later passaged cells. Our study has provided evidence for passage-related changes in key endothelial functions. The donor age-related differences in this in vitro aging process suggests that in vitro endothelial culture can serve as a biomarker for in vivo aging. Nonhuman primates can provide a model for investigating such aging-related biological characteristics.
INTRODUCTION
BIOLOGICAL CHANGES during aging and age-associated diseases have increasingly become major health issues in modern society (Hayflick, 2000a, 2000b.) With advancement of medical research, the lifespan of human population has increased steadily. More than 75% of people living in developed countries live more than 75 years. It is anticipated that by 2050, the average life expectancy will reach 82 years (Hayflick, 2000a, 2000b.) However, we still know very little about biological mechanisms that are responsible for age-related changes. On the other hand, enormous progress has been achieved with our understanding of age-associated degenerative diseases including coronary artery disease (CAD), diabetes, hypertension, dementia, osteoporosis, etc. While current medical advancement may be able to slow the progression of many of these diseases, we are still ineffective in slowing or stopping the degenerative processes. This is largely due to lack of knowledge about normal biological changes during aging.
One of the major obstacles in aging research is the long study period required to reach an end point in human populations. Some small animal models that have shorter lifespans are useful models, but some fundamental mechanistic differences in key aging process exist between small animals and humans. For example, humans have much longer lifespans, yet, human chromosomes contain only 5–15 kb of the repeat sequence TTAGGG at their ends (telomeres), which dictates the replicative capacity of cells. In contrast, mice have a much shorter lifespan, yet, mice have 20–100 kb of this repeat sequence (Greider, 1996; Morin, 1997; Hemann et al., 2000). However, the mammalian lifespan does seem to be directly associated with replication capability during in vitro culture of cells from the species. Human cells can undergo 30–50 population doublings (PDLs) before becoming senescent, whereas mouse cells start proliferative arrest after only 5–10 PDLs in culture. Therefore, an in vitro cell culture model rather than an in vivo organism model has been used extensively for biomedical and aging research. Although passage-dependent changes in aging research have received close attention, relatively little emphasis has been placed on this important cell behavior in biomedical research that uses cells as hosts or tools. Furthermore, due to the invasiveness of tissue harvesting for cell collection, only limited cell types have been used for investigation of human aging process. It also is difficult to validate in vitro cell culture findings in human subjects in vivo. In contrast, nonhuman primates, such as baboons, resemble humans anatomically, physiologically, and pathologically (Wang et al., 2004). One of the major advantages is that in vivo validation, especially those involving invasive procedures, can be accomplished in well-controlled animal experimental settings. In the current study, we developed an in vitro cell culture model in baboons that can be used for follow-up interventional, longitudinal, and mechanistic in vivo investigations. We explored the culture passagedependent and donor age-dependent changes in functional endothelial markers in baboons and provided important data for general application of endothelial cells cultured in vitro and potentially for an in vitro cell aging model.
MATERIALS AND METHODS
Baboon femoral artery endothelial cell culture
Primary baboon femoral artery endothelial cells (BFAECs) used for this study were isolated from nine baboons maintained at the Southwest National Primate Research Center located at the Southwest Foundation for Biomedical Research in San Antonio. Segments of femoral arteries approximately 1–3 cm in length were collected from baboons by sterile methods during necropsy for medical or other research reasons (Table 1). Although some of these baboons were subjected to a high-fat highcholesterol dietary challenge at certain points of their life for approximately 6 months, all baboons were on normal chow diet for at least 6 months prior to necropsy. None of these baboons were purposely exposed to any infectious agents or toxic agents for any research project. Endothelial cells were harvested no longer than 2 h after the vessels were excised. Cell isolation was based on previous reports with modifications (Shi et al., 2004). The arterial sample was placed in a large sterile dish, and the surface was wiped with 2% antibiotic/antimycotic solution (GIBCO-BRL, Grand Island, NY) in PBS. The artery was then gently cannulated at one end with a short blunt needle and flushed with PBS to remove any blood remaining inside the vessel. It was injected with 0.1% collagenase before the other end was cannulated. The closed artery was incubated at 37°C for 15 min for digestion. After completion of digestion, the vessel was massaged gently, flushed with medium, and the released cells were collected by centrifugation and resuspended in medium. The cells were seeded immediately on 1.0% gelatin-coated culture plates. The medium was F-12K supplemented with 20% fetal calf serum (FCS) (GIBCO-BRL), 75 μg/ml EGCS (Sigma, St. Louis, MO), 50 μg/ml heparin, 10 mM HEPES, 2 mM glutamine, and antibiotics. Confluent cells were passaged by 0.05% trypsin and Versene solution (GIBCO-BRL), and subcultured in a threefold dilution, that is, 1:3 split subculture. The study was approved by Institutional Animal Care and Use Committee (IACUC) of the Southwest Foundation for Biomedical Research.
Table 1.
Animal Information
| ID | Age, years | Gender | Health status |
|---|---|---|---|
| 1X3707 | 22.0 | F | Healthy |
| 17839 | 0.5 | M | Seizure |
| 1X2713 | 24 | F | Healthy |
| 1X2788 | 23 | F | Healthy |
| 1X0281 | 19 | F | Diabetes |
| 1X1956 | 33 | F | Geriatric |
| 16715 | 2 | M | Pneumonia |
| 2X0038 | 30 | M | Geriatric |
| 1X4756 | 18 | F | Fistula/inflammation |
Cell proliferation measurement
Mitotic index, which is used to monitor the progression of cells through the M phase when chromatin condensation occurs, correlates well with the progression of cells through the M phase and cytokinesis. This method is useful to monitor cell cycle progression (Muehlbauer and Schuler, 2003; Yuan et al., 2003). At the end point of the experiment, endothelial cells cultured in coverslip were washed with cold PBS twice and fixed in 1:3 cold acetic acid:methanol solution. Slides were allowed to air dry for 0.5 to 2 h. Cells were stained with 0.1 to 0.2 mg/liter Giemsa stain for 10 min; the Giemsa stain was gently rinsed from the slides by dipping in PBS solution before the slides were air dried. Slides were observed microscopically for the presence of cells with mitotic changes, which were characterized by condensed nuclear material and a lack of nuclear membrane. The data were presented as a percentage of mitotic cells in the total number of cells observed (N = 1,000).
Morphological examination
Endothelial cells at various stages of passage were monitored daily during culture and phase-contrast pictures were taken during microscopic examination (Leica, Deerfield, IL).
Uptake of Dil-LDL by endothelial cells
Cells were seeded in a Lab-Tek culture chamber to 80–90% confluence. We incubated cells for 4 h in growth medium containing 10 mg/ml fluorescence Dil-labeled LDL (Dil-LDL, Biomedical Technologies, Inc., MA) without supplementing FCS but with 5% calf albumin at 37°C for 4 h. The slides were fixed in 2% formaldehyde solution for 20 min at room temperature before being mounted with SlowFade (Molecular Probes, Eugene, OR) and observed under a fluorescence microscope. Quantification of Dil-LDL up-take was carried out by measuring the fluorescent intensities from three randomly selected visual fields (×200) at the 12, 4, and 8 o’clock axes.
Quantification of cell vWF on endothelial cell surface
Cells at different passages were seeded in 100 μl at a density of 5 × 104/ml in 96-well plates. After reaching 80–90% confluence, the cells were fixed with 2% formalin at room temperature for 20 min and then blocked with 2% BSA in PBS containing 1% H2O2 and 0.05% (w/v) Tween-20 at 37°C for 1 h and incubated with antihuman vWF conjugated with HRP (DAKO Cytomation #P0226, Denmark) in a dilution of 1:200. The o-phenylenediamine dihydrochloride (OPD, Sigma, Cat# P 9187) was used as the substrate and the absorbance was read at 492 nm after 20 min color development.
eNOS protein quantification by ELISA
After treatment, the cells were collected and lysed in Tris-EDTA buffer with protease inhibitor, and the cell lysates were used for the estimation of eNOS protein levels as described previously. (Wang et al., 2000). Endothelial cells were lysed with lysis buffer, and soluble proteins were extracted by centrifugation. The total soluble protein concentration in the supernatant was determined by the Bradford method (Bio-Rad, Cat# 500-0006). Extracted proteins were diluted to the same concentration for all samples in the ELISA procedures by using an eNOS ELISA kit (R&D systems, Cat. No. DEN00) (Wang et al., 2003). This assay employs the quantitative enzyme immunoassay technique in which a monoclonal antibody specific for human eNOS has been precoated onto a microplate. We have used this method to measure eNOS protein in baboon arterial wall extracts (Wang et al., 2003). Standards and samples were pipetted into the wells and any eNOS present was bound by the immobilized antibody. After washing away any unbound substances, an enzyme-linked polyclonal antibody specific for human eNOS was added to the wells. Following a wash to remove any unbound antibody–enzyme reagent, a substrate solution was added to the wells and color developed in proportion to the amount of eNOS bound in the initial step. The color development was stopped after 10 min and the intensity of the color was measured. The eNOS concentration of each sample was calculated from a standard curve.
Quantification of cell adhesion molecules on endothelial cell surface
Cells at different passages were seeded in 100 μl at a density of 5 × 104/ml in 96-well plates. After 24 h, the cells were exposed to 10 ng/ml TNF-α, or 10 ng/ml Il-1β or 1 μg/ml LPS (Sigma) for 4 and 24 h. We fixed the cells with 2% formalin at room temperature for 20 min and then blocked with 2% BSA in PBS containing 1% H2O2 and 0.05% (w/v) Tween-20 at 37°C for 1 h. The plates were incubated with goat polyclonal antibodies against human E-selectin (R&D Systems, Minneapolis, MN, Cat #BBA19), VCAM-1 (Cat# BBA19) and ICAM-1 (Cat #BBA17) in a dilution of 1:500 at 4°C overnight. After incubation with secondary antibody conjugated with peroxidase (anti-goat IgG Fab2, Sigma, Cat# A5420) in 1:5,000 at 37°C for 1 h, OPD solution was added. Optical density was read at 492 nm after 20 min color development.
Statistical analysis
Data were expressed as mean ± SEM. A student t-test was used to compare the average differences between two groups. ANOVA was applied for comparisons of three groups or more. A two-tailed P < 0.05 was regarded as statistically significant. All data reported are representatives of three independent experiments with duplicate sets for each run.
RESULTS
Characteristics of baboon donors
Baboon femoral arterial endothelial cells were collected from baboons necropsied for various reasons. As shown in Table 1, the ages of the animals varied from 6 months to 30 years (approximately equivalent to humans at 18 months to 90 years of age). Three adult female baboons were euthanized for surgical research projects and one for geriatric health reasons. The other five baboons, including two adult females, two young males, and one aged male were euthanized for other health reasons.
Morphological and growth changes of subculturing
While cells from all ages of baboons exhibited the characteristic cobble stone feature of endothelial cells during early passages of culture, during later passages (from ninth passage or after the 27th PDL), cells started to become flattened, enlarged, and less responsive to growth factors (Fig. 1). While it normally took 2–3 days to reach confluence when cells were seeded in a 1:3 split at passages up to 6–7, it often took 5–7 days to reach confluence for cells at the 8–10th passages. For cells older than 10 passages, confluence was never reached within 7 days. In selected typical passages, we found that mitotic indices were not significantly different between the second and fourth passage. However, from the sixth passage, the cultures became less confluent within the same time period, and the cells gradually became flattened and enlarged to a significant degree at the 10th passage (Fig. 1). The one young animal (6 months old) had a more robust growth pattern than four old animals (22 to 33 years old) as indicated by mitotic index values (Table 2) and microscopic observations.
FIG. 1.
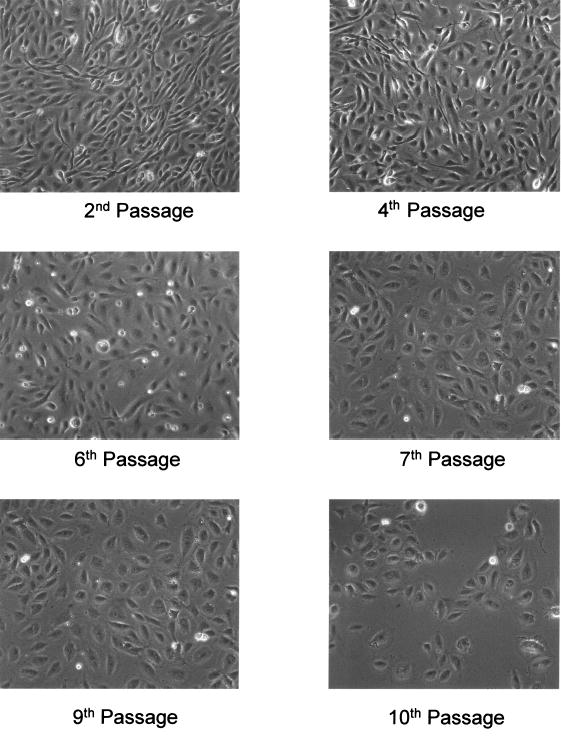
Light microscopic observations of baboon femoral arterial endothelial cells (BFAECs, ID 2X0038) cultured to passage numbers 2, 4, 6, 8, 9, and 10, which are equivalent to cell population doublings of 6, 12, 18, 24, 27, and 30. The BFAECs were collected from a 2-year-old baboon (ID# 1 × 16715, Table 1). As shown on the figure, endothelial cells at the 9th or 10th passage had enlarged cytoplasmic volume, and diminished extent of characteristic cobble stone feature.
Table 2.
Changes in Mitotic Indices of BFAECs During Passaging
| ID | Age, years | Third passage | Sixth passage | Ninth passage |
|---|---|---|---|---|
| 1X3707 | 22.0 | 5.63 ± 0.34 | 4.93 ± 0.52 | 1.93 ± 0.14* |
| 1X2788 | 23 | 4.87 ± 0.21 | 5.03 ± 0.19 | 2.73 ± 0.39* |
| 17893 | 0.5 | 7.43 ± 0.48 | 6.63 ± 0.67 | 2.13 ± 0.12* |
| 2X0038 | 30 | 6.13 ± 0.64 | 4.23 ± 0.17 | 0.89 ± 0.44* |
| 1X1956 | 33 | 4.01 ± 0.24 | 3.73 ± 0.25 | 0.33 ± 0.11* |
P < 0.05 compared to third passage. Mitotic index is the number of mitotic cells in 1,000 total cells.
Effect of culture passages on Dil-LDL uptake
Uptake of LDL by endothelial cells is regarded as one of the characteristic features of endothelial cells and is often used to assess the functionality of endothelial cells. In our study, we also evaluated the uptake of Dil-LDL by endothelial cells of selected passages. As shown in Fig. 2, there was no microscopic difference in the capacity of Dil-LDL uptake by endothelial cells of the third (nine PDLs), sixth (18 PDLs), and ninth (27 PDLs) passage. To semiquantitatively analyze the Dil-LDL uptake, we used the ImagePro software to measure the red fluorescent color intensity from three randomly selected visual fields at the 4, 8, and 12 o’clock position of the slide. For example, the intensity of intracellular Dil-LDL in endothelial cells harvested from a 6-month-old baboon decreased approximately 30% (from 496 ± 42 to 342 ± 48 pixels, P = NS). We also noted that endothelial cells from this young baboon appeared to have higher Dil-LDL uptake (496 ± 42) than those of older baboons (e.g., 321 ± 21 for a 30-year-old baboon) at the third passage. The same trend persisted for both the sixth and ninth passages.
FIG. 2.
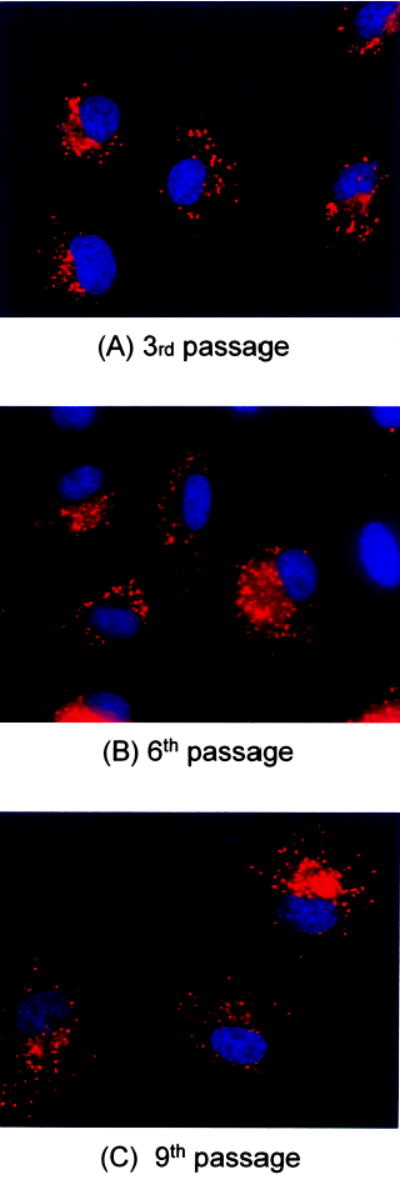
Fluorescent microscopic view of BFAECs at the third (A), sixth (B), and ninth (C) passages. The BFAECs were collected from a 6-month-old baboon (ID# 17839, Table 1). The red color was Dil-LDL phagocytosed by endothelial cells and the nuclei were stained blue. The Dil-LDL at a concentration of 10 mg/ml was incubated with endothelial cells for 4 h at 37°C before stained by 1 μg/ml DAPI (blue) and viewed under Nikon upright fluorescence microscoe at magnification of ×400.
Effect of culture passages on vWF expression
We measured vWF expression by anti-vWF antibody conjugated with HRP. No significant loss of vWF expression was observed up to the ninth passage (Fig. 3).
FIG. 3.
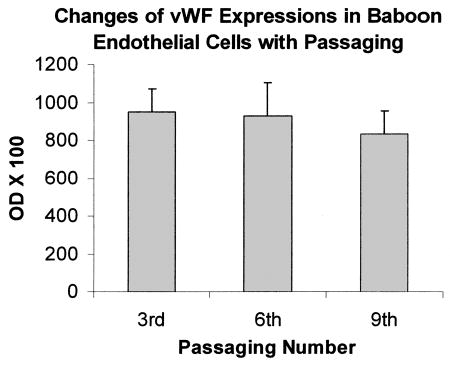
Mean ± SEM percent changes in expressions of vWF in baboon endothelial cells at the third, sixth, and ninth passage.
Effects of passage on endothelial cell eNOS production
Endothelial NO production mainly through eNOS enzymatic reaction plays a key role in maintaining functional endothelial integrity. Endothelial dysfunction is often associated with reduced eNOS expression. Therefore, we measured eNOS protein levels from BFAECs cultured to different generations. As shown in Fig. 4, there was almost a linear decline in eNOS protein levels from the 2nd to 10th passage during in vitro culture (P < 0.001). The eNOS protein levels at the 10th passage (4.6 ± 2.1 pg/μg total protein) were only 13% of the levels at the second to fourth passage (35.7 ± 2.8 pg/μg total protein). Although the consistent decline was uniform for endothelial cells from all ages of baboons, the rate of decline varied according the baboon’s age at the time of necropsy. The reduction for a 22-year-old baboon was 93% (from 38.1 pg/μg total proteins at the second to fourth passages to 2.8 pg/μg total protein at the 10th passage), the reduction for endothelial cells collected from 2-year-old baboon was 79% after the same number of PDLs (from 44.3 pg/μg total protein to 9.4 pg/μg total protein). In this sample of only nine baboons, this donor agedependent difference in eNOS expression with culture passage was not statistically significant.
FIG. 4.
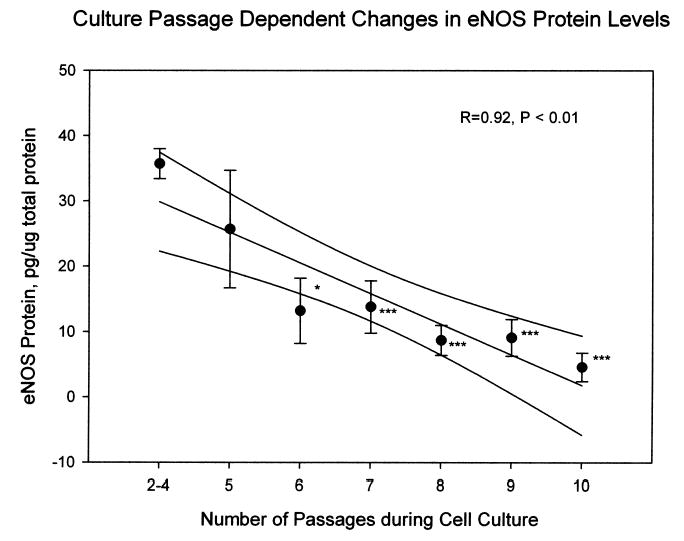
Association between the number of culture passages and eNOS protein levels. The data points were the mean ± SEM of endothelial cells from nine baboons. The curved lines above and below the linear fit line mark the 95% confidence interval for the correlation. *P < 0.05, ***P < 0.001, by comparison with the eNOS levels at two to four passages.
Effect of culture passage on adhesion molecule expression to cytokine stimulation
We measured VCAM-1, ICAM-1, and E-selectin at baseline levels from cells at the third, sixth, and ninth passages. We found little change on the basal level expression in relation to the number of culture passages. In response to stimulation by TNFα and IL-1β, expression of adhesion molecules tended to be stimulated more in cells at the sixth and ninth passages, although the differences did not reach statistical significance (Fig. 5). The change in E-selectin was more prominent at 4 h (Fig. 5A) compared to those at 24 h (Fig. 5B). Response to LPS was not present as we reported previously (Shi et al., 2004). Compared with the endothelial cells collected from a young baboon (19 years old), endothelial cells from a geriatric baboon (30 years old) appeared to have higher increase in CAM expression in response to these cytokine stimulation, although the difference was not statistically significant. For example, at the third to fourth passage, TNFα stimulation increased E-selectin expression by threefold after 4 h exposure in a 30-year-old baboon, but only 2.1 fold in a 19-year-old baboon. The same trends were also seen when cells were stimulated by IL-1β. However, this apparent donor age-related change diminished with the number of culture passages.
FIG. 5.
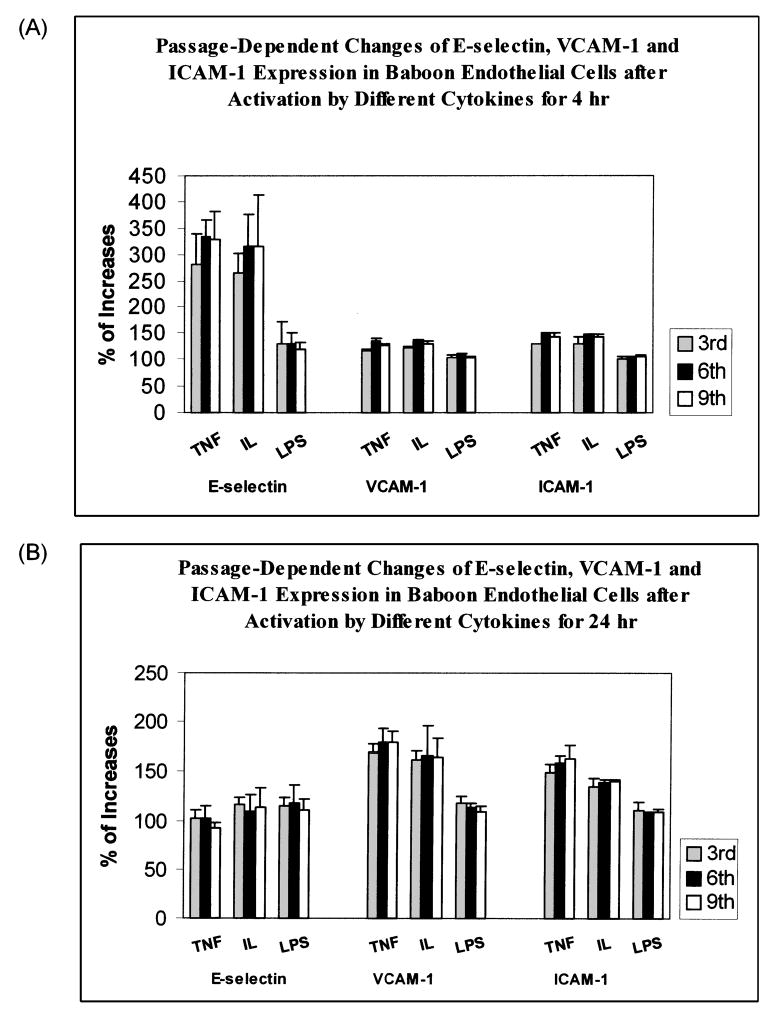
Mean ± SEM percent changes in expressions of E-selectin, VCAM-1, and ICAM-1 by baboon endothelial cells under the stimulation of 10 ng/ml TNFα, or 10 ng/mL IL-1β, or 1 μg/ml LPS. BFAECs were cultured up to third, sixth, and ninth passages before they were stimulated by these cytokines for 4 h (A) and 24 h (B).
DISCUSSION
The results are biologically significant in two aspects. First, the observation of culture passage-dependent reduction in eNOS protein levels is highly relevant to experiments that use cultured endothelial cells for functional studies, because eNOS has a key role in maintaining the normal endothelial function (Cines et al., 1998). A significant reduction in eNOS expression at later passages of the cells would render these endothelial cells less capable in responding to external stimuli. Studies, such as those investigating mechanisms for endothelial apoptosis, would be significantly affected by this passage dependent decline in eNOS. The combination of the donor age dependency and passage-dependent decline in eNOS levels may explain variable outcomes, at times opposite outcomes, for the same treatment when using cultured endothelial cells, or other cells, within or between studies. Hence, valid interpretation of results requires reference to passages and donor ages. Second, our data with baboon endothelial cells have demonstrated the age-dependent changes in several functional features of cultured endothelial cells (Yang et al., 1999; O’Hare et al., 2001), suggesting that baboons may be used for development of in vitro cell culture aging model.
In this study, we also found that most baboon endothelial cells may reach senescence during in vitro culture after approximately 30 PDLs or 10 passages for adult baboons. This growth capacity is shorter than that of human endothelial cells, which typically reach 40–50 PDLs (or ~15 passages) before cell senescence inhibits confluence (Rubin, 1997; Campisi, 2001). Our findings are consistent with observations of cells obtained from other animals with shorter lifespan than humans (Rubin, 1997; Campisi, 2001; Hornsby, 2003). Baboons have approximately 1/3 of human life expectancy. While the endothelial cells obtained from the 6-month-old and 2-year-old baboons tended to go through more PDLs (~36 PDLs, or 12 passages) before reaching senescence than those of older baboons, the small number of animals in this study makes the interpretation of this observation inconclusive. It is clear, however, that the time required to reach confluence is much prolonged at later culture passages compared to early passages. These findings are consistent with the hypothesis that intrinsic control of cell doubling capacity in vitro may reflect intrinsic control of the aging process in vivo, and baboon cell culture may be developed to serve as a useful in vitro model for aging.
Despite clear deterioration in cell growth capacity and eNOS production, the capacity of Dil-LDL uptake does not appear to be affected by the number of passages. However, endothelial cells of younger baboons tended to have higher uptake of Dil-LDL than those of older baboons and to express fewer adhesion molecules. A study with a larger number of donor animals at different ages will be needed to address this issue statistically.
In addition to vascular aging, aging process in immune system may also play a major role in degenerative vascular diseases, such as inflammatory responses in atherosclerosis. Like all other tissues and organs, information on immune system aging is also limited (Aspinall, 2000). However, nonhuman primates share very similar aging process with humans in relation to telomere shortening in leukocytes (Lee et al., 2002; Baerlocher et al., 2003) cytokine production (Mascarucci et al., 2002), lymphocyte subpopulation differentiation and macrophages (Nam et al., 1998; Lloberas and Celada, 2002; Aspinall, 2003; Damjanovich et al., 2003; Issa, 2003)—all of which appear to decrease with aging although double positive CD4+ and CD8+ T cells appear to increase (Lee et al., 2003). It would be interesting to explore the aging-related changes in immune systems in conjunction with vascular systems with relevance to degenerative vascular diseases. In summary, our study has demonstrated both donor age and in vitro cell population doubling dependency in baboon endothelial morphological and functional changes in vitro. They highlight that any findings using in vitro cell culture, especially endothelial cells, will require reference to the donors’ ages and number of passages. Our data also suggest that baboons may serve a good animal model for in vitro aging studies in which cells can be obtained longitudinally through biopsy and interventional strategies can be applied.
Acknowledgments
The project was supported by NIH Grants P01 HL028972, P51 RR013986, and R01 HL-06653. We thank Jane F. Vande-Berg and Catherine Jett for technical support.
References
- ASPINALL R. Longevity and the immune response. Biogerontology. 2000;1:273–278. doi: 10.1023/a:1010046532657. [DOI] [PubMed] [Google Scholar]
- ASPINALL R. Age-related changes in the function of T cells. Microsc Res Technol. 2003;62:508–513. doi: 10.1002/jemt.10412. [DOI] [PubMed] [Google Scholar]
- BAERLOCHER GM, MAK J, ROTH A, RICE KS, LANSDORP PM. Telomere shortening in leukocyte subpopulations from baboons. J Leukoc Biol. 2003;73:289–296. doi: 10.1189/jlb.0702361. [DOI] [PubMed] [Google Scholar]
- CAMPISI J. From cells to organisms: Can we learn about aging from cells in culture? Exp Gerontol. 2001;36:607–618. doi: 10.1016/s0531-5565(00)00230-8. [DOI] [PubMed] [Google Scholar]
- CINES DB, POLLAK ES, BUCK CA, LOSCALZO J, ZIMMERMAN GA, MCEVER RP, POBER JS, WICK TM, KONKLE BA, SCHWARTZ BS, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. [PubMed] [Google Scholar]
- DAMJANOVICH S, GASPAR R, JR, BENE L, JENEI A, MATYUS L. Signal transduction in T lymphocytes and aging. Exp Gerontol. 2003;38:231–236. doi: 10.1016/s0531-5565(02)00205-x. [DOI] [PubMed] [Google Scholar]
- GREIDER CW. Telomere length regulation. Annu Rev Biochem. 1996;65:337–365. doi: 10.1146/annurev.bi.65.070196.002005. [DOI] [PubMed] [Google Scholar]
- HAYFLICK L. The future of ageing. Nature. 2000a;408:267–269. doi: 10.1038/35041709. [DOI] [PubMed] [Google Scholar]
- HAYFLICK L. New approaches to old age. Nature. 2000b;403:365. doi: 10.1038/35000303. [DOI] [PubMed] [Google Scholar]
- HEMANN MT, HACKETT J, A IJ, GREIDER CW. Telomere length, telomere-binding proteins, and DNA damage signaling. Cold Spring Harb Symp Quant Biol. 2000;65:275–279. doi: 10.1101/sqb.2000.65.275. [DOI] [PubMed] [Google Scholar]
- HORNSBY PJ. Replicative senescence of human and mouse cells in culture: Significance for aging research. Mech Ageing Dev. 2003;124:853–855. doi: 10.1016/s0047-6374(03)00173-8. [DOI] [PubMed] [Google Scholar]
- ISSA JP. Age-related epigenetic changes and the immune system. Clin Immunol. 2003;109:103–108. doi: 10.1016/s1521-6616(03)00203-1. [DOI] [PubMed] [Google Scholar]
- LEE WW, NAM KH, TERAO K, AKARI H, YOSHIKAWA Y. Age-related increase of peripheral CD4+ CD8+ double-positive T lymphocytes in cynomolgus monkeys: Longitudinal study in relation to thymic involution. Immunology. 2003;109:217–225. doi: 10.1046/j.1365-2567.2003.01646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE WW, NAM KH, TERAO K, YOSHIKAWA Y. Age-related telomere length dynamics in peripheral blood mononuclear cells of healthy cynomolgus monkeys measured by Flow FISH. Immunology. 2002;105:458–465. doi: 10.1046/j.1365-2567.2002.01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LLOBERAS J, CELADA A. Effect of aging on macrophage function. Exp Gerontol. 2002;37:1325–1331. doi: 10.1016/s0531-5565(02)00125-0. [DOI] [PubMed] [Google Scholar]
- MASCARUCCI P, TAUB D, SACCANI S, PALOMA MA, DAWSON H, ROTH GS, LANE MA, INGRAM DK. Cytokine responses in young and old rhesus monkeys: Effect of caloric restriction. J Interferon Cytokine Res. 2002;22:565–571. doi: 10.1089/10799900252982043. [DOI] [PubMed] [Google Scholar]
- MORIN GB. Telomere control of replicative lifespan. Exp Gerontol. 1997;32:375–382. doi: 10.1016/s0531-5565(96)00164-7. [DOI] [PubMed] [Google Scholar]
- MUEHLBAUER PA, SCHULER MJ. Measuring the mitotic index in chemically-treated human lymphocyte cultures by flow cytometry. Mutat Res. 2003;537:117–130. doi: 10.1016/s1383-5718(03)00076-7. [DOI] [PubMed] [Google Scholar]
- NAM KH, AKARI H, TERAO K, ITAGAKI S, YOSHIKAWA Y. Age-related changes in major lymphocyte subsets in cynomolgus monkeys. Exp Anim. 1998;47:159–166. doi: 10.1538/expanim.47.159. [DOI] [PubMed] [Google Scholar]
- O’HARE MJ, BOND J, CLARKE C, TAKEUCHI Y, ATHERTON AJ, BERRY C, MOODY J, SILVER AR, DAVIES DC, ALSOP AE, et al. Conditional immortalization of freshly isolated human mammary fibroblasts and endothelial cells. Proc Natl Acad Sci USA. 2001;98:646–651. doi: 10.1073/pnas.98.2.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUBIN H. Cell aging in vivo and in vitro. Mech Ageing Dev. 1997;98:1–35. doi: 10.1016/s0047-6374(97)00067-5. [DOI] [PubMed] [Google Scholar]
- SHI Q, WANG J, WANG XL, VANDEBERG JL. Comparative analysis of vascular endothelial cell activation by TNF-alpha and LPS in humans and baboons. Cell Biochem Biophysics. 2004;40:289–303. doi: 10.1385/CBB:40:3:289. [DOI] [PubMed] [Google Scholar]
- WANG J, FELUX D, VANDEBERG J, WANG XL. Discordance of endothelial nitric oxide synthase in the arterial wall and its circulating products in baboons: Interactions with redox metabolism. Eur J Clin Investig. 2003;33:288–295. doi: 10.1046/j.1365-2362.2003.01143.x. [DOI] [PubMed] [Google Scholar]
- WANG XL, SIM AS, WANG MX, MURRELL GA, TRUDINGER B, WANG J. Genotype dependent and cigarette specific effects on endothelial nitric oxide synthase gene expression and enzyme activity. FEBS Lett. 2000;471:45–50. doi: 10.1016/s0014-5793(00)01356-9. [DOI] [PubMed] [Google Scholar]
- WANG XL, WANG J, SHI Q, CAREY KD, VANDEBERG JL. Arterial wall determined risk factors to vascular diseases —A nonhuman primate model. Cell Biochem Biophys. 2004;40:371–388. doi: 10.1385/CBB:40:3:371. [DOI] [PubMed] [Google Scholar]
- YANG J, CHANG E, CHERRY AM, BANGS CD, OEI Y, BODNAR A, BRONSTEIN A, CHIU CP, HERRON GS. Human endothelial cell life extension by telomerase expression. J Biol Chem. 1999;274:26141–26148. doi: 10.1074/jbc.274.37.26141. [DOI] [PubMed] [Google Scholar]
- YUAN L, YU WM, QU CK. DNA damage-induced G2/M checkpoint in SV40 large T antigen-immortalized embryonic fibroblast cells requires SHP-2 tyrosine phosphatase. J Biol Chem. 2003;278:42812–42820. doi: 10.1074/jbc.M305075200. [DOI] [PubMed] [Google Scholar]


