Abstract
Autosomal dominant optic atrophy (DOA) is the most common form of hereditary optic neuropathy. DOA presents in the first decade of life and manifests as progressive vision loss. In DOA retinal ganglion cells and the optic nerve degenerate by an unknown mechanism. The gene mutated in DOA, Optic Atrophy Type 1 (OPA1), encodes a dynamin-related GTPase implicated in mitochondrial fusion and maintenance of the mitochondrial network and genome. Here, we determine which cell types in the normal retina and the optic nerve express OPA1. In the normal rat retina, OPA1 is expressed in the ganglion cell layer as well as in the outer plexiform layer, the inner nuclear layer, and the inner plexiform layer. In the ganglion cell layer, OPA1 is expressed predominantly in retinal ganglion cells. By contrast, OPA1 protein is low or undetectable in astrocytes and oligodendrocytes of the optic nerve. Additionally, OPA1 protein is present in axonal mitochondria. Last, OPA1 expression is present in mitochondria of processes and cell bodies of purified retinal ganglion cells and of the RGC-5 cell line. Thus, OPA1 is predominantly expressed in retinal ganglion cells of the normal rat retina and axons of the optic nerve. These findings may explain the selective vulnerability of retinal ganglion cells to OPA1 loss of function.
Keywords: immunohistochemistry, retinal ganglion cells, mitochondria, dominant optic atrophy, dynamin-related GTPase
Dynamin is a large GTPase regulating vesicular traffic and endocytosis at the plasma membrane (van der Bliek et al., 1993; Sweitzer and Hinshaw, 1998; Hinshaw, 2000). Dynamin-like proteins have been shown to regulate mitochondrial morphology, which is determined by organelle fusion and fission. These include Saccharomyces cerevisiae Dnm1p and its orthologs, Caenorhabditis elegans DRP1 and mammalian Dlp1/Drp1. Proteins of this group are associated with the mitochondrial outer membrane and mitochondrial fission processes (Smirnova et al., 1998, 2001; Bleazard et al., 1999; Labrousse et al., 1999; Yoon et al., 2001). Another group includes S. cerevisiae Mgm1p (Jones and Fangman, 1992; Guan et al., 1993; Shephard and Yaffe et al., 1999; Wong et al., 2000) and Schizosaccharomyces pombe Msp1p (Pelloquin et al., 1998, 1999), both of which are involved in mitochondrial fusion and in maintenance of the mitochondrial genome and network (Jones and Fangman, 1992; Pelloquin et al., 1998; Shepard and Yaffe, 2001). OPA1, the human ortholog of Mgm1p/Msp1p, has recently been identified as a dynamin-related GTPase (Alexander et al., 2000; Delettre et al., 2000).
Dominant optic atrophy (DOA) is the most common form of hereditary optic neuropathy. The prevalence of DOA is 1:50,000 in most populations, with symptoms manifesting in the first decade of life (Alexander et al., 2000; Delettre et al., 2000, 2002). The clinical characteristics of DOA are a loss of visual acuity, development of central and bilateral atrophy of the optic nerve, and color vision defects in the blue hues, all due to a progressive degeneration of retinal ganglion cells (Jaeger, 1966; Kline and Glaser, 1979; Hoyt, 1980). The gene responsible for DOA was recently mapped to human chromosome 3q28 and is designated Optic Atrophy Type 1(OPA1) (Delettre et al., 2000, 2002). The subcellular localization of OPA1 has been assigned primarily to the mitochondrial inner membrane, the intermembrane space, and the cristae. OPA1 has also been observed in the mitochondrial outer membrane, although this finding remains controversial (Olichon et al., 2001; Satoh et al., 2003).
We have previously demonstrated that in the central nervous system mouse OPA1 (mOPA1) is widely expressed in the brain, especially in neurons of the olfactory bulb, cerebral cortex, piriform cortex, hypothalamus, hippocampus, red nucleus, cochlear nucleus, motor trigeminal nucleus, facial nucleus, cerebellar nucleus, and Purkinje cells (Misaka et al., 2002). In addition, mOPA1 protein was clearly observed in the dendrites and soma of neuronal cells, as well as in astrocytes and meningeal cells. In the eye, recent evidence indicates that OPA1 is present in several retinal cell types and in the optic nerve (Aijaz et al., 2004), but its distribution in the retina remains controversial. To lay the groundwork for functional studies of OPA1 in the retina and optic nerve, we performed a detailed immunohistochemical analysis of the distribution of OPA1 protein in the normal rat retina and optic nerve.
MATERIALS AND METHODS
Tissue preparation
Rats were treated in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the ARVO Statement on the Use of Animals in Ophthalmic Research. Animals were sacrificed by intraperitoneal injection of euthanasia cocktail (xylazine, 100 mg/ml and ketamine, 100 mg/ml). Both eyes were enucleated and the anterior segments removed. The posterior segments containing the optic nerve were fixed in 4% paraformaldehyde in 0.1 M phosphate buffer (PB), pH 7.4, for 30 minutes at room temperature. The retinas and optic nerve were dissected from the choroids and fixed under the same conditions for 2 hours at 4°C. After several washes with PB, small pieces were cut from the retina and left in 70% ethanol overnight. Tissues were dehydrated with graded ethanols and embedded in wax (Ju and Neufeld, 2002). For immunoblot analysis, the retinas and optic nerve were dissected from the choroids, immediately frozen in liquid nitrogen, and stored at −70°C until use.
Retrograde labeling of retinal ganglion cells
Male Sprague-Dawley rats at 3 months of age were used for retrograde labeling with Fluoro-Gold (Fluorochrome, Englewood, CO) (Kawai et al., 2001). One week before sacrifice, Fluoro-Gold (5%, 2.4 μl/injection) diluted in saline was microinjected bilaterally into the superior colliculi of anesthetized rats immobilized in a stereotactic apparatus. Fluoro-Gold is taken up by axon terminals of retinal ganglion cells and bilaterally transported retrogradely to their somata in the retina, where the marker persists for at least 3 weeks without significant fading or leakage. Fluoro-Gold-labeled retinal ganglion cells were visualized in retinal sections by fluorescence microscopy.
Preparation of retinal suspension
Tissue was dissociated enzymatically to make a single cell suspension, essentially as described by Leifer et al. (1984) and Huettner and Baughman (1986). Briefly, the tissue was incubated at 34°C for 30 minutes in a papain solution (16.5 U/ml; Worthington, Lakewood, NJ) in Dulbecco’s phosphate-buffered saline (DPBS, Irvine Scientific, Santa Ana, CA) containing L-cysteine (Sigma, St. Louis, MO). The tissue was then triturated sequentially in a solution containing ovomucoid (2 mg/ml; Roche Biochemicals, Indianapolis, IN), DNase (0.004%; Sigma), and bovine serum albumin (BSA; 1 mg/ml; Sigma). After centrifugation at 800g, cells were rewashed in another ovomucoid/BSA solution (10 mg/ml).
Purification of retinal ganglion cells
Retinal ganglion cells were purified by immunopanning as described previously (Barres et al., 1988; Meyer-Franke et al., 1995). Approximately 15,000 purified cells were seeded on 24-well plates coated first with poly-D-lysine (PDL; 70 kDa, 10 μg/ml; Sigma) and then with laminin (10 μg/ml; Sigma) in neurobasal medium. Retinal ganglion cells were cultured in serum-free defined growth medium containing BDNF (50 μg/ml; Sigma), CNTF (10 μg/ml; Sigma), insulin (5 μg/ml; Sigma), and forskolin (10 μg/ml; Sigma). The growth medium was replaced by removing half of the culture medium and adding half a volume of fresh medium every 3 days.
Culture of RGC-5 cells
The rat retinal ganglion cell line, RGC-5, transformed with adenovirus carrying E1A was cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin (Sigma) at 5% CO2 and 37°C (Krishnamoorthy et al., 2001; Aoun et al., 2003).
Transfection of RGC-5 cells
For transfection of RGC-5 cells, 100 μl of Rat Neuron Nucleofector Solution (Amaxa, Gaithersburg, MD) was mixed with 2 × 106 cells and then 1 μg pDsRed2-Mito vector (ClonTech, Palo Alto, CA) was electroporated using a Nucleofector Device (Amaxa). The transfection efficiency was ~70%. DNA for electroporation was purified using an endotoxin-free plasmid preparation kit (Qiagen, Valencia, CA).
RNA interference
For the silencing of the OPA1 gene in HeLa cells, RNAi experiments were done as described (Elbashir et al., 2001). The target sequence for the OPA1 siRNA, 5′-AAGTTATCAGTCTGAGCCAGGTT-3′, from position 1810 –1833 of OPA1 open reading frame (GenBank access. no. AB011139), was purchased from Dharmacon (Lafayette, CO) (Olichon et al., 2003). Transfection of HeLa cells with the siRNA duplex (100 nM) was performed with Oligofectamine reagent (Invitrogen, La Jolla, CA). Scramble II Duplex siRNA (Dharmacon) was used as a negative control. Three days after transfection, live cells were stained with MitoTracker Red CMXRos (100 nM; Molecular Probes, Eugene, OR) for 20 minutes at 37°C. The cells were fixed in 0.5% glutaraldehyde (Ted Pella, Redding, CA) in PBS for 30 minutes at 4°C and counterstained with Hoechst 33342 (1 μg/ml, Molecular Probes). Mitochondrial morphology was scored by fluorescence deconvolution microscopy.
Immunoblot analysis
Polyclonal rabbit anti-mOPA1 antibody were directed against amino acids 938 –960 of mouse OPA1 protein was generated and peptide affinity-purified as previously described (Misaka et al., 2002). This polyclonal antibody cross-reacts with human and rat OPA1 protein.
Brain tissue from adult C57BL/6J mice or retina and optic nerve from adult Sprague-Dawley rats were homogenized using a glass-Teflon Potter homogenizer in ice-cold PBS supplemented with a mixture of complete protease inhibitors (Roche Biochemicals) and then centrifuged at 500g for 10 minutes at 4°C to remove debris. For HeLa and RGC-5 cultures, cells were homogenized in a glass-Teflon Potter homogenizer in lysis buffer containing 20 mM Hepes, pH 7.5, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 0.5% CHAPS plus complete protease inhibitors (Roche Biochemicals). The supernatant was mixed with SDS-PAGE sample buffer and boiled for 10 minutes. Equivalent amounts of protein (20 μg) for each sample were loaded onto 4 –12% precast poly-acrylamide gradient gels (Invitrogen). The proteins were electrotransferred to a nitrocellulose membrane in Tris-glycine-methanol transfer buffer. The membrane was blocked for 1 hour at room temperature in PBS containing 5% nonfat dry milk and 0.05% Tween-20 and then incubated for 15 hours at 4°C with anti-OPA1 antibodies (1: 1,000) in blocking solution. The membrane was then rinsed with 0.05% Tween-20 in PBS four times and incubated for 1 hour at room temperature with peroxidase-conjugated goat antirabbit IgG (Amersham, Arlington Heights, IL) (1:2,000). The blot was developed by Super-Signal West Femto Maximun Sensitivity Substrate (Pierce, Rockford, IL) according the manufacturer’s recommendations and exposed to Hyperfilm (Amersham).
To determine the specificity of OPA1 antibody, we pre-absorbed the antibody with a specific blocking peptide or a recombinant OPA1 protein. The blocking peptide (5 μg/ml) corresponding to amino acids 938 –960 of mOPA1 (CDLKKVREIQEKLDAFIEALHQEK) or recombinant OPA1 (5 μg/ml) were incubated with the OPA1 antibody (1:500) for 1 hour at room temperature before incubation with the nitrocellulose membrane and then the immunoblot was performed as described above.
Recombinant OPA1 protein was generated by expression in SF9 (Spodoptera frugiperda) insect cells using Bac-to-Bac baculovirus system (Invitrogen). Briefly, human OPA1 gene was cloned into pFastBac HT vector (Invitrogen), which was used to generate an expression bacmid in DH10Bac (Invitrogen) E. coli cells. SF9 cells were transfected with the expression bacmid to generate lower titer virus. After four rounds of amplification the higher titer virus was used to infect SF9 cells. The infected SF9 cells were grown for 3 days and collected by centrifugation and lysed in Triton X-100 and sonication. Recombinant OPA1 protein was then purified by binding to the Ni-chelating HiTrap column (Amersham). Further purification was carried out using a Superdex 200 gel filtration column Akta-FPLC (Amersham). The protein was stored at − 80°C.
Immunohistochemistry and immunocytochemistry
For OPA1 immunohistochemistry, we prepared 7-μm thick, rehydrated wax sections as described above. Sections were incubated with solutions from the Tyramide Signal Amplification Kit (Molecular Probes), according to the manufacturer’s instructions. Briefly, tissues were per-meabilized with 0.1% Triton X-100/PBS for 10 minutes at room temperature and washed with PBS. To quench endogenous peroxidase activity, tissues were incubated with quenching buffer (Amplication buffer/0.0015% H2O2) for 1 hour at room temperature. To prevent nonspecific background, tissues were incubated with 1% blocking solution (BSA/PBS) for 1 hour at room temperature and then with the primary antibody against OPA1 for 16 hours at 4°C. After several wash steps, the tissue was incubated with the secondary antibody, peroxidase-conjugated goat anti-rabbit IgG, for 1 hour at room temperature and then washed with PBS. The tissue sections were incubated with the Tyramide working solution for 10 minutes at room temperature. After PBS wash, the sections were incubated with Hoechst 33342 (1 μg/ml, Molecular Probes) for 5 minutes at room temperature and then mounted on glass slides with FluoroGuard Antifade Reagent (Bio-Rad, Hercules, CA).
For OPA1 immunocytochemistry, retinal ganglion cells and RGC-5 cells were fixed with 0.5% glutaraldehyde for 30 minutes at 4°C to preserve mitochondrial morphology. Cells were then briefly rinsed with PBS and incubated with 1% sodium borohydride for 30 minutes at room temperature to quench autofluorescence. After permeabilization with 0.1% Triton X-100/PBS for 15 minutes at room temperature, cells were washed with PBS and then incubated in Tyramide Signal Amplification Kit (Molecular Probes), according to the manufacturer’s instructions as described above.
To determine the specificity of OPA1 antibody in sections, the blocking peptide (5 μg/ml) or recombinant OPA1 (5 μg/ml) were incubated with the OPA1 antibody (1: 1,000) for 1 hour at room temperature before incubation with retinal sections and then immunohistochemistry was performed as described above.
Double immunohistochemistry
To identify cell types expressing OPA1, we performed double immunohistochemistry in normal optic nerve tissue. The following additional primary antibodies were added to histological sections labeled with OPA1-specific antibody as indicated above: mouse anti-neurofilament 200 monoclonal antibody (Clone N52; 1:400; Sigma), mouse anti-cytochrome c monoclonal antibody (1:100, Chemicon, Temecula, CA), mouse anti-glial fibrillary acidic protein monoclonal antibody (GFAP; 1:400; Sigma), and the mouse O4 monoclonal antibody (1:400; Chemicon). The primary antibodies were incubated for 16 hours at 4°C. After rinsing in PBS for 30 minutes, sections were incubated with a secondary antibody, goat anti-mouse IgG conjugated with Alexa Fluor 594 or goat anti-mouse IgM conjugated with Alexa Fluor 555 (1:200; Molecular Probes) for 1 hour at room temperature. After PBS wash, the sections were incubated with Hoechst 33342 (1 μg/ml, Molecular Probes) for 5 minutes at room temperature and then mounted on glass slides with FluoroGuard Antifade Reagent (Bio-Rad).
Representative sections of all samples were stained simultaneously to assure that samples were treated identically. Controls were performed by eliminating the primary antibody from the incubation medium. Images were captured under fluorescence deconvolution microscopy using a Zeiss Axiovert 100M microscope equipped with a high performance CCD camera. Images were acquired using emission filters of 457 nm, 528 nm, or 617 nm and collected by SlideBook 4.0 software (Intelligent Imaging Innovations, Santa Monica, CA) and exported as PhotoShop files (Adobe Systems, San Jose, CA).
RESULTS
Biochemical analysis of OPA1 protein expression
First, we examined whether OPA1 is expressed in the normal rat retina and optic nerve. For this reason, cell lysates were prepared from the rat retina and the optic nerve. Extracts from whole mouse brain were used as a positive control (Misaka et al., 2002). By immunoblot, the OPA1 antibody recognizes two major bands in extracts of brain, retina, and optic nerve (Fig. 1A). A similar protein banding pattern has previously been described and is attributed to sequential proteolytic processing of OPA1 (Misaka et al., 2002; McQuibban et al., 2003). The major bands have approximate molecular weights of 90 and 80 kDa (Fig. 1A). A similar banding pattern was detected in mixed retinal ganglion cell cultures and RGC-5 cells (Fig. 1B) and these molecular weights agree with our previously published data (Misaka et al., 2002). To determine the specificity of OPA1 antibody, we first performed immunoblot analysis using recombinant OPA1 protein. The OPA1 antibody clearly recognized recombinant OPA1 protein with an approximate molecular weight of 100 kDa (Fig. 1C). In contrast, when extracts from whole mouse brain or retina were incubated with preabsorbed OPA1 antibody/peptide or OPA1 antibody/recombinant protein complex, no protein bands were detected, thus confirming the specificity of our OPA1 antibody (data not shown). In addition, HeLa cells were transfected with OPA1 siRNA or scramble II siRNA, as negative control. At 72 hours after transfection, live cells were loaded with MitoTracker to label mitochondria and with Hoechst to identify nuclei. As shown in Figure 1Da, control cells revealed a tubular, interconnected mitochondrial network. By contrast, OPA1 knockdown by siRNA silencing resulted in a dramatic breakdown of mitochondrial network (Fig. 1Db). Instead of the typical tubular network, mitochondria were found as isolated, punctate, small organelles. Immunoblotting with OPA1 antibody of all lysates from these samples indicates that the OPA1 protein doublet is dramatically decreased in cells transfected with OPA1 siRNA (Fig. 1Dc). Immunoblots were reprobed to confirm equal protein loading (Fig. 1Dc). Taken together, these data indicate that the OPA1 antibody used in this study is specific for OPA1.
Fig. 1.
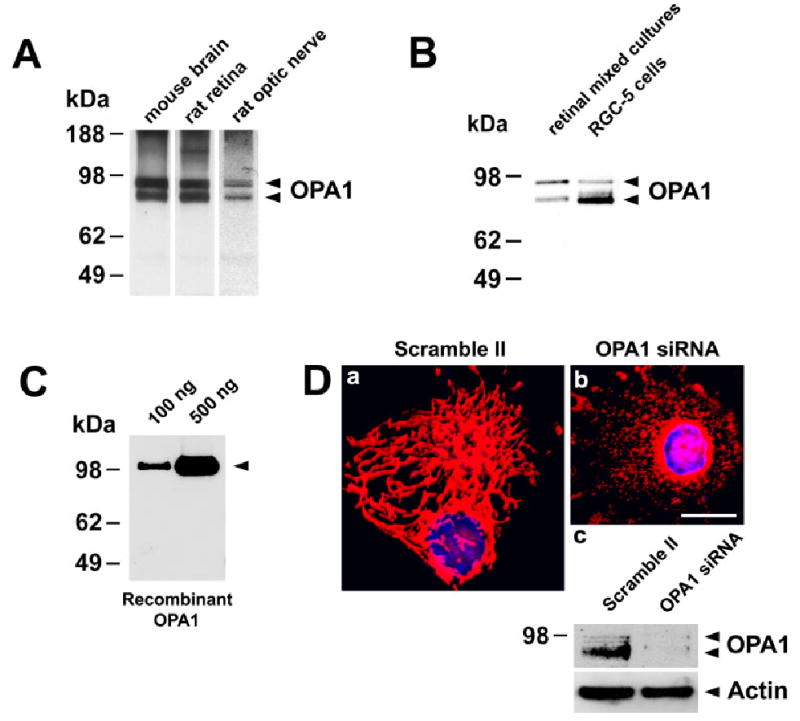
A: OPA1 expression in the normal retina and optic nerve. Immunoblot of extracts from mouse brain, rat retina, and optic nerve probed with OPA1 antibody. B: OPA1 expression in the normal retinal mixed cultures and RGC-5 cells shown by immunoblotting. The arrows show the positions, based on comparison with size standards, of the 90 and 80 kDa forms of OPA1. C: Immunoblotting of recombinant human OPA1 protein using OPA1 antibody. D: HeLa cells transfected with scramble II, control siRNA (a), or with OPA1 siRNA (b). Mitochondrial morphology and nuclei were identified by colabeling with MitoTracker Red and Hoechst. (c) OPA1 immunoblot of extracts from HeLa cells transfected with scramble II siRNA or OPA1 siRNA. The blot was stripped and reprobed with anti-actin antibody to ensure similar protein loading. Scale bar = 10 μm in a (applies to a,b).
OPA1 expression in the normal rat retina
To determine which cell types express OPA1, retinas from young rats were isolated and sections were immunostained using a polyclonal anti-OPA1 antibody. When the primary antibody was omitted, as a control for OPA-1 immunohistochemistry, there was no labeling by the secondary antibody in the normal rat retina (Fig. 2A). When the antibody was preabsorbed by incubation with peptide or recombinant protein (data not shown), no OPA1 immunoreactivity was detected (Fig. 2A1). By contrast, sections incubated with anti-OPA1 antibody showed the most intense OPA1 immunoreactivity in processes of the outer plexiform layer (OPL), the inner nuclear layer (INL), the inner plexiform layer (IPL), and the ganglion cell layer (GCL) (Fig. 2B). In the GCL, OPA1 was expressed in retinal ganglion cells (RGCs) (Fig. 2B–L).
Fig. 2.
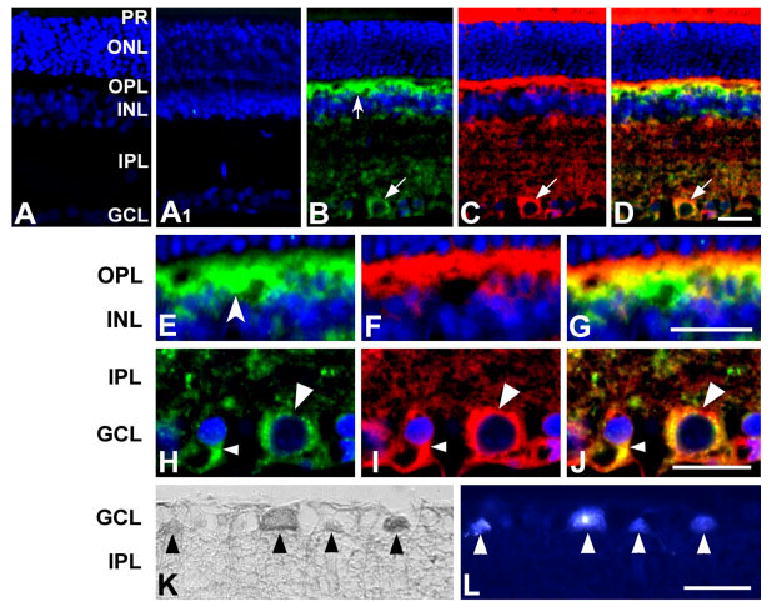
Cellular localization of OPA1 in the normal rat retina. A: Control. When the primary antibody was omitted, there was no binding of the secondary antibody. A1: Immunohistochemistry of rat retina using OPA1 antibody preabsorbed with OPA1 peptide. No OPA1 immunoreactivity was detected. B–G: OPA1 (B,E; green) and cytochrome c (C,F; red) double immunohistochemistry. OPA1 immunoreactivity (OPA1-IR) was seen in the OPL, INL, IPL, and GCL. Note that neurons were positive for OPA1 in the OPL (concave arrow) and GCL (arrow). Cytochrome c, a marker for the mitochondrial intermembrane space, colocalized with OPA1 (D). Higher magnification showed that OPA1-IR is present in horizontal cells (concave arrowhead) in the OPL (E) and OPA1-IR colocalized with cytochrome c-IR in cells with large (large arrowhead) or small (small arrowhead) sized soma (J). K,L: OPA1 (K, HRP-DAB) and Fluoro-Gold (L) double labeling. Neurons containing OPA1-IR were colabeled by Fluoro-Gold (arrowheads), indicating that retinal ganglion cells in the GCL contained OPA1 protein. HRP-DAB, horseradish peroxidase-diaminobenzidine; IR, immunoreactivity; PR, photoreceptor; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. Scale bars = 20 μm in D (applies to A–D), G (applies to E–G), J (applies to H–J), L (applies to K,L).
To confirm the mitochondrial localization of OPA1, retinal sections were coimmunostained with anti-cytochrome c and anti-OPA1 antibodies. As indicated in Figure 2B–J, OPA1 immunolabeling overlaps with the cytochrome c immunostaining pattern. Higher magnification showed that OPA1 immunoreactivity was present in the processes and cell bodies of the horizontal cells in the OPL (Fig. 2E–G) and that OPA1 was present in mitochondria of retinal ganglion cell bodies (Fig. 2H–J).
To verify that RGCs indeed express OPA1, we retrogradely labeled retinal ganglion cells in vivo using Fluoro-Gold. After labeling, retinas were isolated, embedded in wax, and sections were immunostained with anti-OPA1 antibody (Fig. 2K). Remarkably, all neurons containing OPA1 immunoreactivity were colabeled by Fluoro-Gold (Fig. 2L), indicating that RGCs express OPA1 protein. OPA1 immunoreactivity was absent in photoreceptors, bipolar cells, amacrine cells, Müller cells, astrocytes, and microglia (Fig. 2, Table 1).
TABLE 1.
Distribution of OPA1 in the Normal Rat Retina
| Retinal cells | |
|---|---|
| Photoreceptor | − |
| Outer plexiform layer | + |
| Horizontal cells | + |
| Bipolar cells | − |
| Amacrine cells | − |
| Inner plexiform layer | + |
| Ganglion cells | + |
| Displaced amacrine cells | − |
| Müller cells | − |
| Astrocytes | − |
| Microglia | − |
, present; −, not present.
OPA1 expression in isolated rat retinal ganglion cells and RGC-5 cells
We next determined whether isolated retinal ganglion cells expressed OPA1 protein. If expressed, this would suggest that OPA1 was synthesized in the RGCs in a cell-autonomous fashion and not produced in a paracrine manner. To test this idea, RGCs were isolated and purified using a sequential immunopanning method (Barres et al., 1988; Meyer-Franke et al., 1995), and then processed for OPA1 immunocytochemistry. Figure 3A shows that OPA1 was expressed in the cell body and processes of primary RGCs. In addition, we determined the subcellular localization of OPA1 in a line of transformed retinal ganglion cells, RGC-5. Cells were first transfected with the pDsRed2-Mito expression vector, which targets the red fluorescent DsRed protein to the inner mitochondrial membrane. Two days after transfection, cells were fixed and immunostained with anti-OPA1 antibodies. In RGC-5 cells, OPA1 immunoreactivity colocalized with the DsRed2-Mito fluorescent signal (Fig. 3B–D). Higher magnification revealed that OPA1 immunoreactivity overlapped with the mitochondrial signal (Fig. 3E–J). It is noteworthy that OPA1 staining was detected in a slightly spotted pattern in mitochondria, as evidenced in the overlapping OPA1 and DsRed-Mito signal (Fig. 3G,J). This finding suggests that OPA1 may localize to sites within mitochondria.
Fig. 3.
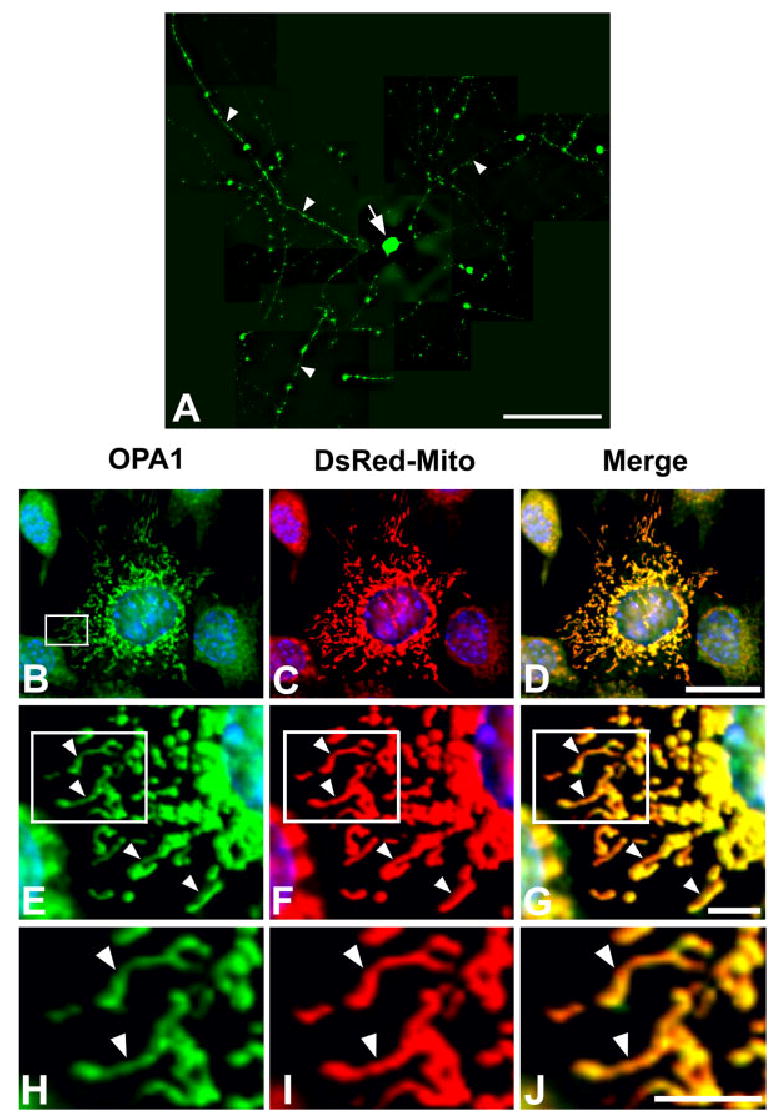
OPA1 immunoreactivity in cultured retinal ganglion cells and RGC-5 cells. A: OPA1 immunocytochemistry. OPA1-IR was present at high levels in the processes (arrowheads) and cell body (arrow) in a retinal ganglion cell. B–J: OPA1 (B,E,H) and DsRed2-Mito (C,F,I) double labeling. Higher magnification shows that OPA1 was present in DsRed-Mito-labeled mitochondria with long tubular forms in RGC-5 cells (merge, D,G,J; arrowheads). Scale bars = 100 μm in A; 20 μm in D (applies to B–D); 10 μm in G (applies to G–E), J (applies to H–J).
OPA1 expression in the optic nerve
We next asked whether OPA1 was expressed in the optic nerve. As shown in Figure 4, OPA1 is highly expressed in longitudinal sections of the optic nerve. To define more precisely whether OPA1 was expressed in nerve fibers rather than in surrounding cell types, cross-sections were immunostained with anti-OPA1 antibodies plus antibodies against GFAP (an astrocyte marker), O4 (an oligodendrocyte marker), or neurofilament (an axonal marker) (Fig. 4D–L). Remarkably, no OPA1 immunoreactivity was found in cells positive for GFAP or O4, as determined by the lack of overlapping fluorescent signals (Fig. 4D–I). By contrast, neurofilament and OPA1 immunoreactivity coincided, as evidenced in the merged images of green and red fluorescent signals (Fig. 4J–L). These data indicate that OPA1 is expressed in neurons rather than in surrounding cells. This result was confirmed by the lack of OPA1 immunoreactivity in astrocytes, oligodendrocytes, and microglia (data not shown) in the normal rat optic nerve (Table 2).
Fig. 4.
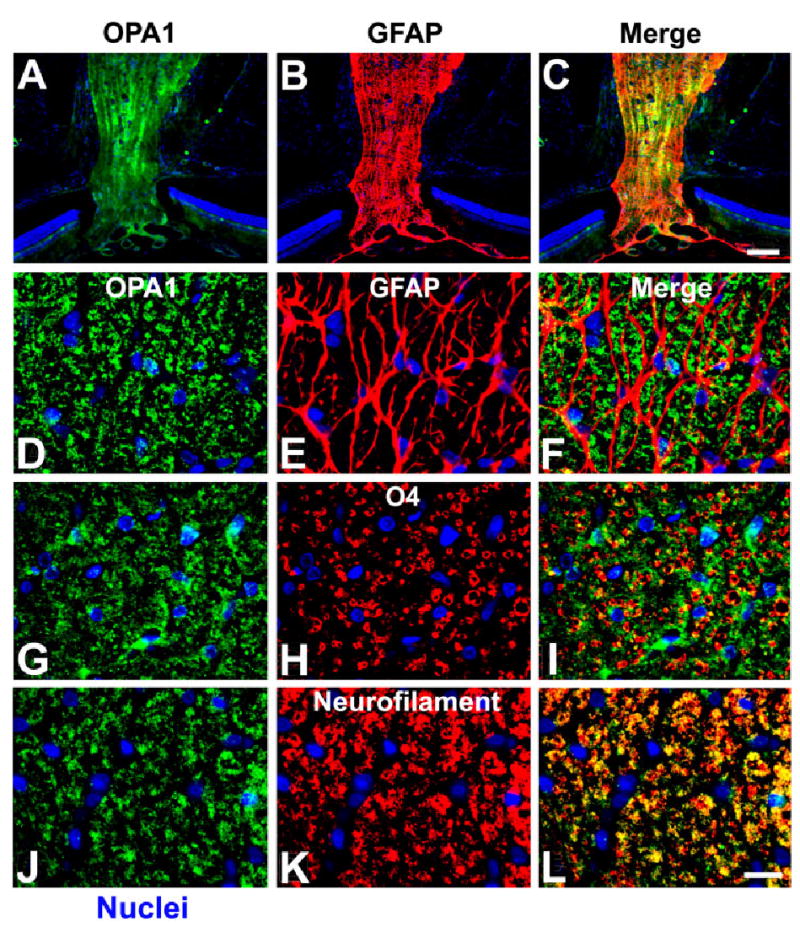
Cellular localization of OPA1 in the normal rat optic nerve. A–F: OPA1 (A,D) and GFAP (B,E) double immunohistochemistry. Cells positive for OPA1 were not positive for GFAP, an astrocyte marker (F). G–I: OPA1 (G) and O4 (H) double immunohistochemistry. OPA1 cells were not positive for O4, an oligodendrocyte marker (I). J–L: OPA1 (J) and neurofilament (K) double immunohistochemistry. OPA1-positive cells were also positive for neurofilament, indicating that OPA1 was present in axons of the optic nerve (L). Scale bars = 100 μm in C (applies to A–C); 20 μm in L (applies to D–L).
TABLE 2.
Distribution of OPA1 in the Normal Rat Optic Nerve
| Optic nerve cells | |
|---|---|
| Astrocytes | − |
| Oligodendrocytes | − |
| Microglia | − |
, present; −, not present.
DISCUSSION
In the mouse CNS, OPA1 expression has previously been described in neurons of the olfactory bulb, cerebral cortex, piriform cortex, hypothalamus, hippocampus, red nucleus, cochlear nucleus, motor trigeminal nucleus, and facial nucleus as well as in cerebellar Purkinje cells (Misaka et al., 2002). In addition, recent studies suggest that OPA1 is present in several cell types and in several layers of the rodent retina although there is not universal agreement as to the cell types involved (Aijaz et al., 2004). To clarify these findings and to establish a basis for functional analysis of OPA1 in the retina and optic nerve, we determined its expression pattern in normal rat retina and optic nerve. As shown here, OPA1 was present in the OPL, INL, IPL, and GCL of the rat retina. Notably, OPA1 was mainly present in retinal ganglion cells (RGCs). In the normal rat optic nerve, OPA1 was present in axonal mitochondria but not in glial cells.
It was previously shown that OPA1 is ubiquitously expressed in several tissues but is most abundant in the retina (Alexander et al., 2000; Pesch et al., 2001). Aijaz et al. (2004) demonstrated that OPA1 is expressed in the GCL as well as in the OPL, INL, and IPL of the mammalian retina and in the optic nerve of the adult mouse and human. Interestingly, Pesch et al. (2004) found that the OPA1 gene and its protein is present in retina ganglion cells and displaced amacrine cells in the retina of the adult mouse and rat. In the present study, we demonstrated that OPA1 is expressed in the OPL, INL, IPL, and GCL in the normal rat retina but that it is mainly present in RGCs of the GCL. We raised an anti-mOPA1 antibody in rabbits immunized with mOPA1 residues 938–960 (Misaka et al., 2002). Notably, Aijaz et al. (2004) and Pesch et al. (2004) obtained similar results using an antibody against a 24 or 18 amino acid region of the C-terminus of human OPA1 protein, respectively, indicating that the distribution pattern of OPA1 in the retina is similar among various mammalian species. Our OPA1 antibody clearly reacted with recombinant OPA1 protein of ~100 kDa, which is the expected size of the engineered OPA1 protein (Misaka et al., 2002). In addition, no OPA1 protein was detected in the normal rat retina using preabsorbed OPA1 antibody/peptide or OPA1 antibody/recombinant protein complex, indicating that the OPA1 antibody used in this study is specific.
In the present study, OPA1 immunoreactivity was most intense in the mitochondria in processes and cell bodies of cultured RGCs. Since OPA1 is widely expressed in many tissues, the reason that RGCs and not other cell types are primarily affected by OPA1 mutations which cause DOA is unknown. Although the biochemical function of OPA1 in RGCs remains to be explored, it is possible that OPA1 mutation in retinal ganglion cell axons causes breakdown of mitochondrial connectivity and consequent dysfunction in electrophysiological activity in the optic nerve.
While this article was under review, Pesch et al. (2004) found that OPA1 was absent from mitochondria-rich nerve fibers and optic nerve in the adult rat. Aijaz et al. (2004) recently reported that OPA1 is expressed in the adult mouse and human optic nerve. However, the subcellular localization of OPA1 in the optic nerve was unknown. Here, we showed that OPA1 immunoreactivity was present in the axonal mitochondria. However, OPA1 was not found in glial cells, such as astrocytes, oligodendrocytes, and microglial cells in the normal rat optic nerve, suggesting that the selective sensitivity to OPA1 function in the optic nerve depends on axonal mitochondria of the RGCs. We have previously demonstrated that GFAP-positive cells that appear to be Bergman glia highly express mOPA1 in primary cultures of dissociated cerebellar cells, but that OPA1 is not detectable by in situ hybridization or immunohistochemistry in these cells (Misaka et al., 2002), suggesting that expression of mOPA1 is upregulated in glial cells in culture. Perhaps, OPA1-negative cells in the optic nerve could also upregulate OPA1 under certain culture conditions.
In summary, our findings demonstrate that OPA1 immunoreactivity is localized in the GCL as well as in the OPL, INL, and IPL of the retina. Most important, we found that OPA1 is predominantly expressed in rat RGCs. OPA1 in the optic nerve was present in axonal mitochondria. These results suggest that mutant OPA1 may contribute to mitochondrial dysfunction in the pathophysiology of RGCs manifesting DOA.
Footnotes
Grant sponsor: National Institutes of Health (NIH)/National Institute of Neurological Disorders and Stroke (NINDS); Grant number: RO1 NS44314; Grant number: RO1 NS047456; Grant sponsor: NIH/National Eye Institute (NEI); Grant number: ROI EY016164 (to E.B.-W.); Grant sponsor: NIH/National Institute of Child Health and Human Development; Grant number: PO1 HD29587; Grant sponsor: NIH/NEI; Grant number: RO1 EY05477; Grant number: RO1 EY09024; Grant sponsor: NIH/NINDS: RO1 NS41207 (to S.A.L.).
References
- Aijaz S, Erskine L, Jeffery G, Bhattacharya SS, Votruba M. Developmental expression profile of the optic atrophy gene product: OPA1 is not localized exclusively in the mammalian retinal ganglion cell layer. Invest Ophthalmol Vis Sci. 2004;45:1667–1673. doi: 10.1167/iovs.03-1093. [DOI] [PubMed] [Google Scholar]
- Alexander C, Votruba M, Pesch UE, Thiselton DL, Mayer S, Moore A, Rodriguez M, Kellner U, Leo-Kottler B, Auburger G, Bhattacharya SS, Wissinger B. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet. 2000;26:211–215. doi: 10.1038/79944. [DOI] [PubMed] [Google Scholar]
- Andrews RM, Griffiths PG, Johnson MA, Turnbull DM. Histochemical localisation of mitochondrial enzyme activity in human optic nerve and retina. Br J Ophthalmol. 1999;83:231–235. doi: 10.1136/bjo.83.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoun P, Simpkins JW, Agarwal N. Role of PPAR-γ ligands in neuroprotection against glutamate-induced cytotoxicity in retina ganglion cells. Invest Ophthalmol Vis Sci. 2003;44:2999 –3004. doi: 10.1167/iovs.02-1060. [DOI] [PubMed] [Google Scholar]
- Barres BA, Silverstein BE, Corey DD, Chun LL. Immunological, morphological, and electrophysiological variation among retinal ganglion cells purified by panning. Neuron. 1988;1:791– 803. doi: 10.1016/0896-6273(88)90127-4. [DOI] [PubMed] [Google Scholar]
- Bleazard W, McCaffery JM, King EJ, Bale S, Mozdy A, Tieu Q, Nunnari J, Shaw JM. The dynamin-related GTPase Dnm1 regulates mitochondrial fission in yeast. Nat Cell Biol. 1999;1:298 –304. doi: 10.1038/13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossy-Wetzel E, Barsoum MJ, Godzik A, Schwarzenbacher R, Lipton SA. Mitochondrial fission in apoptosis, neurodegeneration and aging. Curr Opin Cell Biol. 2003;15:1–11. doi: 10.1016/j.ceb.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Delettre C, Lenaers G, Griffoin JM, Gigarel N, Lorenzo C, Belenguer P, Pelloquin L, Grosgeorge J, Turc-Carel C, Perret E, Astarie-Dequeker C, Lasquellec L, Arnaud B, Ducommun B, Kaplan J, Hamel CP. Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat Genet. 2000;26:207–210. doi: 10.1038/79936. [DOI] [PubMed] [Google Scholar]
- Delettre C, Griffoin JM, Kaplan J, Dollfus H, Lorenz B, Faivre L, Lenaers G, Belenguer P, Hamel CP. Mutation spectrum and splicing variants in the OPA1 gene. Hum Genet. 2001;109:584 –591. doi: 10.1007/s00439-001-0633-y. [DOI] [PubMed] [Google Scholar]
- Delettre C, Lenaers G, Pelloquin L, Belenguer P, Hamel CP. OPA1 (Kjer type) dominant optic atrophy: a novel mitochondrial disease. Mol Genet Metab. 2002;75:97–107. doi: 10.1006/mgme.2001.3278. [DOI] [PubMed] [Google Scholar]
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotides RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494 – 498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- Guan K, Farh L, Marshall TK, Deschenes RJ. Normal mitochondrial structure and genome maintenance in yeast requires the dynamin-like product of the MGM1 gene. Curr Genet. 1993;24:141–148. doi: 10.1007/BF00324678. [DOI] [PubMed] [Google Scholar]
- Hinshaw JE. Dynamin and its role in membrane fission. Annu Rev Cell Dev Biol. 2000;16:483–519. doi: 10.1146/annurev.cellbio.16.1.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell N. Leber hereditary optic neuropathy: potential opportunities/potential pitfalls for drug therapy of optic nerve degenerative disorders. Drug Dev Res. 1999;46:34 – 43. [Google Scholar]
- Hoyt CS. Autosomal dominant optic atrophy: a spectrum of disability. Ophthalmology. 1980;87:245–251. doi: 10.1016/s0161-6420(80)35247-0. [DOI] [PubMed] [Google Scholar]
- Huettner JE, Baughman RW. Primary culture of identified neurons from the visual cortex of postnatal rats. J Neurosci. 1986;6:3044 –3060. doi: 10.1523/JNEUROSCI.06-10-03044.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger W. Hereditary optic atrophies in childhood. J Genet Hum. 1966;15:312–321. [PubMed] [Google Scholar]
- Johnston PB, Gaster RN, Smith VC, Tripathi RC. A clinicopathologic study of autosomal dominant optic atrophy. Am J Ophthalmol. 1979;88:868 –875. doi: 10.1016/0002-9394(79)90565-8. [DOI] [PubMed] [Google Scholar]
- Jones BA, Fangman WL. Mitochondrial DNA maintenance in yeast requires a protein containing a region related to the GTP-binding domain of dynamin. Genes Dev. 1992;6:380 –389. doi: 10.1101/gad.6.3.380. [DOI] [PubMed] [Google Scholar]
- Ju WK, Neufeld AH. Cellular localization of cyclooxygenase-1 and cyclooxygenase-2 in the normal mouse, rat, and human retina. J Comp Neurol. 2002;452:392–399. doi: 10.1002/cne.10400. [DOI] [PubMed] [Google Scholar]
- Kawai SI, Vora S, Das S, Gachie E, Becker B, Neufeld AH. Modeling of risk factors for the degeneration of retinal ganglion cells after ischemia/reperfusion in rats: effects of age, caloric restriction, diabetes, pigmentation, and glaucoma. FASEB J. 2001;15:1285–1287. doi: 10.1096/fj.00-0666fje. [DOI] [PubMed] [Google Scholar]
- Kline LB, Glaser JS. Dominant optic atrophy. The clinical profile. Arch Ophthalmol. 1979;97:1680 –1686. doi: 10.1001/archopht.1979.01020020248013. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy RR, Agarwal P, Prasanna G, Vopat K, Lambert W, Sheedlo HJ, Pang IH, Shade D, Wordinger RJ, Yorio T, Clark AF, Agarwal N. Characterization of a transformed rat retinal ganglion cell line. Mol Brain Res. 2001;86:1–12. doi: 10.1016/s0169-328x(00)00224-2. [DOI] [PubMed] [Google Scholar]
- Labrousse AM, Zappaterra MD, Rube DA, van der Bliek AM. C. elegans dynamin-related protein DRP-1 controls severing of the mitochondrial outer membrane. Mol Cell. 1999;4:815– 826. doi: 10.1016/s1097-2765(00)80391-3. [DOI] [PubMed] [Google Scholar]
- Leifer D, Lipton SA, Barnstable CJ, Masland RH. Monoclonal antibody to Thy-1 enhances regeneration of processes by rat retinal ganglion cells in culture. Science. 1984;224:303–306. doi: 10.1126/science.6143400. [DOI] [PubMed] [Google Scholar]
- Lipton SA. Retinal ganglion cells, glaucoma and neuroprotection. 2001;131:712–718. [PubMed] [Google Scholar]
- McQuibban GA, Saurya S, Freeman M. Mitochondrial membrane remodelling regulated by a conserved rhomboid protease. Nature. 2003;423:537–541. doi: 10.1038/nature01633. [DOI] [PubMed] [Google Scholar]
- Meyer-Franke A, Kaplan MR, Pfrieger FW, Barres BA. Characterization of the signaling interactions that promote the survival and growth of developing retinal ganglion cells in culture. Neuron. 1995;15:805–819. doi: 10.1016/0896-6273(95)90172-8. [DOI] [PubMed] [Google Scholar]
- Misaka T, Miyashita T, Kubo Y. Primary structure of a dynamin-related mouse mitochondrial GTPase and its distribution in brain, subcellular localization, and effect on mitochondrial morphology. J Biol Chem. 2002;277:15834 –15842. doi: 10.1074/jbc.M109260200. [DOI] [PubMed] [Google Scholar]
- Neufeld AH, Sawada A, Becker B. Inhibition of nitric-oxide synthase 2 by aminoguanidine provides neuroprotection of retinal ganglion cells in a rat model of chronic glaucoma. Proc Natl Acad Sci U S A. 1999;96:9944 –9948. doi: 10.1073/pnas.96.17.9944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olichon A, Emorine LJ, Descoins E, Pelloquin L, Brichese L, Gas N, Guillou E, Delettre C, Valette A, Hamel CP, Ducommun B, Lenaers G, Belenguer P. The human dynamin-related protein OPA1 is anchored to the mitochondrial inner membrane facing the inter-membrane space. FEBS Lett. 2002;523:171–176. doi: 10.1016/s0014-5793(02)02985-x. [DOI] [PubMed] [Google Scholar]
- Olichon A, Baricault L, Gas N, Guillou E, Delettre C, Valette A, Belenguer P, Lenaers G. Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J Biol Chem. 2003;278:7743–7746. doi: 10.1074/jbc.C200677200. [DOI] [PubMed] [Google Scholar]
- Pelloquin L, Belenguer P, Menon Y, Ducommun B. Identification of a fission yeast dynamin-related protein involved in mitochondrial DNA maintenance. Biochem Biophys Res Commun. 1998;251:720 –726. doi: 10.1006/bbrc.1998.9539. [DOI] [PubMed] [Google Scholar]
- Pelloquin L, Belenguer P, Menon Y, Gas N, Ducommun B. Fission yeast Msp1 is a mitochondrial dynamin-related protein. J Cell Sci. 1999;112:4151– 4161. doi: 10.1242/jcs.112.22.4151. [DOI] [PubMed] [Google Scholar]
- Pesch UE, Leo-Kottler B, Mayer S, Jurklies B, Kellner U, Apfelstedt-Sylla E, Zrenner E, Alexander C, Wissinger B. OPA1 mutation in patients with autosomal dominant optic atrophy and evidence for semi-dominant inheritance. Hum Mol Genet. 2001;10:1359 –1368. doi: 10.1093/hmg/10.13.1359. [DOI] [PubMed] [Google Scholar]
- Pesch UE, Fries JE, Bette S, Kalbacher H, Wissinger B, Alexander C, Kohler K. OPA1, the disease gene for autosomal dominant optic atrophy, is specifically expressed in ganglion cell and intrinsic neurons of the retina. Invest Ophthalmol Vis Sci. 2004;45:4217– 4225. doi: 10.1167/iovs.03-1261. [DOI] [PubMed] [Google Scholar]
- Quigley HA. Neuronal death in glaucoma. Prog Retin Eye Res. 1999;18:39 –57. doi: 10.1016/s1350-9462(98)00014-7. [DOI] [PubMed] [Google Scholar]
- Satoh M, Hamamoto T, Seo N, Kagawa Y, Endo H. Differential sublocalization of the dynamin-related protein OPA1 isoforms in mitochondria. Biochem Biophys Res Commun. 2003;300:482– 493. doi: 10.1016/s0006-291x(02)02874-7. [DOI] [PubMed] [Google Scholar]
- Shepard KA, Yaffe MP. The yeast dynamin-like protein, Mgm1p, functions on the mitochondrial outer membrane to mediate mitochondrial inheritance. J Cell Biol. 1999;144:711–720. doi: 10.1083/jcb.144.4.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnova E, Shurland DL, Ryazantsev SN, van der Bliek AM. A human dynamin-related protein controls the distribution of mitochondria. J Cell Biol. 1998;143:351–358. doi: 10.1083/jcb.143.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnova E, Griparic L, Shurland DL, van der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell. 2001;12:2245–2256. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweitzer SM, Hinshaw JE. Dynamin undergoes a GTP-dependent conformational change causing vesiculation. Cell. 1998;93:1021–1029. doi: 10.1016/s0092-8674(00)81207-6. [DOI] [PubMed] [Google Scholar]
- van der Bliek AM, Redelmeier TE, Damke H, Tisdale EJ, Meyerowitz EM, Votruba M, Moore AT, Bhattacharya SS. Clinical features, molecular genetics, and pathophysiology of dominant optic atrophy. J Med Genet. 1998;35:793–800. doi: 10.1136/jmg.35.10.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ED, Wagner JA, Gorsich SW, McCaffery JM, Shaw JM, Nunnari J. The dynamin-related GTPase, Mgm1p, is an intermembrane space protein required for maintenance of fusion competent mitochondria. J Cell Biol. 2000;151:341–352. doi: 10.1083/jcb.151.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon Y, Pitts KR, McNiven MA. Mammalian dynamin-like protein DLP1 tubulates membranes. Mol Biol Cell. 2001;12:2894 –2905. doi: 10.1091/mbc.12.9.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]


