Abstract
Intercellular transfer of proteins is a mode of communication between cells that is crucial for certain physiological processes. Chemotherapy is the treatment of choice for ~50% of all cancers. However, multidrug resistance mediated by drug-efflux pumps such as P-glycoprotein (Pgp) minimizes the effectiveness of such therapy in a large number of patients. A new study demonstrates the functional intercellular transfer of Pgp. Non-genetic transfer of the multidrug resistance phenotype raises fascinating questions about the mechanism and regulation of cell-surface membrane-protein-mediated spread of traits.
Transfer of membrane proteins between cells
Biologists have studied the role of diffusible molecules in communication between cells for almost a century. More recently, the clustering of distinct ligands or receptors at the tips of specific cellular extensions has been shown to offer a sophisticated and efficient communication network. However, several recent studies suggest that the wholesale transfer of membrane proteins might be commonplace in biology. Intercellular transfer of membrane proteins [1] has been shown to be an integral part of neuronal and immunological synapses, signals for organogenesis and tumor metastasis [2] and the spreading of the glycosylphosphatidylinositol (GPI)-anchored prion PrPc [3] (Figure 1). The immunological synapse, where the exchange of membrane proteins is rampant [1], is being recognized increasingly as central to immune cell communication. For example, antigen-presenting dendritic cells secrete vesicles called exosomes that carry major histocompatibility complex (MHC) proteins to T cells via a pathway that is under the control of cytokines and chemokines [4]. Similarly, the chemokine receptor CCR5 is released from the peripheral blood mononuclear cells in microparticles (0.1–2.0 μm), resulting in the transfer of CCR5 to CCR5-negative cells [5]. CCR5 is the principal coreceptor for the HIV-1 virus and thus such transfer of a receptor is of considerable concern. Finally, convincing evidence has been provided for the functional role of ‘tunneling nanotubes’ in complex networks [6] through which a significant exchange of membrane components occurs between a variety of cells (e.g. 721.221 B, rat PC12 and HEK293T cells).
Figure 1.
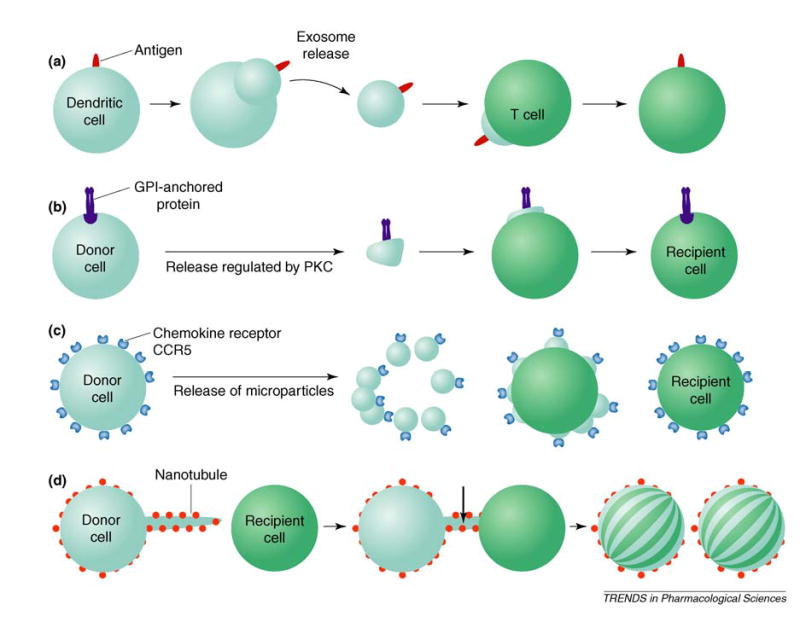
Mechanisms for the intercellular transfer of membrane proteins: (a) exosome-mediated transfer of antigens; (b) release of glycosylphosphatidylinositol (GPI)-anchored proteins such as the prion PrPc; (c) release of chemokine receptors such as CCR5 and CD81 on microparticles by the donor cell in response to diverse stimuli; and (d) transfer of membrane proteins through short-lived tunneling nanotubes formed on a variety of cells. Abbreviation: PKC, protein kinase C.
Multidrug resistance and P-glycoprotein
Classical multidrug resistance is attributed to the elevated expression of ATP-dependent drug-efflux pumps ABCB1 [also known as P-glycoprotein (Pgp)], ABCC1 [also known as multidrug resistance-associated protein (MRP1)] and ABCG2 [also known as breast cancer-resistance protein (BCRP) and mitoxantrone-resistance protein (MXR)], all of which belong to the superfamily of ATP-binding cassette (ABC) transporters [7]. Efflux mediated by ABC drug transporters leads to decreased cellular accumulation of anti-cancer drugs, which is a main cause of the limited success of the currently applied chemotherapy regimens. Pgp, a product of the ABCB1 (previously known as MDR1) gene, is one of the most extensively studied ABC drug transporters. Pgp transports chemically dissimilar drugs that act on diverse targets [8]. The expression of ABCB1 at high levels has been documented in many types of tumors, such as breast cancer, sarcoma, neuroblastoma and acute myelogenous leukemia.
There are several paths a ‘naïve’ cancer cell can take to increase the expression of ABCB1 (Figure 2). In tumors derived from epithelial tissues that normally contain Pgp (e.g. cancers of the kidney, liver, colon and brain), cells can exhibit intrinsic resistance. Other tumors can become resistant during the course of chemotherapy, through the induction of ABCB1 expression or the selection of cells that overexpress ABCB1. Cancer cells are frequently heterogeneous with respect to ABCB1 expression and those cells with higher levels of Pgp have a selective advantage during the progression of the disease. Thus, a significant proportion of malignant cells might already be prepared to cope with the additional stress of chemotherapy at the time of diagnosis. Now, the recent results of Levchenko et al. [9] suggest that, by simply accepting Pgp from a donor cell, cancer cells can use yet another mechanism to acquire resistance to chemotherapeutic drugs.
Figure 2.
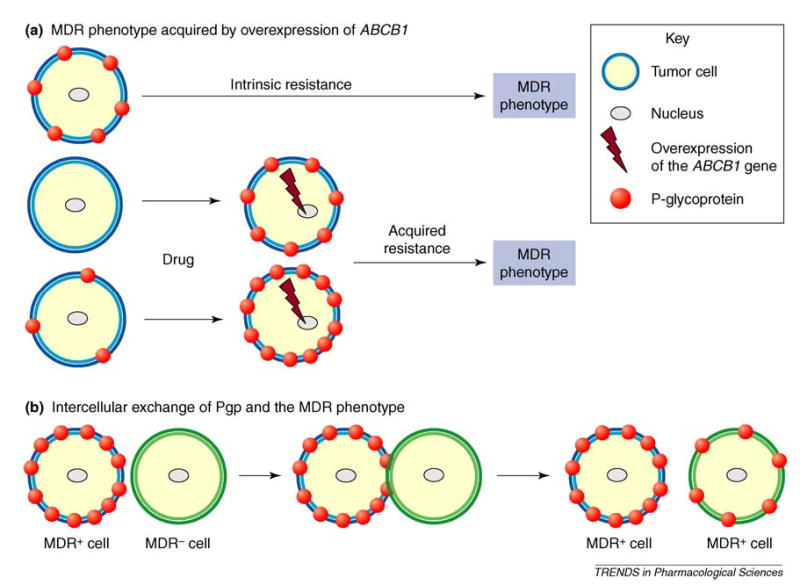
Possible ways cells can develop P-glycoprotein (Pgp)-mediated multidrug resistance (MDR). (a) Tumor cells are either intrinsically resistant to drugs or acquire resistance on exposure to cytotoxic therapy. In both instances MDR is a consequence of the overexpression of the ABCB1 gene product. (b) Drug-sensitive (MDR−) cells acquire the MDR phenotype by the intercellular transfer of Pgp by an unknown mechanism but it is most probably mediated by relatively large (>0.8 μM) membrane microparticles (Figure 1c) and requires cell–cell contact with donor MDR+ cells [9] (for clarity only cell–cell contact is shown). It should be noted that the physiological relevance of this mechanism remains to be determined.
Intercellular transfer of Pgp
Levchenko and colleagues [9] studied the appearance of resistant clones in co-cultures of sensitive and resistant versions of human neuroblastoma [BE(2)-C] and adenocarcinoma (MCF-7) cells (for a list of cell types see Table 1 in [9]). Within a few hours following in vitro mixture of parental (Pgp-negative) cells with multidrug-resistant (Pgp-positive) derivatives, the authors detected a new population of cells that expressed intermediate levels of Pgp. This subpopulation could have emerged from the sensitive or the resistant cells through partial gain or loss of Pgp, respectively. Sensitive cells are not expected to promptly upregulate intrinsic Pgp levels in the absence of drug substrates. Nevertheless, to distinguish between the progenies of the two cell lines, sensitive cells were stably transfected with green fluorescent protein (GFP), and Pgp was monitored using a specific monoclonal antibody (MRK-16) labeled with a red fluorophore. The newly emergent subpopulation contained double-labeled cells. The double-labeled cells were multidrug resistant, despite low levels of ABCB1 mRNA, suggesting that these cells acquire the drug-resistant phenotype through the intercellular transfer of functional Pgp molecules (see Figures 7–16 in [9] for information on several control experiments).
Although these results add another dimension to the ways cells can acquire a particular cell-surface protein-mediated phenotype such as multidrug resistance, they also raise several questions. From a physiological standpoint, the challenge is to demonstrate convincingly that Pgp transfer occurs in vivo. Although Levchenko et al. [9] provide some evidence to support this possibility, it must be noted that their experimental set-up invariably involved the in vitro culturing of cells. In addition to being a drug-efflux pump, Pgp has been shown to confer resistance to apoptosis induced by many chemotherapeutic drugs. It will be interesting to determine whether the inhibitory effect of Pgp on caspase activation [10], which does not require ATP hydrolysis activity [11], can be transferred to acceptor cells. The observed slower growth of acceptor cells following transfer of Pgp suggests the involvement of additional proteins or factors. Is Pgp particularly prone to intercellular transfer or are other plasma membrane transport or channel proteins also transferable? It will be important to decipher the mechanism of transfer of membrane transport proteins, which appears most likely to be mediated by large membrane microparticles and requires cell–cell contact [9]. Are there biochemical (e.g. phosphorylation), chemotactic or physical signals that induce transfer of proteins? Does the proposed transfer of membrane proteins form part of a regulated pathway? Transfer of proteins such as Pgp with a long cell-surface half-life [12] might be easier to detect compared with those proteins that have a shorter half-life. Because the transfer is of a non-genetic nature, the expression of Pgp in acceptor cells is unstable and requires constant selection pressure (presence of anti-cancer drug) or the presence of resistant donor cells. Importantly, further studies using lineage markers (not subject to the proposed transfer) will have to be undertaken to distinguish definitively between functional transfer of proteins and cell fusion.
Clinical consequences of the intercellular transfer of Pgp
Resistance to chemotherapy in metastatic cancers has been correlated with the overexpression of energy-dependent efflux pumps [7]. It now appears that one such drug efflux pump, Pgp, might be transferred from drug-resistant cells to sensitive cells [9]. The implications of this phenomenon are difficult to predict because there are still key gaps in our understanding of this process. For example, it is not known whether there is a large redundancy in the number of Pgp molecules on the surface of drug-resistant cells. The efficiency of the transfer will depend on the density of Pgp molecules at the cell surface of donor cells. Thus, the clinical significance of this mechanism will depend on whether transfer of Pgp at critical levels occurs in vivo. Intercellular transfer of Pgp provides a good model system to address the broader question of the mechanisms by which membrane proteins are transferred between cells. However, without the detailed understanding of underlying principles and the clinical relevance of intercellular Pgp transfer, it is unlikely that this finding will have an immediate impact on current treatment protocols or strategies to overcome multidrug resistance.
References
- 1.Davis DM, et al. The protean immune cell synapse: a supramolecular structure with many functions. Semin Immunol. 2003;15:317–324. doi: 10.1016/j.smim.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Darland DC, D’Amore PA. Cell–cell interactions in vascular development. Curr Top Dev Biol. 2001;52:107–149. doi: 10.1016/s0070-2153(01)52010-4. [DOI] [PubMed] [Google Scholar]
- 3.Liu T, et al. Intercellular transfer of the cellular prion protein. J Biol Chem. 2002;277:47671–47678. doi: 10.1074/jbc.M207458200. [DOI] [PubMed] [Google Scholar]
- 4.Zitvogel L, et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 5.Mack M, et al. Transfer of the chemokine receptor CCR5 between cells by membrane-derived micro particles: a mechanism for cellular human immunodeficiency virus 1 infection. Nat Med. 2000;6:769–775. doi: 10.1038/77498. [DOI] [PubMed] [Google Scholar]
- 6.Rustom A, et al. Nanotubular highways for intercellular organelle transport. Science. 2004;303:1007–1010. doi: 10.1126/science.1093133. [DOI] [PubMed] [Google Scholar]
- 7.Gottesman MM, et al. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 8.Ambudkar SV, et al. P-glycoprotein: from genomics to mechanism. Oncogene. 2003;22:7468–7485. doi: 10.1038/sj.onc.1206948. [DOI] [PubMed] [Google Scholar]
- 9.Levchenko A, et al. Intercellular transfer of P-glycoprotein mediates acquired multidrug resistance in tumor cells. Proc Natl Acad Sci U S A. 2005;102:1933–1938. doi: 10.1073/pnas.0401851102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnstone RW, et al. Multiple physiological functions for multidrug transporter P-glycoprotein? Trends Biochem Sci. 2000;25:1–6. doi: 10.1016/s0968-0004(99)01493-0. [DOI] [PubMed] [Google Scholar]
- 11.Tainton KM, et al. Mutational analysis of P-glycoprotein: suppression of caspase activation in the absence of ATP-dependent drug efflux. Cell Death Differ. 2004;11:1028–1037. doi: 10.1038/sj.cdd.4401440. [DOI] [PubMed] [Google Scholar]
- 12.Gribar JJ, et al. Functional characterization of glycosylation-deficient human P-glycoprotein using a vaccinia virus expression system. J Membr Biol. 2000;173:203–214. doi: 10.1007/s002320001020. [DOI] [PubMed] [Google Scholar]


