Abstract
The Yop virulon enables extracellularly located Yersinia, in close contact with a eukaryotic target cell, to inject bacterial toxic proteins directly into the cytosol of this cell. Several Ysc proteins, forming the Yop secretion apparatus, display homology with proteins of the flagellar basal body. To determine whether this relationship could extend to the regulatory pathways, we analyzed the influence of flhDC, the master regulatory operon of the flagellum, on the yop regulon. In an flhDC mutant, the yop regulon was up-regulated. The transcription of virF and the steady-state level of the transcriptional activator VirF were enhanced. yop transcription was increased at 37°C and could also be detected at a low temperature. Yop secretion was increased at 37°C and occurred even at a low temperature. The Ysc secretion machinery was thus functional at room temperature in the absence of flagella, implying that in wild-type bacteria, FlhD and/or FlhC, or the product of a gene downstream of flhDC, represses the yop regulon. In agreement with this notion, increased expression of flhDC in wild-type bacteria resulted in the oversecretion of flagellins at room temperature and in decreased Yop secretion at 37°C.
The Yop virulon enables extracellularly located Yersinia, in close contact with a eukaryotic target cell, to inject directly into the cytosol of this cell bacterial toxic proteins called Yop effectors. This type III system allows the three Yersinia species that are pathogenic for humans (Yersinia pestis, Y. enterocolitica, and Y. pseudotuberculosis) to resist the nonspecific immune response of the host, in particular by inhibiting phagocytosis and by inducing macrophage apoptosis (3, 8). The Yop virulon, encoded by a large virulence plasmid called pYV in Y. enterocolitica, consists basically of a dozen secreted proteins, the Yop effectors and their translocators, as well as a secretion apparatus composed of the Ysc proteins (6). Yop secretion occurs at 37°C in vivo after contact with a eukaryotic target cell and in vitro when the bacteria are placed in a rich medium deprived of Ca2+ ions. Transcription of the ysc and yop genes is strongly thermoinduced; the thermoregulation results from the interplay between a transcriptional activator, VirF (5), and the chromatin structure (31). The expression of the yop virulon is controlled first by temperature, but the expression of some of its genes is reinforced by the action of VirF (21), whose synthesis is also thermoregulated (5). Temperature, by modifying the structure of the chromatin, is thought to dislodge a repressor, presumably the histone-like protein YmoA (7), bound on promoter regions of VirF-sensitive genes and of some other thermoregulated genes (31).
Y. enterocolitica organisms are peritrichously flagellated bacteria that are motile only when the temperature is below 30°C, so that once again, temperature is a key environmental factor for the Yersinia lifestyle. With regard to motility, the temperature-sensitive regulation of various flagellar genes has been reported even if it is still not fully understood (17, 18). The well-characterized regulation of flagellum biosynthesis in Escherichia coli and Salmonella enterica serovar Typhimurium is subjected to hierarchy, allowing sequential gene expression (25). At the top of the hierarchy is the master operon, composed of the genes flhD and flhC. FlhD and FlhC together form a heterotetrameric transcriptional activator that binds to class II operons (23) and is required for the expression of all other flagellar genes (1). The second level of hierarchy includes genes necessary for the early assembly of the flagellum and a flagellum-specific σ factor, fliA, required for the transcription of level III genes. Class III includes genes for late morphogenesis, motor rotation, and chemotactic signaling. Although there is actually no direct evidence, it is believed that Y. enterocolitica has a flagellar regulatory cascade that is similar to that of E. coli and Salmonella serovar Typhimurium.
Several Ysc proteins that are required for Yop secretion display homology with flagellar proteins. Structural similarities between type III secretion apparatuses and flagella were reported for Salmonella SPI-1 (19) and the Shigella needle-like structure (4, 38). Given that the Y. enterocolitica type III structure and flagellum are related and subjected to alternative expression, there could be a link between the expression of both structures. Since σ28 is not required for transcription of the ysc and yop genes (15), we tested here the effect of silencing the master operon on Yop secretion.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are described in Table 1. E. coli LK111, received from M. Zabeau (Ghent, Belgium), was used for standard genetic manipulations. E. coli SM10λpir+, constructed by Miller and Mekalanos (28), was used to deliver the mobilizable plasmids in Y. enterocolitica.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant featuresa | Reference or source |
|---|---|---|
| Y. enterocolitica strains and pYV plasmids | ||
| MRS40(pYV40) | Wild type | 33 |
| MRS40(pAB4052) | yopE52 | 29 |
| SBY40(pYV40) | flhDΔ44-119 flhCΔ1-124 | This work |
| SBY40(pAB4052) | yopE52 flhDΔ44-119 flhCΔ1-124 | This work |
| KNG22703(pSW2276) | yscNΔ169-177 | 40 |
| SBY22703(pSW2276) | yscNΔ169-177 flhDΔ44-119 flhCΔ1-124 | This work |
| W22703(pGC1153) | virF::pGCS904 | 21 |
| MI1024(pYV1024) | fliA::aphA3 | 15 |
| Other plasmids | ||
| pBSSK(−) | Cloning vector | Stratagene |
| pGCS757 | virF-cat transcriptional fusion in pTZ19R plus oriTRK2 | 5 |
| pGEX-KG | Cloning vector generating translational fusions with GST | Pharmacia Biotech |
| pGY10 | 4.3-kb EcoRI fragment containing flhDC genes of Y. enterocolitica 8081v cloned in pTM100 | 44 |
| pKNG101 | Suicide vector; oriR6Kpir oriTRK2 | 16 |
| pPW107 | pBC19R plus kanamycin resistance cassette | P. Wattiau and G. R. Cornelis, unpublished data |
| pSBY10 | flhDC mutator obtained by cloning the mutated flhD′C′ allele from pSBY9 digested with SalI and XbaI in the same sites of pKNG101 | This work |
| pSBY5 | pGEX-KG producing a hybrid GST-YscJ′ protein (191-bp fragment of yscJ [nucleotides 23-214]) | This work |
| pSBY6 | PCR-amplified flhD and flhC cloned opposite plac in pBluescript SK(−) | This work |
| pSBY7 | StyI deletion in pSBY6 | This work |
| pSBY9 | Cloned kanamycin resistance cassette in the StyI site of pSBY7 blunted with the Klenow fragment of polymerase | This work |
| pSI55 | plac, sycE (bp −25 to stop), and yopE (bp −21 to stop) cloned in the same orientation in pTM100 | I. Stainier and G. R. Cornelis, unpublished data |
| pTM100 | pACYC184 plus oriTRK2 (medium-copy-number, mobilizable vector) | 26 |
Deletions indicate the base pairs deleted from the gene; e.g., in flhDΔ44-119, bp 44 to 119 are deleted from flhD. oriTRK2, origin of transfer of plasmid RK2; oriR6K, origin of replication of plasmid R6K.
Bacteria were grown routinely in tryptic soy broth (Oxoid) and plated on tryptic soy agar (Oxoid), sometimes supplemented with 20 mM MgCl2 and 20 mM sodium oxalate. For induction of the yop regulon, Y. enterocolitica was inoculated to an optical density at 600 nm of 0.1; cultivated in brain heart infusion (BHI; Remel, Lenexa, Kans.) broth supplemented with 4 mg of glucose ml−1, 20 mM MgCl2, and 20 mM sodium oxalate (BHI-Ox) for 2 h at room temperature (RT); shifted to 37°C; and incubated for 4 h. Secretion at a low temperature was tested under the same culture conditions after 6 h of growth at 28°C. To study the effect of Ca2+ on Yop secretion, BHI was supplemented with 4 mg of glucose ml−1 and 5 mM CaCl2. Selective agents were used at the following concentrations: 35 μg of nalidixic acid ml−1, 0.4 mM sodium arsenite, 50 μg of kanamycin ml−1, 50 μg of ampicillin ml−1, 100 μg of streptomycin ml−1, and 5% (wt/vol) sucrose.
Mobility assay.
A 10-μl portion of an overnight culture was inoculated onto semisolid plates (0.3% agar). The capacity of each strain to spread beyond the inoculation point was monitored after 16 h at 28°C.
Molecular cloning and mutagenesis.
To inactivate flhDC genes, we replaced the central part of the operon with a kanamycin resistance cassette. The flhDC locus was amplified from the chromosome of MRS40(pYV40) by PCR with oligonucleotides MIPA 617 (5′-CCGGAATTCATGTATAAAATGAGTACG-3′) and MIPA 618 (5′-AATAAGCTTTCAAACTGCGCGTCTAA-3′), which were designed to include the ATG of flhD and the stop codon of flhC. The PCR product was cloned in the direction opposite that of plac in pBluescript KS(−) digested with SmaI, giving pSBY6. The insert of pSBY6 was sequenced. A StyI deletion that eliminates the last 219 bp of flhD and the first 359 bp of flhC was generated, giving pSBY7. The kan gene was amplified with MIPA 609 (5′-GTGTGATATCAGGGCGCAAGGGCTGCTAAA-3′), MIPA 610 (5′-GCGCGATATCAATTCAGAAGAACTCGTCAA-3′), and pPW107 as a template (P. Wattiau and G. R. Cornelis, unpublished data) and ligated to pSBY7 digested with StyI and blunted with the Klenow fragment of polymerase, giving pSBY9. The 1.3-kb insert of pSBY9 digested with SalI and XbaI was cloned in the corresponding sites of suicide plasmid pKNG101 to give pSBY10. The mutated allele was then introduced into Y. enterocolitica MRS40(pYV40), KNG22703(pSW2276), or MRS40(pAB4052) by allelic exchange as described by Kaniga et al. (16). The mutation was then confirmed by PCR.
SDS-PAGE analysis of proteins and immunoblotting.
Yop proteins (Yops) were precipitated from culture supernatants by overnight precipitation with trichloroacetic acid (final concentration, 10% [wt/vol]), washed with acetone, and resuspended in Laemmli buffer (20) at various concentrations (see figure legends). Electrophoresis in 12% (wt/vol) polyacrylamide gels (polyacrylamide gel electrophoresis [PAGE]) in the presence of sodium dodecyl sulfate (SDS) was performed as described by Laemmli (20). After electrophoresis, proteins were either stained with Coomassie brillant blue or transferred by electroblotting to a nitrocellulose membrane. Immunoblotting was carried out by using antiflagellin polyclonal antibodies (gifts from G. Wauters), anti-YscJ (MIPA 66; this work), anti-YopN (MIPA 48), anti-YopE (MIPA 25), anti-VirF (MIPA 29), anti-PhoE (gift from J. Tommassen), or anti-YopB monoclonal antibody (9B7). Secondary antibodies conjugated to horseradish peroxidase (Dako) were detected with Supersignal chemiluminescence substrate (Pierce).
Preparation of polyclonal antibodies against YscJ.
Polyclonal anti-YscJ antibodies were raised against a fusion of glutathione S-transferase (GST) with YscJ. The coding region for the hydrophilic periplasmic domain of YscJ (Leu23 to Ser214) was amplified from plasmid pYV40 by PCR with oligonucleotides MIPA 608 (5′-CGGAATTCTTTATACCGGAATTAGT-3′) and MIPA 607 (5′-TTAAAGCTTATGACTCTTCACTCACTTG-3′), MIPA 608 creating an EcoRI site and MIPA 607 creating a HindIII site (underlined). The PCR product digested with EcoRI and HindIII was cloned in pGEX-KG (Pharmacia Biotech) in the corresponding sites to generate a translational fusion with GST (pSBY5). The hybrid GST fusion was produced and purified basically as described by Smith and Johnson (37) and Pharmacia Biotech. An 800-μg portion of the purified fusion protein was used to immunize a rabbit.
RNA extraction and Northern blot analysis.
Total RNA of Y. enterocolitica was extracted as described by Lambert de Rouvroit et al. (21). This was done after 4 h of growth at RT or 2 h at RT and 2 h at 37°C. Electrophoresis and transfer were done as described by Cornelis et al. (5), and hybridization was done with yop gene DNA by using DIG High Prime labeling and detection starter kit II (Boehringer Mannheim).
Chloramphenicol acetyltransferase assay.
Chloramphenicol acetyltransferase was assayed by the spectrophotometric method of Shaw (34).
RESULTS
Yop secretion is deregulated in an flhDC mutant.
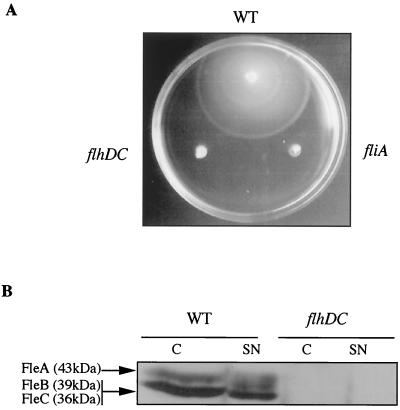
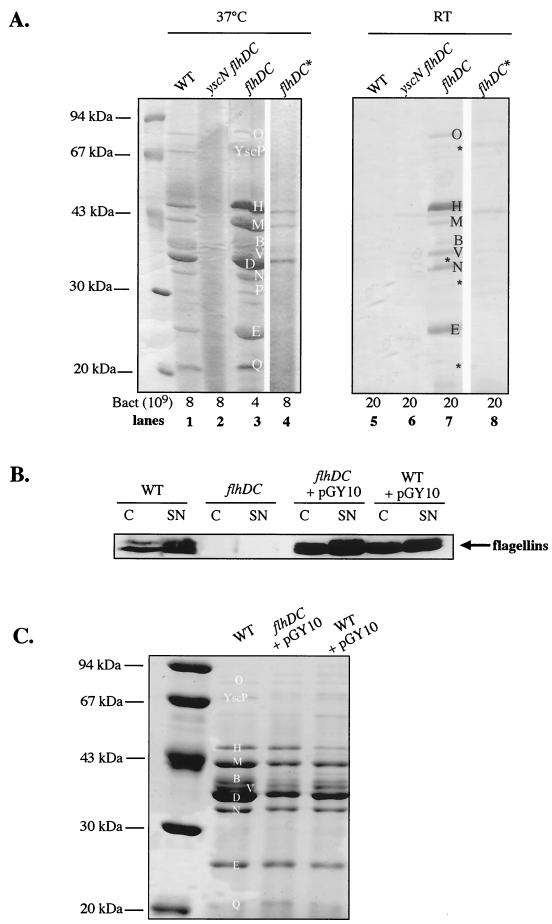
An flhDC knockout mutant of Y. enterocolitica MRS40 was constructed by deleting 219 bp of flhD (codon 44 to stop) and 359 bp of flhC (start to codon 124). As expected, the flhDC mutant bacteria were not motile at 28°C (Fig. 1A), and flagellins were not detected in bacteria or in the supernatants of cultures grown at RT in a rich medium deprived of Ca2+ ions (BHI-Ox) (Fig. 1B). At 37°C in BHI-Ox, mutant bacteria secreted larger amounts of Yops than wild-type bacteria. In Fig. 2A, the lane loaded with supernatants of flhDC mutant bacteria contains more Yops than the lane loaded with supernatants of wild-type bacteria, in spite of the fact that a smaller amount was loaded (lanes 1 and 3). This increased secretion of Yops at 37°C led us to test whether the flhDC mutant would secrete Yops at RT. While a wild-type strain was unable to secrete Yops at RT in BHI-Ox, the flhDC mutant was able to do so (Fig. 2A, lanes 5 and 7). However, we must emphasize that the amount of Yops secreted by mutant bacteria at RT was smaller than the amount secreted by wild-type bacteria at 37°C. Moreover, some of the proteins that are normally detected by Coomassie brilliant blue staining in the supernatants of cultures induced at 37°C were detected only by immunoblotting in the supernatants of flhDC mutant bacteria grown at RT (YscP, YopD, YopP, and YopQ) (Fig. 2A, lane 7, asterisks, and data not shown).
FIG. 1.
Motility phenotype of the flhDC mutant. (A) Motility assay with a BHI-Ox semisolid plate inoculated with Y. enterocolitica MRS40(pYV40) (wild type [WT]), SBY40(pYV40) (flhDC), and MI1024(pYV1024) (fliA). (B) Western blot analysis with antiflagellin polyclonal antibodies of proteins from whole-cell extracts (C) or from culture supernatants (SN) of Y. enterocolitica MRS40(pYV40) (WT) or SBY40(pYV40) (flhDC) grown for 6 h at RT in BHI-Ox. Lanes were loaded with 8 × 108 bacteria or the supernatant from 10 × 1010 bacteria. The three Y. enterocolitica flagellins are indicated on the left.
FIG. 2.
Yop and flagellin secretion by flhDC mutant bacteria and by bacteria overexpressing flhDC. (A) SDS-PAGE and Coomassie brilliant blue staining of proteins from the supernatants of Ca2+-deprived cultures of Y. enterocolitica MRS40(pYV40) (wild type [WT]) (lanes 1 and 5), SBY22703(pSW2276) (yscN flhDC) (lanes 2 and 6), SBY40(pYV40) (flhDC) (lanes 3 and 7), and SBY40(pYV40) with a compensatory mutation (flhDC*) (lanes 4 and 8) after 2 h at RT and 4 h at 37°C (lanes 1 to 4) or 6 h at RT (lanes 5 to 8). The number of bacteria corresponding to the volume of supernatant loaded is given below the panels. Lanes 4 and 8 (flhDC*) were separated from the others because they were not from the same gel. (B) Western blot analysis with antiflagellin polyclonal antibodies of proteins from whole-cell extracts (C) or culture supernatants (SN) of Y. enterocolitica MRS40(pYV40) (WT) or SBY40(pYV40) (flhDC) containing pGY10 or MRS40(pYV40) (WT) containing pGY10 and grown for 6 h at RT in BHI-Ox. Lanes were loaded with 8 × 108 bacteria or the supernatant from 10 × 109 bacteria. (C) SDS-PAGE and Coomassie brilliant blue staining of proteins from the supernatants of Ca2+-deprived cultures of Y. enterocolitica MRS40(pYV40) (WT), SBY40(pYV40) (flhDC) containing pGY10, and MRS40(pYV40) (WT) containing pGY10 after 2 h at RT and 4 h at 37°C. Lanes were loaded with the supernatant from 8 × 109 bacteria. In panels A and C, the positions of the Yops (YopO, -H, -M, -B, -D, -N, -E, and -Q), LcrV (V), and YscP (P) and of proteins that cannot be detected by Coomassie brilliant blue staining (asterisks) are indicated.
To exclude the possibility that the secretion observed in the flhDC mutant was due to lysis, we introduced a yscN mutation into the flhDC background. The yscN gene encodes an ATPase that is required for the secretion process (40). The double mutant was unable to secrete proteins after growth at RT or at 37°C (Fig. 2A, lanes 2 and 6), demonstrating that the observed secretion was true Ysc secretion. We can exclude the possibility that this secretion was due to the second type III machinery discovered in Y. enterocolitica O:8 (14). Indeed, the Ysa system is not present in serotype O:9 strains (10).
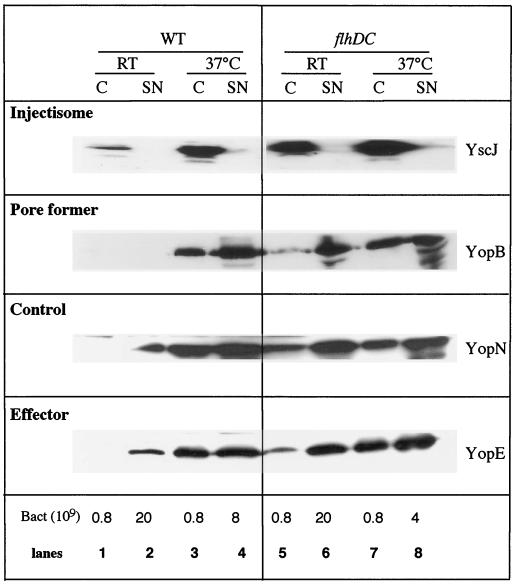
The study of the expression of the Yop virulon was pursued by semiquantitative Western blot analysis with antibodies directed against various components of the system (Fig. 3 and data not shown). At RT, the flhDC mutant systematically produced more of the proteins tested than wild-type Y. enterocolitica (Fig. 3, compare lanes 1 and 5), the difference being in the range of eightfold. At 37°C, steady-state levels of these proteins were doubled, and the percentage of secretion (secreted proteins/total proteins) was increased about threefold (Fig. 3, compare lanes 3 and 4 and lanes 7 and 8). These results are in agreement with what we found by diluting twice the protein sample of the flhDC mutant for Coomassie brilliant blue staining (Fig. 2A, lanes 1 and 3). Taken together, these data show that in an flhDC background, Yop secretion not only is increased at 37°C but also occurs at RT. We should, however, point out that the very high sensitivity of chemiluminescence examination of immunoblots sometimes allows detection of some Yops in the supernatants of wild-type bacteria grown at RT.
FIG. 3.
Western blot analysis of intra- and extracellular proteins. Shown is immunodetection (anti-YscJ, -YopN, and -YopE polyclonal antibodies or anti-YopB monoclonal antibody) of proteins from whole-cell extracts (C) (lanes 1, 3, 5, and 7) and culture supernatants (SN) (lanes 2, 4, 6, and 8) of Y. enterocolitica MRS40(pYV40) (wild type [WT]) (lanes 1 to 4) or SBY40(pYV40) (flhDC) (lanes 5 to 8) grown for 6 h at RT (lanes 1, 2, 5, and 6) or 2 h at RT and 4 h at 37°C (lanes 3, 4, 7, and 8) in BHI-Ox. The number of bacteria loaded or the number of bacteria corresponding to the volume of supernatant loaded is given below the panels.
Like the wild-type, the mutant did not secrete Yops in the presence of Ca2+ at both temperatures. It was restricted for growth in the absence of Ca2+ only at 37°C (data not shown).
The phenotype of the flhDC mutant was highly unstable. Rapidly, the flhDC mutant became unable to secrete Yops at RT, and secretion at 37°C returned to normal or was even below normal (Fig. 2A, lanes 4 and 8). However, these revertants did not produce flagellins and were not motile (data not shown). Storage at −80°C and preculturing in the presence of Ca2+ did not solve the problem, suggesting that compensatory mutations occurred during the growth phase preceding the experiments. For each experiment, the phenotype of the mutant was therefore checked, and the mutant needed to be reconstructed several times during the course of this work.
Complementation of the flhDC mutation.
To complement the flhDC mutation, we used the pGY10 construct of Young et al. (44). This construct contains a 4.3-kb EcoRI fragment of Y. enterocolitica 8081v encoding flhDC. As shown in Fig. 2B, the introduction of pGY10 into the flhDC background restored the secretion of flagellins at RT in BHI-Ox and even led to increased synthesis of flagellins compared to that seen in a wild-type strain. In contrast, Yop secretion at 37°C was decreased to below the level observed with wild-type bacteria (Fig. 2C). Thus, the flhDC clone complemented the flhDC mutation and influenced Yop secretion in the expected way. The introduction of pGY10 into the wild-type background also led to a decrease in Yop secretion at 37°C, concomitant with an increase in flagellin production at RT (Fig. 2B and C). In the culture supernatants of these bacteria, flagellins could be detected even after Coomassie brilliant blue staining of SDS gels, while in the culture supernatants of wild-type bacteria, they could be detected only by immunoblotting (Fig. 2B and data not shown).
Other phenotypes of the flhDC mutant.
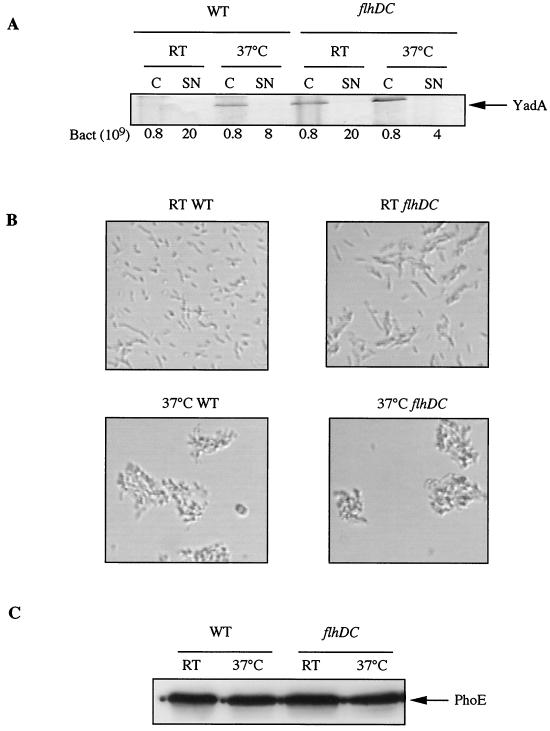
At 37°C, Y. enterocolitica bacteria tend to agglutinate as a result of the presence of YadA at the bacterial surface (35). The polymeric YadA adhesin, which is encoded by a gene that is also dependent on the transcriptional activator VirF (36), was detected by Coomassie brilliant blue staining and SDS-PAGE in flhDC bacteria at RT and 37°C, while it was detected in wild-type bacteria only at 37°C (Fig. 4A). In good agreement with the appearence of YadA at RT, Y. enterocolitica flhDC mutant bacteria tended to agglutinate at RT (Fig. 4B, upper right panel).
FIG. 4.
Other phenotypes of the flhDC mutant. (A) SDS-PAGE and Coomassie brilliant blue staining of proteins from whole-cell extracts (C) and culture supernatants (SN) of Y. enterocolitica MRS40(pYV40) (wild type [WT]) and SBY40(pYV40) (flhDC) grown for 6 h at RT (RT) or 2 h at RT and 4 h at 37°C (37°C) in BHI-Ox. The number of bacteria loaded or the number of bacteria corresponding to the volume of supernatant loaded is given below the panel. (B) Microscopic views (×40) showing total cultures of Y. enterocolitica MRS40(pYV40) (WT) (left panels) or SBY40(pYV40) (flhDC) (right panels) fixed and stained with crystal violet after 6 h at RT (upper panels) or 2 h at RT and 4 h at 37°C (lower panels) in BHI-Ox. (C) Western blot analysis with anti-PhoE polyclonal antibodies of proteins from whole-cell extracts of Y. enterocolitica MRS40(pYV40) (WT) or SBY40(pYV40) (flhDC) grown for 6 h at RT (RT) or 2 h at RT and 4 h at 37°C (37°C) in BHI-Ox. A total of 8 × 108 bacteria were loaded in each lane.
Although FlhD and FlhC are not known to be global regulators (see Discussion), we wondered whether their effect in Yersinia was centered only on the Yop virulon or whether it was more global. A comparison of the cellular protein patterns of wild-type and flhDC Yersinia bacteria grown at RT and 37°C revealed no obvious difference (data not shown). Furthermore, the levels of production of the PhoE porin, tested by Western blotting as a control, were the same at the two temperatures and in the two strains (Fig. 4C). Thus, the effect of the flhDC mutation on the Yop virulon is not global.
VirF is regulated by FlhD and FlhC.
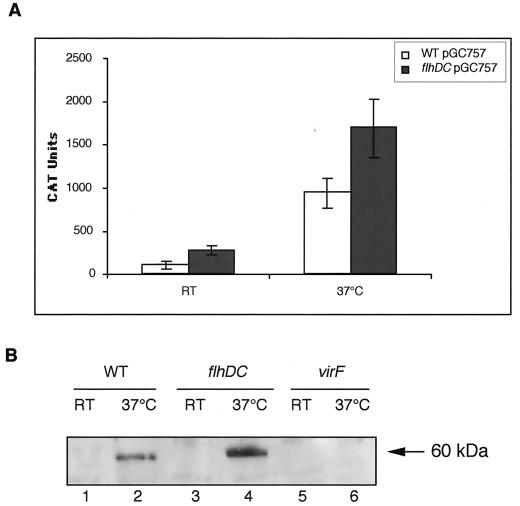
One hypothesis was that FlhD, FlhC, or FlhDC may affect directly or indirectly the expression of the transcriptional activator VirF. In order to study the expression of the virF gene, we measured the chloramphenicol acetyltransferase activity governed by a plasmid-borne virF-cat operon fusion in wild-type and flhDC bacteria. virF-cat was expressed to a higher level in flhDC mutant bacteria than in wild-type bacteria both at RT (2.5-fold) and at 37°C (1.8-fold) (Fig. 5A). These values were significantly different, and the difference could even have been underestimated given the high frequency of loss of the phenotype. This effect was also apparent at the protein level (Fig. 5B). Anti-VirF antibodies detected a band of about 60 kDa that was present in wild-type bacteria grown at 37°C but not in virF mutant bacteria. This band, which could correspond to either a dimer of VirF (the monomer is 30 kDa) or VirF associated with another protein, clearly showed increased production in flhDC bacteria grown at 37°C. This band could even be observed from time to time in mutant bacteria grown at RT. We conclude from these two types of experiments that the virF gene is a target of FlhD, FlhC, or FlhDC.
FIG. 5.
Effect of the flhDC mutation on VirF. (A) Chloramphenicol acetyltransferase (CAT) activity was measured in extracts of Y. enterocolitica MRS40 (wild type [WT]) and SBY40 (flhDC) cured of the pYV plasmid and carrying pGC757, which encodes a virF-cat operon fusion. Bacteria were grown for 6 h at RT (RT) or 2 h at RT and 4 h at 37°C (37°C) in BHI-Ox. CAT activity was expressed in arbitrary units per unit of optical density of the bacterial suspension at 600 nm. The values represent the averages obtained from four independent experiments, including the standard deviations between the measurements. (B) Western blot analysis with anti-VirF polyclonal antibodies of proteins from whole-cell extracts of Y. enterocolitica MRS40(pYV40) (WT) (lanes 1 and 2), SBY40(pYV40) (flhDC) (lanes 3 and 4), and W22703(pGC1153) (virF) (lanes 5 and 6) grown for 6 h at RT (lanes 1, 3, and 5) or 2 h at RT and 4 h at 37°C (lanes 2, 4, and 6) in BHI-Ox. A total of 8 × 108 bacteria were loaded in each lane.
FlhD and FlhC affect transcription of the yop genes.
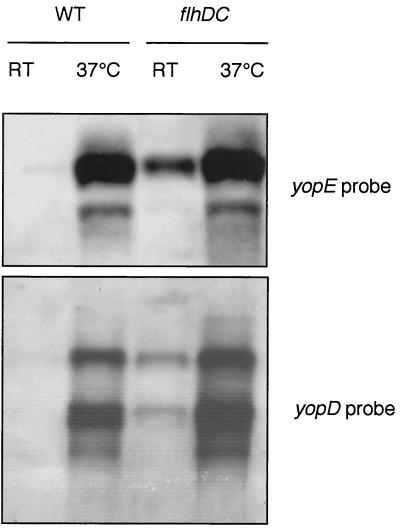
Because of the effect observed on VirF, we studied the transcription of yopE and yopD. Northern blotting was performed on cultures grown for 4 h at RT and on cultures grown for 2 h at RT and 2 h at 37°C (Fig. 6). yopE and yopD mRNAs were clearly detected in flhDC mutant bacteria grown at RT but not in wild-type bacteria grown at RT. For cultures incubated at 37°C, the amounts of yopE and yopD mRNAs were also increased in the flhDC mutant bacteria, but the effect was not so marked. We conclude from these results that FlhD and FlhC act negatively on transcription of the Yop virulon elements.
FIG. 6.
Effect of the flhDC mutation on the transcription of yopE and yopD. Shown is Northern blot analysis of yopE and yopD in Y. enterocolitica MRS40(pYV40) (wild type [WT]) or SBY40(pYV40) (flhDC) grown for 4 h at RT (RT) or 2 h at RT and 2 h at 37°C (37°C). The yopE transcript was detected with a PCR product amplified with oligonucleotides MIPA 538 (5′-GCCCCCATGGAAATATCATCATTTATTTCTACAT-3′) and MIPA 539 (5′-CCGGAATTCGCCCCTTGTTTTTATCC-3′), and the yopD transcript was detected with MIPA 829 (5′-CGGGGATCCATGACAATAAATATCAAGACAGAC-3′) and MIPA 830 (5′-CGCGTCGACTCAGACAACACCAAAAGC-3′).
FlhD and FlhC control the synthesis of the Ysc injectisome.
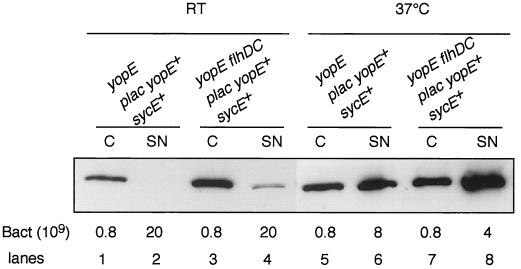
We have seen that FlhD and FlhC control the synthesis of some Ysc proteins, namely, YscJ, YscN, and YscP (Fig. 3 and data not shown). To assess the role of FlhD and FlhC in the entire secretion apparatus, we took advantage of pSI55, a medium-copy-number plasmid in which sycE and yopE are tandemly positioned and transcribed exclusively from a lac promoter (I. Stainier and G. R. Cornelius, unpublished data). This arrangement uncouples the effects of the flhDC mutation on the substrates and on the secretion machinery. Plasmid pSI55 was introduced into a yopE mutant and into a yopE flhDC double mutant, and intra- and extracellular pools of YopE were analyzed by immunoblotting after growth at RT or at 37°C. Surprisingly, at RT, there was more intrabacterial YopE in flhDC mutant bacteria than in wild-type bacteria (about 5.4-fold more) (Fig. 7). Some YopE was secreted by flhDC mutant bacteria but not by wild-type bacteria. At 37°C, the YopE steady-state level was increased 1.8-fold in flhDC mutant bacteria compared to wild-type bacteria. These results show that the mutation in flhDC leads to up-regulation of the synthesis of Ysc components and confirm that the Ysc apparatus is functional at RT in flhDC mutant bacteria. They also suggest that the flhDC mutation can influence the stability of YopE.
FIG. 7.
FlhD and FlhC control the synthesis of the Ysc injectisome. Shown is Western blot analysis of YopE in whole-cell extracts (C) (lanes 1, 3, 5, and 7) and supernatants (SN) (lanes 2, 4, 6, and 8) of Y. enterocolitica MRS40(pAB4052) (yopE) (lanes 1, 2, 5, and 6) and SBY40(pAB4052) (yopE flhDC) (lanes 3, 4, 7, and 8) carrying pSI55 (p lac yopE sycE) and grown for 6 h at RT (lanes 1 to 4) or 2 h at RT and 4 h at 37°C (lanes 5 to 8) in BHI-Ox. The number of bacteria loaded or the number of bacteria corresponding to the volume of supernatant loaded is given below the panel.
DISCUSSION
We showed here that the Yop virulon is deregulated in an flhDC mutant to the extent that Yops were efficiently secreted at a low temperature. Yop secretion was enhanced at 37°C, and Y. enterocolitica bacteria became able to decorate themselves with YadA at a low temperature. The introduction of a plasmid carrying flhDC in wild-type or flhDC backgrounds resulted in a decrease in Yop secretion at 37°C and in an increase in flagellin synthesis, strengthening the antagonism between those two processes. Hence, there is a cross-control between type III secretion and the assembly of the flagellum in Y. enterocolitica. The structures of the two systems are thus not only evolutionarily related but also functionally related. This observation of mutually exclusive synthesis suggests either that building the two structures simultaneously leads to interference or that their assembly requires a common element. This exclusion could also be relevant for the infection process. Indeed, Young et al. (42) demonstrated that motility allows Y. enterocolitica to migrate to host cells and to initiate contact but is not essential for invasion. At a later stage, the Yop virulon enables Yersinia to overcome the defense mechanisms of the host and to survive in the lymphoid tissues (6). This situation of an antagonistic expression of flagella and type III apparatuses appears to be quite different from what has been observed in Salmonella. Indeed, in S. enterica serovar Typhimurium, fliZ, the gene immediately downstream of fliA, positively regulates invasion gene expression (24).
The flhDC mutant is the first Yersinia mutant ever described that is able to secrete Yops at RT. However, the mutant was very unstable, and it had to be reconstructed several times during the course of this study. There is no obvious explanation for this instability, since the mutant bacteria secreted Yops at RT only when Ca2+ was depleted from the medium, and they were always propagated in high Ca2+ concentrations. Moreover, Yop secretion at a low temperature was not accompanied by growth restriction. Nevertheless, the phenotype of Yop secretion at RT in the presence of low Ca2+ concentrations is rapidly lost in the presence of compensatory mutations. To understand better this instability, it would be helpful to map where the mutations occur, but this task would not likely be an easy one.
A clear target of the negative regulation by FlhD and/or FlhC or a downstream regulator (see below for more details) is the transcriptional activator VirF, whose expression and steady-state levels were enhanced in the flhDC mutant. The effect on VirF could not explain completely the phenotype of the flhDC mutant because (i) some type III genes have VirF-independent expression (21, 39) and (ii) overproduction of VirF in a wild-type strain at a low temperature does not lead to Yop secretion and thus does not mimic the flhDC mutation phenotype (21). Hence, the negative regulation must also operate directly on the expression of ysc and yop genes. We observed that at 37°C the effect of the flhDC mutation on yop transcription was not as strong as expected with regard to the increase in Yop secretion. Nevertheless, transcription could be detected at a low temperature in the mutant.
The effect of FlhD and/or FlhC on the Yop virulon could be indirect and mediated via the product of a downstream gene in the flagellar regulation cascade. We can eliminate all the class III components that require σ28 for expression because Iriarte et al. (15) showed that this sigma factor does not influence ysc and yop gene expression. We also confirmed that the phenotype of the fliA mutant is clearly different from that of the flhDC mutant (data not shown). However, we cannot exclude the possibility that FlhD and/or FlhC act via a class II component. However, this possibility would not be easy to investigate. Indeed, Furness et al. (11) showed for Proteus mirabilis a negative-feedback effect of a class II flagellum export defect on flhDC expression, suggesting that the phenotype of a class II mutant could indirectly correspond to an flhDC mutant phenotype. Moreover, Liu and Matsumura (22) demonstrated that some class II genes can be transcribed via σ28 in E. coli. Finally, the sequences of most of the class II genes in Y. enterocolitica are still unknown. However, if the expression of a fliZ homolog is under the control of FlhDC in Y. enterocolitica, FliZ could be responsible for this regulation, as it is in Salmonella (24), but in the other direction.
The master operon was previously shown to be implicated in the regulation of nonflagellar genes: FlhD affects cell division in E. coli (30) and regulates phospholipase expression and secretion in Serratia liquefaciens (13) as well as in Y. enterocolitica (43), and the flhDC operon is also required for the expression of at least two nonflagellar products involved in lipolysis and hemolysis in Xenorhabdus nematophilus (12) and is necessary to initiate host cell invasion by Y. enterocolitica (42). FlhD and FlhC now appear to be involved in the regulation of the Yop virulon in Y. enterocolitica. Thus, not only are FlhD and FlhC flagellar regulators but also their effect is not so broad as to warrant characterizing them as global regulators.
The expression of the E. coli flhDC operon is activated by H-NS (2), which itself responds to a number of physiological and environmental signals. In Shigella and in enteroinvasive E. coli, the same H-NS protein controls the temperature-dependent expression of virulence genes by repressing the in vivo transcription of virF at below 32°C (9). Since histone-like YmoA has been shown to be a negative regulator of the Y. enterocolitica virF gene at a low temperature (7), one may wonder whether it has a stimulatory role in motility. We could not detect any link between YmoA and motility in Y. enterocolitica (data not shown), but this result could be explained as described below.
How can the new data presented here be integrated into the regulation network of Y. enterocolitica? At temperatures below 37°C, the conformation of the pYV plasmid is maintained with a specific architecture involving bends that are thought to be stabilized by the histone-like protein YmoA (32). This configuration keeps the ysc and yop genes in a repressed state. One could hypothesize that an unknown factor (X), responsible for this configuration, could be FlhD and/or FlhC (direct effect) or the product of an FlhDC-regulated gene (indirect effect). One reason to postulate that the X factor acts upstream of YmoA is that ymoA mutants express yop genes at a low temperature but do not secrete Yops at a low temperature (7), but the flhDC mutant does the latter. Exposure to 37°C has an effect on DNA supercoiling and bending which would dislodge the X factor and YmoA, promoting transcription and resulting in subsequent yop induction. In the flhDC mutant, the X factor would be missing, and there would be no topological constraint on the pYV plasmid: transcription could take place at RT. This model links FlhDC to supercoiling and suggests that thermoregulation of motility might also involve changes in DNA topology. In support of this hypothesis is the fact that high osmolarity, another factor known to influence DNA topology, inhibits the motility of Y. enterocolitica (44). The Y. enterocolitica Yst enterotoxin offers another example of the link between temperature, osmolarity, and presumably supercoiling: at low osmolarity, yst is expressed only at a low temperature, but at high osmolarity, yst is expressed at 37°C (27).
After this report was submitted, another study analyzing secretion by the different type III secretion systems in Y. enterocolitica 8081 appeared (41). In the latter report, wild-type bacteria were shown to secrete some Yops (YopO, YopH, YopP, and YopE) at 28°C, and this secretion was not increased in an flhDC mutant background. As expected, wild-type bacteria secreted all the Yops at 37°C in the presence of low Ca2+ concentrations, but this secretion was not enhanced in an flhDC background. These results clearly differ from ours, and it is important to emphasize that they were obtained with a strain of biotype 1B (serotype O:8), while our results were obtained with a strain of biotype 2 (serotype O:9). Biotype 2 (serotype 3) and biotype 4 (serotype 9) strains are known to differ in many respects from biotype 1B (serotype 8) strains, including the lack of the Ysa system in the former (10). Understanding the discrepancy between the two reports would probably require a strict comparison of the two strains under the same experimental conditions.
Acknowledgments
We are very grateful to Virginia Miller for supplying pGY10, to Isabelle Stainier and Pierre Wattiau for sharing the unpublished pSI55 and pPW107 plasmids, to Jan Tommassen for supplying the anti-PhoE antibodies, and to Georges Wauters for supplying the antiflagellin antibodies. We also thank Marie Monteforte for help during the preparation of the manuscript.
S.B. was the recipient of a Van Eessel ICP fellowship, and M.-N.M. was funded by the Belgian Fonds pour la Formation à la Recherche dans l'Industrie et dans l'Agriculture (FRIA). This work was supported by the Belgian Fonds National de la Recherche Scientifique Médicale (Convention 3.4595.97); the Direction Générale de la Recherche Scientifique Communauté Française de Belgique (Action de Recherche Concertée 94/99-172); the Interuniversity Poles of Attraction Program—Belgian State, Prime Minister's Office, Federal Office for Scientific, Technical and Cultural Affairs (PAI 4/03); and the EU TMR network (FMRX-CT98-0164).
Sophie Bleves and Marie-Noëlle Marenne contributed equally to this work.
REFERENCES
- 1.Bartlett, D. H., B. B. Frantz, and P. Matsumura. 1988. Flagellar transcriptional activators FlbB and FlaI: gene sequences and 5′ consensus sequences of operons under FlbB and FlaI control. J. Bacteriol. 170:1575-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertin, P., E. Terao, E. H. Lee, P. Lejeune, C. Colson, A. Danchin, and E. Collatz. 1994. The H-NS protein is involved in the biogenesis of flagella in Escherichia coli. J. Bacteriol. 176:5537-5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bleves, S., and G. R. Cornelis. 2000. How to survive in the host: the Yersinia lesson. Microbes Infect. 2:1451-1460. [DOI] [PubMed] [Google Scholar]
- 4.Blocker, A., P. Gounon, E. Larquet, K. Niebuhr, V. Cabiaux, C. Parsot, and P. Sansonetti. 1999. The tripartite type III secreton of Shigella flexneri inserts IpaB and IpaC into host membranes. J. Cell Biol. 147:683-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornelis, G., C. Sluiters, C. L. de Rouvroit, and T. Michiels. 1989. Homology between VirF, the transcriptional activator of the Yersinia virulence regulon, and AraC, the Escherichia coli arabinose operon regulator. J. Bacteriol. 171:254-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornelis, G. R., A. Boland, A. P. Boyd, C. Geuijen, M. Iriarte, C. Neyt, M. P. Sory, and I. Stainier. 1998. The virulence plasmid of Yersinia, an antihost genome. Microbiol. Mol. Biol. Rev. 62:1315-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cornelis, G. R., C. Sluiters, I. Delor, D. Geib, K. Kaniga, C. Lambert de Rouvroit, M. P. Sory, J. C. Vanooteghem, and T. Michiels. 1991. ymoA, a Yersinia enterocolitica chromosomal gene modulating the expression of virulence functions. Mol. Microbiol. 5:1023-1034. [DOI] [PubMed] [Google Scholar]
- 8.Cornelis, G. R., and H. Wolf-Watz. 1997. The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells. Mol. Microbiol. 23:861-867. [DOI] [PubMed] [Google Scholar]
- 9.Falconi, M., B. Colonna, G. Prosseda, G. Micheli, and C. O. Gualerzi. 1998. Thermoregulation of Shigella and Escherichia coli EIEC pathogenicity. A temperature-dependent structural transition of DNA modulates accessibility of virF promoter to transcriptional repressor H-NS. EMBO J. 17:7033-7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foultier, B., P. Troisfontaines, S. Müller, F. R. Opperdoes, and G. R. Cornelis. J. Mol. Evol., in press. [DOI] [PubMed]
- 11.Furness, R. B., G. M. Fraser, N. A. Hay, and C. Hughes. 1997. Negative feedback from a Proteus class II flagellum export defect to the flhDC master operon controlling cell division and flagellum assembly. J. Bacteriol. 179:5585-5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Givaudan, A., and A. Lanois. 2000. flhDC, the flagellar master operon of Xenorhabdus nematophilus: requirement for motility, lipolysis, extracellular hemolysis, and full virulence in insects. J. Bacteriol. 182:107-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Givskov, M., L. Eberl, G. Christiansen, M. J. Benedik, and S. Molin. 1995. Induction of phospholipase- and flagellar synthesis in Serratia liquefaciens is controlled by expression of the flagellar master operon flhD. Mol. Microbiol. 15:445-454. [DOI] [PubMed] [Google Scholar]
- 14.Haller, J. C., S. Carlson, K. L. Pederson, and D. E. Pierson. 2000. A chromosomally encoded type III secretion pathway in Yersinia enterocolitica is important in virulence. Mol. Microbiol. 36:1436-1446. [DOI] [PubMed] [Google Scholar]
- 15.Iriarte, M., I. Stainier, A. V. Mikulskis, and G. R. Cornelis. 1995. The fliA gene encoding sigma 28 in Yersinia enterocolitica. J. Bacteriol. 177:2299-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaniga, K., I. Delor, and G. R. Cornelis. 1991. A wide-host-range suicide vector for improving reverse genetics in gram-negative bacteria: inactivation of the blaA gene of Yersinia enterocolitica. Gene 109:137-141. [DOI] [PubMed] [Google Scholar]
- 17.Kapatral, V., and S. A. Minnich. 1995. Co-ordinate, temperature-sensitive regulation of the three Yersinia enterocolitica flagellin genes. Mol. Microbiol. 17:49-56. [DOI] [PubMed] [Google Scholar]
- 18.Kapatral, V., J. W. Olson, J. C. Pepe, V. L. Miller, and S. A. Minnich. 1996. Temperature-dependent regulation of Yersinia enterocolitica Class III flagellar genes. Mol. Microbiol. 19:1061-1071. [DOI] [PubMed] [Google Scholar]
- 19.Kubori, T., Y. Matsushima, D. Nakamura, J. Uralil, M. Lara-Tejero, A. Sukhan, J. E. Galan, and S. I. Aizawa. 1998. Supramolecular structure of the Salmonella typhimurium type III protein secretion system. Science 280:602-605. [DOI] [PubMed] [Google Scholar]
- 20.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 21.Lambert de Rouvroit, C., C. Sluiters, and G. R. Cornelis. 1992. Role of the transcriptional activator, VirF, and temperature in the expression of the pYV plasmid genes of Yersinia enterocolitica. Mol. Microbiol. 6:395-409. [PubMed] [Google Scholar]
- 22.Liu, X., and P. Matsumura. 1996. Differential regulation of multiple overlapping promoters in flagellar class II operons in Escherichia coli. Mol. Microbiol. 21:613-620. [DOI] [PubMed] [Google Scholar]
- 23.Liu, X., and P. Matsumura. 1994. The FlhD/FlhC complex, a transcriptional activator of the Escherichia coli flagellar class II operons. J. Bacteriol. 176:7345-7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lucas, R. L., C. P. Lostroh, C. C. DiRusso, M. P. Spector, B. L. Wanner, and C. A. Lee. 2000. Multiple factors independently regulate hilA and invasion gene expression in Salmonella enterica serovar Typhimurium. J. Bacteriol. 182:1872-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macnab, R. M. 1996. Flagella and motility, p. 123-145. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 26.Michiels, T., and G. R. Cornelis. 1991. Secretion of hybrid proteins by the Yersinia Yop export system. J. Bacteriol. 173:1677-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mikulskis, A. V., I. Delor, V. H. Thi, and G. R. Cornelis. 1994. Regulation of the Yersinia enterocolitica enterotoxin Yst gene. Influence of growth phase, temperature, osmolarity, pH and bacterial host factors. Mol. Microbiol. 14:905-915. [DOI] [PubMed] [Google Scholar]
- 28.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mills, S. D., A. Boland, M. P. Sory, P. van der Smissen, C. Kerbourch, B. B. Finlay, and G. R. Cornelis. 1997. Yersinia enterocolitica induces apoptosis in macrophages by a process requiring functional type III secretion and translocation mechanisms and involving YopP, presumably acting as an effector protein. Proc. Natl. Acad. Sci. USA 94:12638-12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pruss, B. M., and P. Matsumura. 1996. A regulator of the flagellar regulon of Escherichia coli, flhD, also affects cell division. J. Bacteriol. 178:668-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rohde, J. R., J. M. Fox, and S. A. Minnich. 1994. Thermoregulation in Yersinia enterocolitica is coincident with changes in DNA supercoiling. Mol. Microbiol. 12:187-199. [DOI] [PubMed] [Google Scholar]
- 32.Rohde, J. R., X. S. Luan, H. Rohde, J. M. Fox, and S. A. Minnich. 1999. The Yersinia enterocolitica pYV virulence plasmid contains multiple intrinsic DNA bends which melt at 37°C. J. Bacteriol. 181:4198-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarker, M. R., C. Neyt, I. Stainier, and G. R. Cornelis. 1998. The Yersinia Yop virulon: LcrV is required for extrusion of the translocators YopB and YopD. J. Bacteriol. 180:1207-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaw, W. V. 1975. Chloramphenicol acetyltransferase from chloramphenicol-resistant bacteria. Methods Enzymol. 43:737-755. [DOI] [PubMed] [Google Scholar]
- 35.Skurnik, M., I. Bolin, H. Heikkinen, S. Piha, and H. Wolf-Watz. 1984. Virulence plasmid-associated autoagglutination in Yersinia spp. J. Bacteriol. 158:1033-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skurnik, M., and P. Toivanen. 1992. LcrV is the temperature-regulated activator of the yadA gene of Yersinia enterocolitica and Yersinia pseudotuberculosis. J. Bacteriol. 174:2047-2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith, D. B., and K. S. Johnson. 1988. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene 67:31-40. [DOI] [PubMed] [Google Scholar]
- 38.Tamano, K., S. Aizawa, E. Katayama, T. Nonaka, S. Imajoh-Ohmi, A. Kuwae, S. Nagai, and C. Sasakawa. 2000. Supramolecular structure of the Shigella type III secretion machinery: the needle part is changeable in length and essential for delivery of effectors. EMBO J. 19:3876-3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wattiau, P., B. Bernier, P. Deslee, T. Michiels, and G. R. Cornelis. 1994. Individual chaperones required for Yop secretion by Yersinia. Proc. Natl. Acad. Sci. USA 91:10493-10497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woestyn, S., A. Allaoui, P. Wattiau, and G. R. Cornelis. 1994. YscN, the putative energizer of the Yersinia Yop secretion machinery. J. Bacteriol. 176:1561-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Young, B. M., and G. M. Young. 2002. YplA is exported by the Ysc, Ysa, and flagellar type III secretion systems of Yersinia enterocolitica. J. Bacteriol. 185:1324-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Young, G. M., J. L. Badger, and V. L. Miller. 2000. Motility is required to initiate host cell invasion by Yersinia enterocolitica. Infect. Immun. 68:4323-4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Young, G. M., D. H. Schmiel, and V. L. Miller. 1999. A new pathway for the secretion of virulence factors by bacteria: the flagellar export apparatus functions as a protein-secretion system. Proc. Natl. Acad. Sci. USA 96:6456-6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Young, G. M., M. J. Smith, S. A. Minnich, and V. L. Miller. 1999. The Yersinia enterocolitica motility master regulatory operon, flhDC, is required for flagellin production, swimming motility, and swarming motility. J. Bacteriol. 181:2823-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]