Abstract
RpoS (σS) in Escherichia coli is a stationary-phase-specific primary sigma factor of RNA polymerase which is 330 amino acids long and belongs to the eubacterial σ70 family of proteins. Conserved domain 1.1 at the N-terminal end of σ70 has been shown to be essential for RNA polymerase function, and its deletion has been shown to result in a dominant-lethal phenotype. We now report that a σS variant with a deletion of its N-terminal 50 amino acids (σSΔ1-50), when expressed in vivo either from a chromosomal rpoS::IS10 allele (in rho mutant strains) or from a plasmid-borne arabinose-inducible promoter, is as proficient as the wild type in directing transcription from the proU P1 promoter; at three other σS-dependent promoters that were tested (osmY, katE, and csiD), the truncated protein exhibited a three- to sevenfold reduced range of activities. Catabolite repression at the csiD promoter (which requires both σS and cyclic AMP [cAMP]-cAMP receptor protein for its activity) was also preserved in the strain expressing σSΔ1-50. The intracellular content of σSΔ1-50 was regulated by culture variables such as growth phase, osmolarity, and temperature in the same manner as that described earlier for σS, even when the truncated protein was expressed from a template that possessed neither the transcriptional nor the translational control elements of wild-type rpoS. Our results indicate that, unlike that in σ70, the N-terminal domain in σS may not be essential for the protein to function as a sigma factor in vivo. Furthermore, our results suggest that the induction of σS-specific promoters in stationary phase and during growth under conditions of high osmolarity or low temperature is mediated primarily through the regulation of σS protein degradation.
The σ factor is a subunit of RNA polymerase in all eubacteria that confers on the enzyme the property of promoter specificity in the initiation of transcription. Based on amino acid sequence similarity as well as organization of the cognate promoters, two families of σ factors have been identified (19); the σ70 family is by far the larger one. The prototypic example of this family is Escherichia coli RpoD or σ70 (with 613 amino acid residues), the primary or housekeeping σ factor in this organism, which is essential for its viability.
Four conserved regions, numbered 1 to 4 beginning from the N-terminal end, have been identified in the σ70 family of proteins; some of these are further divided into distinct subregions (19). Regions 2 and 4 are the most highly conserved among different members of the σ70 family, and these regions are involved in recognition of and binding to promoter DNA by the enzyme. On the other hand, subregion 1.1 appears to be conserved only in the housekeeping or indispensable σ proteins of different bacteria, as well as in σS (see below). In σ70, subregion 1.1 (comprising the segment from approximately residues 30 through 100) has been suggested to function as a mask in free σ protein for DNA-binding domains 2 and 4, so that the latter are exposed only when the σ factor is associated with the core enzyme, that is, the other subunits of the RNA polymerase holoenzyme (6). Other studies have suggested that subregion 1.1 is also required for (i) the initial binding of σ70 to the core enzyme (10, 26); (ii) masking of other core-binding regions in the free σ70 subunit that are later involved in a holoenzyme interface (10); (iii) influencing the efficiency of transcription initiation, perhaps by constraining the holoenzyme to assess the fitness of a promoter by its −10 and −35 sequences (7, 40, 42); and (iv) imparting stability to the protein in vivo (42). Consistent with these findings, mutant versions of σ70 with a deletion of subregion 1.1 confer a dominant-lethal phenotype in vivo (5, 34).
In addition to σ70, E. coli cells possess a second primary sigma factor, RpoS or σS (with 330 amino acid residues), that is encoded by rpoS and that is important for stationary-phase gene expression and survival (reviewed in references 12 and 14). As a member of the σ70 family, σS also has conserved regions 1 through 4. The N-terminal stretch of 60 amino acid residues of σS has been reported to share moderate sequence similarity with domain 1.1 of σ70 (19, 25), and both are also rich in acidic amino acid residues; however, the function of this region in σS is not known. An alignment of these two segments of σS and σ70 is shown in Fig. 1.
FIG. 1.
Similarity between the N-terminal region of σS and domain 1.1 of σ70. In the alignment shown, individual amino acids are represented in the one-letter code, and plus symbols and colons are used to indicate identity and conservative substitution (within one or another of the following groups: D, E, N, or Q; S or T; G or A; and I, V, L, or M), respectively, between the two proteins. When necessary, gaps (dashes) have been introduced in the sequences to maximize the similarity in alignment. Sequence numbers of the N- and C-terminal residues of each of the two polypeptide stretches are given in parentheses. Residues in σ70 that are highly conserved among the primary sigma factors of both gram-negative and gram-positive bacteria (42) are underlined.
The genes of the σS regulon are induced under different environmental conditions, such as the stationary phase of growth, low pH, high osmolarity, or low incubation temperature, consequent to an elevation of the cytoplasmic concentration of σS under each of these conditions (12, 14). Cellular σS content itself is determined by the interplay of a complex set of regulatory mechanisms that operate at the levels of rpoS transcription and translation as well as the stability of the protein.
We have found in this study that a mutant σS protein which is missing its N-terminal 50 amino acid residues retains in vivo activity at σS-regulated promoters. Our results obtained with strains expressing the mutant protein also suggest that environmental regulation of cellular σS content may occur primarily at the posttranslational level.
MATERIALS AND METHODS
Bacterial strains and plasmids.
E. coli K-12 strains that were used in this study are listed in Table 1. Five plasmids, pHYD275, pHYD373, pETF, pBAD24, and pHYD408, were used in this study. The first two are IncW-based single-copy-number plasmids encoding trimethoprim resistance and carrying the lacZ reporter gene (as an operon fusion) downstream of the proU P1 promoter of, respectively, E. coli and Salmonella enterica serovar Typhimurium (4, 28). The third is a ColE1 replicon encoding ampicillin resistance and carrying the E. coli rpoS+ gene (39). Plasmid vector pBAD24, which is also ColE1 based and encodes ampicillin resistance, is designed for achieving l-arabinose (Ara)-induced expression of target genes cloned into its multiple-cloning-site region (11). Plasmid pHYD408 was contructed in this study by cloning into the appropriate sites in pBAD24 a SalI-HindIII fragment obtained by PCR from the mutant rpoS locus of strain GJ875 (see below). This PCR was done with a pair of primers (5′-TATG GTCGA CACATGGTTACGCTTTGG-3′ and 5′-TCGT AAGC TTTCTGACAGATGCTTACTT-3′) whose sequences (to the 3′ side of the residue marked in bold in each case) correspond, respectively, to the sequence near one end of IS10 and to the sequence immediately downstream of the termination codon of rpoS; the SalI and HindIII recognition sites, respectively, provided in the two primers are italicized.
TABLE 1.
E. coli K-12 strains used in this study
| Strain | Genotypea | Reference or source |
|---|---|---|
| MC4100 | Δ(argF-lac)U169 rpsL150 relA1 araD139 flbB5301 deoC1 ptsF25 | 8 |
| RH90 | MC4100 rpoS359::Tn10 | 21 |
| RH100b | MC4100 Δ(nlpD-rpoS)360 zgc-3251::Tn10 | 13 |
| GJ146 | MC4100 ΔputPA101 proP222 Δ(zfi-900::Tn10-proU)233 Δ(pyr-76::Tn10) rpoS365::IS10 | 9 |
| GJ829 | GJ146 ilv-3164::Tn10Kan | This study |
| GJ830 | GJ146 rho-4 ilv-3164::Tn10Kan | This study |
| GJ862 | Like MC4100 | 27 |
| GJ863 | MC4100 rho-4 | 27 |
| GJ875 | GJ862 rpoS365::IS10 zgc-912::Tn10dCm | This study |
| GJ876 | GJ862 zgc-912::Tn10dCm | This study |
| GJ884 | GJ862 csiD::lac(Kan) | 27 |
| GJ885 | GJ863 csiD::lac(Kan) | 27 |
| GJ888 | GJ862 osmY::lac(Kan) | 27 |
| GJ889 | GJ863 osmY::lac(Kan) | 27 |
| GJ893 | GJ884 rpoS365::IS10 zgc-912::Tn10dCm | This study |
| GJ894 | GJ888 rpoS365::IS10 zgc-912::Tn10dCm | This study |
| GJ2731 | GJ885 rpoS365::IS10 zgc-912::Tn10dCm | This study |
| GJ2732 | GJ889 rpoS365::IS10 zgc-912::Tn10dCm | This study |
| GJ2755 | GJ863 rpoS365::IS10 zgc-912::Tn10dCm | This study |
| GJ2756 | GJ862 ara+zac-3093::Tn10Kan rpoS359::Tn10 | This study |
| GJ2758 | GJ875 ara+zac-3093::Tn10Kan | This study |
| GJ2765 | GJ863 zgc-912::Tn10dCm | This study |
| GJ2770 | GJ862 [λ katE::lac(Kan)] | This study |
| GJ2771 | GJ863 [λ katE::lac(Kan)] | This study |
| GJ2778 | RH100 ara+ | This study |
| GJ2780 | GJ2778 csiD::lac(Kan) | This study |
| GJ2781 | GJ2778 osmY::lac(Kan) | This study |
| GJ2782 | GJ2778 [λ katE::lac(Kan)] | This study |
| GJ2785 | GJ884 ara+zac-3051::Tn10 | This study |
| GJ2787 | GJ885 Δ(nlpD-rpoS)360 zgc-3251::Tn10 | This study |
| GJ2788 | GJ889 Δ(nlpD-rpoS)360 zgc-3251::Tn10 | This study |
| GJ2789 | GJ2770 rpoS365::IS10 zgc-912::Tn10dCm | This study |
| GJ2790 | GJ2771 rpoS365::IS10 zgc-912::Tn10dCm | This study |
| GJ2791 | GJ2771 Δ(nlpD-rpoS)360 zgc-3251::Tn10 | This study |
Genotype designations are like those in the work of Berlyn (2). All strains are F−. In the strains listed, the following mutations were transduced from strains previously described: rho-4 from CGSC5072 (E. coli Genetic Stock Center); ilv-3164::Tn10Kan, zac-3093::Tn10Kan, and zac-3051::Tn10 from CAG18599, CAG12131, and CAG12095, respectively (35); csiD::lac(Kan) from DW12 (21); osmY::lac(Kan) (previously called csi-5::lac) from R0151-a (13); and λ katE::lac(Kan) from KT1008EL (38).
The Tn10 allele in RH100 was originally designated zfi-3251::Tn10, based on the calibration in an earlier edition of the E. coli K-12 linkage map.
Media and growth conditions.
For routine experiments, Luria-Bertani (LB) medium (22) and glucose (Glu)-minimal A medium (22) were used as the nutrient and defined media, respectively, and the incubation temperature for growth was 37°C. MacConkey lactose agar was obtained from Difco. Cultures for β-galactosidase assays were incubated with shaking (i) until mid-exponential phase (A600 of 0.4 to 0.8) or until 1 h after entry into stationary phase; (ii) at 30 or 10°C; and (iii) in LB medium or low-osmolarity K-tryptone medium (27), the latter supplemented when necessary with NaCl to 0.3 M. Unless otherwise indicated, Ara induction experiments (11) were performed by supplementation of the growth medium with 0.2% Ara (or 0.2% Glu, as a control). Concentrations of antibiotics used were as described earlier (20).
Immunoblot analysis.
The procedures used for electrophoresis of cell extracts on 10% polyacrylamide gels with sodium dodecyl sulfate, electroblotting to a polyvinylidene difluoride membrane, staining with Ponceau S, and sequential treatments with blocking reagent, primary antibody, alkaline phosphatase-conjugated secondary antibody, and chromogenic substrates were essentially as previously described (32). As part of the protocol, 2.5 μl of rabbit anti-σS antiserum (kindly provided by Regine Hengge-Aronis) was preadsorbed at 4°C overnight with 1 ml of a sonicated cell extract (containing approximately 2 mg of protein) of either the rpoS::Tn10 strain RH90 or the ΔrpoS strain RH100 in 50 mM Tris-Cl buffer (pH 8) containing 1 mM each EDTA and phenylmethylsulfonyl fluoride; following centrifugation at 12,000 × g for 20 min at 4°C, the supernatant was recovered and used in a total volume of 10 ml as the primary antibody preparation.
Other experimental techniques.
The procedures used for phage P1 transduction (8), transposon tagging with Tn10dCm and genetic mapping (22), and experiments involving DNA manipulations (32) were as described previously. Three primers, 5′-GAACCAGTTCAACACGCT-3′, 5′-ACCGAGGTAATGCGCTCGT-3′, and 5′-CCGATGGGCATCGAC-3′, which were specific, respectively, for the 5′ end, the middle, and the 3′ end of rpoS, were used for PCR amplification and DNA sequence determination of the rpoS locus. β-Galactosidase assays were performed by the method of Miller (22); enzyme specific activity values are reported in Miller units.
RESULTS
An rpoS::IS10 insertion mutant with rho-modulated σS activity in vivo.
It was shown earlier that the P1 promoter of the E. coli proU operon is σS regulated both in vivo and in vitro (20, 27-29); its in vivo activity (for example, in a wild-type strain such as GJ862) (Table 2) can be assayed by using plasmid pHYD275, which is a very-low-copy-number replicon that carries P1 upstream of the lacZ reporter gene (4). The starting point for this study were the unexpected observations (Table 2) that lac expression from pHYD275 was abolished in one of our laboratory strains, GJ829, but that it was restored in an isogenic derivative, GJ830, that was defective in the rho gene (encoding the transcription termination factor Rho; for a review, see reference 31). The rho mutation had little effect in a wild-type strain (Table 2, compare values for GJ862 and GJ863; see also reference [27]). An rpoS::Tn10 derivative of GJ830 was also negative for pHYD275-driven lac expression (data not shown), indicating that the rho mutation acted specifically and in a σS-dependent manner to restore proU P1-lac expression in GJ829.
TABLE 2.
Effect of rpoS-rho interaction on proU P1-lac expressiona
| Strain | Genotype | β-Galactosidase sp act with the following plasmid and conditions:
|
|||||
|---|---|---|---|---|---|---|---|
| pHYD275 (E. coli P1-lac)
|
pHYD373 (S. enterica P1-lac)
|
||||||
| No NaCl, 30°C | NaCl, 30°C | No NaCl, 10°C | No NaCl, 30°C | NaCl, 30°C | No NaCl, 10°C | ||
| GJ862 | rpoS+rho+ | 63 | 275 | 962 | 4 | 4 | 54 |
| GJ863 | rpoS+rho | 126 | 322 | 885 | 51 | 69 | 463 |
| GJ829 | rpoS::IS10 rho+ | 7 | 14 | 56 | 1 | 2 | 2 |
| GJ830 | rpoS::IS10 rho | 157 | 467 | 1,360 | 26 | 104 | 849 |
Cultures were grown to mid-exponential phase in trimethoprim-supplemented K-tryptone medium without or with 0.3 M NaCl at 30 or 10°C for β-galactosidase assays. Enzyme specific activities are reported in Miller units (22).
We used classical genetic techniques (22) first to obtain a new transposon Tn10dCm insertion allele (designated zgc-912::Tn10dCm) 85% cotransducible with the locus in GJ829 that confers rho-modulated P1 -lac expression and then to map the chloramphenicol resistance (Cmr) marker to the 62-min region of the chromosome (data not shown). The Cmr marker exhibited 85% linkage to rpoS, raising the possibility that the mutation in GJ829 that affects P1-lac expression is itself an rpoS allele.
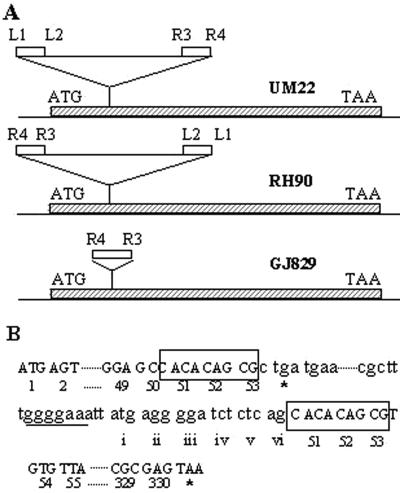
The linked Cmr marker also allowed us to construct, from wild-type strain GJ862, an isogenic pair of derivatives, GJ875 and GJ876, that carried, respectively, the mutant locus of GJ829 and its wild-type allele. PCR amplification of the rpoS gene from the two strains by using a pair of primers specific to the 5′ and 3′ ends of the gene (1.2 kb apart) indicated that the GJ829 mutation is associated with an insertion of 1.3 kb of DNA in the rpoS locus (data not shown). DNA sequence analysis revealed it to be an IS10 insertion disrupting the rpoS open reading frame after codon 53 (Fig. 2), and the mutation was designated rpoS365::IS10. The stock of strain GJ146 that had served as the immediate ancestor for GJ829 and GJ830 was also shown to carry the rpoS::IS10 mutation (data not shown); since GJ146 had been derived during several steps of Tets selection following successive transductions with Tn10 insertions (9), we presume that the IS10 transposition must have occurred subsequently, during routine strain maintenance, spontaneously, and unselected from one of the original Tn10-bearing loci in the chromosome. Incidentally, the site of rpoS365::IS10 insertion was identical to that for each of two different rpoS::Tn10 insertions oriented opposite one another and sequenced earlier (15) in strains RH90 and UM22 (Fig. 2), indicating that it is a hot-spot site for transpositions involving IS10 and its derivative composite transposons. Furthermore, data from an earlier study (27) indicated that the rho mutation does not restore proU P1-lac expression in strains carrying the rpoS359::Tn10 mutation of RH90.
FIG. 2.
Molecular description of the rpoS365:: IS10 insertion. (A) The positions (and orientations) of rpoS::Tn10 insertions previously characterized (15) in strains UM22 and RH90 are compared with those of the rpoS:: IS10 insertion in strain GJ829 (this study). The coding region of rpoS is shown as a hatched bar, and IS10 sequences (including those found in Tn10) are shown as open bars. Representations of Tn10 and IS10 are not to scale. To facilitate comparison, the four distinct ends of IS10 present in Tn10 (3) are marked as L1 and L2 (from IS10-L) and R3 and R4 (from IS10-R). (B) DNA sequence of the rpoS:: IS10 insertion, with particular reference to the sequence in the vicinity of the ends of IS10. Codons of rpoS are indicated by arabic numerals (and stop codons are indicated by asterisks); the 9-bp duplications flanking the ends of IS10 (whose sequence is shown in lowercase letters) are boxed. Codons from IS10 that are predicted to encode an N-terminal extension of the σSΔ1-50 peptide are indicated by small roman numerals, and the polypurine stretch (putative ribosome-binding site motif) is underlined.
A plasmid (pETF) carrying the rpoS+ gene (39) was able to complement the rpoS::IS10 mutation for pHYD275-directed lac expression in the rho+ strain GJ829 (data not shown).
Evidence for the synthesis of an σS polypeptide with a deletion of its N-terminal 50 amino acids (σSΔ1-50) in rpoS::IS10 rho mutants.
There were two conceivable explanations, not mutually exclusive, for the above results. Transcription initiating from proU P1 of S. enterica is subject to premature termination (that is, attenuation), and this phenomenon is known to be Rho dependent (27, 28). It was therefore possible that the rpoS::IS10 insertion mutation somehow accentuated the effect of Rho factor on transcription initiating from E. coli proU P1 as well. For example, even in an rpoS+ background, lacZ reporter gene expression from S. enterica proU P1 (on plasmid pHYD373) is absent in a rho+ strain (GJ862) and is stimulated in a rho mutant (GJ863) (27) (Table 2); in the rpoS::IS10 rho+ and rho derivatives (GJ829 and GJ830, respectively), lac expression from pHYD373 was virtually indistinguishable from that in the corresponding rpoS+ strains (Table 2).
The second explanation (31) is that the rho mutation acted only to relieve the polarity effect of the IS10 insertion in rpoS. The location of the rpoS::IS10 insertion is such that (i) translation initiating from the start codon of rpoS will be terminated immediately within the insertion element after codon 53, and (ii) a potential start site for translation exists (with a polypurine stretch situated upstream of the putative initiation codon stretch to serve as a ribosome-binding site) proceeding outward from the other end of IS10 to produce an in-frame fusion of six codons of IS10 with codons 51 to 330 of rpoS (Fig. 2). (The latter feature is absent from either of the Tn10 insertions at this same site in strains RH90 and UM22.) It was therefore possible that the rho mutation permitted transcription initiating from the rpoS promoter (and upstream promoters) to proceed through IS10 into the 3′ end of the rpoS gene and that the N-terminally truncated mutant σS polypeptide (hereafter referred to as σSΔ1-50) synthesized under these conditions contributed, either alone or in combination with the σS N-terminal fragment from positions 1 to 53 [σS(1-53)] to proU P1 expression in vivo.
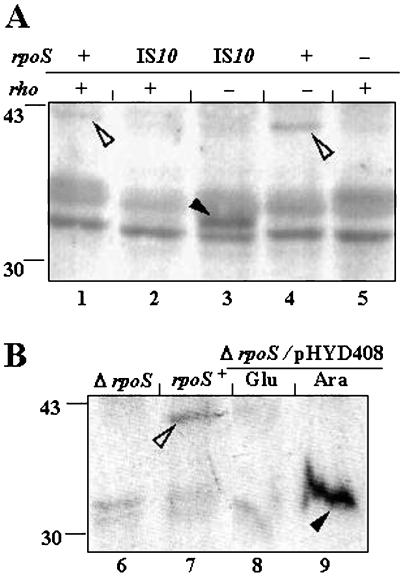
As a test of this second model, an immunoblot experiment was performed with anti-σS antiserum against cell extracts prepared from rho+ and rho derivatives of the rpoS+ and rpoS::IS10 strains (Fig. 3A). An immunoreactive protein corresponding to σS was present in the rpoS+ strains and was more prominent in the rho mutant (Fig. 3A, lane 4) than in the rho+ strain (lane 1), perhaps reflecting increased readthrough in the former from the promoter(s) for gene nlpD situated upstream of rpoS (17); the σS band was absent in the rpoS::IS10 strains (lanes 2 and 3) as well as in the rpoS::Tn10 mutant control (lane 5). On the other hand, a new protein cross-reacting with the anti-σS antiserum and whose faster migration was consistent with that for the postulated σSΔ1-50 polypeptide was detected only in the cell extract from the rpoS::IS10 rho derivative (Fig. 3A, lane 3). These observations lent support to the suggestion that, in a rho background, an N-terminally truncated σS protein was expressed from the chromosomal rpoS::IS10 allele.
FIG. 3.
Identification by immunoblotting of σSΔ1-50 expressed from the chromosome of an rpoS::IS10 strain (A) and from plasmid pHYD408 by Ara induction in a ΔrpoS strain (B). Anti-σS antiserum was used for immunoblot analysis of cell extracts (of cultures grown at 30°C in LB medium until 1 h after entry into stationary phase) from strains with different combinations of chromosomal rpoS and rho alleles (A) or from rho+ strains without or with plasmid pHYD408 (B). Cultures of the pHYD408 derivative were supplemented with either Glu or Ara and ampicillin. Strains were as follows: lane 1, GJ876; lane 2, GJ875; lane 3, GJ2755; lane 4, GJ2765; lane 5, RH90; lane 6, RH100; lane 7, GJ862; and lanes 8 and 9, GJ2782/pHYD408. To the left of each panel are shown the positions of migration of two marker proteins with sizes in kilodaltons. Open and closed arrowheads identify, respectively, bands corresponding to wild-type σS and σSΔ1-50.
σSΔ1-50 expression from a heterologous promoter.
As a further test of the model invoking a function for σSΔ1-50, we designed experiments to address the question of whether the putative IS10-encoded translation initiation signals are indeed proficient for the expression of the truncated protein from a heterologous inducible promoter in rho+ strains and, if so, whether the protein exhibits sigma activity in vivo. For this purpose, we used the thermostable high-fidelity Pwo DNA polymerase enzyme to amplify by PCR, from the chromosome of the rpoS::IS10 strain GJ875, an 0.85-kb DNA segment comprising the coding region of the putative σSΔ1-50 peptide along with the adjacent 43 bp from the end of IS10. The DNA segment was then cloned downstream of the Ara-inducible promoter in plasmid vector pBAD24 (11), and the resulting plasmid was designated pHYD408.
An immunoblot experiment with anti-σS antiserum was undertaken to demonstrate Ara-induced synthesis from the pHYD408 template of the N-terminally truncated σSΔ1-50 polypeptide (Fig. 3B). As in the previous experiment, an immunoreactive protein corresponding to wild-type σS was detected only in cells of the rpoS+ strain (Fig. 3B, lane 7) and was absent from those of the ΔrpoS derivatives (lanes 6, 8, and 9). Instead, a cross-reacting protein whose electrophoretic mobility was consistent with that expected for σSΔ1-50 was present in abundance in Ara-grown (Fig. 3B, lane 9) but not Glu-grown (lane 8) cells of the ΔrpoS/pHYD408 derivative.
In experiments aimed at testing in vivo σ activity, we were able to show that plasmid pHYD408 could also direct substantial lac expression, specifically in an Ara-inducible manner, from the E. coli proU P1 promoter on pHYD275 in rho+ strains that carried the rpoS::IS10, the rpoS359::Tn10, or the ΔrpoS mutation on the chromosome (Table 3). These results provide additional support for the model invoking σ activity in vivo for the σSΔ1-50 protein and furthermore suggest that σS(1-53) encoded by the chromosomal rpoS::IS10 allele may not be essential for this purpose (since considerable proU P1-lac expression was elicited in the ΔrpoS background as well).
TABLE 3.
Ara-induced expression of proU P1 from pHYD275 in pHYD408 derivatives of rpoS rho+ strainsa
| Strain | Chromosomal rpoS genotype | β-Galactosidase sp act with:
|
|
|---|---|---|---|
| Glu | Ara | ||
| GJ2758 | rpoS::IS10 | 17 | 783 |
| GJ2756 | rpoS::Tn10 | 11 | 447 |
| GJ2778 | ΔrpoS | 10 | 263 |
Cultures were incubated in LB medium supplemented with trimethoprim, ampicillin, and either Glu or Ara at 30°C until 1 h after entry into stationary phase for β-galactosidase assays. Enzyme specific activities are reported in Miller units (22).
Recognition of other σS-dependent promoters by σSΔ1-50.
With the aid of the respective promoter-lac operon fusions, we examined the in vivo activity of σSΔ1-50 (expressed either from the chromosomal rpoS::IS10 allele in a rho background or by Ara induction of ΔrpoS rho+ strains carrying plasmid pHYD408) on the promoters of three other σS-dependent genes, katE, osmY, and csiD. Although each of the two methods used to obtain the expression of σSΔ1-50 protein had their individual shortcomings—namely, a nonspecific reduction, associated with the pleiotropic rho mutation, of lac expression from promoters other than proU P1 even in control rpoS+ strains (Table 4) (see also reference 27) and considerable overproduction of the mutant protein in the Ara induction experiment—we reasoned that their potential confounding effects might not overlap.
TABLE 4.
Activity of σSΔ1-50 on σS-specific promotersa
| Promoter-lac fusion | β-Galactosidase sp act in the following strain:
|
|||||||
|---|---|---|---|---|---|---|---|---|
|
rpoS+
|
ΔrpoS
|
rpoS::IS10
|
ΔrpoS rho+/pHYD408 with:
|
|||||
| rho+ | rho | rho+ | rho | rho+ | rho | Glu | Ara | |
| katE | 772 | 533 | 16 | 33 | 38 | 141 | 23 | 586 |
| osmY | 415 | 348 | 14 | 14 | 10 | 46 | 13 | 141 |
| csiD | 948 | 612 | 4 | 21 | 48 | 214 | 3 | 324b |
Cultures were incubated in LB medium (supplemented, for pHYD408 derivatives, with ampicillin and either Glu or Ara) at 30°C until 1 h after entry into stationary phase for β-galactosidase assays. Enzyme specific activities are reported in Miller units (22). Strains used for each promoter-lac fusion study were as follows (in the order rpoS+ rho+, rpoS+ rho, rpoS::IS10 rho+, rpoS::IS10 rho, ΔrpoS rho+, and ΔrpoS rho): katE, GJ2770, GJ2771, GJ2789, GJ2790, GJ2782, and GJ2791; osmY, GJ888, GJ889, GJ894, GJ2732, GJ2781, and GJ2788; and csiD, GJ884, GJ885, GJ893, GJ2731, GJ2780, and GJ2787.
Value obtained with 0.02% Ara (see Fig. 4).
The results from the experiments involving the rpoS::IS10 rho strains indicated that, unlike the situation with proU P1, the other three promoters were transcribed to the extent of only about 15 to 30% in the presence of σSΔ1-50 synthesized from the chromosomal allele, compared to their activities in the isogenic rho rpoS+ strains (Table 4). Nevertheless, in vivo lac expression with σSΔ1-50 was higher than that in the corresponding rho ΔrpoS strains. The Lac phenotypes of the different promoter-lac fusion strains (rho+ and rho, in combination with rpoS+, rpoS::IS10, and ΔrpoS) on MacConkey lactose agar plates correlated well with the β-galactosidase specific activities reported in Table 4 (data not shown).
On the other hand, when σSΔ1-50 was overproduced in the ΔrpoS rho+ strains by Ara induction from pHYD408, the level of expression of each of the three lac fusions was much higher and, at least for katE, comparable to that in the corresponding haploid rpoS+ rho+ derivatives (Table 4). A substantial level of expression was also obtained for each of the lac fusions with Ara induction of pHYD408 transformants of rpoS::IS10 rho+ derivatives (data not shown).
In the course of these studies, we observed that whereas the activities of other promoters, such as katE, in the pHYD408 derivatives (ΔrpoS) were unaffected when the concentration of Ara as an inducer was varied between 0.2% (routinely used) and 0.02% (data not shown), the expression of csiD-lac was maximal at 0.02% Ara and then progressively declined with increasing sugar concentrations (Fig. 4). Even in a control rpoS+ strain, GJ2785/pBAD24 (in which, as expected, the synthesis of σS could occur without Ara) (Fig. 4), csiD-lac expression was progressively repressed by Ara at concentrations above 0.02% (Fig. 4). It is known that the csiD promoter requires both σS and cyclic AMP (cAMP)-cAMP receptor protein for its activity (21), and the present results therefore suggest (i) that the promoter is catabolite repressed in the rpoS+ strain at Ara concentrations above 0.02% and (ii) that a holoenzyme with the σSΔ1-50 subunit is also proficient for catabolite repression at this promoter.
FIG. 4.
β-Galactosidase specific activities in a pair of csiD::lac strain derivatives: GJ2785 (rpoS+) carrying plasmid pBAD24 (vector) and GJ2780 (ΔrpoS) carrying plasmid pHYD408 (with the gene for σSΔ1-50). Cultures were grown at 30°C in LB medium supplemented with ampicillin and the indicated concentrations of Ara until 1 h after entry into stationary phase. Enzyme specific activities are reported in Miller units (22). White bars, GJ2785/pBAD24; black bars, GJ2780/pHYD408.
Environmental regulation of σSΔ1-50.
As mentioned above, it is believed that the regulation of cellular σS levels by environmental variables, such as growth phase, temperature, and osmolarity, is achieved by a combination of transcriptional, translational, and posttranslational control mechanisms (12, 14). On the other hand, Zgurskaya et al. (43) suggested that the increase in σS content in stationary-phase cells may be accounted for solely by the increased stability of σS, and Becker et al. (1) showed that the increase under these conditions occurs even in the absence of rpoS transcriptional or translational regulation.
In the present study, when we used the chromosomal rpoS::IS10 rho strain GJ830, we observed that lac reporter gene expression from proU P1 of E. coli or S. enterica was induced both by elevated osmolarity and by growth at 10°C and that the magnitude of such induction (4- and 10-fold, respectively) was comparable to that in the rpoS+ strains (Table 2). Given that the cis regulatory sequences for native rpoS expression have been separated from the coding region of σSΔ1-50 by the IS10 insertion in GJ830, these results raised the possibility that environmental regulation of σSΔ1-50 synthesis could occur even in the absence of such sequences, but alternative explanations were not excluded by the data.
We therefore examined whether the cellular content of σSΔ1-50 could be regulated by the same environmental cues as those reported earlier for wild-type σS (12, 14), even when the former is expressed by Ara induction from pHYD408, that is, from a template devoid of the transcriptional and translational controls of native rpoS. The intracellular concentrations of σSΔ1-50 were assayed both directly by immunoblotting with anti-σS antiserum (Fig. 5) and indirectly by determination of lac reporter gene expression from the proU P1 and katE promoters (Table 5).
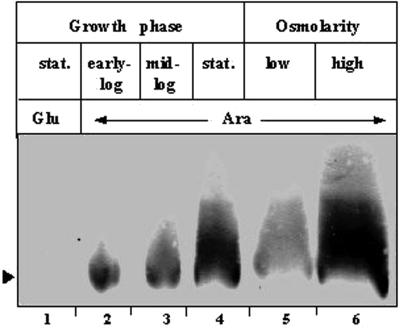
FIG. 5.
Environmental regulation of cellular σSΔ1-50 content. Anti-σS antiserum was used for immunoblot analysis of cell extracts of ΔrpoS strain GJ2782 carrying plasmid pHYD408 and grown with ampicillin and Ara supplementation in LB medium for the growth phase regulation studies to an A600 of 0.38 (lane 2), 0.7 (lane 3), or 1.2 (lane 4) or in K-tryptone medium without (lane 5) or with (lane 6) added 0.3 M NaCl for the osmotic regulation studies to an A600 of about 0.4. A control culture was grown in LB medium with Glu supplementation to an A600 of 1.2 (lane 1). stat., stationary phase. The arrowhead identifies the band corresponding to σSΔ1-50.
TABLE 5.
Environmental regulation with σSΔ1-50a
| Variable(s) | Culture condition | β-Galactosidase sp act for lac fusion with the following promoter in the indicated strain:
|
|||
|---|---|---|---|---|---|
|
proU P1
|
katE
|
||||
| rpoS+ | ΔrpoS/pHYD408 | rpoS+ | ΔrpoS/pHYD408 | ||
| Temp and growth phase | 30°C, log | 63 | 27 | 110 | 50 |
| 30°C, stationary | 536 | 263 | 772 | 586 | |
| 10°C, log | 279 | 263 | 1,686 | 645 | |
| Osmolarity | No NaCl | 63 | 21 | 297 | 65 |
| 0.3 M NaCl | 275 | 193 | 576 | 333 | |
Cultures for β-galactosidase assays were grown in LB medium for experiments involving growth phase (log or mid-exponential phase and 1 h after entry into stationary phase) and temperature as variables and in K-tryptone medium (to mid-exponential phase at 30°C) for those involving osmolarity as the variable. For strain derivatives with the proU P1-lac fusion (on plasmid pHYD275), the medium was supplemented with trimethoprim; for derivatives with plasmid pHYD408, it was supplemented with ampicillin and Ara. Enzyme specific activities are reported in Miller units (22). Strains used were as follows (in the order rpoS+ and ΔrpoS): for proU P1-lac, GJ862 and GJ2778 (each with pHYD275); and for katE-lac, GJ2770 and GJ2782.
The data from the immunoblotting experiment indicated that the levels of σSΔ1-50 expressed following Ara induction in a ΔrpoS strain increase progressively from the early exponential to the stationary growth phase (Fig. 5, lanes 2 through 4) and that the levels are also increased by growth under conditions of elevated osmolarity (Fig. 5, compare lanes 5 and 6).
In the indirect assay (Table 5), lacZ reporter gene expression from the proU P1 and katE promoters was significantly higher in the stationary growth phase than in the exponential growth phase. The comparisons were done with cultures that had been subjected to Ara induction for both equal lengths of time and the same numbers of generations of growth. Similarly, the osmotic inducibility of both promoters was observed in derivatives expressing σSΔ1-50 from pHYD408 to the same extent as in the rpoS+ strain. Finally, both proU P1-lac transcription and katE-lac transcription directed by σSΔ1-50 were substantially higher in cultures grown at 10°C than in those grown at 30°C, and the magnitude of low-temperature induction was equivalent to that seen with wild-type σS. The significance of these findings for the understanding of σS synthesis and turnover mechanisms is discussed below.
DISCUSSION
Properties of σSΔ1-50 as a σ factor.
Previous studies on σ70 showed that its N-terminal domain 1.1 is essential for the protein to function as a σ factor of RNA polymerase in bacterial cells (5, 6, 34). The present study demonstrates that a mutant version of σS (σSΔ1-50) in which the N-terminal 50 amino acids have been deleted is still capable of directing transcription in vivo from a variety of σS-dependent promoters.
The data presented in Table 2 suggest that chromosomally expressed σSΔ1-50 may be as proficient as wild-type σS in its ability to initiate transcription from the E. coli and S. enterica proU P1 promoters. On the other hand, the in vivo activities of three other promoters, katE, csiD, and osmY, in the presence of σSΔ1-50 were only fractions (15 to 30%) of the values obtained with σS (Table 4).
Two alternative explanations, not mutually exclusive, are possible for the latter finding. The first is that it represents a defect in σS activity associated with the N-terminal truncation (that can be overcome at least partially by increased expression of the mutant protein from plasmid pHYD408). With a K173E mutant of σS, Becker et al. (1) had earlier analogously demonstrated normal activity at one promoter (csiD) and three- to fivefold reduced activities at several others (osmY, bolA, and otsB). The second explanation is that of a confounding effect of the rho mutation on some σS-dependent promoters. As reported earlier (27) and as is also clear from the data in Tables 2 and 4, even in an rpoS+ background the rho mutation has opposite effects on expression from proU P1 (increase) and expression from the csiD, osmY, or katE promoter (decrease).
In vitro experiments to assess the binding affinity of σSΔ1-50 for core RNA polymerase and to determine the transcription activity of the reconstituted enzyme at the various promoters will help address these questions. Catabolite repression at csiD was preserved under conditions where the promoter was active in vivo with σSΔ1-50, suggesting that an interaction of RNA polymerase bearing the mutant subunit with cAMP-cAMP receptor protein is normal (21).
σSΔ1-50 and environmental regulation of σS-specific promoters.
As mentioned above, the intracellular σS concentration varies according to the growth conditions and is regulated by different mechanisms, including those that operate at the steps of rpoS transcription, rpoS translation, and σS proteolysis (12, 14). There also exists substantial evidence for the notion that regulation of the intracellular σS content explains the regulation of expression of genes of the σS regulon by environmental variables, such as osmolarity (13, 16), growth phase (16, 41), and temperature (36), or by factors such as Hfq (23, 24) and ClpXP (33).
In the present study, therefore, the first suggestion that σSΔ1-50 may exhibit normal environmental regulation even in the absence of the rpoS transcriptional and translational control signals came from the data in Table 2, which showed that osmotic and cold induction of proU P1 was unaffected in the chromosomal rho rpoS::IS10 strain (in which the rpoS expression control sequences, although still retained in cis, are separated from the coding region for σSΔ1-50 by IS10).
This possibility was then validated in experiments in which σSΔ1-50 was designed to be expressed from a plasmid construct (pHYD408) that completely lacked the rpoS transcriptional and translational control sequences. Although Ara-induced synthesis of σSΔ1-50 occurred at higher-than-physiological levels in these experiments, environmental regulation by growth phase, low temperature, and high osmolarity was demonstrated by direct immunoblotting and indirect reporter gene expression assays. The nature and extent of regulation so identified for σSΔ1-50 are similar to those described earlier for wild-type σS (12, 14).
Since normal environmental regulation of σSΔ1-50 could be demonstrated even in the absence of the transcriptional and translational control elements of wild-type rpoS, our data suggest that alternative mechanisms, such as those affecting proteolytic degradation of σS, may be the primary determinants for regulation under these conditions. The σSΔ1-50 protein retains the σS turnover element, which has been implicated in the regulation by RssB of ClpXP-catalyzed degradation of the protein (1).
Although regulation at the level of σS proteolysis was suggested earlier as one of several mechanisms involved in osmotic and stationary-phase induction of the cognate σS-specific promoters (12), its relative importance in either of these phenomena had so far not been defined. Nor had such a mechanism been postulated to participate in the phenomenon of low-temperature induction, since previous studies had described roles for only DsrA RNA and Hfq protein in the stimulation of rpoS translation during low-temperature growth (18, 30, 36, 37).
In summary, therefore, three major conclusions that have emerged from the present investigation are that (i) the N-terminal region of σS is not essential for in vivo sigma activity on at least one promoter (proU P1), although its removal leads to an apparent partial reduction of activity at three other promoters; (ii) environmental regulation of the σS regulon is largely unaffected even in the absence of cis elements needed for the transcriptional and translational control of rpoS; and (iii) the N-terminal region is also not necessary for the posttranslational regulation of σS.
Acknowledgments
We thank Jon Beckwith, Carol Gross, Regine Hengge-Aronis, Akira Ishihama, and Kan Tanaka for making available various strains and plasmids that were used in this study. The anti-σS antiserum was kindly provided by Regine Hengge-Aronis. The assistance of Mehar Sultana and N. Nagesh with oligonucleotide synthesis and DNA sequencing, respectively, is acknowledged. The immunoblot experiment whose results are reported in Fig. 3A was performed by R. Harinarayanan.
REFERENCES
- 1.Becker, G., E. Klauck, and R. Hengge-Aronis. 1999. Regulation of RpoS proteolysis in Escherichia coli: the response regulator RssB is a recognition factor that interacts with the turnover element in RpoS. Proc. Natl. Acad. Sci. USA 96:6439-6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berlyn, M. K. B. 1998. Linkage map of Escherichia coli K-12, edition 10: the traditional map. Microbiol. Mol. Biol. Rev. 62:814-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chalmers, R., S. Sewitz, K. Lipkow, and P. Crellin. 2000. Complete nucleotide sequence of Tn10. J. Bacteriol. 182:2970-2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dattananda, C. S., K. Rajkumari, and J. Gowrishankar. 1991. Multiple mechanisms contribute to osmotic inducibility of proU operon expression in Escherichia coli: demonstration of two osmoresponsive promoters and of a negative regulatory element within the first structural gene. J. Bacteriol. 173:7481-7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dombroski, A. J., W. A. Walter, and C. A. Gross. 1993. Amino-terminal amino acids modulate σ-factor DNA-binding activity. Genes Dev. 7:2446-2455. [DOI] [PubMed] [Google Scholar]
- 6.Dombroski, A. J., W. A. Walter, M. T. Record Jr., D. A. Siegele, and C. A. Gross. 1992. Polypeptides containing highly conserved regions of transcription initiation factor σ70 exhibit specificity of binding to promoter DNA. Cell 70:501-512. [DOI] [PubMed] [Google Scholar]
- 7.Gopal, V., and D. Chatterji. 1997. Mutations in the 1.1 subdomain of Escherichia coli sigma factor σ70 and disruption of its overall structure. Eur. J. Biochem. 244:613-618. [DOI] [PubMed] [Google Scholar]
- 8.Gowrishankar, J. 1985. Identification of osmoresponsive genes in Escherichia coli: evidence for participation of potassium and proline transport systems in osmoregulation. J. Bacteriol. 164:434-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gowrishankar, J., P. Jayashree, and K. Rajkumari. 1986. Molecular cloning of an osmoregulatory locus in Escherichia coli: increased proU gene dosage results in enhanced osmotolerance. J. Bacteriol. 168:1197-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gruber, T. M., D. Markov, M. M. Sharp, B. A. Young, C. Z. Lu, H. J. Zhong, I. Artsimovitch, K. M. Geszvain, T. M. Arthur, R. R. Burgess, R. Landick, K. Severinov, and C. A. Gross. 2001. Binding of the initiation factor σ70 to core RNA polymerase is a multistep process. Mol. Cell 8:21-31. [DOI] [PubMed] [Google Scholar]
- 11.Guzman, L.-M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hengge-Aronis, R. 2000. The general stress response in Escherichia coli, p.161-178. In G. Storz and R. Hengge-Aronis (ed.), Bacterial stress responses. American Society for Microbiology, Washington, D.C.
- 13.Hengge-Aronis, R., R. Lange, N. Henneberg, and D. Fischer. 1993. Osmotic regulation of rpoS-dependent genes in Escherichia coli. J. Bacteriol. 175:259-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishihama, A. 2000. Functional modulation of Escherichia coli RNA polymerase. Annu. Rev. Microbiol. 54:499-518. [DOI] [PubMed] [Google Scholar]
- 15.Ivanova, A., M. Renshaw, R. V. Guntaka, and A. Eisenstark. 1992. DNA base sequence variability in katF (putative sigma factor) gene of Escherichia coli. Nucleic Acids Res. 20:5479-5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lange, R., and R. Hengge-Aronis. 1994. The cellular content of the σS subunit of RNA polymerase in Escherichia coli is controlled at the levels of transcription, translation, and protein stability. Genes Dev. 8:1600-1612. [DOI] [PubMed] [Google Scholar]
- 17.Lange, R., and R. Hengge-Aronis. 1994. The nlpD gene is located in an operon with rpoS on the Escherichia coli chromosome and encodes a novel lipoprotein with a potential function in cell wall formation. Mol. Microbiol. 13:733-743. [DOI] [PubMed] [Google Scholar]
- 18.Lease, R. A., and M. Belfort. 2000. A trans-acting RNA as a control switch in Escherichia coli: DsrA modulates function by forming alternative structures. Proc. Natl. Acad. Sci. USA 97:9919-9924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lonetto, M., M. Gribskov, and C. A. Gross. 1992. The σ70 family: sequence conservation and evolutionary relationships. J. Bacteriol. 174:3843-3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manna, D., and J. Gowrishankar. 1994. Evidence for involvement of proteins HU and RpoS in transcription of the osmoresponsive proU operon in Escherichia coli. J. Bacteriol. 176:5378-5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marschall, C., V. Labrousse, M. Kreimer, D. Weichart, A. Kolb, and R. Hengge-Aronis. 1998. Molecular analysis of the regulation of csiD, a carbon starvation-inducible gene in Escherichia coli that is exclusively dependent on σS and requires activation by cAMP-CRP. J. Mol. Biol. 276:339-353. [DOI] [PubMed] [Google Scholar]
- 22.Miller, J. H. 1992. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 23.Muffler, A., D. Fischer, and R. Hengge-Aronis. 1996. The RNA-binding protein HF-I, known as a host factor for phase Qβ RNA replication, is essential for rpoS translation in Escherichia coli. Genes Dev. 10:1143-1151. [DOI] [PubMed] [Google Scholar]
- 24.Muffler, A., D. D. Traulsen, D. Fischer, R. Lange, and R. Hengge-Aronis. 1997. The RNA-binding protein HF-I plays a global regulatory role which is largely, but not exclusively, due to its role in expression of the σS subunit of RNA polymerase in Escherichia coli. J. Bacteriol. 179:297-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mulvey, M. R., and P. C. Loewen. 1989. Nucleotide sequence of katF of Escherichia coli suggests KatF protein is a novel σ transcription factor. Nucleic Acids Res. 17:9979-9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagai, H., and N. Shimamoto. 1997. Regions of the Escherichia coli primary sigma factor σ70 that are involved in interaction with RNA polymerase core enzyme. Genes Cells 2:725-734. [DOI] [PubMed] [Google Scholar]
- 27.Rajkumari, K., and J. Gowrishankar. 2001. In vivo expression from the RpoS-dependent P1 promoter of the osmotically regulated proU operon in Escherichia coli and Salmonella enterica serovar Typhimurium: activation by rho and hns mutations and by cold stress. J. Bacteriol. 183:6543-6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rajkumari, K., A. Ishihama, and J. Gowrishankar. 1997. Evidence for transcription attenuation rendering cryptic a σS-dependent promoter of the osmotically regulated proU operon of Salmonella typhimurium. J. Bacteriol. 179:7169-7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rajkumari, K., S. Kusano, A. Ishihama, T. Mizuno, and J. Gowrishankar. 1996. Effects of H-NS and potassium glutamate on σS- and σ70-directed transcription in vitro from osmotically regulated P1 and P2 promoters of proU in Escherichia coli. J. Bacteriol. 178:4176-4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Repoila, F., and S. Gottesman. 2001. Signal transduction cascade for regulation of RpoS: temperature regulation of DsrA. J. Bacteriol. 183:4012-4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richardson, J. P., and J. Greenblatt. 1996. Control of RNA chain elongation and termination, p. 822-848. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. American Society for Microbiology, Washington, D.C.
- 32.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 33.Schweder, T., K.-H. Lee, O. Lomovskaya, and A. Matin. 1996. Regulation of Escherichia coli starvation sigma factor by ClpXP protease. J. Bacteriol. 178:470-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharp, M. M., C. L. Chan, C. Z. Lu, M. T. Marr, S. Nechaev, E. W. Merritt, K. Severinov, J. W. Roberts, and C. A. Gross. 1999. The interface of σ with core RNA polymerase is extensive, conserved, and functionally specialized. Genes Dev. 13:3015-3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singer, M., T. A. Baker, G. Schnitzler, S. M. Deischel, M. Goel, W. Dove, K. J. Jaacks, A. D. Grossman, J. W. Erickson, and C. A. Gross. 1989. A collection of strains containing genetically linked alternating antibiotic resistance elements for genetic mapping of Escherichia coli. Microbiol. Rev. 53:1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sledjeski, D. D., A. Gupta, and S. Gottesman. 1996. The small RNA, DsrA, is essential for the low temperature expression of rpoS during exponential growth in Escherichia coli. EMBO J. 15:3993-4000. [PMC free article] [PubMed] [Google Scholar]
- 37.Sledjeski, D. D., C. Whitman, and A. Zhang. 2001. Hfq is necessary for regulation by the untranslated RNA DsrA. J. Bacteriol. 183:1997-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanaka, K., K. Handel, P. C. Loewen, and H. Takahashi. 1997. Identification and analysis of the rpoS-dependent promoter of katE, encoding catalase HPII in Escherichia coli. Biochim. Biophys. Acta 1352:161-166. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka, K., Y. Takayanagi, N. Fujita, A. Ishihama, and H. Takahashi. 1993. Heterogeneity of principal σ factor in Escherichia coli: the rpoS gene product, σ38, is a second principal σ factor of RNA polymerase in stationary phase Escherichia coli. Proc. Natl. Acad. Sci. USA 90:3511-3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vuthoori, S., C. W. Bowers, A. McCracken, A. J. Dombroski, and D. M. Hinton. 2001. Domain 1.1 of the σ70 subunit of Escherichia coli RNA polymerase modulates the formation of stable polymerase/promoter complexes. J. Mol. Biol. 309:561-572. [DOI] [PubMed] [Google Scholar]
- 41.Weichart, D., R. Lange, N. Henneberg, and R. Hengge-Aronis. 1993. Identification and characterization of stationary phase-inducible genes in Escherichia coli. Mol. Microbiol. 10:407-420. [PubMed] [Google Scholar]
- 42.Wilson, C., and A. J. Dombroski. 1997. Region 1 of σ70 is required for efficient isomerization and initiation of transcription by Escherichia coli RNA polymerase. J. Mol. Biol. 267:60-74. [DOI] [PubMed] [Google Scholar]
- 43.Zgurskaya, H. I., M. Keyhan, and A. Matin. 1997. The σS level in starving Escherichia coli cells increases solely as a result of its increased stability, despite decreased synthesis. Mol. Microbiol. 24:643-651. [DOI] [PubMed] [Google Scholar]