Abstract
This article distinguishes between normal and pathological aging, provides an interdisciplinary context, and then considers a sample case of cognitive aging. Developmental influences on cognition include the physiological infrastructure, genetic predispositions, and environmental influences. Different types of longitudinal studies are distinguished, and contrasting findings of cross-sectional and longitudinal are examined in the sample case of the Seattle Longitudinal Study. Also considered is the longitudinal context for intervention studies and the role of longitudinal family studies in assessing rate of aging and generational differences in rates of aging. Finally, attention is given to the role of longitudinal studies in the early detection of risk for dementia in advanced age.
In this article I distinguish between normal and pathological aging, provide some historical context, discuss shifts in relevant methodological paradigms, provide an interdisciplinary context, and then consider a sample case of cognitive aging in greater detail.
A distinction is made between age related declines that should be attributed either to neuropathology or to disuse and obsolescence. But aging can also be considered as development in domains such as experience and wisdom, and we must distinguish between successful and unsuccessful aging.
The study of adult development originated in the early mental testing movement. Cross-sectional findings of substantial age-related declines from early to late adulthood soon motivated the development of longitudinal studies. Other methodological paradigm shifts of importance to longitudinal inquiry have involved advances in the measurement of age, confirmatory factor analysis, and treatment of age as the dependent variable.
I then present a model that considers the roles of age-related changes in the physiological infrastructure, genetic predispositions, and environmental influences. Different types of longitudinal studies are distinguished, and contrasting findings of cross-sectional and longitudinal studies are examined in the sample case of the Seattle Longitudinal Study.
Selected findings from this study are presented to discuss the complex interaction of longitudinal age changes and cohort differences. The latter introduces the role of longitudinal family studies in assessing rate of aging and generational differences in rates of aging. Also, I briefly consider the longitudinal context for intervention studies designed to slow the rate of aging.
And, finally, I discuss the role of longitudinal studies in the early detection of risk for dementia in advanced age, which involves linkages between studies of normal and pathological aging as well as attention to genetic markers of dementia.
NORMAL AND PATHOLOGICAL AGING
There is considerable controversy in the developmental sciences regarding whether it is possible to conceptualize aging free of age-related diseases (cf. Solomon, 1999). There are vast individual differences in the age of onset, the particular disease(s) an individual experiences, and the severity of chronic disease in old age. Nevertheless, one of the concomitants of reaching advanced ages is the presence of more or less disabling chronic disease. It may be more useful, therefore, to distinguish between those aspect of aging that involve decline and/or functional losses and those processes that can be characterized as developmental gains occurring with advancing age.
Aging as Decline
One of the most popular models guiding discussions of human aging and behavior involves the assumption that there is an accelerating linear decline for most behaviors past an asymptote occurring somewhere in adolescence or young adulthood. This model is informed primarily by cross-sectional data. It does not account for the fact that different behaviors have been found to have divergent developmental courses through adulthood or that longitudinal data often have nonlinear growth curves in adulthood.
The substantial age differences observed at a given point in time that often, but not always, favor young adults over the elderly can perhaps be best accounted for by three co-occurring phenomena: neuropathology, disuse, and obsolescence.
Neuropathology
It has been observed that there are age changes in volume of various brain structures occurring in normal individuals with advancing age (Gunning-Dixon & Raz, 2003; Raz, Rodrigue, & Acker, 2003). Excess volumetric decline in brain tissues may indeed be a precursor of clinically diagnosable neuropathology. These brain changes are associated as well with decline in cognitive functioning. Recent work in our laboratory has shown that excess cognitive change can be identified as early as 14 years prior to neuropsychological diagnosis of cognitive impairment (Schaie, Caskie, & Willis, 2004). Hence, with increasing age, there will also be an increase in the proportion of individuals who behavior is impaired due to the precursors of neuropathology.
Disuse
Following the principles of selective optimization and compensation (cf. Baltes, 1997; Baltes & Baltes, 1990), we would expect that aging individuals would progressively increase their efforts on maintaining those behaviors that are most adaptive and meaningful for their particular life situation. Because some skills and behaviors are neglected in this process, we would expect increasing proportions of elderly to show decline that may well be reversible (cf. Willis, 2001).
Obsolescence
In a rapidly changing society, skills that are acquired at earlier points in life may quickly become obsolete, particularly in the presence of rapid technological change (Charness & Schaie, 2003; Pew & Van Hemel, 2004; Willis & Dubin, 1990). The resultant obsolescence, in turn, leads to the avoidance of behaviors and social or work roles that now depend on more effective behaviors or higher skill levels than those attained asymptotically in young adulthood. Enhancing this obsolescence is a tendency of employers to give preference to younger employees for on-the-job training or other updating experiences (cf. Czaja, 2001; Schaie & Schooler, 1998). Hence, in the age-comparative literature older groups will have successively larger proportions of members who have not declined but appear obsolete when compared with their younger peers.
Aging as Development
Not all comparisons of the young and the old result in findings that are unfavorable for the elderly. There are a number of time-dependent processes that accompany living for a long time that result in favorable outcomes. Of particular interest here is the fact that it requires time to acquire the experience necessary for complex processes and societal roles, something that is often discussed and researched under the topic of wisdom (Staudinger, Maciel, Smith, & Baltes, 1998; Sternberg & Lubart, 2001). But there are other behavioral developments that include the adoption or neglect of favorable life styles as well as the development of flexible response styles and appropriate management of stress and emotional conflicts. All of the latter developments have been studied with the intention of differentiating successful from unsuccessful aging (cf. Rowe & Kahn, 1987). Identification of the major influences involved in this differentiation, often first discernible in midlife, requires long-term longitudinal data (cf. Arbuckle, Maag, Pushkar, & Chaikelson, 1998; Schaie, 1984; Schaie & Hofer, 2001; Willis & Reid, 1999).
SOME HISTORICAL CONTEXT
Longitudinal studies of behavioral change over the life span did not occur in a vacuum. They originated from the early mental testing movement and the age-comparative studies that presented difficulties in interpreting the course of individual development.
The Mental Testing Movement: Binet and Terman
Early research in developmental psychology began with the investigation of intellectual competence. Objectives of this line of research included devising orderly procedures for the removal of mentally retarded children from the public schools (Binet & Simon, 1905) and studying the distribution of individual differences in the interest of demonstrating their Darwinian characteristics (Galton, 1869). The methods to describe developmental status of individuals were brought to the United States by Lewis Terman with his introduction of the widely used Stanford–Binet Intelligence Test (1916) and the concepts of mental age and the IQ.
Empirical studies of intelligence that followed investigated how complex mental functions were acquired early in life (Brooks & Weintraub, 1976). Soon interest arose in following intellectual development beyond childhood, beginning with the theoretical expositions of G. Stanley Hall (1922), H. L. Hollingsworth (1927), and Sidney Pressey (Pressey, Janney, & Kuhlen, 1939). These authors raised questions concerned with identifying the age of attaining peak performance levels, maintenance or transformation of intellectual structures, and decremental changes thought to occur from young adulthood to old age.
Early Age-Comparative Studies of Adults
Developmental studies began with comparing the characteristics of groups of individuals of different ages at one point in time (cross-sectional studies). In his original standardization of the Binet tests for American use, Terman (1916) assumed that intellectual development reached a peak at age 16 and would then remain level throughout adulthood. However, large-scale studies of American soldiers using the Army Alpha Intelligence Test during World War I (Yerkes, 1921) suggested that the peak level of intellectual functioning for young adults, on average, might already be reached by age 13.
Other empirical studies questioned these inferences. One of the most influential cross-sectional studies, by Jones and Conrad (1933), collected data on most inhabitants of a New England community between the ages of 10 and 60 years. Age differences found in this study were quite substantial on some of the subtests of the Army Alpha Intelligence Test but not on others. In a similar fashion, Wechsler’s (1939) initial standardization studies for the Wechsler–Bellevue Adult Intelligence scales found that growth of intelligence does not cease in adolescence. In fact, peak ages were found to differ for various aspects of intellectual functioning, and decrements at older ages were clearly not uniform across different subtests. The progressive shift in peak age of performance was perhaps the first harbinger of the now familiar problem of cohort differences that compromise the utility of cross-sectional inquiry to provide a model of developmental phenomena.
SHIFT IN METHODOLOGICAL PARADIGMS
Next, I consider the shifts in methodological paradigms that have characterized the study of human development. Those covered here are the shift from cross-sectional to longitudinal study designs, advances in the measurement of change, the shift from exploratory to confirmatory (hypothesis-testing) methods of factor analysis, and the reconceptualization of calendar age from its status as an independent (causal) variable to that of a dependent variable or temporal scale along which an outcome occurs.
Cross-Sectional to Longitudinal Design
In the late 1920s some developmental psychologists began to realize that age-comparative studies did not permit studying the association of antecedent and consequent variables over time in order to discover the mechanisms that accounted for individual development. Panels of children and their parents were, therefore, selected with the intent of systematic follow-up across time (e.g., Berkeley Growth Study; Eichorn, Clausen, Haan, Honzik, & Mussen, 1981).
By the 1950s reports appeared on longitudinal studies of individuals who had initially been studied as children or young adults and who had now reached middle adulthood (e.g., Bayley & Oden, 1955; Jarvik, Kallman, & Falek, 1962; Owens, 1953, 1959). Findings from these studies provided strong evidence that most abilities were maintained at least into midlife and that some abilities remained stable into early old age. These findings clearly contrasted with the results of the earlier cross-sectional literature, including my own findings (Schaie, 1958, 1959).
Further analyses of the conflicting evidence from cross-sectional and longitudinal data suggested that cross-sectional data representing age differences can model change over time only in the case of a perfectly stable environment and the absence of cohort differences (Ryder, 1965; Schaie, 1965). Although both cross-sectional and longitudinal approaches face a variety of different validity threats (cf. Schaie, 1977, 2004), it is clear that longitudinal data are preferred under most circumstances (cf. Baltes & Nesselroade, 1979).
The principal advantage of longitudinal studies is their ability to furnish information on intraindividual change in contrast to cross-sectional studies that provide information only on interindividual differences. Five distinct rationales for longitudinal studies have been suggested by Baltes and Nesselroade (1979; also see Schaie, 1983). They include the direct identification of intraindividual change, the identification of interindividual variability in intraindividual change, the interrelationships among intraindividual changes, the analysis of determinants of intraindividual change, and the analysis of intraindividual variability in the determinants of intraindividual change.
Advances in the Measurement of Change
The second paradigm shift with major impact on the study of adult development occurred in the field of measurement of change, which is so essential for defining developmental transitions. It began with some heated debates of the problem that measurement imperfections (i.e., deviations of observed scores from true scores) were likely to cumulate in gain (or loss) scores comparing multiple measurements of the same individuals (Lord, 1956; Thorndike, 1924). During the 1960s many developmentalists despaired about whether it was even possible to assess change adequately (cf. Cronbach & Furby, 1970; Harris, 1963). However, difference scores have always been important in studies of adult development that require tests of hypotheses about directional change. Fortunately, the problem of the unreliability of difference scores is usually confined to two-point studies, hence, leading to increasing preference for multiple occasion studies (cf. Nesselroade, Stigler, & Baltes, 1980; Rogosa, Brandt, & Zimowsky, 1982; Willett, 1989).
When longitudinal studies are conducted over long periods of time with multiple measurements, it is possible to apply powerful methods of linear growth curve modeling that allow separating patterns of individual change over time from the group averages that had previously represented the primary focus of inquiry. These methods also allow incorporating covariates and predictors of different forms of development. Multivariate growth curve methods were first introduced by Tucker (1958). But more powerful computational resources were required to implement currently popular approaches of multilevel modeling (cf. Bryk & Raudenbush, 1987; Rogosa & Willett, 1985; Rudinger & Rietz, 2001; Willett & Sayer, 1994). These methods are particularly useful because differences in genetic predisposition and environmental exposure may result in longitudinal aging patterns that differ markedly for subsets of the population, including groups of individuals with either favorable or unfavorable life experiences.
Exploratory to Confirmatory Factor Analysis
The method of exploratory factor analysis was developed as a way of organizing domains of variables such that a minimum number of latent constructs could explain and represent a large universe of observable behaviors in a psychologically meaningful fashion (e.g., Thurstone, 1947). A perennial problem of exploratory factor analysis has been the fact that there are an infinite number of alternate sets of equations that can account equally well for the regression of the latent factors on a particular set of observed variables.
The introduction of formal methods of confirmatory factor analysis and structural equation modeling facilitated the use of this method for testing hypotheses regarding developmental change. Thus, it is now possible to examine formally the proposition that there are differences in psychological constructs across samples of different age or within the same sample over time (cf. Reinert, 1970; Sörbom & Jöreskog, 1978).
It is now possible to assess systematically the invariance (stability) of the regression of the latent constructs on the observed variables. In studies of adult development such invariance is a singular prerequisite for the comparison of individuals and groups over long periods of time or the comparison of groups of different individuals who differ in salient characteristics. Confirmatory factor analysis can also be used to test hypotheses about the differentiation and dedifferentiation of psychological domains across the adult life span (Baltes & Lindenberger, 1997; Maitland, Intrieri, Schaie, & Willis, 2000; Reinert, 1970; Schaie, 2000; Schaie, Maitland, Willis, & Intrieri, 1998).
Age: From Independent to Dependent Variable
Another paradigmatic shift has occurred suggesting that developmentalists should treat chronological (calendar) age as a dependent rather than as an independent variable. First introduced conceptually by Wohlwill (1973), behavioral scientists soon began to realize that the study of age or duration time as a dependent variable could be operationalized via methods of survival or event-time analysis (Allison, 1984; Schaie, 1989; Singer & Willett, 1991). This approach has also been important in cognitive and health psychology, because the prediction of morbidity and mortality by means of earlier behavioral characteristics requires not only the definition of end points but also the timing (i.e., age) at which such end points are most likely to occur (e.g., Bosworth, Schaie, Willis, & Siegler, 1999). We have begun to recognize that the passage of time and getting older cannot have any causal property for any observed behavior change. Instead, we seek to identify and understand those causal variables and covariates that provide the mechanisms for change. Given these considerations, chronological age then becomes a scale on which we can arrange the timing of developmental events conditional to the specific characteristics of the individual under observation.
TYPES OF LONGITUDINAL STUDIES
Three different formats may be found in the literature. The first involves the adult follow-up of samples collected for studies of child development, the second explicitly covers the entire period of adulthood, and the third involves studies that begin by obtaining samples of the old or very old (cf. Schaie & Hofer, 2001).
From Early Childhood to Adulthood
The earliest longitudinal studies depict panels whose members had been recruited at birth or in early childhood and who were successively followed into young adulthood and midlife (e.g., the Berkeley Growth and Guidance Studies; Eichorn et al., 1981). These studies have been historically important, and they also can inform regarding those early influences that may differentially affect behavioral outcomes at later life stages. However, because no such studies were designed to continue into middle or old age, they often lack information on critical variables, such as the early precursors of cardiovascular disease that may be unimportant in childhood but may have important predictive value for later life.
From Young Adulthood to Midlife and Beyond
Other studies explicitly wish to cover the entire range of adulthood. They often begin as a cross-sectional study covering a wide age range and then continue as a multiple-birth cohorts (cf. Schaie, 1996, 2005). Such studies focus on variables and processes occurring in young adulthood and midlife that suspected to contribute to the divergent developmental paths seen in old age. As new relationships among developmental processes are discovered and as new assessment tools become available, long-range data from such studies also allow postdicting developmental processes for variables that could not be assessed at earlier measurement occasions. Hence, these studies as they persist over long periods of time may give us an understanding of gains occurring from young adulthood into middle age as well as provide early predictions of risk of late-life decline and pathologies.
Studies Originating in Later Life
The largest number of longitudinal studies of adults have actually originated when participants were at advanced ages. These studies were initiated to understand the vast individual differences in aging patterns and in neuropathology in the elderly. Such studies have typically recruited their samples in the late 60s or early 70s, but there are also studies that have provided initial looks at octogenarians and even centenarians. Noteworthy examples of such studies are the Duke Longitudinal Study (Palmore, Busse, Maddox, Nowlin, & Siegler, 1985), the Swedish Betula Study (Nilsson et al., 1997), the German Berlin Aging Study (BASE; Baltes & Mayer, 1999), and the Victoria Longitudinal Study (Hultsch, Hertzog, Dixon, & Small, 1998).
THE SAMPLE CASE OF COGNITIVE DEVELOPMENT: A CONCEPTUAL MODEL
I now illustrate some of the advantages of longitudinal studies of development by examining some design features and data from the Seattle Longitudinal Study (SLS; Schaie, 1996, 2005). To begin, I describe a conceptual model of adult cognitive development that is currently guiding this study and that also provides an illustration of the interdisciplinary context that informs longitudinal studies of behavioral development (see Figure 1).
FIGURE 1.
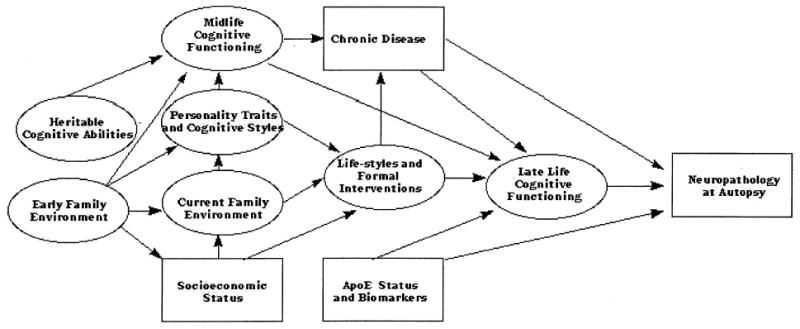
Conceptual model of factors associated with adult cognitive development. From Developmental Influences on Adult Intelligence: The Seattle Longitudinal Study (p. 8), by K. W. Schaie, 2005, New York: Oxford University Press. Copyright by Oxford University Press. Adapted with permission.
Cognitive development from early adulthood to old age cannot be understood adequately without examining such development within the context of changing environmental influences and changes in individuals’ physiological infrastructure. The schematic displayed in Figure 1 indicates how these influences might operate over the adult life course. The schematic contains two end points: The first concerns the level of late life cognitive functioning, and a second end point represents the status of the cortex at life’s end. The latter end point describes the physiological infrastructure required for the maintenance of cognitive functioning, most reliably determinable only at post mortem. Although future work might include imaging techniques at earlier life stages, the conceptual model uses the typical conventions of path models. Rectangles identify those individual indicators that are observed directly, whereas ovals indicate the latent constructs inferred from sets of observed variables (not specified in this heuristic model).
The initial bases for the development of adult intelligence must be attributed to both heritable (genetic) influences and early environmental influences experienced within the home of the biological parents. The older behavior genetic literature suggests that much of the early environmental variance is not shared (e.g., Plomin & Daniels, 1987), but there is recent retrospective evidence that some early shared environmental variance influences later cognitive performance (Schaie & Zuo, 2001). Both genetic and early environmental factors are thought to influence midlife cognitive functioning. Both shared and not-shared environmental influences in early life exert influences on midlife social status (Nguyen, 2000). But environmental influences do not cease in adulthood. Indeed, attributes of the current family environment account for a substantial share of variance in midlife cognitive performance (Schaie & Zuo, 2001). Genetic factors are also likely to be implicated in the rate of cognitive decline in adulthood. Thus far the best-studied gene in this context is the Apo-E gene, one of whose alleles (e4) is a risk factor for Alzheimer’s disease. Apo-E status is, therefore, added as a factor; the expression of the gene is probably not at issue prior to midlife.
The causal influences that determine the level of intellectual functioning in late life as well as cortical status at autopsy are also specified in the model. The direct influences implicated, in addition to genes whose expression is turned on in late life, most likely originate in midlife. They include level of midlife cognitive functioning, midlife life styles, and the incidence and severity of chronic disease. But there are indirect influences attributable to the effects of midlife cognitive function and life styles on chronic disease, as well as shared family influences on midlife cognition and of social status on midlife life styles.
Some of the paths shown in Figure 1 represent concurrent observations that would allow alternative reciprocal causal directions. However, most of the paths specified by the model represent antecedent–consequent relationships that require longitudinal data for their estimation and understanding. Over the course of the SLS most of the influences specified in this model either have already been systematically investigated or are currently under investigation.
THE SLS
The SLS began in 1956 with a cross-sectional inquiry of the relation between flexibility–rigidity and cognitive abilities over the age range from 20 to 70 years (Schaie, 1958). It was converted to a multiple-cohort longitudinal study in 1963, and it has been continued in 7-year intervals, adding new random samples from the membership of a large health maintenance organization (HMO). In the longitudinal part of the study members are followed until death or dropout, and our oldest study participant is now 100 years old. Figure 2 shows the sampling plan for data collected from 1956 through 1998.
FIGURE 2.
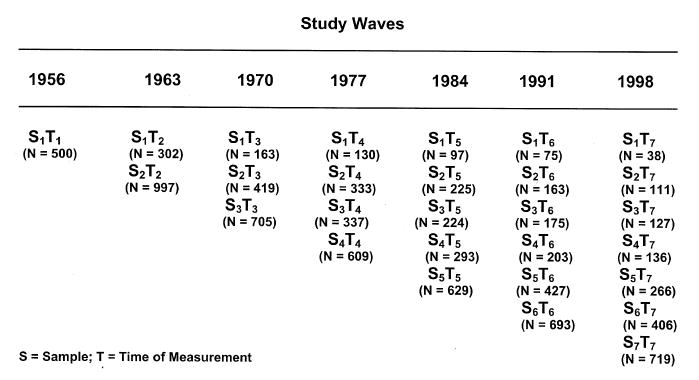
Design of the SLS.
Study Objectives
Although the initial objective of the SLS was to contribute to the problem of differentiating age changes and age differences, efforts soon turned to the identification of antecedents of individual differences in aging. Influences studied in the SLS include the following: occurrence of chronic disease, life styles and leisure activities, cognitive styles, personality traits, environment in family of origin and current family, and health behaviors. Given the multicohort nature of our sample we then studied generational differences in performance level and rate of decline at comparable ages. With the collaboration of behavior geneticists we then began to pursue the role of family similarity in cognition, including the impact of family environment on cognition (e.g., Schaie et al., 1993; Schaie & Zuo, 2001). The family study is now in its third wave, generating longitudinal data that can be compared to our original longitudinal panel.
Having identified some of the antecedents of favorable and unfavorable change in adult cognition, we began to design cognitive intervention studies to slow or reverse cognitive decline in old age (Willis & Schaie, 1986), with studies of long-range effects up to 14 years (Schaie, 2005). And, finally, we have investigated precursors of cognitive impairment with neuropsychological studies and identification of ApoE gene status. These studies are beginning to contribute to the early identification of persons at risk for dementia.
Abilities Studied
The following six multiply marked cognitive ability factors were studied:
Verbal Comprehension is the ability to understand ideas expressed in words. It indicates the range of a person’s passive vocabulary used in activities wherein information is obtained by reading or listening.
Spatial Orientation is the ability to visualize and mentally manipulate spatial configurations in two or three dimensions, to maintain orientation with respect to spatial objects, and to perceive relationships among objects in space. This ability is important in tasks that require deducing one’s physical orientation from a map or visualizing what objects would look like when assembled from pieces.
Inductive Reasoning is the ability to recognize and understand novel concepts or relationships; it involves the solution of logical problems—to foresee and plan. Thurstone and Thurstone (1949) proposed that persons with good reasoning ability could solve problems, foresee consequences, analyze situations on the basis of past experience, and make and carry out plans according to recognized facts.
Numeric Ability is the ability to understand numerical relationships, to work with figures, and to solve simple quantitative problems rapidly and accurately.
Perceptual Speed is the ability to find figures, make comparisons, and carry out other simple tasks involving visual perception with speed and accuracy.
Verbal Memory is the ability that involves memorization and recall of meaningful language units primarily measured by memorizing lists.
LONGITUDINAL AGE CHANGES
We now contrast the longitudinal age changes obtained by studying intraindividual change over time with age differences measured for different age groups at one point in time for the six abilities averaged across the same cohorts used for the intraindividual change estimates.
Note first that in the cross-sectional data, reflecting interindividual age differences, four of the six factors show consistent negative age differences (see Figure 3). They are statistically significant for Inductive Reasoning, Spatial Orientation, and Perceptual Speed at age 46 and for Verbal Memory at age 39. The magnitude of age difference from the youngest to the oldest group amounts to approximately 2 SDs on average. The remaining two factors, Numeric Facility and Verbal Ability, have a very different profile. They both show positive age differences until midlife, with less than .5 SDs negative differences thereafter, such that persons in advanced old age, on average, are at a higher level than the youngest age group.
FIGURE 3.
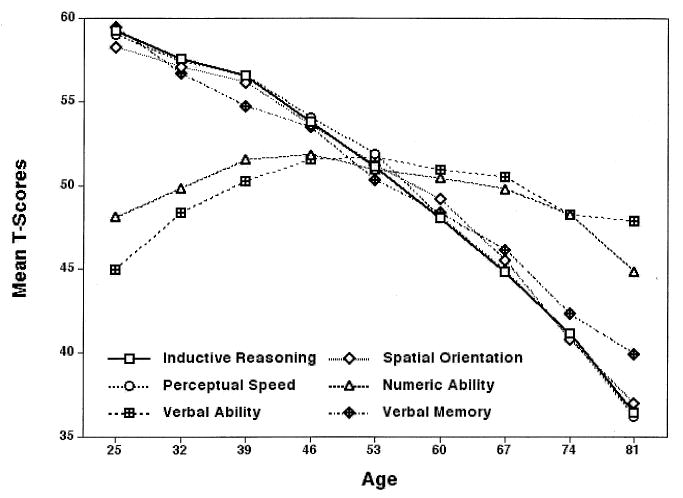
Age-difference patterns of six cognitive abilities (from Schaie, 2004).
Now we examine the age changes as obtained from intraindividual data (shown in Figure 4). These gradients are centered on the actually observed mean for the average age group in our sample (age 53). A rather different picture emerges for the longitudinal data. For these ability factor scores, earliest reliably observed decline over 7 years occurs for Perceptual Speed and Numeric Facility by age 60; for Inductive Reasoning, Spatial Orientation, and Verbal Memory by age 67; and for Verbal Ability only by age 81.
FIGURE 4.

Estimated age changes from 7-year intraindividual data for six cognitive abilities. From Developmental Influences on Adult Intelligence: The Seattle Longitudinal Study (p. 127), by K. W. Schaie, 2005, New York: Oxford University Press. Copyright 2005 Oxford University Press. Reprinted with permission.
Contrasting the Cross-Sectional and Longitudinal Findings
The cross-sectional data obviously misinform if they were to be taken as estimates of decline in cognitive abilities. In the longitudinal data both the peak of performance and the age at which significant average decline is first noted occurs much later. But there are also other surprises. Perhaps the most dramatic difference between the cross-sectional and longitudinal findings is for Numeric Facility. Here we see only minor differences among age groups in the cross-sectional data in contrast to the longitudinal data that show that there is early and steep decline for this ability.
How can we explain these differences? The major culprits here are cohort or generational differences. That is, if there is a positive cohort difference, then in cross-sectional studies older people will look as if they had declined from an earlier peak performance even though they have remained stable. By contrast, if there are negative cohort differences, older persons will appear to have remained stable in cross-sectional studies even though they have experienced marked decline from their peak. To address this issue we turn next to the study of generational differences.
STUDYING GENERATIONAL DIFFERENCES
Multicohort studies are required to determine whether there are differences in performance level obtained by successive cohorts at identical ages. Alternatively, we can compare biologically related individuals if we have access to data collected at similar ages for parents and offspring.
Cohort Studies
Cross-national studies of successive cohorts of school children have found substantial increments in intellectual performance over the past century (Flynn, 1987). Similar findings have occurred in the SLS. However, our ability-specific data suggest that cohort differences may occur in a multidirectional manner. Our latest findings for the six cognitive abilities described earlier are presented in Figure 5.
FIGURE 5.
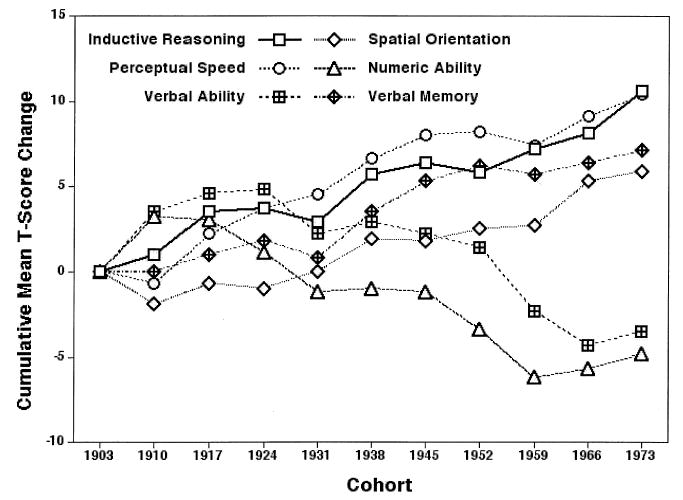
Cohort difference gradients for the six cognitive abilities (from Schaie, 2004).
Over the 70-year range covered by our cohort data, substantial positive and linear cohort differences were observed for the Inductive Reasoning and Perceptual Speed abilities (SD ≈ 1). A similar, albeit less steep, positive difference pattern occurred for Spatial Orientation (SD = .6) and Verbal Memory (SD = .7). A modest negative gradient (SD ≈ .05) was found for Numeric Facility, and there was a modest concave gradient with recent declines for Verbal Ability. These are exactly the abilities that appear flat in cross-sectional studies (as previously mentioned).
Family Studies
We also examined generational differences in cognitive abilities using the parent–offspring data from our family study (Schaie, Plomin, Willis, Gruber-Baldini, & Dutta, 1992). To permit matching parents and offspring at similar ages we reported data on five single-marker ability tests: Verbal Meaning, Space, Reasoning, Number, and Word Fluency (Schaie, 1985). Figure 6 shows differences between the parent and offspring generations; a bar above the zero line indicates the advantage of the offspring over their parents, and a bar below the zero line indicates an advantage for the parents.
FIGURE 6.
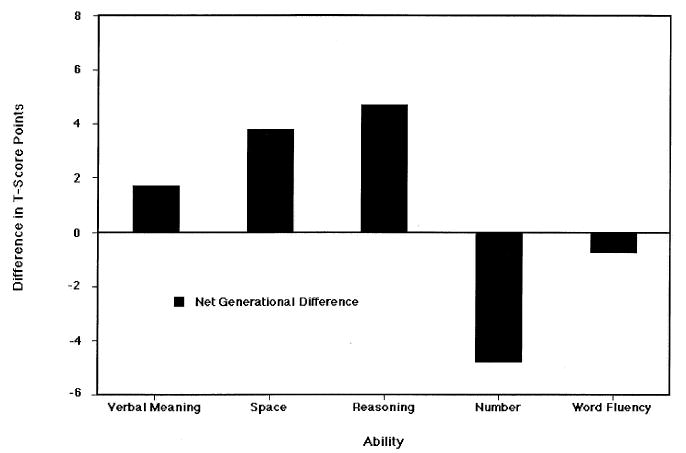
Generational differences between parents and offspring in T-score points. From Developmental Influences on Adult Intelligence: The Seattle Longitudinal Study (p. 335), by K. W. Schaie, 2005, New York: Oxford University Press. Adapted with permission.
Consistent with the general population data, the offspring generation performed significantly better than their parents at comparable ages on Verbal Meaning, Space, and Reasoning, whereas the parent generation did better than their offspring on Number and Word Fluency.
Rate of Cognitive Change
Longitudinal studies also allow the investigation of cohort and generational differences in rate of cognitive change. A policy-relevant aspect of our generational studies is the possibility of asking whether the rate of aging has changed across successive generations. Recent policy debates in the United States and Europe have asked the question whether Social Security and other pension systems will remain viable when the baby boomers reach retirement. A straightforward solution for this problem would be to raise the age at which pensions are now paid (cf. Crystal & Shea, 2002). However, such an approach requires the assumption that the next generation is able to work to later ages because the rate of aging has slowed. That is, will the next generation decline physically and mentally more slowly than their parents?
The most direct test of whether the rate of cognitive aging has slowed is provided by the comparison of persons with their biologically related adult offspring at approximately the same ages. We now have a relevant data set in the SLS. To obtain approximate age equivalence, we compared the 1970 and 1977 test scores of the parents with the 1990 and 1997 test scores of the adult offspring. Figure 7 shows the 7-year change from the mid-60s to the early 70s. As previously shown, the offspring generation has typically higher levels of performance than their parents. More noteworthy, however, is the fact that although the parent generation declined significantly over the 7-year period on five of six abilities, the offspring generation showed decline on only two of six abilities.
FIGURE 7.
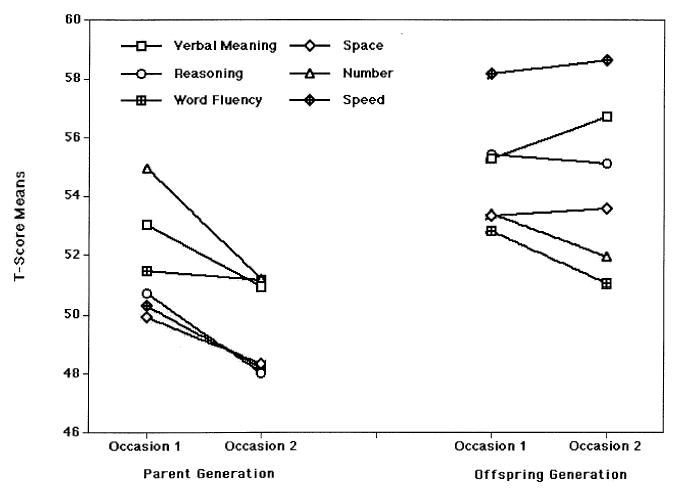
Generational differences between parents and offspring by cohort. From Developmental Influences on Adult Intelligence: The Seattle Longitudinal Study (p. 336), by K. W. Schaie, 2005, New York: Oxford University Press. Reprinted with permission.
COGNITIVE INTERVENTIONS TO SLOW AGING
Longitudinal studies lend themselves to the design and implementation of cognitive interventions because they can identify mechanisms involved in age-related change. They also allow the determination of whether any obtained training gain is a function of remediating decline from a previous higher level of functioning or whether they simply “teach old dogs new tricks.” The latter, of course, is not to be eschewed lightly because training gains in cognitive skills may well be important in facilitating obsolescence-reducing activities by the elderly. Important issues in intervention research are the determination of the specific targets of the intervention, the question of whether there is transfer of training to the broader dimensions being targeted, and whether there is maintenance of the effects of the intervention over time (see Willis, 2001).
Results of Cognitive Training
In the SLS, we conducted a cognitive training study as part of our 1984, 1991, and 1998 cycles. Participants age 64 and older were screened on whether they had remained stable or had experienced significant decline on the Inductive Reasoning and Spatial Orientation factors over the previous 14 years. Individuals who had declined only on one ability were assigned to training on that ability, whereas those who had remained stable or who had declined on both abilities received random assignment to the two training conditions. One-on-one strategy training was conducted in five 1-hr sessions. Figure 8 shows training gains for the three replications. Training resulted in approximately a .5 SD gain (Schaie, 2005; Willis & Schaie, 1986). Training was most effective for those who declined. Of that group 40% were returned to the level of performance they had experienced 14 years earlier.
FIGURE 8.
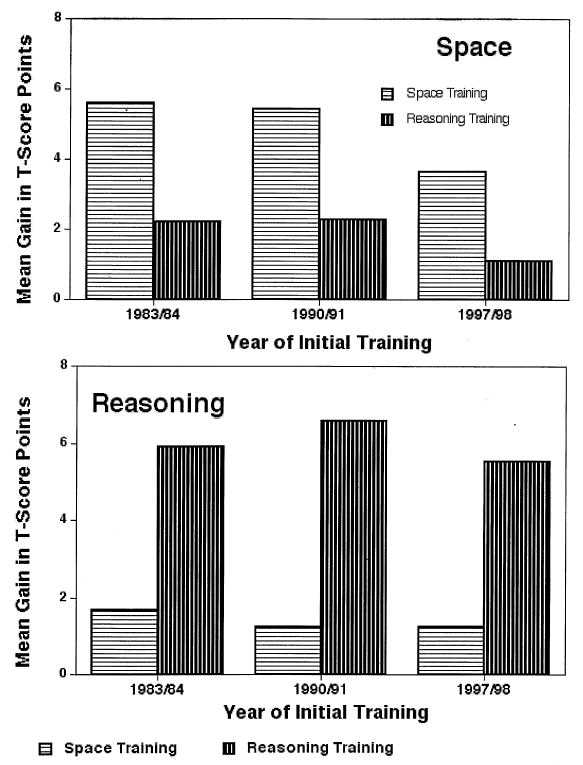
Replicated training gain for intervention and control groups (from Schaie, 2004).
Maintenance of Training Gain
The intervention literature is full of reports of immediate gains following brief interventions (pretest–posttest gains). What is of greater concern, however, is whether training gains have any long-lasting effects. This is an area in which only longitudinal studies can provide relevant findings. In the SLS we have now followed trained individuals over 7- and 14-year intervals (Schaie, 2005). Figure 9 shows changes in performance on Inductive Reasoning from initial pretest prior to training to 14 years after training, as compared with a control group that received training on Spatial Orientation. As expected there is further average decline from the first training (M age = 72) to the second pretest (at age 79) and from the second to the third posttest (at age 86). But the trained group even at the oldest age still raised above the initial level, whereas the control group dropped precipitously.
FIGURE 9.
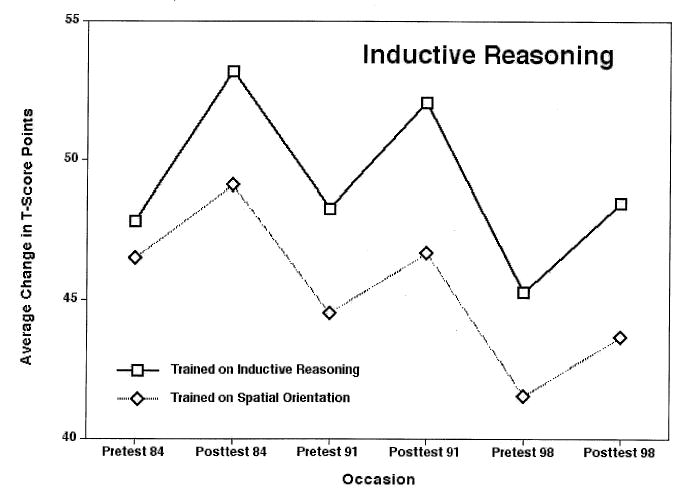
Piecewise growth curve models from baseline prior to training to 14-year posttest. From Developmental Influences on Adult Intelligence: The Seattle Longitudinal Study (p. 185), by K. W. Schaie, 2005, New York: Oxford University Press. Reprinted with permission.
EARLY DETECTION OF RISK OF DEMENTIA
Longitudinal data from the SLS have also been useful in identifying variables that might assist in the early detection of risk for eventual dementia in old age. We pursued this question by expanding our study of community dwelling persons in which we added a diagnostic neuropsychological battery for participants over age 60 and by collecting data on the ApoE gene to determine its role in cognitive impairment.
Neuropsychology Studies in Community Dwelling Persons
New questions arise in scientific inquiry that could best be addressed if data were available over time periods. One way of dealing with this problem is to study concurrent data that include the new variables of interest and the variables for which data are available across time. We can thus postdict; that is, we can estimate what a longitudinal study participant’s score would have been on the data not available for prior occasions. Dwyer (1937) took this approach to project standard neuropsychological measures into the factor space of our ability battery by means of extension factor analysis. By knowing the relationship between the measures that were not available at an earlier time and those that have been given throughout our study, we can then estimate the performance on the unmeasured variables at earlier data points. This procedure allowed us to determine how far back we could have predicted eventual diagnoses of dementia by estimating earlier performance on neuropsychological tests. Our data suggest that it would have been possible, for many persons, to predict the eventual occurrence of dementia at least 14 years prior to the time the actual neuropsychological diagnosis of cognitive impairment occurred (Schaie et al., 2004).
Genetic Studies: The ApoE Gene
The role of the ApoE gene as a risk factor for Alzheimer’s disease has been known for some time (Saunders, 2000). In particular, it has been found that presence of the e4 allele is a precursor of dementia. If that is the case, then we should expect excess decrement in cognitive abilities during a portion of the preclinical period. Our longitudinal data allow the requisite analyses, and we find that indeed there is a significantly greater rate of decline over 7 years in individuals with the e4 allele, particularly so for individuals with the e4/2 and e4/4 pairings (Revell & Schaie, 2004; Schaie, 2005).
CONCLUSIONS AND FUTURE DIRECTIONS
In this article I summarized some of the advantages of longitudinal studies of adult development. I provided some of the background and the rationale regarding why cross-sectional methods will not, in most cases, lead to a useful model if questions are to be investigated of intraindividual change, individual aging trajectories, or antecedent–consequent relationships. I also provided examples from the SLS to show how cohort and generation effects cause misleading interpretations of cross-sectional findings. Examples of the use of longitudinal data for estimating rates of age change and generational differences in such rates were given. Also considered were the utility of longitudinal data in intervention studies and in the prediction cognitive impairment.
Many of the earlier longitudinal studies of adult development were typically based on single markers of the constructs of interest. Current research practice, however, demands that latent constructs should be multiply marked. Hence, we are likely to see increasing emphasis on the study of structural invariance across age and time (cf. Meredith, 1993; Schaie, 2000). Also, in the area of measurement we can expect an increase in multivariate applications of item response theory.
With respect to study design I expect an increase in short-term longitudinal studies in those phases of adulthood where rapid change is likely to occur, whether it be during menopause or in advanced old age. More closely spaced data points would allow the powerful applications of latent growth curve modeling (cf. Rudinger & Rietz, 2001) but would allow the powerful designs derived from the age–cohort–period model (cf. Schaie & Willis, 2002, chap. 5).
As mentioned earlier, longitudinal data are required to study the antecedent–consequent relationships that will eventually lead us from the description of old age to a determination of the mechanism that underlie age changes. Once these mechanisms are identified we will be on the way to developing the interventions necessary to ensure successful aging for most or all of the population.
Finally, I mention the important role of public archives for longitudinal studies of adult development. Even the most persistent investigators cannot follow their study participants from birth to death, and they cannot gather sufficiently large and representative data to permit effective studies of subpopulations. It is, therefore, important that resources be available to document and deposit data from long-term studies so that they can be combined into larger data sets or further explored by other investigators. Examples of public web sites providing such data access include the National Archives of Computerized Data on Aging Research (http://www.icpsr.umich.edu/NACDA), the public data archive of the SLS (http://geron.psu.edu/sls), and the Murray Center at Radcliffe College (http://www.radcliffe.edu/murray).
References
- Allison, P. D. (1984). Event history analysis: Regression for longitudinal event data Beverly Hills, CA: Sage.
- Arbuckle Y, Maag U, Pushkar D, Chaikelson JS. Individual differences in trajectory of intellectual development over 45 years of adulthood. Psychology and Aging. 1998;13:663–675. doi: 10.1037//0882-7974.13.4.663. [DOI] [PubMed] [Google Scholar]
- Baltes PB. On the incomplete architecture of human ontogenesis: Selection, optimization and compensation as foundations of developmental theory. American Psychologist. 1997;52:366–381. doi: 10.1037//0003-066x.52.4.366. [DOI] [PubMed] [Google Scholar]
- Baltes, P. B., & Baltes, M. M. (1990). Psychological perspectives on successful aging: The model of selective optimization with compensation. In P. B. Baltes & M. M. Baltes (Eds.), Successful aging: Perspectives from the behavioral sciences (pp. 1–34). Cambridge, England: Cambridge University Press.
- Baltes PB, Lindenberger U. Emergence of a powerful connection between sensory and cognitive functions across the adult life span: A new window to the study of cognitive aging. Psychology and Aging. 1997;12:12–21. doi: 10.1037//0882-7974.12.1.12. [DOI] [PubMed] [Google Scholar]
- Baltes, P. B., & Mayer, K. U. (1999). The Berlin Aging Study: Aging from 70 to 100 New York: Cambridge University Press.
- Baltes, P. B., & Nesselroade, J. R. (1979). The developmental analysis of individual differences on multiple measures. In J. R. Nesselroade & H. W. Reese (Eds.), Life-span developmental psychology: Methodological issues (pp. 1–40). New York: Academic.
- Bayley N, Oden MH. The maintenance of intellectual ability in gifted adults. Journal of Gerontology. 1955;10:91–107. doi: 10.1093/geronj/10.1.91. [DOI] [PubMed] [Google Scholar]
- Binet A, Simon T. Méthodes nouvelles pour le diagnostic du niveau intellectuel des anormaux [New methods for the diagnosis of intellectual level of abnormal persons] L’Année Psychologique. 1905;11:191–199. [Google Scholar]
- Bosworth HB, Schaie KW, Willis SL, Siegler IC. Age and distance to death in the Seattle Longitudinal Study. Research on Aging. 1999;21:723–738. [Google Scholar]
- Brooks, J., & Weintraub, M. (1976). A history of infant intelligence testing. In M. Lewis (Ed.), Origins of intelligence (pp. 19–58). New York: Plenum.
- Bryk AS, Raudenbush SW. Application of hierarchical linear models to assessing change. Psychological Bulletin. 1987;101:147–158. [Google Scholar]
- Charness, N., & Schaie, K. W. (Eds.). (2003). Impact of technology on the aging individual New York: Springer.
- Cronbach LJ, Furby L. How should we measure change—or should we? Psychological Bulletin. 1970;74:68–80. [Google Scholar]
- Crystal, S., & Shea, D. (Eds.). (2002). Economic outcomes in later life: Public policy, health and cumulative advantage. Annual Review of Gerontology and Geriatrics, Vol. 22
- Czaja, S. J. (2001). Technological change and the older worker. In J. E. Birren & K. W. Schaie (Eds.), Handbook of the psychology of aging (5th ed., pp. 547–568). San Diego, CA: Academic.
- Dwyer PS. The determination of the factor loadings of a given test from the known factor loadings of other tests. Psychometrika. 1937;2:173–178. [Google Scholar]
- Eichorn, D. H., Clausen, J. A., Haan, N., Honzik, M. P., & Mussen, P. H. (1981). Present and past in middle life New York: Academic.
- Flynn JR. Massive gains in 14 nations: What IQ tests really measure. Psychological Bulletin. 1987;101:171–191. [Google Scholar]
- Galton, F. (1869). Hereditary genius London, England: Macmillan.
- Gunning-Dixon FM, Raz N. Neuroanatomical correlates of selected executive functions in middle-aged and older adults: A prospective MRI study. Neuropsychologia. 2003;41:1929–1941. doi: 10.1016/s0028-3932(03)00129-5. [DOI] [PubMed] [Google Scholar]
- Hall, G. S. (1922). Senescence, the last half of life New York: Appleton. [DOI] [PMC free article] [PubMed]
- Harris, C. W. (Ed.). (1963). Problems in measuring change Madison: University of Wisconsin Press.
- Hollingsworth, H. L. (1927). Mental growth and decline: A survey of developmental psychology New York: Appleton.
- Hultsch, D. F., Hertzog, C., Dixon, R. A., & Small, B. J. (1998). Memory changes in the aged New York: Cambridge University Press.
- Jarvik LF, Kallman FJ, Falek A. Intellectual changes in aged twins. Journal of Gerontology. 1962;17:289–294. doi: 10.1093/geronj/17.3.289. [DOI] [PubMed] [Google Scholar]
- Jones HE, Conrad HS. The growth and decline of intelligence: A study of a homogeneous group between the ages of ten and sixty. Genetic Psychology Monographs. 1933;13:223–298. [Google Scholar]
- Lord FM. The measurement of growth. Educational and Psychological Measurement. 1956;16:421–437. [Google Scholar]
- Maitland SB, Intrieri RC, Schaie KW, Willis SL. Gender differences in cognitive abilities: Invariance of covariance and latent mean structure. Aging, Neuropsychology and Cognition. 2000;7:32–53. [Google Scholar]
- Meredith W. Measurement invariance, factor analysis and factorial invariance. Psychometrika. 1993;58:525–543. [Google Scholar]
- Nesselroade JR, Stigler SM, Baltes PB. Regression towards the mean and the study of change. Psychological Bulletin. 1980;88:622–637. [Google Scholar]
- Nilsson LG, Bäckman L, Erngrund K, Nyberg L, Adolfsson R, Bucht G, Karlsson S, et al. The Betula prospective cohort study: Memory, health, and aging. Aging, Neuropsychology, and Cognition. 1997;4:1–32. [Google Scholar]
- Nguyen, H. T. (2000). Environmental complexity factors: A study of familial similarities and differences Unpublished doctoral dissertation, Pennsylvania State University, University Park.
- Owens WA., Jr Age and mental abilities: A longitudinal study. Genetic Psychology Monographs. 1953;48:3–54. [PubMed] [Google Scholar]
- Owens WA., Jr Is age kinder to the initially more able? Journal of Gerontology. 1959;14:334–337. doi: 10.1093/geronj/14.3.334. [DOI] [PubMed] [Google Scholar]
- Palmore, E., Busse, E. W., Maddox, G. L., Nowlin, J. B., & Siegler, I. C. (1985). Normal aging (Vol. 3). Durham, NC: Duke University Press.
- Pew, R. W., & Van Hemel, S. B. (Eds.). (2004). Technology for adaptive aging Washington, DC: National Academy Press. [PubMed]
- Plomin R, Daniels D. Why are two children in the same family so different from each other? Behavioral and Brain Sciences. 1987;10:1–16. [Google Scholar]
- Pressey, S. L., Janney, J. E., & Kuhlen, R. G. (1939). Life: A psychological survey New York: Hayer.
- Raz N, Rodrigue KM, Acker JD. Hypertension and the brain: Vulnerability of the prefrontal regions and executive function. Behavioral Neuroscience. 2003;117:1169–1180. doi: 10.1037/0735-7044.117.6.1169. [DOI] [PubMed] [Google Scholar]
- Reinert, G. (1970). Comparative factor analytic studies of intelligence through the human life span. In L. R. Goulet & P. B. Baltes (Eds.), Life-span developmental psychology: Research and theory (pp. 468–485). New York: Academic.
- Revell, A. J., & Schaie, K. W. (2004, April). Domain-specific cognitive deficits associated with ApoE genotype by age group in community-dwelling elderly Poster presented at the biennial Cognitive Aging Conference, Atlanta, GA.
- Rogosa D, Brandt D, Zimowsky M. A growth curve approach to the measurement of change. Psychological Bulletin. 1982;92:726–748. [Google Scholar]
- Rogosa D, Willett JB. Understanding correlates of change by modeling individual differences in growth. Psychometrika. 1985;50:203–228. [Google Scholar]
- Rowe JW, Kahn RL. Human aging: Unusual and successful aging. Science. 1987;237:143–149. doi: 10.1126/science.3299702. [DOI] [PubMed] [Google Scholar]
- Rudinger, G., & Rietz, W. (2001). Structural equation modeling in longitudinal research on aging. In J. E. Birren & K. W. Schaie (Eds.), Handbook of the psychology of aging (5th ed., pp. 29–52). San Diego, CA: Academic.
- Ryder NB. The cohort as a concept in the study of social changes. American Sociological Review. 1965;30:843–861. [PubMed] [Google Scholar]
- Sanders AM. Apolipoprotein E and Alzheimer disease: An update on genetic and functional analyses. Journal of Neuropathology and Experimental Neurology. 2000;59:751–758. doi: 10.1093/jnen/59.9.751. [DOI] [PubMed] [Google Scholar]
- Schaie, K. W. (1958). Rigidity–flexibility and intelligence: A cross-sectional study of the adult life-span from 20 to 70. Psychological Monographs, 72(9, Whole No. 462).
- Schaie KW. Cross-sectional methods in the study of psychological aspects of aging. Journal of Gerontology. 1959;14:208–215. doi: 10.1093/geronj/14.2.208. [DOI] [PubMed] [Google Scholar]
- Schaie KW. A general model for the study of developmental problems. Psychological Bulletin. 1965;64:91–107. doi: 10.1037/h0022371. [DOI] [PubMed] [Google Scholar]
- Schaie, K. W. (1977). Quasi-experimental designs in the psychology of aging. In J. E. Birren & K. W. Schaie (Eds.), Handbook of the psychology of aging (pp. 39–58). New York: Van Nostrand Reinhold.
- Schaie, K. W. (1983). What can we learn from the longitudinal study of adult psychological development? In K. W. Schaie (Ed.), Longitudinal studies of adult psychological development (pp. 1–19). New York: Guilford.
- Schaie KW. Midlife influences upon intellectual functioning in old age. International Journal of Behavioral Development. 1984;7:463–478. [Google Scholar]
- Schaie, K. W. (1985). Manual for the Schaie–Thurstone Adult Mental Abilities Test (STAMAT) Palo Alto, CA: Consulting Psychologists Press.
- Schaie KW. The hazards of cognitive aging. Gerontologist. 1989;29:484–493. doi: 10.1093/geront/29.4.484. [DOI] [PubMed] [Google Scholar]
- Schaie, K. W. (1996). Intellectual development in adulthood: The Seattle Longitudinal Study New York: Cambridge University Press.
- Schaie, K. W. (2000). Longitudinal and related methodological issues in the Swedish Twin Registry. In B. Smedby, I. Lundberg, & T. I. A. Sørensen (Eds.), Scientific evaluation of the Swedish Twin Registry (pp. 62–74). Stockholm, Sweden: Swedish Council for Planning and Coordination of Research.
- Schaie, K. W. (2005). Developmental influences on adult intelligence: The Seattle Longitudinal Study New York: Oxford University Press.
- Schaie KW, Caskie GIL, Revell AJ, Willis SL, Kaszniak AW, Teri L. Extending neuropsychological assessment into the Primary Mental Ability factor space. Aging, Neuropsychology and Cognition. 2004;11 doi: 10.1080/13825580590969343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaie, K. W., & Hofer, S. M. (2001). Longitudinal studies in research on aging. In J. E. Birren & K. W. Schaie (Eds.), Handbook of the psychology of aging (5th ed., pp. 55–77). San Diego, CA: Academic.
- Schaie KW, Maitland SB, Willis SL, Intrieri RL. Longitudinal invariance of adult psychometric ability factor structures across seven years. Psychology and Aging. 1998;13:8–20. doi: 10.1037/0882-7974.13.1.8. [DOI] [PubMed] [Google Scholar]
- Schaie, K. W., Plomin, R., Willis, S. L., Gruber-Baldini, A., & Dutta, R. (1992). Natural cohorts: Family similarity in adult cognition. In T. Sonderegger (Ed.), Psychology and aging: Nebraska Symposium on Motivation, 1991 (pp. 205–243). Lincoln, NE: University of Nebraska Press. [PubMed]
- Schaie, K. W., Plomin, R., Willis, S. L., Gruber-Baldini, A. L., Dutta, R., & Bayen, U. (1993). Family similarity in adult intellectual development. In J. J. F. Schroots (Ed.), Aging, health and competence: The next generation of longitudinal research (pp. 183–198). Amsterdam: Elsevier.
- Schaie, K. W., & Schooler, C. E. (Eds.). (1998). Impact of the work place on older persons New York: Springer.
- Schaie, K. W., & Willis, S. L. (2002). Adult development and aging Upper Saddle River, NJ: Prentice-Hall.
- Schaie KW, Willis SL, Caskie GIL. The Seattle Longitudinal Study: Relation between personality and cognition. Aging, Neuropsychology and Cognition. 2004;11:304–324. doi: 10.1080/13825580490511134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaie, K. W., & Zuo, Y. L. (2001). Family environments and adult cognitive functioning. In R. L. Sternberg & E. Grigorenko (Eds.), Context of intellectual development (pp. 337–361). Mahwah, NJ: Lawrence Erlbaum Associates, Inc.
- Singer JD, Willett JB. Modeling the days of our lives: Using survival analysis when designing and analyzing longitudinal studies of duration and the time of events. Psychological Bulletin. 1991;110:268–290. [Google Scholar]
- Solomon, D. H. (1999). The role of aging processes in age-dependent diseases. In V. L. Bengtson & K. W. Schaie (Eds.), Handbook of theories of aging (pp. 133–152). New York: Springer.
- Sörbom, D., & Jöreskog, K. G. (1978). Confirmatory factor analysis with model modification Chicago: International Educational Services.
- Staudinger UM, Maciel AG, Smith J, Baltes PB. What predicts wisdom-related performance? A first look at personality, intelligence, and facilitative experiential contexts. European Journal of Personality. 1998;12:1–17. [Google Scholar]
- Sternberg, R. J., & Lubart, T. I. (2001). Wisdom and creativity. In J. E. Birren & K. W. Schaie (Eds.), Handbook of the psychology of aging (5th ed., pp. 500–522). San Diego, CA: Academic.
- Terman, L. M. (1916). The measurement of intelligence Boston: Houghton.
- Thorndike EL. The influence of chance imperfections of measures upon the relation of initial scores to gains or loss. Journal of Experimental Psychology. 1924;7:225–232. [Google Scholar]
- Thurstone, L. L. (1947). Multiple factor analysis Chicago: University of Chicago Press.
- Thurstone, L. L., & Thurstone, T. G. (1949). Examiner manual for the SRA Primary Mental Abilities Test Chicago: Science Research Associates.
- Tucker LR. Determination of parameters of a functional relation by factor analysis. Psychometrika. 1958;23:19–23. [Google Scholar]
- Wechsler, D. (1939). The measurement of adult intelligence Baltimore: Williams & Wilkins.
- Willett JB. Some results on reliability for the longitudinal measurement of change: Implications for the design of studies of individual growth. Educational and Psychological Measurement. 1989;49:587–601. [Google Scholar]
- Willett JB, Sayer AG. Using covariance structure analysis to detect correlations and predictors of individual change over time. Psychological Bulletin. 1994;116:363–381. [Google Scholar]
- Willis, S. L. (2001). Methodological issues in behavioral intervention research with the elderly. In J. E. Birren & K. W. Schaie (Eds.), Handbook of the psychology of aging (5th ed., pp. 78–108). San Diego, CA: Academic.
- Willis, S. L., & Dubin, S. (Eds.). (1990). Maintaining professional competence San Francisco: Jossey-Bass.
- Willis, S. L., & Reid, J. E. (Eds.). (1999). Life in the middle: Psychological and social development in middle age San Diego, CA: Academic.
- Willis SL, Schaie KW. Training the elderly on the ability factors of spatial orientation and inductive reasoning. Psychology and Aging. 1986;1:239–247. doi: 10.1037//0882-7974.1.3.239. [DOI] [PubMed] [Google Scholar]
- Wohlwill, J. (1973). The study of behavioral development New York: Academic.
- Yerkes RM. Psychological examining in the United States Army. Memoirs of the National Academy of Sciences. 1921;15:1–890. [Google Scholar]


