Abstract
Corneal epithelial stem cells are located in the basal layer of the limbus between the cornea and the conjunctiva. Regulation of these limbal epithelial progenitor cells by the stromal niche dictates corneal surface health. To further characterize this process, limbal explants were cultured at the air-fluid interface, termed air-lifting, to stimulate the niche. As compared to submerged cultures, air-lifting significantly promoted epithelial stratification, migration, proliferation, and intrastromal invasion by limbal epithelial cells. Epithelial intrastromal invasion was noted when the limbal, but not corneal, epithelium was recombined with the limbal stroma containing live, but not dead, cells. Invading limbal basal cells displayed up-regulated nuclear expression of p63 and Ki67, down-regulated E-cadherin and cornea-specific keratin 3, and switched expression of β-catenin from intercellular junctions to the nucleus and cytoplasm, indicating the activation of the Wnt/β-catenin pathway. Invaded cells isolated by collagenase from the stroma of air-lifted, but not submerged, explants showed vivid clonal growth on 3T3 fibroblast feeder layers and complete epithelial-mesenchymal transition by expressing nuclear p63 and cytoplasmic S100A4. These findings collectively suggest that epithelial-mesenchymal transition via the Wnt/β-catenin pathway influences the fate of limbal epithelial cells, likely to be progenitor cells, between regeneration and fibrosis when the stromal niche is activated.
Among different adult epithelia, the corneal epithelium and the epidermis have two types of progenitor cells, ie, stem cells (SCs) and transient amplifying cells (TACs), situated at two anatomically separable locations.1 The limbal epithelium of the cornea is enriched in pigmentation, vascularization, and innervation.2 The SCs of the corneal epithelium are located in the basal epithelial layer of the limbus between the cornea and the conjunctiva, whereas the TACs are located at the corneal basal epithelium.3 Such a unique compartmentalization facilitates studies showing differences between limbal SC and corneal TAC in the expression of keratins 3 and 12,3–5 connexin 43 (Cx43)-mediated gap junction intercellular communication,6 and the nuclear transcription factor of p63,7 the cell cycle length,8 differential responses to tumor-promoting phorbol esters,9 cell size,10 and ex vivo expansion supported by 3T3 fibroblast feeder layers11,12 or by amniotic membrane.13
The homeostasis of the corneal epithelium is ultimately maintained by limbal epithelial SCs.14–17 Under the normal uninjured state, limbal SCs are mitotically quiescent and maintained in a specialized limbal stromal microenvironment or niche. However, on corneal epithelial wounding, SCs proliferate to generate SCs and TACs. Clinically, destruction of limbal epithelial SCs or the limbal stromal niche can lead to a pathological state of limbal SC deficiency with severe loss of vision.18 Chronic inflammation in the limbal-deficient stroma is sufficient to cause a detrimental damage to conjunctival limbal autograft transplanted to patients19 or experimental rabbits.20 These findings suggest that the limbal stromal niche is critical in regulating the self-renewal and fate decision of SCs, however the exact mechanism remains elusive. It is also unclear why limbal-deficient corneas also manifest fibrovascular proliferation in the stroma.18
As a first step to investigate the regulation of limbal stromal niche, we have developed a method of isolating a viable limbal epithelial sheet from several species including rabbit.21–23 When adult rabbit corneal epithelium was recombined with the limbal stroma and cultured at the air-fluid interface, termed air-lifting (AL), the level of expression of keratin 3 in the basal epithelium is down-regulated, suggesting that epithelial differentiation of an already differentiated corneal epithelium can be influenced by the limbal stroma to resemble the limbal epithelium.22 Herein, we further demonstrate that AL promotes intrastromal invasion by limbal epithelial progenitor cells in rabbit limbal explants. This phenomenon is dependent on the presence of a viable limbal stromal niche and is mediated by activation of the Wnt/β-catenin pathway. Furthermore, some limbal basal epithelial progenitor cells invading the stroma also undergo complete epithelial-mesenchymal transition (EMT) into fibroblasts. The significance of these findings implying how EMT can be involved in the fate decision of epithelial SC between regeneration and fibrosis during wound healing is further discussed.
Materials and Methods
Reagents
Amphotericin B, Dulbecco’s modified Eagle’s medium, F-12 nutrient mixture (F12), fetal bovine serum, gentamicin, Hanks’ balanced salt solution, HEPES-buffer, phosphate buffered saline (PBS), and 0.25% trypsin/1 mmol/L ethylenediamine tetraacetic acid were purchased from Life Technologies, Inc. (Grand Island, NY). Dispase II powder was obtained from Roche (Indianapolis, IN). Tissue-Tek OCT compound and cryomolds were from Sakura Finetek (Torrance, CA). Other reagents and chemicals including bovine serum albumin, cholera-toxin (subunit A), dimethyl sulfoxide, hydrocortisone, insulin-transferrin-sodium selenite media supplement, mouse-derived epidermal growth factor, sorbitol, fluorescein isothiocyanate-conjugated goat anti-mouse, and tetramethyl-rhodamine isothiocyanate-conjugated rabbit anti-goat secondary antibody were purchased from Sigma (St. Louis, MO). All primary antibodies used in this study are summarized in Table 1. Vybrant DiI solution was from Molecular Probes (Eugene, OR).
Table 1.
Sources of Primary Antibodies
| Antigens | Category | Clone | Dilution | Method | Source |
|---|---|---|---|---|---|
| Pancytokeratins | Mouse monoclonal | AE1/AE3 | 1:200 | IF | DAKO |
| Keratin 3 | Mouse monoclonal | AE5 | 1:100 | IF | Chemicon, Temecula, CA |
| E-Cadherin | Mouse monoclonal | NCH-38 | 1:50 | IHC | DAKO |
| β-Catenin | Mouse monoclonal | β-catenin-1 | 1:50 | IHC | DAKO |
| p63 | Mouse monoclonal | 4A4 | 1:50 | IHC | DAKO |
| Collagen IV | Goat polyclonal | 1:50 | IF | Southern Biotechnology, Birmingham, AL | |
| Ki67 | Mouse monoclonal | MIB-1 | 1:50 | IHC | DAKO |
| S100A4 | Rabbit polyclonal | 1:50 | IHC | DAKO | |
| Vimentin | Mouse monoclonal | V9 | 1:100 | IF | DAKO |
Rabbit Limbal Explant Culture
Animals used in this study were handled according to guidelines described in ARVO statement for the Use of Animals in Ophthalmic and Vision Research. Whole globes enucleated from pigmented Dutch belted rabbits and New Zealand albino rabbit, older than 1 month, were immediately stored in supplemented hormonal epithelial medium (SHEM) at 4°C until use. SHEM was made of an equal volume of HEPES-buffered Dulbecco’s modified Eagle’s medium and F12, 0.5% dimethyl sulfoxide, 10 ng/ml epidermal growth factor, 5 μg/ml insulin, 5 μg/ml transferrin, 5 ng/ml sodium selenite, 0.5 μg/ml hydrocortisone, 10−10 mol/L cholera toxin, 5% fetal bovine serum, 50 μg/ml gentamicin, and 1.25 μg/ml amphotericin B. These eyes were rinsed with PBS three times, and each was equally subdivided into six limbal explants, including 7 mm of cornea and 4 mm of conjunctiva/sclera with the pigmented limbus in between. These explants were seeded on a polycarbonate membrane in 24-mm culture inserts (pore size of 3.0 μm) (Becton Dickinson, Lincoln Park, NJ) and cultured under AL, ie, the fluid level was set at the base of the explant stroma, or submerged mode in SHEM for 7 days.
Migration of Limbal Pigmented Lines and Epithelial Outgrowth from Explants
During the course of 7 days of culturing, epithelial migration was judged by the migration of the limbal pigmented line, which was documented by photography, and by epithelial outgrowth onto the membrane, which was detected by crystal violet staining. The extent of outgrowth was measured by scanning the stained photographs and the outgrowth area was analyzed by Image J (National Institutes of Health, Bethesda, MD).
Tissue Recombination
From six rabbit eye globes, we separated the central cornea of 8 mm in diameter and the corneoscleral rim including 3 mm of the cornea and 3 mm of the conjunctiva/sclera with the pigmented limbus in the middle. From these two tissues, the corneal (K) and the limbal (L) epithelial sheets were isolated by dispase digestion (15 mg/ml at 4°C for 16 hours)24 and then labeled with Vybrant DiI solutions (5 μl in 1 ml of PBS) for 20 minutes at 37°C. After washing gently three times with PBS, each for 5 minutes, these labeled epithelial sheets were recombined with the limbal stroma as previously described.22 The limbal stroma was prepared to include live stromal cells (Ls) or dead stromal cells (Ld), of which the latter was prepared by three rounds of freezing and thawing, each for 10 minutes. Three different tissue recombinants were thus prepared, ie, L/Ls, L/Ld, and K/Ls, and cultured on the aforementioned culture insert containing SHEM under AL for 7 days. The tissue sections were counterstained with 4,6-diamidino-2-phenylindole (DAPI), and labeled cells were photographed with a Nikon TE-2000U Eclipse epi-fluorescent microscope (Nikon, Tokyo, Japan).
Characterization of Invading Epithelial Cells in Limbal Stroma
To further characterize the phenotype of intrastromally invading cells, explants were retrieved at day 2, 4, and 7, and were subjected to frozen sectioning and immunostained as described below. In parallel, the surface epithelial cells were first removed from other submerged or AL explants at day 7 by dispase digestion (15 mg/ml at 4°C for 16 hours).24 The remaining stroma was then washed three times with Hanks’ balanced salt solution, ∼1 mm from the edge of each explant was trimmed off to eliminate any residual epithelial cells, and the remaining tissue was digested by collagenase (1 mg/ml at 37°C for 16 hours).25 Cells thus isolated were seeded on plastic dishes containing SHEM for 48 hours before being subjected to immunostaining as described below.
Clonal Culture on 3T3 Fibroblast Feeder Layers
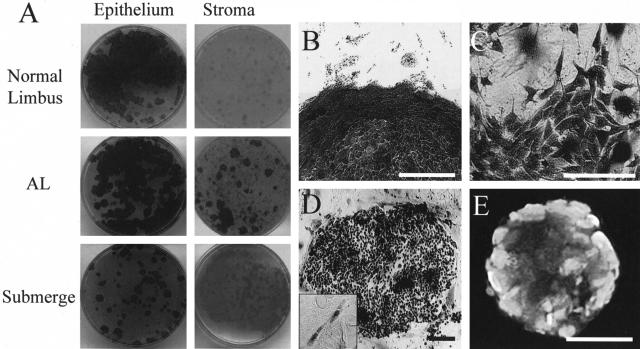
To further characterize whether intrastromally invading cells were truly epithelial progenitor cells, 1 × 104 cells isolated by collagenase treatment from the remaining stroma of the normal control, submerged, and AL explants were seeded on 2 × 104/cm2 mitomycin C-treated 3T3 fibroblasts feeder layers per 60-mm dish as previously described.26,27 After culturing for 10 days, epithelial colonies were visualized by staining with crystal violet and antibodies against p63 and AE1/AE3. For comparison, the clonal culture of a total of 1 × 103 single cells isolated from the surface epithelium removed by dispase digestion from each explant were also tested.
Immunostaining
Each explant, embedded in OCT compound and snap-frozen in liquid nitrogen, was cut into 20 sections (5 μm thick) from the center of the explant to make sure that the epithelial invasion into the stroma was not an artifact caused by cells migrating along the edge of the explant. Both tissue sections and the cells cultured on plastic were then subjected to immunostaining using standard methods of immunohistochemistry or immunofluorescence staining with appropriate dilutions of primary antibody summarized in Table 1 and respective secondary antibody. For anti-p63 antibody, we used clone 4A4, which recognizes all six p63 isotypes.28 This antibody also cross-reacted with p63 protein band of primary human limbal epithelial cells expanded by amniotic membrane, primary cultures of rabbit limbal epithelial cells cultured in SHEM, but not rabbit stromal fibroblasts by Western blot (supplemental data http://www.amjpathol.org).
Briefly, each sample was fixed in cold methanol for 10 minutes at −20°C, permeabilized, and blocked as previously described.29 After blocking, cells were incubated for 1 hour with primary antibody. Specific binding was detected by a fluorescein isothiocyanate-conjugated anti-mouse secondary antibody, counterstained with propidium iodine and mounted in anti-fading solution (Vector Laboratories, Burlingame, CA). Primary antibodies were also detected using an immunoperoxidase protocol (ABC kit Vectastain Elite, Vector Laboratories) and developed with a DAB kit (DAKO, Carpinteria, CA), and some were counterstained with hematoxylin. Substitution of primary antibody with PBS served as negative-staining controls. For double immunostaining, tissue sections were subsequently incubated with the second antibody for 30 minutes. Detection was performed using AEC kit (Sigma) or tetramethyl-rhodamine isothiocyanate-conjugated anti-goat secondary antibody. Images were photographed with a Nikon TE-2000U Eclipse epi-fluorescent microscope (Nikon).
Results
Epithelial Migration and Outgrowth from Limbal Explants
Limbal explants were cultured in SHEM under AL or submerged mode for 7 days. As shown in Figure 1, the limbal pigmented line remained stationary in submerged explants (Figure 1A). In contrast, this pigmented line migrated toward the sclera in AL explants. Migration of limbal pigmented line was progressive during the course of 7 days of culturing (Figure 1B). Epithelial outgrowth from the explant onto the polyester membrane of the insert was noted 2 days after culturing in both conditions. Epithelial outgrowth consisted of a monolayer of small epithelial cells in both conditions (not shown). On day 7, the surface area of the epithelial outgrowth of AL explants, depicted by crystal violet staining was significantly larger than that of submerged explants (P < 0.001) (Figure 1, C and D). These data collectively showed that epithelial migration, as evidenced by the limbal pigmented line, and outgrowth was promoted by AL.
Figure 1.
Epithelial migration and outgrowth of corneo-limbo-conjunctival explant cultures. A: Representative explants on day 5 exhibited that the pigment line at the limbal epithelium remained stationary when cultured in submerged modes. In contrast, the limbal pigment line migrated toward the sclera (white portion) when cultured in the AL mode (arrow). B: Continuous migration of the limbal pigment line of an AL explant toward the sclera (black arrow) from day 3 to day 7. C and D: The surface area of epithelial outgrowth on the membrane in AL explants was significantly more than that of submerged (P < 0.001). Explant location was marked by an asterisk in C.
Intrastromal Invasion of Limbal Epithelium in AL Explants
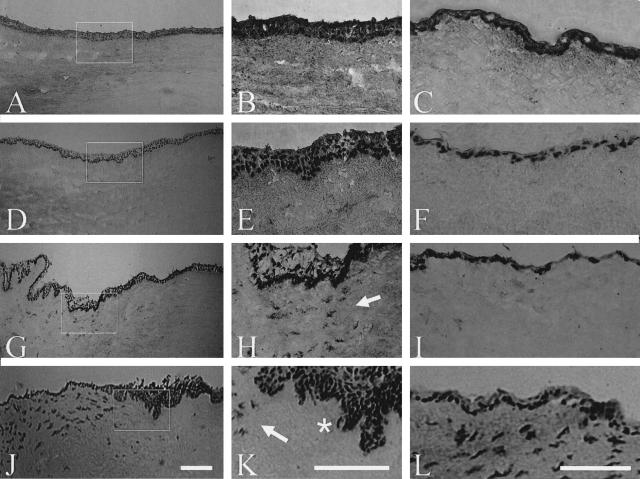
To determine whether the aforementioned epithelial migration and outgrowth from the explants was also coupled with epithelial changes on the explant, we subjected explants retrieved on day 7 to frozen sections and hematoxylin staining. The stromal thickness was more compact in AL explants than that of submerged explants because of AL-induced dehydration (not shown). As expected, the normal cornea control had a stratified epithelium of four to six cell layers and an avascular stroma with keratocytes aligned in a lamellar manner (Figure 2A). The normal limbus control also showed a stratified epithelium with more cell layers and a vascularized stroma with fibroblasts (Figure 2B). The nuclear orientation of basal epithelial cells to the basement membrane was vertical, whereas that of all suprabasal cells were horizontal (Figure 2B). However, the corneal epithelium of the submerged explant became fragile, desquamating, and less adherent, while the cell density of the corneal stroma was markedly reduced (Figure 2C). A similar finding was noted in the limbal epithelium of submerged explants although the stromal cell density was slightly reduced (Figure 2D). In contrast, the epithelial stratification was markedly promoted in both the corneal (Figure 2E) and the limbal (Figure 2F) regions of AL explants. Besides marked stratification, the most striking finding was the appearance of digital invasion of the basal epithelial cells into the limbal stroma, while the cell density of limbal stromal mesenchymal cells was increased after AL (Figure 2F). The nuclear orientation of basal and several layers of suprabasal epithelial cells were vertical in AL explants (Figure 2F). Due to the migration of pigmented cells, there was less pigment in the limbal region of AL explants than submerged explants.
Figure 2.
Increased stratification and intrastromal invasion in AL explants. A: Hematoxylin staining of the normal control cornea showed a stratified epithelium of four to six cell layers and an avascular stroma with cells aligned in a lamellar manner. B: The normal limbus had more epithelial cell layers and a vascularized stroma with cells randomly arranged. C: After 7 days of culturing, the corneal epithelium of the submerged explant was fragile, desquamating, and less adherent, and the cell density of the stroma was markedly reduced. D: Similar finding was noted in the limbal region with the cell density of the stroma was mildly reduced. E and F: In contrast, the epithelial stratification was marked promoted in the corneal (E) and the limbal (F) regions after AL. F: Besides marked stratification, the most striking finding was the appearance of digital invasion of basal epithelial cells into the limbal stroma for AL explants. The cell density of stromal cells appeared to increase after AL. All photographs are taken at the same magnification. Inset shows epithelial morphology under higher magnification. Scale bar, 50 μm.
Epithelial Migration and Intrastromal Invasion in the Conjunctival Region of AL Explants
To determine whether there were any epithelial cells invading the stroma that might have been missed by hematoxylin staining, we performed immunostaining to pancytokeratins using AE1/AE3 monoclonal antibodies. In the conjunctival region, all surface epithelial cells of the normal control (Figure 3A), submerged explants (Figure 3C), and AL explants (Figure 3E) expressed keratins and hence were positive for AE1/AE3 staining. Importantly, there were clusters of epithelial cells in the stroma of the conjunctival region of AL explants (Figure 3E, arrows), but not in that of submerged explants (Figure 3C). To determine whether these invaded cells were of a corneal epithelial lineage, we performed immunostaining against keratin 3, which is specific for cornea type epithelial differentiation.3 As reported,3 keratin 3 expression was negative in the conjunctival region of the normal control (Figure 3B). Keratin 3 was expressed by all epithelial cells on the surface of submerged (Figure 3D) and AL (Figure 3F) explants, indicating that they were derived from limbal progenitor cells via migration. Furthermore, keratin 3 was also expressed by epithelial cells invading into the conjunctival stroma of AL explants (Figure 3F, arrows), further supporting that these invading epithelial cells were also derived from limbal epithelial progenitor cells. These epithelial cells were not contiguous with surface epithelial cells as judged by hematoxylin staining of tangential sections in the limbus (Figure 3G), between the limbus and the conjunctiva (Figure 3H), and in the conjunctiva (Figure 3I).
Figure 3.
Expression of cytokeratins in the conjunctival region of explants. Expression of pancytokeratins and keratin 3 in the conjunctival region was studied by immunostaining using AE1/AE3 (A, C, and E) and AE5 (B, D, and F) antibodies, respectively. As expected, the surface epithelium of normal (A) and submerged (C), and AL (E) were all positive to AE1/AE3. Keratin 3 was not expressed in the normal conjunctiva (B), but was expressed in the surface epithelium of both explants (D and F). Interestingly, clusters of epithelial cells that expressed cytokeratins were found in the conjunctival stroma of AL (E, arrows) explants, which also expressed keratin 3 (F, arrows). Tangential section from the limbus to the conjunctiva (G–I) showed the same clusters (E and F), indicating that it was not continuous with the surface epithelium. All photographs of B to I are taken at the same magnification. Scale bars, 100 μm.
Intrastromal Invasion of Tissue Recombinants
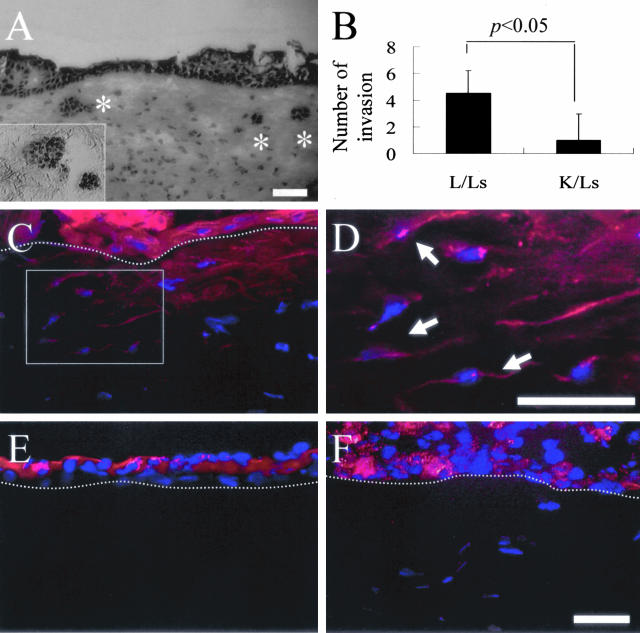
To further establish if invaded cells were derived from corneal or limbal progenitor cells, and if such invasion depended on live stromal cells, corneal (K) and limbal (L) epithelial sheets were isolated by dispase, and recombined with the limbal stroma that contained live cells (Ls) or dead cells (Ld) as previously described.22 After culturing in SHEM under AL conditions for 7 days, hematoxylin staining of the tissue section showed readily observed intrastromal invasion which was markedly increased in L/Ls recombinants (Figure 4A, asterisk), but not in L/Ld recombinants (not shown). The number of clusters of invading epithelial cells, confirmed by p63-positive staining (Figure 4A, inset), was significantly different between L/Ls and K/Ls (Figure 4B; n = 5, P < 0.05). When DiI-labeled epithelial sheets were recombined in the same manner and cultured for 4 days, only L/Ls have red fluorescence-labeled cells in the superficial stroma (L/Ls) (Figure 4C). These labeled cells (C, inset) adopted a slender morphology as observed under higher magnification (Figure 4D). No red fluorescence-labeled cells were found in L/Ld and K/Ls (Figure 4, E and F, respectively), and no DAPI-labeled cells were found in the stroma of L/Ld (Figure 4E). Because it is known that Dil labeling resists intercellular transfer,30 we thus confirmed that limbal epithelial cells invaded into the limbal stroma under AL, and that such epithelial invasion was dependent on live limbal stromal cells.
Figure 4.
Intrastromal invasion of epithelial cells in tissue recombinants. Corneal (K) and limbal (L) epithelial sheets were isolated by dispase and recombined with the limbal stroma that contained live cells (Ls) or dead cells (Ld). A: After culturing under AL for 7 days, hematoxylin staining of the tissue section showed intrastromal invasion only in L/Ls recombinant (asterisk), but not in K/Ls or L/Ld recombinants (not shown). A: Furthermore these invading cells expressed nuclear p63 (inset). B: The difference of intrastromal invasion between L/Ls and K/Ls was statistically significant (n = 5, P < 0.05). C: When DiI-labeled epithelial sheets were recombined in the same manner and cultured for 4 days, only L/Ls have red fluorescence-labeled cells in the superficial stroma (L/Ls). C and D: These labeled cells (C, inset) adopted a slender morphology under higher magnification (D, arrows). E: No DAPI-labeled cells in the stroma of L/Ld. E and F: No red fluorescence-labeled cells were found in L/Ld and K/Ls, respectively. The dotted line indicates the border of epithelium. Micrographs of C, E, and F are taken at the same magnification. Scale bar, 50 μm.
Characterization of Intrastromally Invading Limbal Basal Epithelial Cells in AL Explants
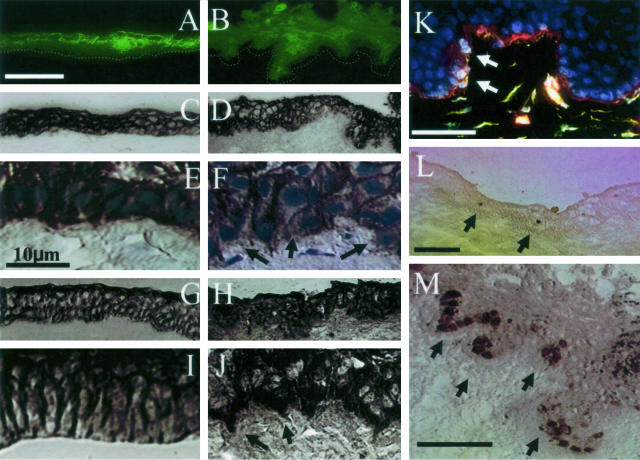
In the limbal region, as reported,3 keratin 3 was expressed by suprabasal epithelial cell layers, but not by basal epithelial progenitor cells (Figure 5A). However, such a negative staining to keratin 3 of basal epithelial cells was extended to include suprabasal cells in AL explants (Figure 5B). The increasing negative expression of keratin 3 suggested activation of limbal epithelial progenitor cells. Because there was also a change in the nuclear orientation to the basement membrane in AL explants (Figure 2), we would like to characterize the phenotype of limbal basal epithelial cells by immunostaining with antibodies to markers known to be associated with the Wnt/β-catenin pathway. Staining to E-cadherin was noted characteristically in the intercellular junctions of all basal and suprabasal epithelial cell layers of the normal limbus (Figure 5, C and E). In contrast, such an intercellular staining of E-cadherin was lost in invading basal epithelial cells, especially those located at the invading tip in AL explants (Figure 5, D and F; arrows). Staining to β-catenin was also noted in the intercellular junctions of all basal and suprabasal epithelial cells of the normal limbus (Figure 5, G and I). In contrast, the staining of β-catenin became localized in the nucleus in some basal epithelial cells of AL explants (Figure 5, H and J). Double staining to collagen type IV and vimentin showed that vimentin-positive cells were clustered in the region where collagen IV was degraded (Figure 5K, arrows). Positive nuclear staining of Ki67, which is a proliferative marker, was infrequently noted in the normal limbal basal epithelium (Figure 5L), but was vividly found in the invading tip of AL explants (Figure 5M, indicated by arrows). These changes were not observed in submerged explants (not shown). Collectively, these results indicated the activation of Wnt/β-catenin pathway at the limbal explant by AL.
Figure 5.
Characterization of invaded limbal basal epithelial cells in AL explants. A: Cytoplasmic staining of K3 keratin was noted in all suprabasal, but not basal, epithelial cells of the normal limbus. B: In contrast, negative staining of K3 keratin extended from basal to several suprabasal epithelial layers in the limbal region of AL explants. The dotted line indicates the basement membrane. C and E: Intercellular staining of E-cadherin was noted in all basal and suprabasal epithelial cell layers of the normal limbus. D and F: In contrast, E-cadherin staining was negative in some basal epithelial cells, especially at the tip of those invading cell in AL explants (arrows). G and I: Intercellular staining of β-catenin was also noted in basal and suprabasal epithelial cells of the normal limbus. H and J: In contrast, staining of β-catenin was located in the nucleus and cytoplasm of basal and suprabasal epithelial cells in AL explants (arrows). K: Double staining of collagen IV and vimentin showed that vimentin-positive basal cells were preferentially located in the collagen IV-degraded area (red, collagen IV; green, vimentin; and blue, nuclei; arrows). L and M: Nuclear staining of Ki67 was infrequently noted in the normal limbus (L, arrows), but vividly found in the tip of invading limbal basal epithelial cells of AL explants (M, arrows). All photographs of A to D and G and H are taken at the same magnification, so are E, F, I, and J. Scale bars, 50 μm.
Expression of p63 in AL Explants
Knowing that limbal epithelial progenitor cells were activated in AL explants, we then studied the expression of p63, an epithelium-specific transcription factor31 claimed to be expressed predominantly by the basal cell compartment of a variety of epithelial tissues28 including limbal epithelial SCs.7 Nuclear expression of p63 was reported to be primarily negative in normal human cornea, but positive in the human limbus.7 We noted that p63 was expressed by basal and some suprabasal epithelial cells of the normal rabbit cornea (Figure 6; A to C). Nuclear staining of p63 was noted in basal epithelial cells of submerged explants (not shown). In contrast, nuclear p63 staining was markedly promoted in basal and several layers of suprabasal epithelial cells of both the corneal and the limbal regions of AL explants at day 2 (Figure 6, D and F), day 4 (Figure 6; G to I), and day 7 (Figure 6; J to L), especially in the invading areas (Figure 6; E, H, and K). It should be noted that p63 staining was negative in the limbal stroma of the normal control (Figure 6B). To our surprise, positive staining of p63 was noted in many cells in the limbal stroma subjacent to the invading basal epithelium of AL explants especially at day 4 and day 7 (Figure 6, H and K, respectively, indicated by arrows). Some limbal basal epithelial cells at the invading tip also adopted a slender and wavy shape at day 7 (Figure 6K, asterisk). Furthermore, they appeared to be slender and wavy in shape as observed under a higher magnification especially in the conjunctival stroma at day 7 (Figure 6L). These results indicated that epithelial progenitor cells were expanded in number in the corneal and limbal regions of AL explants. The emergence of p63-expressing cells in the limbal stroma subjacent to the invading epithelial progenitor cells further suggested that some limbal epithelial progenitor cells underwent EMT into mesenchymal cells in AL explants.
Figure 6.
Time course expression of p63 in AL explants. Nuclear staining of p63 was noted in basal and some suprabasal epithelial cells of the normal cornea (A), limbus (B), and conjunctiva (C). p63 staining was promoted in the limbal region (D) of AL explants from day 2 (D–F). Although p63 staining was negative in the normal limbal stroma (B), positive staining of p63 was found in some cells of the limbal stroma of AL explants clearly from day 4 (G and H), adjacent to the invading limbal basal epithelium from day 4, but no p63-positive cells were in the conjunctival stroma (I). Some limbal basal epithelial cells at the invading tip also had slender and wavy positive staining of p63 at day 7 (K, asterisk). Furthermore, a higher magnification showed that positive p63 staining was slender and wavy in the limbal stroma (K). p63-positive cells in the stroma were more at day 4 at the limbal location but more at day 7 in the conjunctival location. There was no counterstaining. Scale bars, 100 μm.
Changes of Limbal Stromal Cells in AL Explants
To determine whether the increased cellularity in the limbal stroma of AL explants (Figure 2) resulted in part from an increase of cell proliferation, we performed immunostaining to Ki67. Positive nuclear staining to Ki67 was noted in many cells of the limbal stroma of AL explants (Figure 7A), but not in that of submerged explants (Figure 7B). The increased cellularity was indeed of mesenchymal cells as illustrated by the dense vimentin staining in the stroma of the limbal and conjunctival regions, but not in that of the corneal region of AL explants (Figure 7C). These vimentin-positive cells were markedly increased in the stroma adjacent to the digital invasion of the limbal basal epithelial cells (Figure 7D). Collectively, these findings also indicated that stromal cells were increased in the limbal stroma of AL explants.
Figure 7.
Characterization of limbal stromal cells. Nuclear staining to Ki67 was found in many mesenchymal cells in the limbal stroma of AL explants (A, arrow), but not in that of submerged explants (B). Vimentin staining revealed numerous cells in the stroma of the limbal (C, inset) and conjunctival region (C, left of the inset) of the AL explant. Note that there was a marked loss of vimentin staining in the stroma of the cornea (C, far right of the inset). D: Higher magnification of the limbal region of AL explant revealed a marked increase of vimentin-positive cells in the stroma especially adjacent to the digital invasion of the limbal basal epithelial cells. Cells were isolated from the stroma of AL and submerged explants by collagenase digestion and seeded on plastic for 48 hours. E: AE1/AE3-positive cells were observed in the stroma of AL explants. F: Double staining revealed that cells expressing both nuclear p63 and cytoplasmic S100A4 were found in the same culture. Note that some mesenchymal cells expressed S100A4, but not p63. Scale bars, 100 μm.
To determine whether the aforementioned increase of stromal cells in the subepithelial location of AL explants (Figure 7) might be derived from the limbal epithelial progenitor cells through EMT (Figure 6), we isolated cells from the stroma of AL and submerged explants by collagenase digestion and seeded them on plastic containing SHEM. Cells attached well in 5 hours with high viability. At 48 hours, cultures were terminated and subjected to immunostaining. We noted that cells expressing p63 or AE1/AE3 (Figure 7E) were less than 1% of the total cell population isolated from the stroma of AL and submerged explants. Double staining revealed some cells expressed both p63 and S100A4 (Figure 7F) in the limbal stroma of AL explant, but not in that of submerged explants (not shown). These results indicated that invading cells indeed had undergone EMT in the stroma of AL explants.
3T3 Clonal Assay of Limbal Stromal Cells in AL Explants
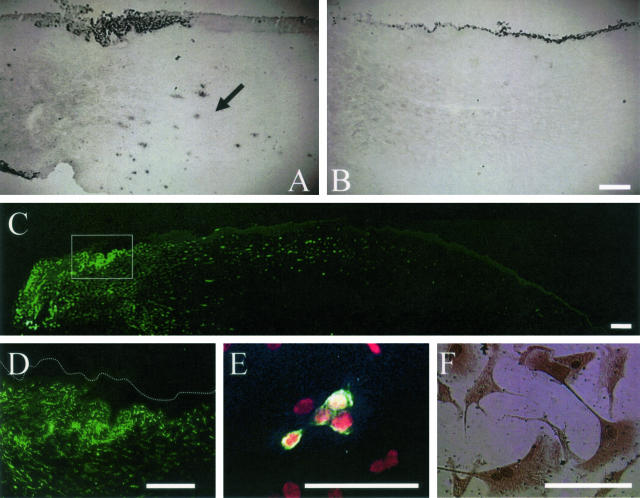
To determine whether invading cells in the limbal stroma of AL explants contained epithelial progenitor cells, we also seeded these cells on 3T3 fibroblast feeder layers. Cells derived from the surface epithelium from the normal control, submerged explants, and AL explants formed many epithelial colonies (Figure 8A, left). Nevertheless, epithelial colonies formed only from cells isolated by collagenase treatment from the remaining stroma of AL explants, but not from those of the normal control and submerged explants (Figure 8A, right). Under higher magnification, epithelial colonies were distinguished by their morphology and smooth contour (Figure 8B). However, colonies formed by mesenchymal cells stained faintly with crystal violet and were noted in the normal control and submerged explants. Under higher magnification, these nonepithelial colonies contained fibroblastic cells and irregular contour (Figure 8C). The majority of cells in the epithelial colony found in AL limbal stroma expressed p63 including fibroblast-like cells (Figure 8D) and also expressed AE1/AE3 (Figure 8E). Similar results were obtained in three sets of experiments.
Figure 8.
Clonal analysis of cells isolated from limbal epithelium and stroma. Cells from the surface epithelium (isolated by dispase) or the remaining stroma (isolated by collagenase) were seeded on 3T3 fibroblast feeder layers. Epithelial colonies were readily detected by crystal violet staining from cells of isolated surface epithelium of the normal control, AL explant, and submerged explant (A, left). Nevertheless, epithelial colonies were not formed by cells isolated from the stroma of the normal control and submerged explants, but formed by AL explants (A, right). B: Epithelial colonies were stained dark with a smooth and distinct border as verified by higher magnification. C: In contrast, fibroblastic colonies were stained faint with an irregular border as verified by higher magnification. Those stromal colonies of AL (B) have clearly p63-positive expression in all of cells (D), and elongated fibroblast-like p63-positive cells were observed out of colony (D, inset). E: These colonies expressed pancytokeratins by AE1/AE3. Scale bars, 50 μm.
Discussion
Distinct anatomical separation of SCs and TACs of the corneal epithelium allows us to investigate how limbal epithelial SC might be regulated by the limbal stromal niche. In this report, we introduced AL as a stimulus to the rabbit limbal explant. Without knowing the actual mechanism, AL has been used to induce epithelial stratification and differentiation of corneal,32,33 conjunctival,32 and epidermal34 epithelial cells on fibroblast-containing collagen gel (ie, organotypic) cultures. Herein, we noted that AL not only promoted epithelial stratification of the corneal epithelium (Figure 2, left), but also induced pronounced epithelial migration, as demonstrated by the limbal pigmented line migration and by the increased epithelial outgrowth from the explant (Figure 1). Migration of limbal pigmented lines has been regarded as an indication that healing of corneal epithelial wounds is proceeded by limbal epithelial progenitor cells.35 However, the direction of pigment migration toward the conjunctival edge is unusual and different from in vivo corneal wound healing, and might be due to in vitro experimentation, eg, different extents of AL in the conjunctiva and the cornea of the explant. Furthermore, the surface epithelium migrating onto the conjunctival region of the explant expressed cornea-specific keratin 3 (Figure 3, left), and the limbal basal epithelial progenitor cells up-regulated Ki67 expression (Figure 5). Collectively, these results indicated that AL-induced migration is coupled with increased proliferation of limbal basal epithelial cells.
Besides epithelial migration and proliferation, AL further induced intrastromal invasion by epithelial cells in limbal explants. Previously, we reported that rabbit corneal and conjunctival epithelial cells invade into the collagen gel of an organotypic culture only when the gel is impregnated with fibroblasts and cultured under AL.32 Because the normal conjunctival epithelium does not express keratin 3,3 the finding that intrastromally invading clusters of epithelial cells in the conjunctival region of the explant expressed keratin 3 (Figure 3, right) supported the notion that they were derived from limbal epithelial progenitor cells. In the limbal region, intrastromal invasion appeared as direct digital invasion by basal epithelial cells in AL explants (Figure 2), but as clusters of epithelial cells in limbal tissue recombinants (L/Ls) (Figure 4). Because dispase, which digests laminin 5 and collagen IV,24 is used in the tissue recombinant but not in the explant, we speculate that such a difference in invasion pattern might result from the presence or absence of basement membrane components at the limbal region. If this interpretation is correct, we would also expect that the regional difference in basement membrane components between the limbal and conjunctival regions36 may also explain why intrastromal invasion appeared as clusters in the conjunctival region of AL explants (Figure 3).
When recombined with the limbal stroma with live cells, the limbal epithelium, which contains both SCs and TACs, was more potent than the corneal epithelium, which contains only TACs, in undergoing intrastromal invasion (Figure 4). This finding strongly suggested that invading limbal epithelial progenitor cells are mainly SCs and not TACs. To further substantiate this view, we examined the expression of p63, an epithelium-specific nuclear transcription factor31 and a putative SC marker for the limbal epithelium12 and keratinocytes.7 The p63 gene also has been suggested to play a major role in maintenance of SC in several organs.28,31,37 Herein, p63 nuclear expression was markedly promoted in both basal and suprabasal epithelial cells at the limbal region of AL explants more so than submerged explants (Figure 6). Furthermore, collagenase-released cells from the limbal stroma of AL, but not submerged, explants, exhibited vivid epithelial clonal growth when seeded on 3T3 fibroblast feeder layers (Figure 8).
In the limbal region, invading basal epithelial progenitor cells lost the expression of keratin 3 and E-cadherin, and up-regulate nuclear and cytoplasmic β-catenin accumulation (Figure 5). Collectively, such phenotypic alteration signifies the activation of Wnt/β-catenin pathway known to govern cellular migration and invasion by dissociating cellular adhesion complexes.38 Importantly, AL also induced a vertical orientation of p63-positive nuclei in invading epithelial cells, and a fibroblastic morphology of p63-positive cells in the limbal stroma (Figure 6). A similar result was also noted in L/Ls, but not K/Ls, tissue recombinants (not shown). The latter phenomenon indicative of EMT by invading epithelial cells was further confirmed by the expression of pancytokeratins and S100A4 in cells expressing p63-positive nuclei that were released by collagenase from the AL limbal stroma (Figure 7). It has been extensively documented that p63 is only expressed by basal and some suprabasal cells of many stratified epithelia including skin, esophagus, and urothelia.37 Co-expression of p63 and α-smooth muscle actin has been found in myoepithelial cells.37 Our study is the first using co-expression of p63 and S100A4 to demonstrate EMT, which is prevalent during embryonic morphogenesis and cancer metastasis. EMT is also regarded as a mechanism explaining tissue fibrosis leading to parenchymal failure in the kidney39 and the lung.40 However, it was believed that fibroblasts in the kidney fibrosis are derived from a differentiated epithelium,39 besides the resident mesenchymal cells and bone marrow-derived progenitor cells.41
Herein, we provided for the first time strong evidence suggesting that EMT induced by AL is primarily preceded with limbal epithelial progenitor cells, most likely SCs. Using tissue recombinants, we further noted that intrastromal invasion took place only if the limbal stroma containing live mesenchymal cells (Figure 4). Such dependency on fibroblasts has also been reported in collagen gel organotypic cultures.32 In AL limbal stroma, there was an increase of cellularity (Figure 2) and Ki67 expression (Figure 7). Previously, it has also been shown that AL has been shown to promote the proliferative activity of fibroblasts in collagen gel by up-regulating MAPK pathway.42 We thus wonder if activation of mesenchymal cells by AL may play an active role in triggering epithelial intrastromal invasion. This possibility was also suggested by increased vimentin expression by stromal cells only in the limbal stroma adjacent to invading epithelial cells, but not in the corneal region where intrastromal invasion did not occur (Figure 7). However, we cannot exclude the possibility that invading epithelial cells may also partake in activating stromal mesenchymal cell activity. Future studies are needed to elucidate how such epithelial-mesenchymal interactions may interplay in the limbal niche regarding the activation of Wnt/β-catenin signaling pathway, giving rise to epithelial invasion and EMT using this model.43,44
Therefore, under AL, limbal basal epithelial progenitor cells may invade into the stroma and adopt two different fates, ie, maintaining the epithelial phenotype or adopting complete EMT to become fibroblasts. The former fate may help explain several pathological states of the eye including subconjunctival inclusion cysts, epithelial downgrowth (invading into the anterior chamber from limbal incision), and epithelial ingrowth in LASIK complications. However, the latter fate provides a new paradigm to explain how the limbal SC population is lost and fibrovascular tissue is formed in a number of diseases manifesting limbal SC deficiency. We have studied the corneal pannus from three human patients with total limbal SC deficiency, and noted that p63-positive cells were present in the stroma (data now shown). If AL simulates wound healing, the experimental model described herein is ideal to explore the question regarding how epithelial SCs might be regulated by the stromal niche in fate decision between regeneration and fibrosis. This explant is devoid of inflammatory responses. Therefore, this model may be used to investigate how additional inflammation may threaten the well being of limbal epithelial SCs and push them toward EMT to generate fibrosis in the limbal stroma in diseases with limbal SC deficiency.
Supplementary Material
Footnotes
Address reprint requests to Scheffer C.G. Tseng, M.D., Ph.D., Ocular Surface Center, 7000 SW 97 Ave., Suite 213, Miami, FL 33173. E-mail: stseng@ocularsurface.com.
Supported by grants from the National Eye Institute, National Institutes of Health, Bethesda, MD (grants EY06819, EY15735, and EY12486); a research grant from TissueTech, Inc.; and an unrestricted grant from Ocular Surface Research and Education Foundation, Miami, FL.
Presented in part at the annual meeting of Association of Research in Vision and Ophthalmology at Ft. Lauderdale, FL, in April 2005.
References
- Lavker RM, Sun T-T. Epidermal stem cells: properties, markers and location. Proc Natl Acad Sci USA. 2000;97:13473–13475. doi: 10.1073/pnas.250380097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg MF, Bron AJ. Limbal palisades of Vogt. Trans Am Ophthalmol Soc. 1982;80:155–171. [PMC free article] [PubMed] [Google Scholar]
- Schermer A, Galvin S, Sun T-T. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986;103:49–62. doi: 10.1083/jcb.103.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WYW, Mui M-M, Kao WW-Y, Liu C-Y, Tseng SCG. Conjunctival epithelial cells do not transdifferentiate in organotypic cultures: expression of K12 keratin is restricted to corneal epithelium. Curr Eye Res. 1994;13:765–778. doi: 10.3109/02713689409047012. [DOI] [PubMed] [Google Scholar]
- Liu C-Y, Zhu G, Converse R, Kao CW-C, Nakamura H, Tseng SCG, Mui M-M, Seyer J, Justice MJ, Stech ME, Hansen GM, Kao WW-Y. Characterization and chromosomal localization of the cornea-specific murine keratin gene Krt1.12. J Biol Chem. 1994;260:24627–24636. [PubMed] [Google Scholar]
- Matic M, Petrov IN, Chen S, Wang C, Dimitrijevich SD, Wolosin JM. Stem cells of the corneal epithelium lack connexins and metabolite transfer capacity. Differentiation. 1997;61:251–260. doi: 10.1046/j.1432-0436.1997.6140251.x. [DOI] [PubMed] [Google Scholar]
- Pellegrini G, Dellambra E, Golisano O, Martinelli E, Fantozzi I, Bondanza S, Ponzin D, McKeon F, De Luca M. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci USA. 2001;98:3156–3161. doi: 10.1073/pnas.061032098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotsarelis G, Cheng SZ, Dong G, Sun T-T, Lavker RM. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57:201–209. doi: 10.1016/0092-8674(89)90958-6. [DOI] [PubMed] [Google Scholar]
- Kruse FE, Tseng SCG. A tumor promoter-resistant subpopulation of progenitor cells is present in limbal epithelium more than corneal epithelium. Invest Ophthalmol Vis Sci. 1993;34:2501–2511. [PubMed] [Google Scholar]
- Romano AC, Espana EM, Yoo SH, Budak MT, Wolosin JM, Tseng SC. Different cell sizes in human limbal and central corneal basal epithelia measured by confocal microscopy and flow cytometry. Invest Ophthalmol Vis Sci. 2003;44:5125–5129. doi: 10.1167/iovs.03-0628. [DOI] [PubMed] [Google Scholar]
- Lavker RM, Dong G, Cheng SZ, Kudoh K, Cotsarelis G, Sun TT. Relative proliferative rates of limbal and corneal epithelia. Implications of corneal epithelial migration, circadian rhythm, and suprabasally located DNA-synthesizing keratinocytes. Invest Ophthalmol Vis Sci. 1991;32:1864–1875. [PubMed] [Google Scholar]
- Pellegrini G, Golisano O, Paterna P, Lambiase A, Bonini S, Rama P, De Luca M. Location and clonal analysis of stem cells and their differentiated progeny in the human ocular surface. J Cell Biol. 1999;145:769–782. doi: 10.1083/jcb.145.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meller D, Pires RTF, Tseng SCG. Ex vivo preservation and expansion of human limbal epithelial stem cells on amniotic membrane cultures. Br J Ophthalmol. 2002;86:463–471. doi: 10.1136/bjo.86.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng SCG, Sun T-T. Stem cells: ocular surface maintenance. Brightbill FS, editor. St. Louis: Mosby; 1999:pp 9–18. [Google Scholar]
- Wolosin JM, Xiong X, Schütte M, Stegman Z, Tieng A. Stem cells and differentiation stages in the limbo-corneal epithelium. Prog Retin Eye Res. 2000;19:223–255. doi: 10.1016/s1350-9462(99)00005-1. [DOI] [PubMed] [Google Scholar]
- Kinoshita S, Adachi W, Sotozono C, Nishida K, Yokoi N, Quantock AJ, Okubo K. Characteristics of the human ocular surface epithelium. Prog Retin Eye Res. 2001;20:639–673. doi: 10.1016/s1350-9462(01)00007-6. [DOI] [PubMed] [Google Scholar]
- Lavker RM, Tseng SC, Sun TT. Corneal epithelial stem cells at the limbus: looking at some old problems from a new angle. Exp Eye Res. 2004;78:433–446. doi: 10.1016/j.exer.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Puangsricharern V, Tseng SCG. Cytologic evidence of corneal diseases with limbal stem cell deficiency. Ophthalmology. 1995;102:1476–1485. doi: 10.1016/s0161-6420(95)30842-1. [DOI] [PubMed] [Google Scholar]
- Kenyon KR, Rapoza PA. Limbal allograft transplantation for ocular surface disorders. Ophthalmology. 1995;102:S101–S102. [Google Scholar]
- Tsai RJF, Tseng SCG. Effect of stromal inflammation on the outcome of limbal transplantation for corneal surface reconstruction. Cornea. 1995;14:439–449. [PubMed] [Google Scholar]
- Espana EM, Romano AC, Kawakita T, Di Pascuale M, Smiddy R, Tseng SC. Novel enzymatic isolation of an entire viable human limbal epithelial sheet. Invest Ophthalmol Vis Sci. 2003;44:4275–4281. doi: 10.1167/iovs.03-0089. [DOI] [PubMed] [Google Scholar]
- Espana EM, Kawakita T, Romano A, Di Pascuale M, Smiddy R, Liu CY, Tseng SC. Stromal niche controls the plasticity of limbal and corneal epithelial differentiation in a rabbit model of recombined tissue. Invest Ophthalmol Vis Sci. 2003;44:5130–5135. doi: 10.1167/iovs.03-0584. [DOI] [PubMed] [Google Scholar]
- Kawakita T, Espana EM, He H, Yeh LK, Liu CY, Tseng SC. Calcium-induced abnormal epidermal-like differentiation in cultures of mouse corneal-limbal epithelial cells. Invest Ophthalmol Vis Sci. 2004;45:3507–3512. doi: 10.1167/iovs.04-0266. [DOI] [PubMed] [Google Scholar]
- Espana EM, Romano AC, Kawakita T, Di Pascuale M, Smiddy R, Tseng SC. Novel enzymatic isolation of an entire viable human limbal epithelial sheet. Invest Ophthalmol Vis Sci. 2003;44:4275–4281. doi: 10.1167/iovs.03-0089. [DOI] [PubMed] [Google Scholar]
- Espana EM, He H, Kawakita T, Di Pascuale MA, Raju VK, Liu CY, Tseng SC. Human keratocytes cultured on amniotic membrane stroma preserve morphology and express keratocan. Invest Ophthalmol Vis Sci. 2003;44:5136–5141. doi: 10.1167/iovs.03-0484. [DOI] [PubMed] [Google Scholar]
- Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6:331–337. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- Tseng SCG, Kruse FE, Merritt J, Li D-Q. Comparison between serum-free and fibroblast-cocultured single-cell clonal culture systems: evidence showing that epithelial anti-apoptotic activity is present in 3T3 fibroblast conditioned media. Curr Eye Res. 1996;15:973–984. doi: 10.3109/02713689609017643. [DOI] [PubMed] [Google Scholar]
- Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dotsch V, Andrews NC, Caput D, McKeon F. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998;2:305–316. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- Grueterich M, Espana E, Tseng SC. Connexin 43 expression and proliferation of human limbal epithelium on intact and denuded amniotic membrane. Invest Ophthalmol Vis Sci. 2002;43:63–71. [PubMed] [Google Scholar]
- Gant VA, Shakoor Z, Hamblin AS. A new method for measuring clustering in suspension between accessory cells and T lymphocytes. J Immunol Methods. 1992;156:179–189. doi: 10.1016/0022-1759(92)90024-n. [DOI] [PubMed] [Google Scholar]
- Mills AA, Zheng B, Wang X-J, Vogel H, Roop DR, Bradley A. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398:708–713. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- Chen WYW, Tseng SCG. Differential intrastromal invasion by normal ocular surface epithelia is mediated by different fibroblasts. Exp Eye Res. 1995;61:521–533. doi: 10.1016/s0014-4835(05)80046-6. [DOI] [PubMed] [Google Scholar]
- Zieske JD, Mason VS, Wasson ME, Meunier SF, Nolte CJ, Fukai N, Olsen BR, Parenteau NL. Basement membrane assembly and differentiation of cultured corneal cells: importance of culture environment and endothelial cell interaction. Exp Cell Res. 1994;214:621–633. doi: 10.1006/excr.1994.1300. [DOI] [PubMed] [Google Scholar]
- Bernstam LI, Vaughan FL, Bernstein IA. Keratinocytes grown at the air-liquid interface. In Vitro Cell Dev Biol. 1986;22:695–705. doi: 10.1007/BF02621086. [DOI] [PubMed] [Google Scholar]
- Davanger M, Evensen A. Role of the pericorneal papillary structure in renewal of corneal epithelium. Nature. 1971;229:560–561. doi: 10.1038/229560a0. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Chikama T, Nakamura M, Nishida T. Differential distribution of subchains of the basement membrane components type IV collagen and laminin among the cornea, conjunctiva and amniotic membrane. Cornea. 1999;18:73–79. [PubMed] [Google Scholar]
- Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson RT, Tabin C, Sharpe A, Caput D, Crum C, McKeon F. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714–718. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]
- Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilosi M, Poletti V, Zamo A, Lestani M, Montagna L, Piccoli P, Pedron S, Bertaso M, Scarpa A, Murer B, Cancellieri A, Maestro R, Semenzato G, Doglioni C. Aberrant Wnt/beta-catenin pathway activation in idiopathic pulmonary fibrosis. Am J Pathol. 2003;162:1495–1502. doi: 10.1016/s0002-9440(10)64282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Ishikawa F, Sonoda KH, Hisatomi T, Qiao H, Yamada J, Fukata M, Ishibashi T, Harada M, Kinoshita S. Characterization and distribution of bone marrow-derived cells in mouse cornea. Invest Ophthalmol Vis Sci. 2005;46:497–503. doi: 10.1167/iovs.04-1154. [DOI] [PubMed] [Google Scholar]
- Toda S, Yokoi F, Yamada S, Yonemitsu N, Nishimura T, Watanabe K, Sugihara H. Air exposure promotes fibroblast growth with increased expression of mitogen-activated protein kinase cascade. Biochem Biophys Res Commun. 2000;270:961–966. doi: 10.1006/bbrc.2000.2466. [DOI] [PubMed] [Google Scholar]
- Kim K, Lu Z, Hay ED. Direct evidence for a role of beta-catenin/LEF-1 signaling pathway in induction of EMT. Cell Biol Int. 2002;26:463–476. doi: 10.1006/cbir.2002.0901. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.