Abstract
Purpose.
This study was carried out to determine the biodistribution profiles and tumor localization potential of poly(ethylene oxide) (PEO)-modified poly (β-amino ester) (PbAE) as a novel, pH-sensitive biodegradable polymeric nanoparticulate system for tumor-targeted drug delivery.
Methods.
The biodistribution studies of PEO-modified PbAE and PEO-modified poly(ɛ-caplactone) (PCL), a non-pH sensitive polymer, nanoparticle systems were carried out in normal mice using 111indiumoxine [111In] as a lipophilic radiolabel encapsulated within the polymeric matrix and the distribution of the nanoparticles was studied in plasma and all the vital organs following intravenous administration. Solid tumors were developed on nude mice using human ovarian carcinoma xenograft (SKOV-3) and the change in concentrations of tritium [3H]-labeled paclitaxel encapsulated in polymeric nanoparticles was examined in blood, tumor mass and liver.
Results.
Study in normal mice with a gamma-emitting isotope [111In] provided a thorough biodistribution analysis of the PEO-modified nanoparticulate carrier systems, while the 3H-paclitaxel was useful to understand the change in concentration and tumor localization of anticancer compound directly in major sites of distribution. Both PEO-PbAE and PEO-PCL nanoparticles showed long systemic circulating properties by virtue of surface modification with PEO-containing triblock block copolymer (Pluronic®) stabilizer. While the PCL nanoparticles showed higher uptake by the reticulo-endothelial system (RES), the PbAE nanoparticles effectively delivered the encapsulated payload into the tumor mass.
Conclusions.
PEO-modified PbAE nanoparticles showed considerable passive tumor targeting potential in early stages of biodistribution via the enhanced permeation and retention (EPR) mechanism. This prompts a detailed biodistribution profiling of the nanocarrier for prolonged periods of time to provide conclusive evidence for superiority of the delivery system.
Keywords: biodegradable, pH-sensitive, nanoparticles, poly(β-amino ester), poly(ε-caprolactone), tumor targeting, non-compartmental pharmacokinetics
INTRODUCTION
Poly (β-amino ester)s (PbAE) are synthetic, hydrolytically-degradable, biocompatible cationic polymers that are synthesized using parallel synthetic chemistry by addition reaction between primary or secondary diamines and diol-diacrylates (1, 2). Since there are a number of different types of diamines and diol-diacrylates, PbAE can be engineered to be water-soluble or insoluble, having very high or low cationic charge density, different crystallinity, and degradation kinetics to meet the specific requirements of potential use (2, 3). Given the polycationic nature of these polymers, due to secondary and tertiary amine groups in the backbone, they are particularly suited for intracellular delivery of DNA and oligonucleotides and allow these systems to remain stable in the cytosol due to their ability to buffer the pH of their surroundings (3, 4). Drug delivery systems prepared from the PbAE may be used for polynucleotides, proteins, peptides, antigen, and low molecular weights drugs with intracellular targets (1, 3, 5–7). PbAE degrade under physiological conditions via hydrolysis of their backbone esters to yield small molecular weight bis(β-amino acid) and diol products, which along with the parent polymer, are significantly less toxic than many other polycations, such as polyethyleneimine and poly(L-lysine) (2, 8).
Among United States Food and Drug Administration (FDA)-approved polyesters, poly(ɛ-caprolactone) (PCL) possesses unique properties such as enhanced biocompatibility, higher hydrophobicity, and neutral biodegradation end products that do not disturb the pH balance of the degradation medium (9–13). Over the years, an array of drug delivery systems has been developed using PCL (14–21).
Polymeric nanoparticles made from natural and synthetic polymers have drawn major attention due to higher stability, maneuverability for industrial manufacture, and opportunity for further surface nanoengineering (22–33). They can be tailor-made to achieve both controlled drug release and disease specific localization by tuning the polymer characteristics and surface chemistry (23, 28–30, 34–36). It has been established that nanocarriers can get concentrated preferentially in the tumor mass, inflammatory sites, and at infectious sites by virtue of the enhanced permeability and retention (EPR) effect of the vasculature. Once accumulated at the target site, hydrophobic biodegradable polymeric nanoparticles can act as local drug depot depending upon the make-up of the carrier, thus providing a source for continuous supply of encapsulated therapeutic compound at the disease site, such as solid tumor (23, 28). In order to prolong the systemic circulation times of the nanoparticles and enhance their passive targeting efficiency, various strategies that involve regulation of particle size, surface charge, and creation of a hydrophilic surface “brush” on the nanomatrices have been employed. Majority of reported strategies employ poly(ethylene glycol) (PEG) or poly(ethylene oxide) (PEO) chains for surface modification through physical adsorption during particle formation or by covalent linkage to the core-forming polymer [e.g., copolymer of PEG/PEO with poly(lactic acid)] prior to particle formation (19, 36–41). More recently, the possibility of introducing PEO-containing block copolymers into the nanoparticles matrix has been achieved by intimate blending of the PEO-copolymer with the core-forming polymer prior to nanoparticulate fabrication (42, 43). This method of PEO incorporation is expected to lead to partitioning of the PEO chains on the nanoparticle surface upon hydration and better stability of the PEO chains on the nanoparticle surface.
While considering the investigation of any polymeric nanocarrier system for the biodistribution profile, radio-labeling of the polymer backbone is found to be the most favorable and reliable means for subsequent quantification. This chemical conjugation of the radio-active moiety is strongly dependent on the presence of reactive groups in the polymer. However, for hydrophobic polymers such as PbAE and PCL, which do not have any reactive functional groups, the attachment of radio-label can be difficult. In such cases, the alternative is to encapsulate the radio-marker within the polymeric matrix. Gamma ray emitting labels are usually preferred for evaluating the biodistribution due to higher sensitivity and ease of sample preparation and processing. In certain cases, the bioactive compounds are labeled with beta-emitting species (like tritium) and become helpful in tracing the pathway of distribution of the pharmacologically-active compound in-vivo.
In the present study, we chose Indium-111 (111In) as the label for the nanoparticles mainly due to its shorter half-life (2.8 days) and its availability in the lipophilic form (111In oxyquinoline or 111In-oxine). For tumor uptake and concentration studies, we selected paclitaxel as the most successful cytotoxic agent against solid tumors, which was readily available in tritiated form. A systematic in-vitro investigation of the nanoparticles in tumor cells formed the basis for the current in-vivo studies (44).
MATERIALS AND METHODS
Materials
A representative hydrophobic PbAE (MW ~ 10,000) was synthesized by the addition reaction of 4,4’-trimethyldipiperidine with 1,4-butanediol diacrylate in dimethylformamide for 48 hours at 50oC and purified according to the protocol described earlier (2, 5). PCL with a number average molecular weight of 14,800 Da (as verified by gel-permeation chromatography), was purchased from Polysciences Inc. (Warrington, PA). National Formulary grade Pluronic® F-108 was kindly provided by the Performance Chemical Division of BASF Corporation (Parsipanny, NJ). Paclitaxel was purchased from LC Laboratories (Woburn, MA) and tritiated (3H) paclitaxel with an activity of 3.2 Ci/mmol was purchased from Movravek Biochemicals (Brea, CA). Paclitaxel solution, available as a commercial injection in Cremophore EL-ethanol (50:50 mixture) (Onxol®) was purchased from Ivax Pharmaceuticals (Miami, FL). Indium (111In) oxyquinoline solution was purchased from Amersham Health and had an activity of 1 mCi per ml at calibration time. SKOV-3, human ovarian adenocarcinoma cells, were kindly supplied by Dr. Michael Seiden’s laboratory at Massachusetts General Hospital (Cambridge, MA). All the other chemicals and reagents were of analytical grade and were used as supplied. Deionized distilled water (NanoPure II, Dubuque, IA) was used for all aqueous preparations.
Preparation and Characterization of Radio-labeled Polymeric Nanoparticles
111In-Labeled Polymeric Nanoparticles
The 111In oxyquinoline (or 111In -oxine) is commercially available as an aqueous solution for injection. Prior to incorporation into the nanoparticles, it is necessary to extract 111In-oxine into an organic phase (chloroform). Two ml of chloroform was gently mixed with 250 μl of the 111In-oxine for 10 minutes and allowed to separate. The organic layer was carefully pipetted into two clean glass vials in equal volumes. Contents of one vial were used to dissolve known quantities of PbAE and Pluronic® F-108 (25 mg + 6.5 mg) and the other was used to dissolve PCL and Pluronic® F-108 (25 mg + 6.5 mg). The organic solvent was evaporated under a gentle stream of dry nitrogen. Absolute ethanol and acetone (2.5 ml each) were used to re-dissolve the residues of PbAE and PCL blends with Pluronic® F-108 and 111In-oxine, respectively. This organic solution was introduced into pre-cooled (< 15°C) purified water (12.5 ml, pH adjusted to 7.0) maintained under vigorous magnetic stirring. Gentle stirring was continued for 5 hours to evaporate majority of the organic solvent from the bulk. The nanoparticles were collected by centrifugation (10,000 rpm for 20 min.), washed once with 5 ml of water (pH 7.0) and again collected by centrifugation. The final suspension was made in known volume of sterile phosphate buffered saline (PBS, pH 7.4) and the formulation was ready to be injected.
3H-Paclitaxel Encapsulated Nanoparticles
For preparing drug-loaded nanoparticles, cold paclitaxel (3.5 mg) was dissolved along with the polymers (blends of PbAE-Pluronic® F-108 and PCL-Pluronic® F108) in organic phase (acetone or ethanol) before introduction into aqueous medium. Suitable quantity (15 μCi) of 3H-labeled paclitaxel was incorporated in the organic phase to obtain tritiated nanoparticles and the products were collected and purified as described in the previous section.
PEO-modified PbAE and PCL nanoparticles without any radioactive payload were prepared and diluted suitably in deionized distilled water (pH ~ 7.0). The particle size was determined by Coulter® N4-Plus submicron particle sizer (Coulter Corporation, FL) at multiple scattering angle detection. For zeta potential measurements, a diluted aqueous suspension of nanoparticles was mounted in a 90Plus particle sizer / zetasizer (Brookhaven Instruments, NY) and mean zeta potential was computed using Smoluchowski equation.
In Vivo Experiments
The experimental protocol involving usage of radioactive materials in animals was approved by the Institutional Animal Care and Use Committee and the Office of Environmental Health and Safety at Northeastern University. Female athymic mice (Nu/Nu strain), 4–6 weeks old, weighing about 25 gm were purchased from Charles River Laboratories (Cambridge, MA) and were housed in polycarbonate cages having free access to sterilized rodent pellet diet and water. The animals were allowed to acclimate for at least 48 hours prior to any experiments.
Biodistribution Studies of 111In-Labeled Polymeric Nanoparticles
The nanoparticle biodistribution studies were carried out in female Nu/Nu without any tumor load. The choice of this species was based on the fact that Nu/Nu mice with human ovarian cancer xenograft were subsequently used for paclitaxel accumulation studies. Eight Nu/Nu mice without any xenografted tumors were used per time-point (0.5, 1, 6, 12 and 24 hours) and were accordingly divided into two treatment groups. One group of mice received 111In-labeled PEO-modified PbAE nanoparticles and the second group received 111In-labeled PEO-modified PCL nanoparticles. The formulations were suspended in PBS and contained approximately 10 μCi of 111In dose per mouse. The injections were given through the tail vein as a slow bolus. At periodic time intervals, blood was collected through sino-arbital vein puncture under light anesthesia (with isoflurane). The animals were then sacrificed by cervical dislocation and the vital organs were harvested, washed with sterile PBS, and collected in pre-weighed glass tubes. The radioactivity counts was obtained using a gamma counter (Wallac-Coulter, Miami, FL) and the counts-per-minute (cpm) were converted to Ci units and reported as percentage activity recovered per gram of fluid or tissue.
Tumor Concentrations of Paclitaxel in SKOV-3 Bearing Mice
The SKOV-3 human ovarian carcinoma cells were grown in culture flasks containing RPMI 1640® medium modified with fetal bovine serum and antibiotics. They were harvested and re-suspended in serum-free medium (SFM) before injecting into nude mice. Approximately, two million tumor cells suspended in 100 μl of SFM were injected subcutaneously into the dorsal side of mice under light isoflurane anesthesia. Solid tumors developed within four weeks post-tumor inoculation and once the tumor volume reached approximately 200 mm3, the animals were chosen for experimental treatment.
Four mice were used per time-point (1, 6, 12, 24 and 48 hours) and there were three treatment groups – control (paclitaxel in solution), paclitaxel in PEO-modified PbAE nanoparticles, and paclitaxel in PEO-modified PCL nanoparticles. The formulations were diluted and suspended in sterile PBS and contained approximately 1 μCi of tritium dose per mouse. The injections were given through the tail vein as slow bolus. At periodic time intervals, blood was collected through sino-arbital vein puncture under light anesthesia (with isoflurane). The animals were then sacrificed by cervical dislocation and liver and tumor were harvested. The tissues were washed with PBS and collected in pre-weighed glass tubes. A 10% (w/v) homogenate was prepared in water and 1.5 ml each was added to a scintillation vial. All tissues and fluids (blood) were digested with Scintigest® fluid (from Fisher Scientific, USA, 1 ml, incubation for 2 hours at 50ºC) and decolorized with hydrogen peroxide (200 μl of 30% solution, incubation for 30 min. at 50ºC). Then, scintillation cocktail (ScintiSafe® Econo 1, from Fisher Scientific, USA, 10 ml) was added and the sample was allowed to quench for 2 hours in dark before measuring in a liquid scintillation analyzer (TriCarb 1600TR, Packard Instrument Co., CT). The counts-per-minute were converted into μCi using appropriate calibration curves.
Non-Compartmental Pharmacokinetic Analysis
Non-compartmental pharmacokinetic analysis was performed on the raw data expressing plasma concentration versus time profiles for the nanocarriers and the parameters were calculated using Win-Nonlin® software (Pharsight Corp, Mountain View, CA).
RESULTS AND DISCUSSION
Polymeric Nanoparticle Preparation and Characterization
Spherical nanoparticles having smooth surface and district boundary were obtained by solvent diffusion method. Nanoparticles were formed instantaneously with the newly formed nanosurface being sterically stabilized through the PEO chains of the polymeric stabilizer (Pluronic®). The particles obtained with PbAE were in the range of 100 to 150 nm with a mean diameter of 113 nm. The PbAE nanoparticles without any payload had a surface charge of 46.8 mV (in purified water) and upon drug loading the value was slightly reduced to 39.4 mV. We optimized the formulations at a paclitaxel loading of 20% by weight and achieved an entrapment efficiency of 95% through regulation of the organic-to-aqueous phase ratios and polymer concentrations. The PCL nanoparticles had a mean diameter of about 200 nm and the surface charge was 30.8 mV with 20% by weight paclitaxel loading.
Higher hydrophobicity of PCL and PbAE plays a key role in efficient surface modification strategy using Pluronic® triblock copolymers having a ABA structure of PEO and poly(propylene oxide) (PPO) segments. The evidence for surface modifications of PCL and PbAE nanoparticles with Pluronics and the stability of Pluronic layer has been reported in our earlier publications (17, 44). The surface modification is purely dependent on the hydrophobic interactions between the center-block (PPO) of the stabilizing surfactant and the polymeric core (PCL or PbAE nanocarrier). In the present study, we propose to use Pluronic® F108 having 56 residues of propylene oxide (PO) and 122 residues of ethylene oxide (EO). By blending PCL and PbAE and the Pluronic® copolymers in the right proportion, the hydrophilic PEO side-arms remain in the mobile state as they extend outwards from the particle surface and provide stability to the particle suspension by a repulsion effect through a steric mechanism of stabilization involving both enthalpic and entropic contributions. The end result of such an assembly is a stable, slow-eroding (PEO-PCL) or faster-eroding (PEO-PbAE) colloidal system that is less phagocyte-prone (hence long circulating). This type of surface modification strategy is sufficiently versatile and can be applied to a number of other hydrophobic polymeric systems. Also, there are over 15 different types of Pluronic® copolymers available containing different chains lengths of PEO and PPO and, as such, the nanoparticle surface can be specifically engineered for different applications.
Preparation of Radio-labeled Polymeric Nanoparticles
The solvent displacement method is convenient for preparation of hydrophobic nanoparticles reproducibly and with well-defined and controlled properties. This method is very useful for encapsulation of lipophilic drugs, such as paclitaxel. The encapsulation efficiency in such situations is generally very high (>95%). When there are no chemically-reactive functional groups present on the polymer for exploring labeling through conjugation, encapsulation of the radio-label within the particles is the only viable option. Such methods have been reported extensively in the past for lipophilic drugs that have a radio-active marker conjugated to them. The labeled drug is distributed in the nanomatrix and the delivery system as a whole represents a monolithic assembly. However, there are very few reports for a similar system where a lipophilic radiol-label derivative has been encapsulated. One reported example is of polymeric nanocapsules with the lipophilic radioactive compound dissolved in an oily core (45, 46).
111In is a popular radioactive marker that is used in diagnostic medicine for many reasons: it has a clinically appreciable half-life (2.8 days), available as aqueous injections (111In-chloride and 111In-oxine), it is stable in the systemic environment, and it is simple to process exposed tissues and fluids before counting. The lipophilic derivative (111In-oxine) has been previously reported for studying the biodistribution of biodegradable nanocapsules made from poly(D,L-lactic-co-glycolic acid) (PLGA) and PCL (45, 46).111In-oxine was extracted into olive oil, which formed the core of the nanocapsules that were prepared by solvent evaporation method. As we wanted to utilize the solvent displacement method for the preparation of the nanoparticles (and not nanocapsules), we had to suitably modify the technique. The extraction step was carried out using chloroform (a lipophilic solvent having excellent solubility for both polymers used) with an efficiency of 75%. To prevent the loss of the extracted radioactive compound during evaporation of chloroform, the polymer blends (PbAE + Pluronic® or PCL + Pluronic®) were first dissolved into the 111In-oxine containing chloroform followed by exposure to gentle stream of nitrogen to obtain a sticky residue of the radioactive-polymer blend. If one chooses to follow solvent evaporation technique for preparation of nanoparticles, the organic solution of the polymer blends with 111In- oxine can be directly employed. However, as we chose to follow a well-standardized solvent displacement technique, the solvent for polymer had to be changed from water-immiscible organic solvent (chloroform) to a water-miscible (hence water-displaceable) solvent such as absolute ethanol (for PbAE) or acetone (for PCL). The labeling efficiency was once again excellent (about 90% of the radiolabel being incorporated) as expected for a lipophilic agent. The advantage in the current situation was that the radio-label was distributed within the entire polymeric nanomatrix rather than the surface. For those systems utilizing surface bound radio-label, one is concerned about the stability and subsequent interpretation of the results.
In the case of 3H-labeled paclitaxel, the radio-label was in the form of chemical conjugation with the cytotoxic agent and was incorporated within the nanoparticles as the drug itself to form a monolithic matrix-based nanocarrier system. The incorporation efficiency was about 95%. In both cases, the labeling step was found to be fast, easy and reproducible and binding was expected to be stable within the systemic environment after intravenous administration.
Biodistribution Studies of 111In-Labeled Nanoparticles
As the first step for studying the overall biodistribution pattern of the nanoparticles, we chose to use blank PEO-modified PbAE and PCL nanoparticles with an incorporated radio-label in normal mice. A gamma emitting label is desirable for the purpose as it would drastically reduce the processing and estimation times because for the large volume of tissues samples generated. Additionally, the developed system may also be applied in gamma imaging applications for diagnostic purpose.
The results obtained for the biodistribution profile of 111In-labeled PEO-modified PbAE and PCL nanoparticles are represented in Figures 1 and 2 and Table 1. The two polymers showed different distribution profiles and kinetics depending upon their properties. In general, the both PEO-modified PCL (PEO-PCL) nanoparticles and PEO-modified PbAE (PEO-PbAE) nanoparticles showed higher tendency for accumulation in highly-perfused organs such as liver, spleen and lungs during initial phase and in kidney at the later stages of biodistribution.
Figure 1.

Plasma concentrations versus time profiles of 111indium oxine-labeled poly(ethylene oxide)-modified poly(ɛ-caprolactone) (PCL) nanoparticles (▪) and poly(ethylene oxide)-modified poly(β-amino ester) (PbAE) (♦) nanoparticles in mice.
Figure 2.


Biodistribution profiles of 111indium oxine-labeled poly(ethylene oxide)-modified poly(ɛ-caprolactone) (PCL) nanoparticles (A) and poly(ethylene oxide)-modified poly(β-amino ester) (PbAE) (B) nanoparticles in mice.
Table 1.
Non-Compartmental Pharmacokinetic Parameters from Plasma Concentrations versus Time Profiles after Intravenous Administration of 111In-Labeled PEO-Modified PbAE and PCL Nanoparticles
| Pharmacokinetic Parameter | PEO-PbAE Nanoparticles | PEO-PCL Nanoparticles |
|---|---|---|
| Half-life (h) | 20.55 ± 1.73* | 25.3 ± 0.34 |
| Mean residence time (h) | 21.03 ± 0.69 | 24.0 ± 0.23 |
| Volume of distribution(%(%/g/g)) | 2.49 ± 0.43 | 10.2 ± 0.89 |
| Clearance (%h(%/g)/g) | 0.12 ± 0.06 | 0.43 ± 0.04 |
| Maximum concentration (%/g) | 37.5 ± 1.25 | 51.7 ± 0.83 |
| Area under the curve (h.%/g) | 849.4 ± 48.98 | 236.0 ± 18.40 |
Values are expressed as mean ± SD (n = 4)
During initial phase of distribution, the PEO-PbAE nanoparticles tend to be trapped within the microvasculature of the lungs (Figure 2B). Given the proof that in our study, the size as well as the zeta potential of the two types of nanoparticles were comparable, it present a possibility of the PEO-PbAE nanoparticles undergoing aggregation upon introduction into the systemic circulation. This could be induced by interactions with the components of the plasma or by gradual displacement of the hydrophilic PEO chains from the nanoparticle surfaces. Similarly, for PEO-PCL nanoparticles, the profile represents a rapid localization of a significant percentage of the dose within the liver and spleen through RES uptake (Figure 2A). Previous reports on 111In-oxine injection have proven that upon intravenous administration, it is quickly bound to plasma protein – transferrin (47). Subsequently, the marker is taken up by the liver cells though the transferrin receptor and a progressive elimination of the marker occurs. While for the nanoparticle-encapsulated indium, the elimination proceeds through the uptake of the particle by the liver through a process mediated by opsonization. After the internalization of the nanoparticles, the indium-oxine is released and converted to its hydrophilic form which may then be eliminated through the kidneys.
There was no significant difference between two carriers with respect to the elimination half-life, mean residence time, or the maximum concentrations in the blood (Table 1). The plasma half-life of unmodified (without PEO coating) polymeric nanoparticles usually ranges from 1 to 10 min. (23) but the PEO-surface modification can significantly extend the half-life for longer times as we observe in the present case. About 4 times higher volume of distribution was observed for PEO-PCL nanoparticles due to their extensive extra-vascular distribution in well-perfused tissues such as liver, lungs and kidney (Figure 2A). For the PEO-PbAE nanoparticles, an initial localization was found in lungs which dropped quickly resulting in simultaneous increase the counts for blood and kidney (Figure 2B). An increase in the 111In concentrations in the kidney suggest that there could be a disintegration / dissolution of the nanoparticles occurring at its residence in lung capillary bed – probably due to reduced systemic pH, thus releasing a portion of the radioactive payload.
About 3.5 times higher AUC for PEO-PbAE nanoparticles in the systemic circulation suggest an enhanced performance of the nanocarrier with respect to the systemic presence / bioavailability and a balanced distribution compared to PEO-PCL nanoparticles. A higher magnitude in AUC may also indicate a quicker release of the contents of the PbAE nanomatrix compared to the PCL
Tumor Uptake Studies of Paclitaxel-Containing Nanoparticles
Majority of the injected dose (approx. 60–80%) in case of nanoparticles reached the liver compared to the control (Onxol® injection). The liver uptake was significantly higher for PEO-PCL nanoparticles. PEO-PCL nanoparticles and the control formulation showed prolonged presence in blood for two different reasons (Figure 3). The PEO modification leads to significantly higher circulation times for polymeric nanoparticles due to a decrease in degree of interactions with the plasma proteins and hence reduced clearance from the systemic circulation. Being highly hydrophobic, paclitaxel is extensively protein-bound drug (>99%) and hence when administered in solution form, it exhibited pronounced blood concentrations during the entire study period. The PEO-PbAE nanoparticles were cleared from the systemic compartment with a corresponding increase in tumor concentration (Figures 3 and 4). The trend observed in Figure 1 indicates the long circulating property for PEO-modified nanoparticles. At the same time, in the presence of a compromised (or fenestrated) capillary network (as in a solid tumor), they seem to get cleared more efficiently from the general circulation than PEO-PCL nanoparticles (Figure 3) and show a higher efficiency to get accumulated within the tumor mass predominantly by virtue of EPR mechanism (Figure 4). It is clear from the tumor concentrations of the drug that both the nanoparticles formulations can deliver a significantly higher concentration of the drug into the tumor that the solution form. At 1 hour post-administration, for instance, there was a 5.2 fold higher concentration of paclitaxel in the tumor when delivered in PEO-PbAE nanocarriers and 2.2 fold higher concentrations with PEO-PCL nanocarriers as compared to the aqueous solution. The results were even more pronounced at 5 hours post-administration with almost 23 fold higher paclitaxel concentrations with PEO-PbAE and 8.7 fold higher concentrations with PEO-PCL relative to the aqueous solution formulation.
Figure 3.
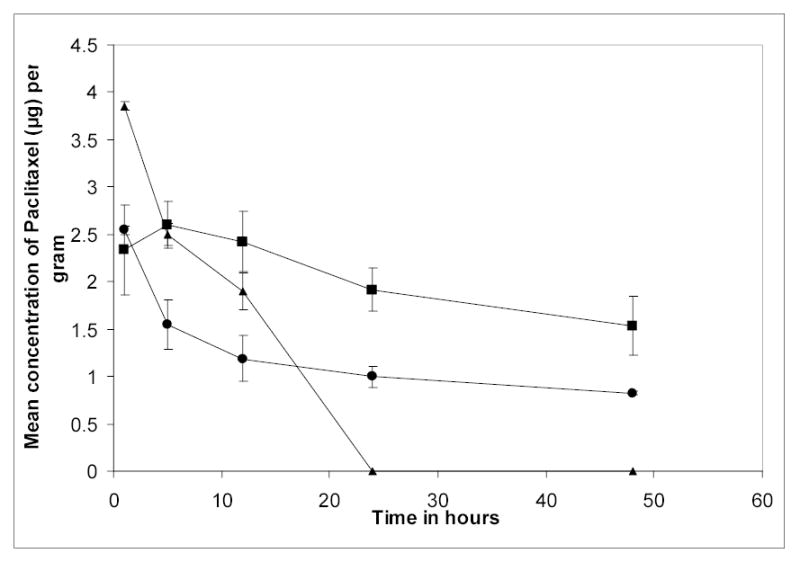
Plasma concentrations versus time profiles of tritiated [3H] paclitaxel upon intravenous administration to SKOV-3 human ovarian adenocarcinoma-bearing nude mice. Paclitaxel was administered in aqueous solution as control (^), in poly(ethylene oxide)-modified poly(ɛ-caprolactone) nanoparticles (▪), and poly(ethylene oxide)-modified poly(β-amino ester) (PbAE) nanoparticles (▴).
Figure 4.

Tumor concentrations as a function of time of uptake of tritiated [3H] paclitaxel upon intravenous administration to SKOV-3 human ovarian adenocarcinoma-bearing nude mice. Paclitaxel was administered intravenously in aqueous solution as control, in poly(ethylene oxide)-modified poly(ɛ-caprolactone) nanoparticles (PCL), and poly(ethylene oxide)-modified poly(β-amino ester) (PbAE) nanoparticles.
PEO-PCL nanocarriers clearly exhibited long-circulating properties in the plasma as shown by the half-life and mean residence time values of 25.3 hours and 24.0 hours, respectively. The long residence time of PEO-PCL nanocarriers in the systemic circulation is evident by an almost 8-fold lower total body clearance value [0.43 versus 3.45 (%h(%/g)/g)] as compared to the free drug. The pharmacokinetic results clearly show that PEO-PCL and PEO-PbAE had long-circulating properties in the systemic circulation and could be targeted to the tumor mass by the EPR effect.
In our earlier studies with PEO-PCL nanoparticles encapsulated with 3H-labeled tamoxifen, similar results were obtained upon intravenous administration in MDA-MB-231 human breast adenocarcinoma-bearing nude mice (17). It is likely that due to its pH-sensitive behavior (increased solubility at pH < 6.5) that the PbAE-based nanocarriers are dissolving rapidly and providing the additional benefit of triggered drug release within the low pH-environment existing within tumor mass leading to high localized drug concentrations(44).
CONCLUSIONS
PEO-modified PbAE and PCL nanoparticles can be prepared successfully by blending the polymers with Pluronic® F-108. The PPO central block gets anchored onto nanoparticle surface more strongly than mere physical adsorption leaving the hydrophilic PEO chains mobile on surface leading to steric stabilization of the nanocarrier. Both types of nanoparticles can be radio-labeled during the manufacturing step through physical entrapment of a lipophilic marker and can be used for determination of biodistribution profile after intravenous administration. The PEO-surface modification rendered both the nanoparticulate carriers long circulating (compared to unmodified nanocarriers) as evident from increased half-lives. PEO-modified PbAE nanoparticles were highly efficient in delivering the cytotoxic cargo to the tumor site through EPR localization, while PEO-modified PCL nanoparticles showed high extravascular distribution. PbAE nanoparticles, being pH-sensitive, could deliver the entire payload more rapidly compared to PCL-based system and hence nanoparticles can be highly efficient carrier systems for cytotoxic agents to achieve rapid tumoricidal action.
Acknowledgments
This study was supported by the National Cancer Institute grant R01-CA095522 from the National Institutes of Health. The authors thank Professor Robert Campbell for the particles size and zeta potential measurements, Professor Richard Deth for the liquid scintillation counter, and Dr. Ralph Loring for the gamma counter. Dr. Michael Seiden and Dr. Duan Zhenfeng of Massachusetts General Hospital are thanked for providing the SKOV-3 tumor cell lines. Dr. Ananthsrinivas Chakilam is acknowledged for his help in carrying out the non-compartmental pharmacokinetic analysis.
References
- 1.D. M. Lynn, D. G. Anderson, A. Akinc, and R. Langer. Deagradable poly (β-amino ester)s for gene delivery. In M. Amiji (ed), Polymeric gene delivery: Principles and applications, CRC Press LLC, Boca Raton, 2005, pp. 227–241.
- 2.Lynn DM, Anderson DG, Putnam D, Langer R. Accelerated discovery of synthetic transfection vectors: parallel synthesis and screening of a degradable polymer library. J Am Chem Soc. 2001;123:8155–6. doi: 10.1021/ja016288p. [DOI] [PubMed] [Google Scholar]
- 3.Akinc A, Anderson DG, Lynn DM, Langer R. Synthesis of poly(beta-amino ester)s optimized for highly effective gene delivery. Bioconjug Chem. 2003;14:979–88. doi: 10.1021/bc034067y. [DOI] [PubMed] [Google Scholar]
- 4.Akinc A, Lynn DM, Anderson DG, Langer R. Parallel synthesis and biophysical characterization of a degradable polymer library for gene delivery. J Am Chem Soc. 2003;125:5316–23. doi: 10.1021/ja034429c. [DOI] [PubMed] [Google Scholar]
- 5.Potineni A, Lynn DM, Langer R, Amiji MM. Poly(ethylene oxide)-modified poly(beta-amino ester) nanoparticles as a pH-sensitive biodegradable system for paclitaxel delivery. J Control Release. 2003;86:223–34. doi: 10.1016/s0168-3659(02)00374-7. [DOI] [PubMed] [Google Scholar]
- 6.Little SR, Lynn DM, Ge Q, Anderson DG, Puram SV, Chen J, Eisen HN, Langer R. Poly-beta amino ester-containing microparticles enhance the activity of nonviral genetic vaccines. Proc Natl Acad Sci U S A. 2004;101:9534–9. doi: 10.1073/pnas.0403549101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berry D, Lynn DM, Sasisekharan R, Langer R. Poly(beta-amino ester)s promote cellular uptake of heparin and cancer cell death. Chem Biol. 2004;11:487–98. doi: 10.1016/j.chembiol.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 8.Lynn DM, Amiji MM, Langer R. pH-responsive polymer microspheres: rapid release of encapsulated material within the range of intracellular pH. Angew Chem Int Ed Engl. 2001;40:1707–1710. [PubMed] [Google Scholar]
- 9.Pitt CG, Marks TA, Schindler A. Biodegradable drug delivery systems based on aliphatic polyesters: application to contraceptives and narcotic antagonists. NIDA Res Monogr. 1981;28:232–53. [PubMed] [Google Scholar]
- 10.Pitt CG, Gratzl MM, Kimmel GL, Surles J, Schindler A. Aliphatic polyesters II. The degradation of poly (DL-lactide), poly (epsilon-caprolactone), and their copolymers in vivo. Biomaterials. 1981;2:215–20. doi: 10.1016/0142-9612(81)90060-0. [DOI] [PubMed] [Google Scholar]
- 11.Pitt CG, Jeffcoat AR, Zweidinger RA, Schindler A. Sustained drug delivery systems. I. The permeability of poly(epsilon-caprolactone), poly(DL-lactic acid), and their copolymers. J Biomed Mater Res. 1979;13:497–507. doi: 10.1002/jbm.820130313. [DOI] [PubMed] [Google Scholar]
- 12.Pitt CG, Gratzl MM, Jeffcoat AR, Zweidinger R, Schindler A. Sustained drug delivery systems II: Factors affecting release rates from poly(epsilon-caprolactone) and related biodegradable polyesters. J Pharm Sci. 1979;68:1534–8. doi: 10.1002/jps.2600681219. [DOI] [PubMed] [Google Scholar]
- 13.Cha Y, Pitt CG. The biodegradability of polyester blends. Biomaterials. 1990;11:108–12. doi: 10.1016/0142-9612(90)90124-9. [DOI] [PubMed] [Google Scholar]
- 14.Sinha VR, Khosla L. Bioabsorbable polymers for implantable therapeutic systems. Drug Dev Ind Pharm. 1998;24:1129–38. doi: 10.3109/03639049809108572. [DOI] [PubMed] [Google Scholar]
- 15.Varela MC, Guzman M, Molpeceres J, del Rosario Aberturas M, Rodriguez-Puyol D, Rodriguez-Puyol M. Cyclosporine-loaded polycaprolactone nanoparticles: immunosuppression and nephrotoxicity in rats. Eur J Pharm Sci. 2001;12:471–8. doi: 10.1016/s0928-0987(00)00198-6. [DOI] [PubMed] [Google Scholar]
- 16.Chawla JS, Amiji MM. Cellular uptake and concentrations of tamoxifen upon administration in poly(epsilon-caprolactone) nanoparticles. AAPS PharmSci. 2003;5:E3. doi: 10.1208/ps050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shenoy DB, Amiji MM. Poly(ethylene oxide)-modified poly(epsilon-caprolactone) nanoparticles for targeted delivery of tamoxifen in breast cancer. Int J Pharm. 2005;293:261–70. doi: 10.1016/j.ijpharm.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Jiao YY, Ubrich N, Marchand-Arvier M, Vigneron C, Hoffman M, Maincent P. Preparation and in vitro evaluation of heparin-loaded polymeric nanoparticles. Drug Deliv. 2001;8:135–41. doi: 10.1080/107175401316906892. [DOI] [PubMed] [Google Scholar]
- 19.Gref R, Luck M, Quellec P, Marchand M, Dellacherie E, Harnisch S, Blunk T, Muller RH. 'Stealth' corona-core nanoparticles surface modified by polyethylene glycol (PEG): influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surf B: Biointerfaces. 2000;18:301–313. doi: 10.1016/s0927-7765(99)00156-3. [DOI] [PubMed] [Google Scholar]
- 20.Williamson MR, Chang HI, Coombes AG. Gravity spun polycaprolactone fibres: controlling release of a hydrophilic macromolecule (ovalbumin) and a lipophilic drug (progesterone) Biomaterials. 2004;25:5053–60. doi: 10.1016/j.biomaterials.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 21.Marchal-Heussler L, Sirbat D, Hoffman M, Maincent P. Poly(epsilon-caprolactone) nanocapsules in carteolol ophthalmic delivery. Pharm Res. 1993;10:386–90. doi: 10.1023/a:1018936205485. [DOI] [PubMed] [Google Scholar]
- 22.Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. J Control Release. 2001;70:1–20. doi: 10.1016/s0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- 23.Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol Rev. 2001;53:283–318. [PubMed] [Google Scholar]
- 24.Alonso MJ. Polymeric nanoparticles: new systems for improving ocular bioavailability of drugs. Arch Soc Esp Oftalmol. 2001;76:453–4. [PubMed] [Google Scholar]
- 25.Muller RH, Mader K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery - a review of the state of the art. Eur J Pharm Biopharm. 2000;50:161–77. doi: 10.1016/s0939-6411(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 26.Jung T, Kamm W, Breitenbach A, Kaiserling E, Xiao JX, Kissel T. Biodegradable nanoparticles for oral delivery of peptides: is there a role for polymers to affect mucosal uptake? Eur J Pharm Biopharm. 2000;50:147–60. doi: 10.1016/s0939-6411(00)00084-9. [DOI] [PubMed] [Google Scholar]
- 27.Kreuter J. Nanoparticles and microparticles for drug and vaccine delivery. J Anat. 1996;189(Pt 3):503–5. [PMC free article] [PubMed] [Google Scholar]
- 28.Kreuter J. Drug targeting with nanoparticles. Eur J Drug Metab Pharmacokinet. 1994;19:253–6. doi: 10.1007/BF03188928. [DOI] [PubMed] [Google Scholar]
- 29.Ravi Kumar M, Hellermann G, Lockey RF, Mohapatra SS. Nanoparticle-mediated gene delivery: state of the art. Expert Opin Biol Ther. 2004;4:1213–24. doi: 10.1517/14712598.4.8.1213. [DOI] [PubMed] [Google Scholar]
- 30.Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003;55:329–47. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 31.Vauthier C, Dubernet C, Fattal E, Pinto-Alphandary H, Couvreur P. Poly(alkylcyanoacrylates) as biodegradable materials for biomedical applications. Adv Drug Deliv Rev. 2003;55:519–48. doi: 10.1016/s0169-409x(03)00041-3. [DOI] [PubMed] [Google Scholar]
- 32.Florence AT, Hussain N. Transcytosis of nanoparticle and dendrimer delivery systems: evolving vistas. Adv Drug Deliv Rev. 2001;50(Suppl 1):S69–89. doi: 10.1016/s0169-409x(01)00184-3. [DOI] [PubMed] [Google Scholar]
- 33.Speiser PP. Nanoparticles and liposomes: a state of the art. Methods Find Exp Clin Pharmacol. 1991;13:337–42. [PubMed] [Google Scholar]
- 34.Benns JM, Kim SW. Tailoring new gene delivery designs for specific targets. J Drug Target. 2000;8:1–12. doi: 10.3109/10611860009009205. [DOI] [PubMed] [Google Scholar]
- 35.Douglas SJ, Davis SS, Illum L. Nanoparticles in drug delivery. Crit Rev Ther Drug Carrier Syst. 1987;3:233–61. [PubMed] [Google Scholar]
- 36.Otsuka H, Nagasaki Y, Kataoka K. PEGylated nanoparticles for biological and pharmaceutical applications. Adv Drug Deliv Rev. 2003;55:403–19. doi: 10.1016/s0169-409x(02)00226-0. [DOI] [PubMed] [Google Scholar]
- 37.Tan JS, Butterfield DE, Voycheck CL, Caldwell KD, Li JT. Surface modification of nanoparticles by PEO/PPO block copolymers to minimize interactions with blood components and prolong blood circulation in rats. Biomaterials. 1993;14:823–33. doi: 10.1016/0142-9612(93)90004-l. [DOI] [PubMed] [Google Scholar]
- 38.Nobs L, Buchegger F, Gurny R, Allemann E. Surface modification of poly(lactic acid) nanoparticles by covalent attachment of thiol groups by means of three methods. Int J Pharm. 2003;250:327–37. doi: 10.1016/s0378-5173(02)00542-2. [DOI] [PubMed] [Google Scholar]
- 39.Kaul G, Amiji M. Long-circulating poly(ethylene glycol)-modified gelatin nanoparticles for intracellular delivery. Pharm Res. 2002;19:1061–7. doi: 10.1023/a:1016486910719. [DOI] [PubMed] [Google Scholar]
- 40.Gbadamosi JK, Hunter AC, Moghimi SM. PEGylation of microspheres generates a heterogeneous population of particles with differential surface characteristics and biological performance. FEBS Lett. 2002;532:338–44. doi: 10.1016/s0014-5793(02)03710-9. [DOI] [PubMed] [Google Scholar]
- 41.Bhadra D, Bhadra S, Jain P, Jain NK. Pegnology: a review of PEG-ylated systems. Pharmazie. 2002;57:5–29. [PubMed] [Google Scholar]
- 42.Csaba N, Gonzalez L, Sanchez A, Alonso MJ. Design and characterisation of new nanoparticulate polymer blends for drug delivery. J Biomater Sci Polym Ed. 2004;15:1137–51. doi: 10.1163/1568562041753098. [DOI] [PubMed] [Google Scholar]
- 43.Csaba N, Caamano P, Sanchez A, Dominguez F, Alonso MJ. PLGA:poloxamer and PLGA:poloxamine blend nanoparticles: new carriers for gene delivery. Biomacromolecules. 2005;6:271–8. doi: 10.1021/bm049577p. [DOI] [PubMed] [Google Scholar]
- 44.D. B. Shenoy, S. Little, R. Langer, and M. M. Amiji. Poly(ethylene oxide)-Modified Poly(-amino ester) Nanoparticles as a pH-Sensitive System for Tumor-Targeted Delivery of Hydrophobic Drugs. 1. In Vitro Evaluations. Mol. Pharm. In press (2005). [DOI] [PMC free article] [PubMed]
- 45.Kedzierewicz F, Thouvenot P, Monot IH, Maincent MP. Influence of different physicochemical conditions on the release of indium oxine from nanocapsules. J Biomed Mater Res. 1998;39:588–593. doi: 10.1002/(sici)1097-4636(19980315)39:4<588::aid-jbm13>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 46.Marchal-Heussler L, Thouvenot P, Hoffman M, Maincent P. Comparison of the biodistribution in mice of 111indium oxine encapsulated intopoly(lactic-co-glycolic)-D,L-85/15 and poly(epsilon caprolactone) nanocapsules. J Pharm Sci. 1999;88:450–453. doi: 10.1021/js980307k. [DOI] [PubMed] [Google Scholar]
- 47.Wochner RD, Adatepe M, Van Amburg A, Potchen EJ. A new method for estimation of plasma volume with the use of the distribution space of indium-113m-transferrin. J Lab Clin Med. 1970;75:711–720. [PubMed] [Google Scholar]


