Abstract
We hypothesized that AMP-activated protein kinase-related kinase 5 (ARK5)/novel kinase family 1 (NUAK1), an AMP-activated protein kinase (AMPK)-related kinase that has been found to be stimulated by protein kinase B (Akt), would be expressed in rat skeletal muscle and activated by electrically elicited contractions, 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR), or insulin. We verified expression of ARK5 in muscle through RT-PCR and Western blot. Cross-reactivity of ARK5 immunoprecipitates with antibodies against phospho-AMPK was increased by ~30% by muscle contractions and ~60% by incubation of muscle with AICAR. AMPK was not detected in the ARK5 immunoprecipitates. Despite the apparent increase in phosphorylation of ARK5 at a site essential to its activation, neither contractions nor AICAR increased ARK5 activity. For muscles from animals injected with saline or insulin, we probed nonimmunoprecipitated samples in sequence for phosphotyrosine (P-Tyr), ARK5, and phosphorylated substrates of Akt (P-AS) and found that the ARK5 band could be precisely superimposed on phosphoprotein bands from the P-Tyr and P-AS blots. In the band corresponding to ARK5, insulin increased P-Tyr content by ~45% and cross-reactivity with the antibody against P-AS by approximately threefold. We also detected ARK5 in phosphotyrosine immunoprecipitates. Our data suggest that increased phosphorylation of ARK5 by muscle contractions or exposure to AICAR is insufficient to activate ARK5 in skeletal muscle, suggesting that some other modification (e.g., phosphorylation on tyrosine or by Akt) may be necessary to its activity in muscle.
Keywords: novel kinase family 1, novel kinase family 2, sucrose nonfermenting protein kinase/AMP-activated protein kinase-related protein kinase, protein kinase B, phosphotyrosine
the amp-activated protein kinase (AMPK) and related kinases regulate metabolism in response to cellular stress (3, 20, 23, 33). In skeletal muscle, regulatory roles for AMP-activated signaling processes have been demonstrated for glucose transport, fatty acid oxidation, insulin sensitivity, and induction of key metabolic enzymes (2, 6, 9, 12, 13, 22, 25, 36, 38). The catalytic subunit of AMPK (AMPKα) is activated allosterically by factors including increases in the concentrations of AMP or AMP analogs and decreases in the phosphocreatine-to-creatine concentration ratio (7, 27, 37). AMPKα is also activated through threonine phosphorylation by an AMPK kinase, which is likely to be serine/threonine kinase 11 (LKB1; see Ref. 21).
In addition to AMPKα isoforms, there are an additional 12 related members of the AMPK catalytic subfamily (21). Of the AMPK family members, only AMPKα isoforms have so far been found to be activated by muscle contractions (29). AMP-activated protein kinase related kinase 5 (ARK5), also known as novel (Nua) kinase family 1 (NUAK1), protein has not previously been detected in rat skeletal muscle (29). However, ARK5 expression in skeletal muscle has been demonstrated by RT-PCR (16). Like AMPKα and all but one of the other members of the AMPK family, ARK5 is activated >10- to 20-fold by phosphorylation of its T-loop threonine by LKB1 (21). Prevention of threonine phosphorylation in the T-loop of ARK5 by mutation of the residue to alanine prevents stimulation of ARK5 activity in the presence of LKB1, whereas mutation of the threonine to glutamate creates a fully active ARK5 mutant (21).
If ARK5 is indeed present in skeletal muscle, it appears to be an attractive candidate for a role in regulation of metabolism. ARK5 is stimulated in vitro by AMP (33) and phosphorylates SAMS peptide, a common synthetic substrate for AMPK family members (21, 33). Uniquely among AMPK family members, ARK5 has been shown to be activated through phosphorylation by protein kinase B (Akt; see Ref. 33). Activation of Akt is central to insulin action (26), and Akt has been shown in some cases (31), although not all instances (5), to be activated by muscle contractions.
We hypothesized that ARK5, which is regulated through T-loop phosphorylation (21) and is a substrate of Akt (33), would be phosphorylated and/or activated during muscle contractions, after incubation of muscle with 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside [AICAR, an AMPK activator (7)], and by exposure of muscle to insulin.
METHODS
Animals and muscle collection.
Animal procedures were approved by the Animal Care Committee of Saint Louis University. Male Wistar rats (~125 g) were obtained from Charles River Laboratories (Wilmington, MA), housed in a temperature-controlled facility with 12:12-h light-dark cycles, and given free access to food and water. Animals were fasted overnight before experiments. Rats were anesthetized by intraperitoneal injections of pentobarbital sodium (50 mg/kg). For each animal (n = 8) in the muscle contraction studies, the extensor digitorum longus (EDL) muscle from one leg was removed and frozen with clamps that were cooled in liquid nitrogen. The sciatic nerve on the contralateral leg was exposed, attached to an electrode, and stimulated at 100 Hz to produce 10 hindlimb muscle contractions of 10 s in duration, one contraction per minute. Immediately after the final contraction, the stimulated EDL was rapidly removed and clamp-frozen.
We evaluated the effects of AICAR (Toronto Research Chemicals) on ARK5 phosphorylation and activity using epitrochlearis muscles. Epitrochlearis muscles were dissected out and allowed to recover for 30 min in oxygenated Krebs-Henseleit buffer containing 8 mM glucose, 0.1% BSA, and 32 mM mannitol, as previously described (9). Muscles were then incubated for 30 min in Krebs-Henseleit buffer containing 8 mM glucose and with 32 mM mannitol or 30 mM mannitol with 2 mM AICAR (9) and finally clamp-frozen. The epitrochlearis incubations were performed at 35°C.
For experiments using insulin, EDL muscles were removed and clamp-frozen 10 min after an intraperitoneal injection of 0.9% saline for control animals (n = 8) or 500 U/kg Iletin II pork insulin (Lilly, Indianapolis, IN) for treated animals (n = 8).
Antibodies.
The ARK5 + sucrose nonfermenting protein kinase/AMP-activated protein kinase-related protein kinase (SNARK) antibodies were raised in rabbits against a peptide with the sequence of KKPRQRESGYYSSPEPS and have been shown to bind recombinant human ARK5 (33). Both human ARK5 and rat ARK5 (accession no. XP_234998) contain amino acid sequences (KKTQQRESGYYSSPERS) corresponding to the immunizing peptide. Additional ARK5 antibodies were raised in chickens (Aves Labs, Tigard, OR) against a sequence of rat ARK5 (YYSSPERSESSE).
Antibodies against phosphorylated Akt substrate (P-AS) proteins that bind to serine- or threonine-phosphorylated (*) motifs of the pattern R-x-R-x-x-[ST*] were obtained from Cell Signaling Technology (Beverly, MA). The P-AS antibody has been previously used to identify proteins that are phosphorylated by Akt after insulin stimulation of adipocytes (14). The Akt target site in human ARK5, RqRirS* (33), is conserved in rat ARK5 and has been shown to bind antibodies against P-AS (34).
Antibodies against phosphorylated T172 of AMPKα were obtained from Cell Signaling Technology. The sequence around this T-loop activation site is similar between AMPKα isoforms (FLRT*SCGSP) and rat ARK5 (FLQT*FCGSP). A family tree for AMPK and the AMPK-related kinases compiled by Lizcano et al. (21) shows the similarity among T-loop sequences of human AMPKα isoforms and ARK5/NUAK1.
Antibodies against Akt phosphorylated on serine-473 (S473) and threonine-308 (T308) were obtained from Cell Signaling Technology. Monoclonal antibodies against phosphotyrosine were obtained from Upstate USA (Charlottesville, VA). Horseradish peroxidase (HRP)-linked goat anti-chicken antibodies were obtained from Aves Labs. HRP-linked goat anti-rabbit and goat anti-mouse antibodies were from Pierce Biotechnology (Rockford, IL).
RT-PCR.
A male Wistar rat weighing ~100 g was killed, and its heart, brain, kidney, liver, and white and red quadriceps muscle tissues were dissected, snap-frozen in liquid nitrogen, and stored at −80°C before total RNA extraction. Tissues were homogenized with a Polytron homogenizer in TRIzol (Life Technologies, Rockville, MD). Precipitated RNA was resuspended in water, heated at 60°C for 10 min, and treated with DNase I (Ambion, Austin, TX). Samples of total RNA were reverse-transcribed to cDNA in RT buffer (25 μg/ml random primer, 2.5 mM MgCl2, 500 μM dNTP mixture, 10 mM dithiothreitol, and 75 U of AMV RT; Promega). After incubation for 60 min at 42°C, the reaction was terminated by heating at 70°C for 15 min. The cDNA obtained was further amplified by PCR with oligonucleotide primers corresponding to the nucleotide sequence (XM_234998) of rat ARK5 (primer 1: TGA ACT GGG GCT ACA AGA GCA G, primer 2: TTT GAA AGG GGA TGG TAA GGC GGG). PCR products were resolved on 1.5% agarose gels, stained with SYBR Green nucleotide (Molecular Probes), and scanned with GelExpert Software (NucleoTech). Two other sets of primers corresponding to the rat ARK5 sequence were also used to generate cDNA from skeletal muscle (primer 1b: AAC CTG TAC CAG AAG GAC AAG TTC, primer 2b: CTG CTG ATG ATC ACA TTG TTA CTG; primer 1c: CTG AAG AAG TCC AAG AAA GAG AAC, primer 2c: AGC CTT CTT ATA CAC CTG AGT CAC).
Immunoprecipitations.
Muscle samples were homogenized in Kontes ground glass tubes in ice-cold buffer containing protease and phosphatase inhibitors (50 mM HEPES, pH 7.4, 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1.5 mM MgCl2, 1 mM EDTA, 10 mM sodium pyrophosphate, 100 mM NaF, 2 mM Na3VO4, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 0.5 μg/ml pepstatin, and 2 mM phenylmethylsulfonyl fluoride) and centrifuged for 10 min at 14,000 g (31) before assay of supernatants for protein content by the bicinchoninic acid method (Pierce Biotechnology). Protein A-Sepharose (Sigma, St. Louis, MO) was hydrated with PBS, incubated at 4°C overnight with end-over-end rotation, and washed three times in PBS. Chicken anti-ARK5 antibodies were similarly incubated overnight with the protein A-Sepharose slurry before washing with PBS. For each muscle homogenate, 100 μg protein in a total volume of 50 μl was added to 50 μl of 50% antibody-linked protein A-Sepharose slurry. After end-over-end rotation at 4°C overnight, beads were washed several times (3), then either used for kinase assays or processed for Western blots of proteins in immunoprecipitates.
Kinase assays.
Kinase reactions were initiated by addition of AMPK assay reagent [40 mM HEPES, pH 7.0, 0.2 mM SAMS peptide (Tocris, Ellisville, IL), 0.2 mM AMP, 80 mM NaCl, 8% glycerol, 0.8 mM EDTA, 0.8 mM dithiothreitol, 5 mM MgCl2, and 0.2 mM ATP containing 2 μCi/sample [γ-32P]ATP (MP Biomedicals, Irvine, CA); see Ref. 36], and reactions were performed for 20 min at 30°C. Reactions were terminated by centrifugation, and samples of supernatants were spotted on Whatman P81 filters. Filters were washed six times in 1% phosphoric acid and one time in acetone, placed in 1 ml of Microscint20 (PerkinElmer, Boston, MA) scintillation fluid, and subjected to scintillation counting (along with known amounts of assay reagent to compute specific activity of ATP) in a Packard TopCount plate reader (PerkinElmer).
Western blot.
Tissue homogenates (prepared as described above) or immunoprecipitates were diluted in Laemmli sample buffer containing dithiothreitol, boiled, run on 10% polyacrylamide gels, and transferred to nitrocellulose for Western blotting as previously described (9). HRP-linked antibodies were detected by exposure of photographic film to enhanced chemiluminescence (Perkin-Elmer), and bands were quantitated by Total Lab software (Nonlinear Dynamics Limited, Newcastle upon Tyne, UK). In many cases, nitrocellulose membranes were stripped of bound antibodies and subsequently probed with different antibodies, as described previously (15). All data from membranes that were probed multiple times are presented together in the order in which the blots were performed.
Statistics.
For contraction and AICAR studies, differences between means for control and contralateral, treated muscles were assessed with paired sample t-tests, with the level of significance set at P < 0.05. For insulin studies, differences between means for control and insulin-injected animals were assessed with t-tests, with the level of significance was set at P < 0.05. All data are presented as means with SE.
RESULTS
ARK5 expression.
We verified that ARK5 was expressed in skeletal muscle using three sets of primers for RT-PCR. ARK5 mRNA was present in all tissues analyzed (heart, kidney, brain, liver, and skeletal muscle; Fig. 1A). ARK5 expression in all of these tissue types has been reported previously (16). The data in Fig. 1A are derived from the use of the first primer set described (primer 1: TGA ACT GGG GCT ACA AGA GCA G, primer 2: TTT GAA AGG GGA TGG TAA GGC GGG). Data from RT-PCR of skeletal muscle RNA using the other two primer sets is not shown, although cDNAs of the expected sizes were obtained.
Fig. 1.
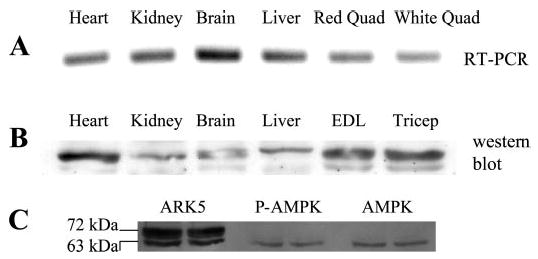
AMP-activated protein kinase-related kinase 5 (ARK5) expression. A: RT-PCR products using primers derived from the rat ARK5 sequence and RNA extracted from heart, kidney, brain, liver, and skeletal muscle. B: Western blot showing presence of ARK5 protein in heart, kidney, brain, liver, and skeletal muscle. C: Western blots for ARK5, phosphorylated (P) AMP-activated protein kinase (AMPK), and AMPK. After the gel was run and transferred, the nitrocellulose membrane was cut into three sections, each of which was incubated separately with the indicated antibodies. The membrane was reassembled before photography of enhanced chemiluminescence (ECL). Quad, quadriceps.
A Western blot using the chicken anti-ARK5 antibody demonstrated the presence of ARK5 protein in heart, kidney, brain, liver, and skeletal muscle (Fig. 1B). There were two prominent ARK5 bands in skeletal muscle, and Fig. 1C shows that the lower of the two ARK5 bands is the same molecular weight as AMPK.
Characterization of antibodies.
Chicken anti-ARK5 antibodies completely immunoprecipitated ARK5 from muscle samples, as shown by the presence of ARK5 (probed with the validated ARK5 + SNARK antibody) in ARK5 immunoprecipitates but not in supernates (Fig. 2A). In contrast, AMPK was found in homogenates before immunoprecipitation but not in ARK5 immunoprecipitates (Fig. 2B). Blots shown in fig 2C demonstrate that antibodies against phosphorylated AMPK (P-AMPK) cross-react with ARK5 immunoprecipitates despite the absence of AMPK in immunoprecipitates. The ARK5 band also cross-reacts with antibodies against P-AS. The ARK5 + SNARK band is precisely superimposable over the P-AMPK and phosphorylated Akt substrate bands. Cross-reactivity with P-AMPK antibody establishes the ARK5 band as likely to be an AMPK family member that is not an AMPKα isoform, as demonstrated by the absence of AMPKα in ARK5 immunoprecipitates. Cross-reactivity with the anti-P-AS antibody suggests that the band immunoprecipitated by anti-ARK5 antibodies is indeed ARK5, the only AMPK family member so far described to be an Akt target (33).
Fig. 2.
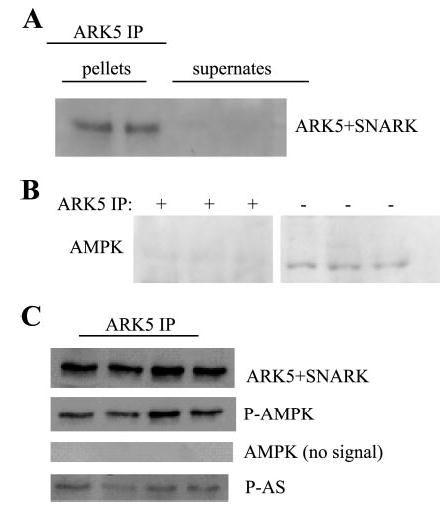
Characterization of antibodies. A: ARK5 antibodies completely immunoprecipitated ARK5 from skeletal muscle homogenates. Anti-ARK5 + sucrose nonfermenting protein kinase/AMP-activated protein kinase-related protein kinase (SNARK) antibodies bound a protein in the ARK5 immunoprecipitates but not in the supernates after immunoprecipitation. B: anti-AMPK antibodies reacted with bands in the whole homogenates (−IP), but not in the ARK5 immunoprecipitates (+IP). Bands shown are from the same blot but were not in adjacent lanes. C: antibodies against P-AMPK and phosphorylated substrates of protein kinase B (P-AS) cross-react with the ARK5 band. AMPK and ARK5 amino acid sequences are similar around the threonine phosphorylation site. ARK5 is the only AMPK family member that is known to be phosphorylated by protein kinase B (Akt).
Effects of muscle contractions.
The ARK5, ARK5 + SNARK, P-AMPK, and phosphorylated Akt substrate bands in immunoprecipitates were all superimposable (Fig. 3A). Cross-reactivity of ARK5 immunoprecipitates with anti-P-AMPK antibodies was increased by ~30% (P < 0.05) by muscle contractions (representative bands in Fig. 3A, quantification in Fig. 3B). AMPK was not detected in the ARK5 immunoprecipitates, but it was present in nonimmunoprecipitated samples. Despite the apparent increased T-loop phosphorylation of ARK5 (Fig. 3B) in contracted muscles, contractions did not alter ARK5 phosphotransferase activity toward SAMS peptide (Fig. 3C).
Fig. 3.
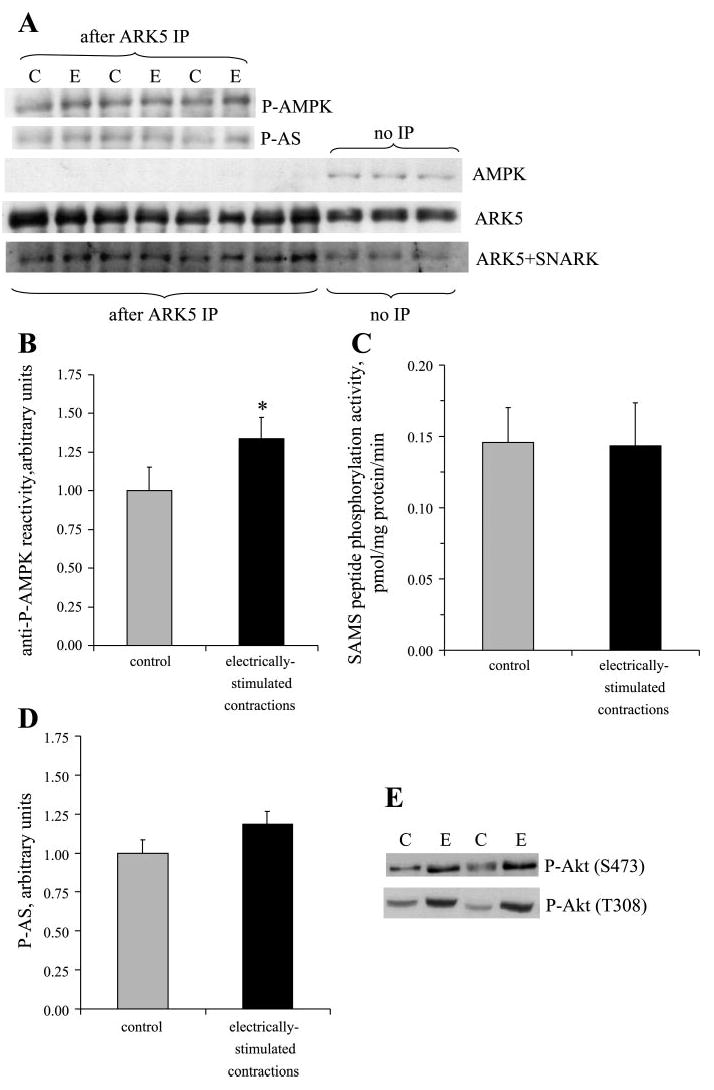
Muscle contractions increase ARK5 phosphorylation but not activity. ARK5 immunoprecipitations were performed for resting control (C) and contralateral electrically stimulated (E) muscles and processed for Western blots or kinase activity. A: immunoblots of ARK5 immunoprecipitates demonstrate binding of antibodies against P-AMPK, and P-AS, but not AMPK. B: quantitation of P-AMPK bands (*P < 0.05, n = 8 muscles/group). C: SAMS peptide (ARK5 substrate) phosphorylation activity (n = 8/group). D: quantitation of P-AS. E: Akt phosphorylation.
Muscle contractions slightly, but not statistically significantly, increased reactivity of the ARK5 immunoprecipitates with the antibody against P-AS (bands in Fig. 3A, quantitation in Fig. 3D). The lack of significant increase in ARK5 that had been phosphorylated by Akt was surprising, because muscle contractions caused approximately twofold increases (Fig. 3E) in activating phosphorylations of Akt on S473 (control 1.0 ± 0.1, contracted 2.2 ± 0.3, relative units, P < 0.05) and T308 (control 1.0 ± 0.1, contracted 2.4 ± 0.5, P < 0.05).
Effects of AICAR.
AICAR stimulated an ~60% increase in reactivity of ARK5 with antibodies against P-AMPK (Fig. 4A). Despite this increased phosphorylation of ARK5, AICAR did not increase ARK5 phosphotransferase activity toward SAMS peptide (Fig. 4B).
Fig. 4.
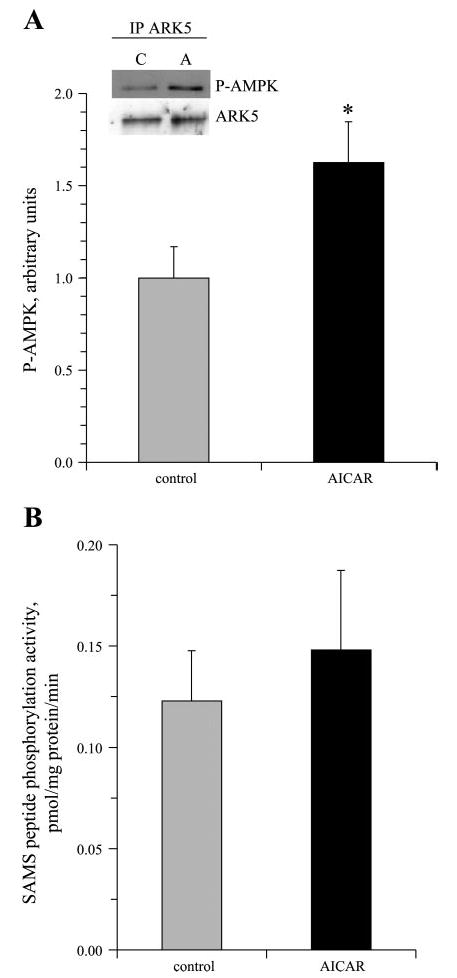
5-Aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) increases ARK5 phosphorylation but not activity. Epitrochlearis muscles were incubated in the absence or presence of 2 mM AICAR, and ARK5 was immunoprecipitated from muscle samples for Western blots for P-AMPK and ARK5 (*P < 0.05, n = 4/group; A) or kinase assays (n = 8/group; B).
Effects of insulin.
Unfortunately, there was a large reduction in recovery of ARK5 in immunoprecipitates from insulin-treated samples (Fig. 5). Because it appeared that the ARK5 antibody would be ineffective in recovery of ARK5 from insulin-treated muscles, we performed Western blots for samples without immunoprecipitation and probed in sequence for phosphotyrosine, ARK5, and P-AS. The ARK5 band corresponded exactly with major bands from both blots against phosphorylated proteins. Insulin stimulated a 45% increase (P < 0.05) in tyrosine phosphorylation of the band corresponding to ARK5 (Fig. 6, A and B). As further evidence that ARK5 can be tyrosine phosphorylated, we recovered ARK5 in samples immunoprecipitated with antibodies against phosphotyrosine (Fig. 6C). Insulin treatment caused a threefold increase in reactivity of the antibody against P-AS with the band that could be overlaid on the ARK5 band (Fig. 6, A and D), which is consistent with the insulin-stimulated increase in Akt S473 phosphorylation (Fig. 6E, control 1.0 ± 0.1, contracted 7.7 ± 1.7, relative units, P < 0.05).
Fig. 5.
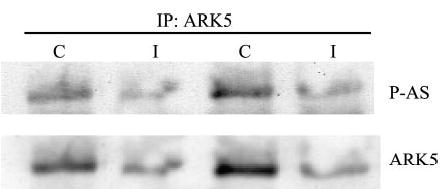
Prior treatment with insulin appears to reduce the affinity of the ARK5 antibody for ARK5. Muscle samples from control rats (C) or rats that were injected with insulin (I) were subjected to Western blot with antibodies against ARK5 and P-AS after immunoprecipitation (IP) with ARK5 antibodies.
Fig. 6.
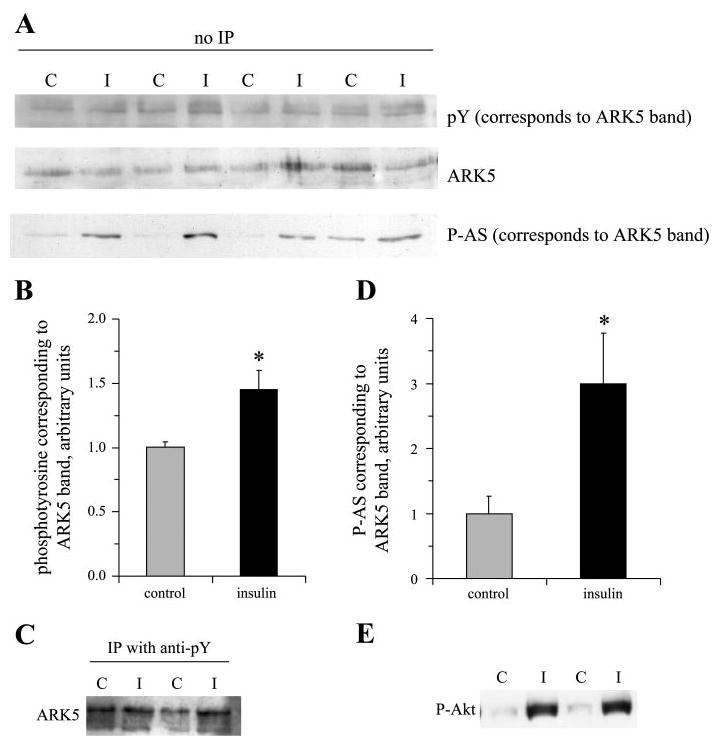
Insulin stimulates phosphorylation of protein bands corresponding to ARK5. Nonimmunoprecipitated muscle samples from control (C) rats or rats that were injected with insulin (I) were subjected to SDS-PAGE, transferred to nitrocellulose, and probed in sequence for phosphotyrosine, ARK5, and P-AS. A: protein bands from the blots. These bands were precisely superimposable. B: insulin-stimulated tyrosine phosphorylation of the protein in the band corresponding to ARK5. C: presence of ARK5 in phosphotyrosine immunoprecipitates. D: increased cross-reactivity of the band corresponding to ARK5 with antibodies against P-AS. E: Akt serine-473 phosphorylation. *P < 0.05, n = 8 rats/group.
DISCUSSION
The new information provided by this study is that ARK5 protein is present in skeletal muscle and is phosphorylated (as demonstrated by cross-reactivity with antibodies against P-AMPK) after muscle contractions or exposure of muscle to AICAR. In addition, insulin appears to stimulate tyrosine phosphorylation of ARK5 and phosphorylation of ARK5 by Akt.
ARK5 expression in skeletal muscle has previously been verified by RT-PCR (16), and ARK5 expression has been demonstrated in several gene chip studies involving human skeletal muscle (accessible through the NCBI Gene Expression Omnibus module by searching for ARK5 AND muscle). In support of the presence of ARK5 in skeletal muscle, we have successfully obtained cDNAs from RNA extracted from rat skeletal muscle by performing RT-PCR using three separate sets of primers derived from nucleotide sequences of rat ARK5. However, it has been reported that antibodies against ARK5/NUAK1, which have been shown to immunoprecipitate recombinant ARK5 (21) and have been reported to immunoprecipitate ARK5 from rat brain (29), did not immunoprecipitate ARK5 protein or activity from rat skeletal muscle (29). These ARK5/NUAK1 antibodies (30) were raised against a peptide sequence of human ARK5 (MEGAAAPVAGDRPDLGLGAPG) that is similar in rat ARK5 (MEGAAAVSAAGDGPAVETGLPG, accession no. XP_234998). The ARK5 + SNARK antibodies used in the current study to demonstrate the presence of ARK5 protein in skeletal muscle have been validated against recombinant ARK5 (33), and these ARK5 + SNARK antibodies recognized the ARK5 band that was immunoprecipitated with our own ARK5 antibodies. Furthermore, the ARK5 antibodies in the current study immunoprecipitated a protein (that was not AMPK) that cross-reacted with antibodies against P-AMPK and P-AS, both of which would be expected for ARK5. It seems unlikely that our antibodies immunoprecipitated a different member of the AMPK family, because the peptide sequence of the immunizing peptide is shared only with ARK5 and SNARK. SNARK, however, does not contain an Akt target site.
In our hands, ARK5 appears to have 63- and 72-kDa forms. Human ARK5 (NP_055655) is 661 amino acids in length, which corresponds to ~76 kDa. Mouse ARK5 (XP_196007) is predicted to contain 658 amino acids. If translation of the rat protein were to occur at the same position as predicted for human and mouse, it would be 660 amino acids long. The human ARK5 gene has been predicted to contain 12 alternative exons, and alternatively spliced ARK5 mRNAs coding for three distinct proteins have been described so far (24). It seems possible that different species and/or tissues might contain ARK5 protein products of various sizes.
Insulin appeared to robustly increase phosphorylation of ARK5 at an Akt target site, which is consistent with the finding of IGF-I-stimulated phosphorylation of ARK5 by Akt (34). Akt phosphorylation of ARK5 is not necessary to basal activity of the kinase but has been found to be essential to the threefold increase in ARK5 activity in cells transfected with constitutively active Akt (33). Akt activity is necessary for the stimulation of ARK5 activity by IGF-I, and a dominant-negative form of ARK5 that cannot be phosphorylated by Akt blocks Akt-mediated IGF-I effects, such as phosphorylation of mammalian target of rapamycin (34). ARK5 is so far the only catalytic AMPK family member that has been described as being regulated by Akt (32–34). For example, AMPKα isoforms do not contain Akt target sites.
As pointed out by Lizcano et al. (21), the Akt phosphorylation site on ARK5 (identical to that corresponding to rat ARK5 amino acid sequence, 740–745 RqRirS) that has been implicated in the control of kinase activity lies far from the activation loop. At present, it is unknown whether ARK5 itself contains an AMP-binding site or whether the reported AMP sensitivity of ARK5 (33) is dependent on associations of ARK5 with other proteins [such as the case for AMPK, for which AMP-binding sites on the γ-subunit have been reported to mediate the allosteric control of the α-subunit by AMP (1)]. It is conceivable that phosphorylation of ARK5 by Akt could play some role in mediation of AMP sensitivity of the kinase or alter the activity of the enzyme through some other means, such as by affecting interactions with other proteins.
The utility of our ARK5 antibody for immunoprecipitations was reduced in muscle that was exposed to insulin. We suspect that this was because of phosphorylation of a residue at or near the epitope to which the antibody binds, although it could have been caused by other means, such as conformational change or coverage of the binding site by another protein. In ARK5, the region around the peptide used to produce the antibody (kkgilkktqqreSgYYsSperSeSselldsnnviiss; peptide antigen underlined) contains six residues with high probability for phosphorylation (http://www.cbs.dtu.dk/services/NetPhos; see Ref. 4), as indicated by capital letters in the sequence. The second tyrosine in the sequence is predicted to be the most likely tyrosyl residue to be phosphorylated in ARK5. The potential phosphorylatable serinyl residues within the region of interest are consensus target sites for casein kinase I, glycogen synthase kinase 3β (GSK3β, two sites), and protein kinase A (PKA). Phosphorylation of these sites by these kinases is probably not stimulated by insulin, because the potential CKI target requires co-phosphorylation of a second serine that is highly unlikely to be phosphorylated, and GSK3β and PKA activities are suppressed in the presence of insulin (8, 17). Cross-reactivity of the anti-phosphotyrosine antibody with the protein band corresponding to ARK5 suggests that ARK5 is phosphorylated on tyrosine, though the specific tyrosyl residue involved and the functional consequence of such phosphorylation is unknown.
AMPK or AMP-activated processes have been implicated in the regulation of multiple metabolic pathways (for review, see Ref. 11), including stimulation of glucose transport (12, 22, 23) and activation of insulin sensitivity (9, 13, 18). If ARK5 plays a role in regulation of muscle metabolism, it seems likely that it would be active in metabolic pathways that are controlled similarly by muscle contractions and insulin. For example, both muscle contractions and insulin stimulate glucose transport (10, 35), and muscle contractions increase the sensitivity of glucose transport to stimulation by insulin (6, 9, 28). Consistent with a role for ARK5 in insulin signaling, the ATM kinase, a wortmannin-sensitive phosphatidylinositol 3-kinase family member that is activated by ARK5 (33), has been shown to mediate some aspects of insulin action [e.g., insulin-stimulated cell-surface localization of GLUT4 (40) and insulin-stimulated protein synthesis (39)], and mutations of ATM are related to insulin resistance (19).
In conclusion, we have used RT-PCR and Western blot to demonstrate expression of ARK5/NUAK1 in rat skeletal muscle. Muscle contractions and incubation of muscle with AICAR appear to increase phosphorylation of the ARK5 T-loop threonine. However, this increased ARK5 phosphorylation was not associated with increased ARK5 activity, suggesting that threonine phosphorylation of ARK5 is insufficient for activation of the kinase in skeletal muscle. Exposure of skeletal muscle to insulin increases tyrosine phosphorylation of a protein corresponding to ARK5 and also increases apparent phosphorylation of ARK5 by Akt. These findings on the ARK5 phosphorylations caused by contractions, incubation of muscle with AICAR, and treatment with insulin could provide the basis for future work regarding the regulation and physiological role of ARK5 in skeletal muscle.
Acknowledgments
We thank Long Nguyen, Bora Lee, and Rebecca Carroll for technical assistance and Dr. John C. Kennell for performing kinase assays.
Current address for A. Suzuki: National Institute for Physiological Sciences, Division of Reproductive/Endocrine Development, Okazaki, Aichi 444-8585, Japan
Footnotes
GRANTS
This research was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant K01 DK-66330 awarded to J. Fisher. P. Oppelt was supported by a Summer Research Award from the Saint Louis University Office of Research Services.
References
- 1.Adams J, Chen ZP, Van Denderen BJ, Morton CJ, Parker MW, Witters LA, Stapleton D, Kemp BE. Intrasteric control of AMPK via the gamma1 subunit AMP allosteric regulatory site. Protein Sci. 2004;13:155–165. doi: 10.1110/ps.03340004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al Khalili L, Chibalin AV, Yu M, Sjodin B, Nylen C, Zierath JR, Krook A. MEF2 activation in differentiated primary human skeletal muscle cultures requires coordinated involvement of parallel pathways. Am J Physiol Cell Physiol. 2004;286:C1410–C1416. doi: 10.1152/ajpcell.00444.2003. [DOI] [PubMed] [Google Scholar]
- 3.Barnes BR, Ryder JW, Steiler TL, Fryer LG, Carling D, Zierath JR. Isoform-specific regulation of 5′ AMP-activated protein kinase in skeletal muscle from obese Zucker (fa/fa) rats in response to contraction. Diabetes. 2002;51:2703–2708. doi: 10.2337/diabetes.51.9.2703. [DOI] [PubMed] [Google Scholar]
- 4.Blom N, Gammeltoft S, Brunak S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol. 1999;294:1351–1362. doi: 10.1006/jmbi.1999.3310. [DOI] [PubMed] [Google Scholar]
- 5.Brozinick JT, Jr, Birnbaum MJ. Insulin, but not contraction, activates Akt/PKB in isolated rat skeletal muscle. J Biol Chem. 1998;273:14679–14682. doi: 10.1074/jbc.273.24.14679. [DOI] [PubMed] [Google Scholar]
- 6.Cartee GD, Young DA, Sleeper MD, Zierath J, Wallberg-Henriksson H, Holloszy JO. Prolonged increase in insulin-stimulated glucose transport in muscle after exercise. Am J Physiol Endocrinol Metab. 1989;256:E494–E499. doi: 10.1152/ajpendo.1989.256.4.E494. [DOI] [PubMed] [Google Scholar]
- 7.Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem. 1995;229:558–565. doi: 10.1111/j.1432-1033.1995.tb20498.x. [DOI] [PubMed] [Google Scholar]
- 8.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 9.Fisher JS, Gao J, Han DH, Holloszy JO, Nolte LA. Activation of AMP kinase enhances sensitivity of muscle glucose transport to insulin. Am J Physiol Endocrinol Metab. 2002;282:E18–E23. doi: 10.1152/ajpendo.2002.282.1.E18. [DOI] [PubMed] [Google Scholar]
- 10.Garetto LP, Richter EA, Goodman MN, Ruderman NB. Enhanced muscle glucose metabolism after exercise in the rat: the two phases. Am J Physiol Endocrinol Metab. 1984;246:E471–E475. doi: 10.1152/ajpendo.1984.246.6.E471. [DOI] [PubMed] [Google Scholar]
- 11.Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology. 2003;144:5179–5183. doi: 10.1210/en.2003-0982. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi T, Hirshman MF, Kurth EJ, Winder WW, Goodyear LJ. Evidence for 5′ AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes. 1998;47:1369–1373. doi: 10.2337/diab.47.8.1369. [DOI] [PubMed] [Google Scholar]
- 13.Iglesias MA, Ye JM, Frangioudakis G, Saha AK, Tomas E, Ruderman NB, Cooney GJ, Kraegen EW. AICAR administration causes an apparent enhancement of muscle and liver insulin action in insulin-resistant high-fat-fed rats. Diabetes. 2002;51:2886–2894. doi: 10.2337/diabetes.51.10.2886. [DOI] [PubMed] [Google Scholar]
- 14.Kane S, Sano H, Liu SC, Asara JM, Lane WS, Garner CC, Lienhard GE. A method to identify serine kinase substrates. Akt phosphorylates a novel adipocyte protein with a Rab GTPase-activating protein (GAP) domain. J Biol Chem. 2002;277:22115–22118. doi: 10.1074/jbc.C200198200. [DOI] [PubMed] [Google Scholar]
- 15.Kaufmann S, Kellner U. Erasure of western blots after autoradiographic or chemiluminescent detection. Methods Mol Biol. 1998;80:223–235. doi: 10.1007/978-1-59259-257-9_22. [DOI] [PubMed] [Google Scholar]
- 16.Kazusa DNA.Research Institute (Japan). Gene/Protein Characteristic Table for KIAA0537 http://www.kazusa.or.jp/huge/gfpage/KIAA0537/[2002].
- 17.Kida Y, Nyomba BL, Bogardus C, Mott DM. Defective insulin response of cyclic adenosine monophosphate-dependent protein kinase in insulin-resistant humans. J Clin Invest. 1991;87:673–679. doi: 10.1172/JCI115045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim J, Solis RS, Arias EB, Cartee GD. Post-contraction insulin sensitivity: relationship with contraction protocol, glycogen concentration, and 5′AMP-activated protein kinase phosphorylation. J Appl Physiol. 2004;284:575–583. doi: 10.1152/japplphysiol.00909.2003. [DOI] [PubMed] [Google Scholar]
- 19.Lavin MF. An unlikely player joins the ATM signalling network. Nat Cell Biol. 2000;2:E215–E217. doi: 10.1038/35046628. [DOI] [PubMed] [Google Scholar]
- 20.Lefebvre DL, Bai Y, Shahmolky N, Sharma M, Poon R, Drucker DJ, Rosen CF. Identification and characterization of a novel sucrose-nonfermenting protein kinase/AMP-activated protein kinase-related protein kinase, SNARK. Biochem J. 2001;355:297–305. doi: 10.1042/0264-6021:3550297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lizcano JM, Goransson O, Toth R, Deak M, Morrice NA, Boudeau J, Hawley SA, Udd L, Makela TP, Hardie DG, Alessi DR. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 2004;23:833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merrill GF, Kurth EJ, Hardie DG, Winder WW. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol Endocrinol Metab. 1997;273:E1107–E1112. doi: 10.1152/ajpendo.1997.273.6.E1107. [DOI] [PubMed] [Google Scholar]
- 23.Mu J, Brozinick JT, Jr, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell. 2001;7:1085–1094. doi: 10.1016/s1097-2765(01)00251-9. [DOI] [PubMed] [Google Scholar]
- 24.NCBI. Homo Sapiens Gene ARK5 Encoding KIAA0537 Gene Product http://www.ncbi.nlm.nih.gov/IEB/Research/Acembly/av.cgi?db=34&c=Gene&l=ARK5, 2004.
- 25.Ojuka EO, Nolte LA, Holloszy JO. Increased expression of GLUT-4 and hexokinase in rat epitrochlearis muscles exposed to AICAR in vitro. J Appl Physiol. 2000;88:1072–1075. doi: 10.1152/jappl.2000.88.3.1072. [DOI] [PubMed] [Google Scholar]
- 26.Pessin JE, Saltiel AR. Signaling pathways in insulin action: molecular targets of insulin resistance. J Clin Invest. 2000;106:165–169. doi: 10.1172/JCI10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ponticos M, Lu QL, Morgan JE, Hardie DG, Partridge TA, Carling D. Dual regulation of the AMP-activated protein kinase provides a novel mechanism for the control of creatine kinase in skeletal muscle. EMBO J. 1998;17:1688–1699. doi: 10.1093/emboj/17.6.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richter EA, Garetto LP, Goodman MN, Ruderman NB. Muscle glucose metabolism following exercise in the rat: increased sensitivity to insulin. J Clin Invest. 1982;69:785–793. doi: 10.1172/JCI110517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakamoto K, Goransson O, Hardie DG, Alessi DR. Activity of LKB1 and AMPK-related kinases in skeletal muscle: effects of contraction, phenformin and AICAR. Am J Physiol Endocrinol Metab. 2004;287:E310–E317. doi: 10.1152/ajpendo.00074.2004. [DOI] [PubMed] [Google Scholar]
- 31.Sakamoto K, Hirshman MF, Aschenbach WG, Goodyear LJ. Contraction regulation of Akt in rat skeletal muscle. J Biol Chem. 2002;277:11910–11917. doi: 10.1074/jbc.M112410200. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki A, Kusakai G, Kishimoto A, Lu J, Ogura T, Esumi H. ARK5 suppresses the cell death induced by nutrient starvation and death receptors via inhibition of caspase 8 activation, but not by chemotherapeutic agents or UV irradiation. Oncogene. 2003;22:6177–6182. doi: 10.1038/sj.onc.1206899. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki A, Kusakai GI, Kishimoto A, Lu J, Ogura T, Lavin MF, Esumi H. Identification of a novel protein kinase mediating Akt survival signaling to ATM. J Biol Chem. 2003;278:48–53. doi: 10.1074/jbc.M206025200. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki A, Lu J, Kusakai G, Kishimoto A, Ogura T, Esumi H. ARK5 is a tumor invasion-associated factor downstream of Akt signaling. Mol Cell Biol. 2004;24:3526–3535. doi: 10.1128/MCB.24.8.3526-3535.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wallberg-Henriksson H, Holloszy JO. Activation of glucose transport in diabetic muscle: responses to contraction and insulin. Am J Physiol Cell Physiol. 1985;249:C233–C237. doi: 10.1152/ajpcell.1985.249.3.C233. [DOI] [PubMed] [Google Scholar]
- 36.Winder WW, Hardie DG. Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol Endocrinol Metab. 1996;270:E299–E304. doi: 10.1152/ajpendo.1996.270.2.E299. [DOI] [PubMed] [Google Scholar]
- 37.Winder WW, Hardie DG. AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol Endocrinol Metab. 1999;277:E1–E10. doi: 10.1152/ajpendo.1999.277.1.E1. [DOI] [PubMed] [Google Scholar]
- 38.Winder WW, Holmes BF, Rubink DS, Jensen EB, Chen M, Holloszy JO. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J Appl Physiol. 2000;88:2219–2226. doi: 10.1152/jappl.2000.88.6.2219. [DOI] [PubMed] [Google Scholar]
- 39.Yang DQ, Kastan MB. Participation of ATM in insulin signalling through phosphorylation of eIF-4E-binding protein 1. Nat Cell Biol. 2000;2:893–898. doi: 10.1038/35046542. [DOI] [PubMed] [Google Scholar]
- 40.Yang DQ, He J. ATM protein kinase participates in GLUT4 translocation by insulin in L6 muscle cells. Diabetologia. 2003;46(Suppl 2):A40–A41. [Google Scholar]


