Abstract
Mucoid, mucA mutant Pseudomonas aeruginosa cause chronic lung infections in cystic fibrosis (CF) patients and are refractory to phagocytosis and antibiotics. Here we show that mucoid bacteria perish during anaerobic exposure to 15 mM nitrite (NO2–) at pH 6.5, which mimics CF airway mucus. Killing required a pH lower than 7, implicating formation of nitrous acid (HNO2) and NO, that adds NO equivalents to cellular molecules. Eighty-seven percent of CF isolates possessed mucA mutations and were killed by HNO2 (3-log reduction in 4 days). Furthermore, antibiotic-resistant strains determined were also equally sensitive to HNO2. More importantly, HNO2 killed mucoid bacteria (a) in anaerobic biofilms; (b) in vitro in ultrasupernatants of airway secretions derived from explanted CF patient lungs; and (c) in mouse lungs in vivo in a pH-dependent fashion, with no organisms remaining after daily exposure to HNO2 for 16 days. HNO2 at these levels of acidity and NO2– also had no adverse effects on cultured human airway epithelia in vitro. In summary, selective killing by HNO2 may provide novel insights into the important clinical goal of eradicating mucoid P. aeruginosa from the CF airways.
Introduction
Pseudomonas aeruginosa is an important pathogen that is most refractory to therapy when it forms biofilms in the airways of cystic fibrosis (CF) patients. CF is caused by mutations in the CF transmembrane conductance regulator (CFTR) gene. Although airway CFTR functions predominantly as an epithelial Cl– channel, it serves to coordinate Na+ absorption and Cl– secretion to produce sufficient airway surface liquid (ASL) for normal mucus clearance (1). Without functional CFTR, isotonic hyperabsorption of ASL, driven by enhanced absorption of Na+ via the epithelial sodium channel, results in ASL depletion, mucus concentration, and the formation of stagnant mucus plaques (2). Another feature of CF ion transport dysfunction is that the ASL may be acidified (pH <6.5) due to defective bicarbonate (HCO3–) ion transport (3).
Two simultaneous seminal studies have recently indicated that the mucus layer lining the CF airway lumen is anaerobic and that robust biofilm formation by P. aeruginosa occurs under such conditions (4, 5). The anaerobic nature of the CF airway mucus reflects the oxygen-consumptive activities of airway epithelia, P. aeruginosa, and other opportunistic pathogens as well as neutrophils that combat infection. As chronic CF lung disease progresses, mucoid, alginate-overproducing strains emerge and become the predominant form (6). Mucoid P. aeruginosa biofilms are inherently resistant to antibiotics (7) and phagocytic neutrophils (8). Although several gene products have been reported to stimulate either a genotypic or phenotypic switch to the mucoid form, the best-characterized mechanism of mucoid conversion in CF isolates is via mutations in mucA, encoding an anti-ς factor (9). Without MucA, the extracytoplasmic ς factor AlgT(U) transcribes genes involved in alginate biosynthesis. Mutations in mucA and mucoid conversion can be triggered in vitro when biofilms are treated with H2O2 at levels similar to those generated by human neutrophils (10), professional phagocytes that are abundant in the CF airways. Approximately 84% of mucoid CF isolates (n = 53) in the US have been shown to possess mucA mutations (11). In contrast, mucoid mucA mutant bacteria are found in approximately 44% of the CF isolates from Australia, although the number of patients studied was substantially less than in the US cohort (12).
An important link between mucoidy and anaerobic metabolism by P. aeruginosa was identified in a study demonstrating that mucoid organisms were incapable of reversion to their nonmucoid, antibiotic- and phagocyte-susceptible counterparts during anaerobic growth (13), results that were confirmed in 2002 by Wyckoff et al. (14). Recently, Worlitzsch and colleagues reported that anaerobic ASL favored production of alginate by P. aeruginosa (4). P. aeruginosa is capable of robust anaerobic growth by respiration using nitrate (NO3–) or nitrite (NO2–) as terminal electron acceptors (5). NO3– and NO2– are present in CF ASL (15–18) and sputum (19), and NO3– levels have been estimated as high as 600 μM (19), concentrations permissive for anaerobic P. aeruginosa growth in vitro and in vivo (20). Still, during anaerobic growth, P. aeruginosa must control the levels of a toxic intermediate of NO2– reduction, NO, by synthesis of protective NO reductase (NOR) (5). This requirement was demonstrated by the observation that overproduction of NO by anaerobic P. aeruginosa biofilms lacking the rhl quorum sensing circuit caused a metabolic suicide of these bacteria, an event that was prevented by a NO scavenger (21).
NO is also produced in normal airway epithelia by 3 different NO synthases (NOSs). Neuronal NOS and eNOS isoforms are constitutively expressed. The third isoform (iNOS) is also constitutively expressed in the airways but is inducible and upregulated in response to proinflammatory cytokines and bacterial LPS (22). Specifically, the iNOS2 class of enzymes contributes most effectively to the antimicrobial armament of the airway. However, in chronic CF, iNOS2 activity is significantly reduced (23), and this defect is thought to contribute to the persistence of airway P. aeruginosa populations.
Herein we describe a novel approach to killing mucoid P. aeruginosa. This is attributable in part to the organism’s extremely low nitrite reductase (NIR) and NOR activity, a defect dependent upon mucA mutations. We investigated the mechanism of this effect in in vitro human studies and explored the therapeutic potential of NO2– in both human and mouse safety and mouse in vivo efficacy studies. Treatment of mucoid, mucA mutant bacteria with NO2– at pH 6.5 under anaerobic conditions, similar to conditions within mucopurulent secretions in the airways of CF patients, led to the death of these organisms. Ultimately, this observation may lead to the development of novel therapies for CF patients colonized with mucoid P. aeruginosa.
Results
CF airway luminal secretion pH is slightly acidic.
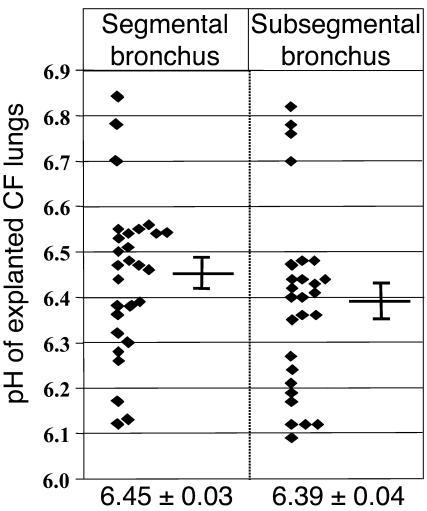
The goal of this study was to test the hypothesis that mucoid P. aeruginosa are far more adept at growing during anaerobic respiration than nonmucoid bacteria, which is based on the fact that mucoid bacteria emerge and predominate during the chronic stages of CF airway disease. However, to accurately test this hypothesis, we wanted to assure that our medium pH was identical to that of the CF airway mucus. Previous in vitro studies suggest that the pH of the CF ASL is less than 6.5 (3). However, it is conceivable that the pH of mucopurulent secretions within CF airways might differ in vivo. Hence, we performed in situ pH measurements of luminal secretions from freshly explanted lungs removed from 9 different CF patients at the time of transplantation. The pH of the secretions was slightly lower than what was observed in vitro: 6.45 ± 0.03 in segmental airways and somewhat lower in more distal subsegmental bronchi (6.39 ± 0.04; Figure 1). These results guided our selection of pH 6.5 for subsequent experiments.
Figure 1.
The pH of CF airway mucus is slightly acidic. In situ pH measurements of mucopurulent airway secretions from CF airways were made as described in Methods. Each measurement is presented as an individual data point in the scatter plots. The mean ± SEM is shown below each panel.
Mucoid, mucA mutant bacteria are sensitive to acidified NO2–.
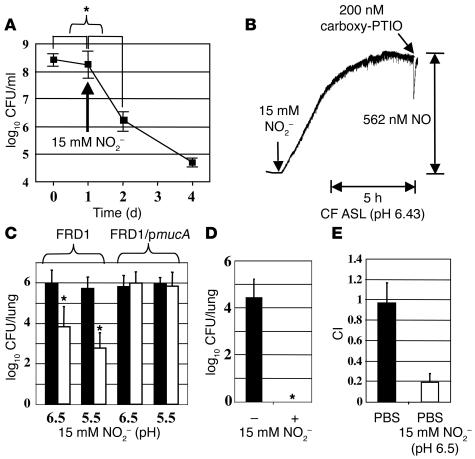
Based upon the slightly acidic pH measurements of segmental and subsegmental bronchi from CF transplant patients discussed above, we elected to grow various well-characterized P. aeruginosa strains at pH 6.5 under strict anaerobic conditions. Thus, upon anaerobic culture of P. aeruginosa at pH 6.5 with 15 mM NO3– (electron acceptor), mucoid P. aeruginosa strain FRD1 grew more slowly than nonmucoid strains PAO1 and FRD1/pmucA. Strain FRD1 is the best characterized mucoid, mucA mutant derived from a CF patient (24). However, no difference in viability patterns was observed (Figure 2A). Using 15 mM NO2–, however, mucoid FRD1 was killed at a rate of approximately 90% per day, while nonmucoid strains PAO1 and FRD1/pmucA remained viable over the 4-day incubation (Figure 2B).
Figure 2.
Mucoid P. aeruginosa FRD1 is selectively killed by NO2– in a pH-dependent manner. Anaerobic growth of P. aeruginosa strains at pH 6.5 using 15 mM NO3– or NO2– as a terminal electron acceptor. Aerobic overnight suspensions of PAO1, FRD1, and FRD1/pmucA were diluted 100-fold for the main anaerobic culture with NO3– (A) or NO2– (B). CFUs were enumerated each day and plotted as linear (A) and logarithmic (B) graphs. (C) NO2– sensitivity versus pH. FRD1 or FRD1/pmucA were seeded onto LB agar buffered at the indicated pH value. After placing a filter disk containing 10 μl of 1 M NO2–, the plates were incubated anaerobically for 48 hours and scanned for viewing the zone of killing. To support anaerobic growth, 15 mM NO3– was included in the media. (D) Mucoid strain FRD1 (gray bars) and nonmucoid FRD1/pmucA (black bars) were incubated together at the indicated ratios (FRD1/pmucA:FRD1) for 5 days in the presence of 15 mM NO2–, pH 6.5, after which CFUs were determined. *P < 0.001 versus CFU in the initial inoculum. (E) Dose-response killing of mucoid strain FRD1 by NO2–. Bacteria were suspended in LB (pH 6.5) with various amounts of NO2– for 24 hours under anaerobic conditions. Survival against NO2– is presented as the percentage of CFU relative to that in initial inoculum. (F) Long-term anaerobic exposure of mucoid strain FRD1 to 15 mM NO2– at pH 6.5. All experiments were performed in triplicate and presented as mean ± SEM.
After discovering this unique NO2– sensitivity of mucA mutant strain FRD1, we also found that NO2– killed these bacteria more effectively at a lower pH (Figure 2C), while little or no killing was observed in strain FRD1/pmucA at pH 6.0–7.5 (Figure 2C).
To test whether NO2– killing of mucoid bacteria occurs in the presence of nonmucoid bacteria, mucoid and nonmucoid P. aeruginosa were mixed and treated with 15 mM NO2– anaerobically. Strain FRD1 consistently lost viability at 3 different bacterial test ratios, while nonmucoid FRD1/pmucA maintained viability (Figure 2D).
We next characterized the dose-effect relationship between NO2– concentration and killing of mucoid bacteria. Figure 2E shows that 90–95% of the bacteria were killed by 15 mM NO2– and the LD50 was approximately 3 mM NO2– after 24 hours. The remaining organisms did not develop resistance to NO2– when we extended our study to 12 days (Figure 2F), demonstrating that all organisms were killed during this time period.
Other mucoid, mucA mutant CF isolates are also sensitive to NO2–.
To test whether NO2– sensitivity is a trait of all mucA mutant mucoid CF isolates, the mucA genes of 94 mucoid clinical isolates recovered from a variety of CF clinics in the US and Canada were sequenced. Of 94 strains, 82 harbored mucA mutations, leading to either premature termination of translation (88%) or a loss of the stop codon (12%), thereby confirming previous findings (13) that mucA mutations are the major reason for mucoid conversion in CF isolates (Figure 3 and Supplemental Table 1; supplemental material available online with this article; doi:10.1172/JCI24684DS1 http://dx.doi.org/10.1172/JCI24684DS1). The most abundant mutations were single-bp deletions that resulted in a frame shift leading to premature termination of translation at bp 441 (the wild-type mucA gene is 585 bp; see Supplemental Table 1 for a detailed breakdown of mucA mutations in each strain). Consistent with the results of Martin et al. (9), approximately 13% of mucoid isolates had a wild-type mucA allele, indicating the presence of other mechanisms or mutations allowing for mucoid conversion. Upon anaerobic treatment with 15 mM NO2– at pH 6.5, almost all of the mucA mutant mucoid isolates (78 of 82 strains) showed increased susceptibility to NO2–, with 74% killed by more than 2 logs relative to the mean of the nonmucoid MucA-proficient organisms (Figure 3). Importantly, 4 strains that were deemed antibiotic resistant were still sensitive to anaerobic treatment of acidified NO2– (Figure 3, arrows). Out of 12, however, 8 mucoid isolates with a wild-type mucA allele were resistant to acidified NO2–. These results suggest that NO2– sensitivity is likely caused by mucA mutations and not by cellular processes associated with alginate overproduction.
Figure 3.
HNO2 sensitivity of 94 different mucoid CF clinical isolates of P. aeruginosa. Aerobic starter cultures of each strain were diluted 100-fold in LB (pH 6.5) supplemented with 15 mM NO2–. The CFU in the inoculum versus that after a 4-day anaerobic incubation were determined. The values for log10 [CFUafter 4 days/CFUinoculum] were calculated and are plotted as the viability index. The mucA gene of each isolate was sequenced, and mucoid strains with wild-type mucA alleles and those harboring mucA mutation were shown at left and right, respectively. Arrows indicate clinical isolates that were found to be highly resistant to amikacin, aztreonam, cefepime, ceftazidime, ciprofloxacin, gentamycin, imipenem, and tobramycin.
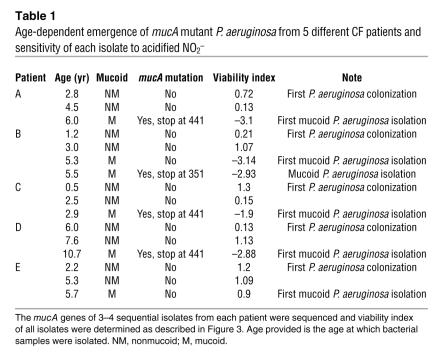
In a separate longitudinal study using P. aeruginosa strains isolated from 5 different young CF patients, we consistently detected mucoid P. aeruginosa as the patient aged, a hallmark of chronic infection (Table 1). As predicted, initial airway colonization of each patient was by nonmucoid P. aeruginosa. Most importantly, however, mucoid variants that were detected in patients A, B, C, and D possessed mutated mucA genes and were all killed by 15 mM NO2– (1.9–3.1 logs). These results indicate that the genotypic and phenotypic switch to the mucoid form that is sensitive to NO2– treatment can occur in patients less than 3 years of age (see patient C).
Table 1.
Age-dependent emergence of mucA mutant P. aeruginosa from 5 different CF patients and sensitivity of each isolate to acidified NO2–
mucA mutations are responsible for NO2– sensitivity.
The above results suggest that mucA mutations could be responsible for the enhanced sensitivity to NO2–. Since mucA mutant bacteria overproduce alginate, we initiated experiments to test whether NO2– sensitivity is caused by mucA mutations and not by alginate production. An isogenic PAO1 mucA mutant, PDO300, whose intact mucA allele was replaced with that of strain FRD1 (mucA22) was also sensitive to NO2– (Table 2). Two FRD1 derived nonmucoid mutants [ΔalgD, lacking GDP-mannose dehydrogenase, and ΔalgT(U), lacking AlgT(U)] were equally sensitive to killing by NO2– (Table 2). We next tested whether NO2– also killed mucB, mucD, and algW mutants of strain PAO1. Other than mucA, the aforementioned genes are the only reported loci that, when inactivated, allow for mucoid conversion in P. aeruginosa (25–27). In contrast to mucA mutant bacteria, these mutants were not sensitive to NO2– (Table 2). Most critically, 5 nonmucoid revertants of mucoid strains were still sensitive to HNO2 (see Table 2). Therefore, our results suggest that NO2– sensitivity is MucA- and not alginate dependent. Finally, the LD50 of NO2– for sensitive strains was almost identical to that for FRD1 (Figure 2E), suggesting that the rate at which these organisms were killed by NO2– was similar to that of strain FRD1.
Table 2.
Correlation between mucA mutations and NO2– sensitivity
HNO2 is required for killing of mucA mutant bacteria; NO and other HNO2-derived intermediates are responsible.
Collectively, our results indicate that the acidic pH approximately 6.5 of the CF airway mucus promotes the generation of NO2– derivative(s) that selectively kill mucA mutant P. aeruginosa. Undoubtedly, these derivatives originate from nitrous acid (HNO2, pKa = 3.3, where pKa is the negative logarithm of the equilibrium constant Ka), whose equilibrium concentration increases with medium acidity. We tested this conjecture by exposing strain FRD1 to 2 different culture conditions with identical HNO2 concentrations of approximately 10 μM (pH 6.5, 15 mM NO2–, and pH 5.5, 1.5 mM NO2–). Under both conditions, equal killing of bacteria was observed, supporting the notion that formation of HNO2 is a prerequisite for killing mucoid P. aeruginosa (Figure 4A). Both mucoid FRD1 and nonmucoid FRD1/pmucA maintained viability at these pH values when no NO2– was added (Figure 4A, left side of each plate).
Figure 4.
NO is involved in killing of mucoid mucA mutant bacteria. (A) Anaerobic sensitivity of P. aeruginosa under conditions allowing for the formation of identical NO levels. Bacteria were incubated anaerobically for 4 days with or without 15 mM and 1.5 mM NO2– at pH 6.5 and 5.5, respectively. To enumerate viable cells, 10 μl of serial dilutions were spotted on LB agar plates and incubated at 37°C for 15 hours. The left and right side of each plate represent control and NO2–-exposed bacteria. (B) NO levels generated by 15 mM NO2– (pH 6.5) over time. Carboxy-PTIO (200 nM) was added, and the stoichiometric decrease in NO signal is shown. The coefficient of determination (r2) between NO concentration and electric current (pA) was 0.9997. (C) Effects of NO scavengers on protection of FRD1 from acidified NO2–. Carboxy-PTIO (5 mM) or deoxyhemoglobin (0.5 mM) was added in the initial media (LB, pH 6.5, 15 mM NO2–). (D) Toxicity of NO toward FRD1 and FRD1/pmucA in LB at pH 6.5. NO (333 parts per million) balanced with argon was continuously bubbled for 24 hours anaerobically and viable bacteria were subsequently enumerated. Results of 4 experiments are shown. In NO-treated FRD1, differences in CFU between 2 time points were statistically significant (*P < 0.01). (E) P. aeruginosa PAO1 (circles) and isogenic sodAsodB double mutants (diamonds) were grown in LB, pH 6.5, containing 15 mM NO2– under aerobic (2 days, dotted lines) and anaerobic (4 days, solid lines) conditions. CFUs were measured daily and plotted in logarithmic scale. For aerobic samples, strains were grown with vigorous shaking (300 rpm).
At such low concentrations, HNO2 is unlikely to directly inflict lethal lesions because it is relatively unreactive and would revert to NO2– upon penetrating bacterial membrane and entering the neutral cytoplasm. However, HNO2 is unstable toward disproportionation that slowly generates NO and NO3–. Consistent with this chemistry, our results revealed that NO accumulated upon addition of NO2– to Luria-Bertani broth (LB) at pH 6.5 (Figure 4B). After a rapid rise, NO levels plateau at approximately 490 nM, which corresponds to the decomposition of a small fraction of added NO2– and indicates that its decomposition is impeded by accumulating NO. The addition of the stoichiometric NO scavenger, 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (carboxy-PTIO), temporarily depleted NO, but the NO level was promptly restored following scavenger consumption. This dynamic behavior directly bears on the bactericidal action of acidified NO2– and is explicable by the well-established 2-step HNO2 decomposition mechanism (Supplemental Table 2). In the first reversible step, a pair of NO and NO2• radicals are generated (Equation 1), but this reaction is so strongly shifted to the left that only 3 nM each of NO and NO2• are present at equilibrium.
Equation
| 1 |
However, the second dimerization/hydrolysis step (Equation 2) removes NO2•, thus shifting the equilibrium of Equation 1 to the right and leading to accumulation of NO to a level at which the reverse NO + NO2• reaction in Equation 1 outcompetes the NO2• dimerization/hydrolysis, making further NO accumulation extremely slow.
Equation
| 2 |
This chemistry is amenable to a kinetic analysis by computer simulation (ref. 28 and Supplemental Table 2) revealing that the initial rate of NO accumulation of approximately 4 μM/h decreases by more than 3 orders of magnitude when NO reaches approximately 500 nM; notably, identical NO accumulation profiles are predicted for 15 mM NO2–, pH 6.5 and 1.5 mM NO2–, pH 5.5, thereby explaining the results in Figure 4A. As observed in Figure 4B, the simulations predict 90% NO depletion in 25 minutes upon the addition of 200 nM carboxy-PTIO and clearly show that out of the 3 intermediates of NO2– decomposition (HNO2, NO2•, and NO), only NO reacts with carboxy-PTIO. If HNO2 reacted, the addition of carboxy-PTIO would cause no change in NO concentration. If NO2• were reactive, we would observe a sharp 200-nM increase in the NO concentration upon carboxy-PTIO addition. Thus, carboxy-PTIO is a selective NO scavenger in our system.
This analysis naturally suggests that NO is involved in the microbicidal action of HNO2. It also allows an experimentally verifiable prediction that carboxy-PTIO should have a strong protective effect when added at millimolar concentrations. As shown in Figure 4C, such protection is indeed observed. The computer modeling analysis shows that, under the conditions in Figure 4C, carboxy-PTIO will persist in solution for several weeks, keeping NO concentrations at picomolar levels. Similarly protective effects were observed with another NO scavenger, deoxyhemoglobin (Figure 4C and ref. 29). These experiments therefore establish that NO was at least potentially 1 direct or indirect contributor to the bactericidal activity of acidified NO2–. This conclusion is strongly supported by the observation that strain FRD1, but not FRD1/pmucA, was killed when an NO/argon gas mixture was bubbled into the bacterial suspension (Figure 4D); a 380-nM solution concentration of NO maintained in this experiment was comparable to that generated by 15 mM NO2– at pH 6.5 (Figure 4B). In contrast, both strains maintained viability when treated with argon gas.
Although a link between NO depletion and the decrease of bactericidal activity of acidified NO2– clearly exists, it is premature to conclude that NO is per se the toxic agent in this system or the only toxic agent. NO depletion, whether through the action of exogenous carboxy-PTIO or endogenous NOR activity, may inhibit several downstream reactions by which HNO2 may kill mucoid P. aeruginosa. These reactions alter the reaction dynamics modeled in buffer alone and may ultimately prove relevant to the observed bactericidal effect of HNO2. For example, high NO levels promote formation of a strong nitrosating intermediate, N2O3 in Equation 1, which can cause “nitrosative stress” by modifying the function of proteins (30). Additionally, NO can react with iron and sulfur species in bacteria to form bioactive S-nitrosothiols (31) that may be potent in killing mucoid P. aeruginosa, an intriguing possibility given that S-nitrosothiol levels are low in the CF airway.
Although we strived for strictly anaerobic conditions, even remotely low levels of O2 could be problematic because of the attendant generation of O2–, which combines with NO to form peroxynitrite (ONOO–), an extremely bactericidal species (32, 33). We used a sodAsodB mutant, devoid of iron- and manganese-cofactored superoxide dismutase. As such, it is extremely sensitive to even endogenously generated O2– (34). Were trace oxygen to be reduced to O2– in the sodAsodB mutant in the presence of NO, then ONOO– would form and kill the sodAsodB mutant much more rapidly than was observed in wild-type organisms. Figure 4E shows that the sodAsodB mutant of strain PAO1 was not sensitive to HNO2 under anaerobic conditions. In contrast and as expected, under aerobic conditions, the sodAsodB mutant was more sensitive to HNO2 (Figure 4E, compare lines shown with diamonds). These results indicate that ONOO– is not formed in our anaerobic cultures and that bacterial killing is not due to this species.
Mucoid mucA mutant bacteria harbor low anaerobic NOR and NIR activity.
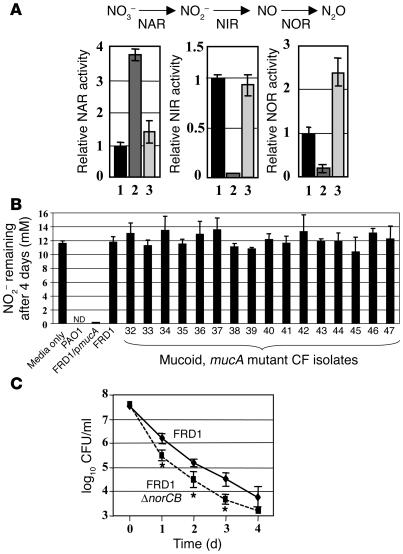
Our next goal was to define the molecular basis of HNO2 sensitivity of mucoid mucA mutant bacteria. We first measured the activity of enzymes involved in P. aeruginosa anaerobic respiration, including NO3– reductase (NAR), NIR, and NOR (Figure 5A). Strain FRD1 possessed approximately 3.7-fold greater NAR activity compared with nonmucoid FRD1/pmucA and PAO1. However, strain FRD1 possessed only 4% and approximately 20% the NIR and NOR activity, respectively, of strain PAO1 (Figure 5A). Interestingly, FRD1/pmucA possessed approximately 2-fold higher NO consuming activity compared with strain PAO1 (Figure 5A), suggesting a positive correlation of NOR activity with the presence of multiple copies of wild-type MucA. The reduced NOR activity in strain FRD1 explains in part the very limited capacity for removal of NO by this organism and, hence, its greater sensitivity to purified NO. The lack of NIR activity in strain FRD1 explained the failure of NO2– to support anaerobic growth of this strain. In addition, the low NIR activity in strain FRD1 led to the constancy of HNO2 levels in the culture medium and the attendant increase in NO levels compared with strains that metabolize NO2–. Strikingly, there was no significant loss of NO2– from the culture medium after 4 days at pH 6.5, suggesting that there was no biological reduction of NO2– in strain FRD1 and 16 different mucoid, mucA mutant CF isolates assessed for NO2– sensitivity (Supplemental Table 1) and remaining NO2– levels (Figure 5B). Moreover, these data also demonstrate that NO formed from HNO2 at this pH and NO2– concentration in the presence of mucA mutant organisms was virtually all recycled to NO2–, supporting a mechanism for NIR-deficient mutant bacteria killing that involves NO oxidation and formation of NO+ equivalents. These data confirm the lack of respiratory NIR activity in mucoid, mucA mutant P. aeruginosa (Figure 5B). In contrast, supernatants of wild-type strain PAO1 and FRD1/pmucA had little or no media NO2– remaining (Figure 5B).
Figure 5.
Significantly reduced NIR and NOR activities in mucA mutant P. aeruginosa account for the sensitivity to HNO2. (A) Relative activities of NAR, NIR, and NOR in FRD1 (lane 2) and FRD1/pmucA (lane 3), normalized to that of the well-characterized laboratory strain PAO1 (lane 1). Each assay was performed in triplicate, and the mean ± SEM is presented. A schematic diagram of the P. aeruginosa anaerobic respiration pathway is shown. Bacteria were grown in LB (pH 6.5) containing 100 mM NO3– for 15 hours under anaerobic conditions. (B) P. aeruginosa strains were incubated anaerobically in LB containing 15 mM NO2–, pH 6.5. After 4 days at 37°C, NO2– levels were measured in triplicate. All other mucA mutant clinical isolates were among those described in Supplemental Table 1. For the media-only control, no bacteria were used. ND, not detected. (C) Anaerobic sensitivity of FRD1 and FRD1 norCB mutant exposed to NO2– at pH 6.5. Experimental conditions were identical to those used in Figure 2B. Δ, deletion of a specific gene. *P < 0.01 versus FRD1.
Finally, because NOR activity was significantly reduced in mucoid bacteria, we postulated that mucoid organisms completely devoid of NOR activity would be more sensitive to HNO2. Figure 5C demonstrates that a norCB mutant of strain FRD1 was approximately 10-fold more sensitive to acidified NO2– than were wild-type organisms.
HNO2 kills mucoid P. aeruginosa in in vitro anaerobic biofilms and fresh sputum isolates from CF patients.
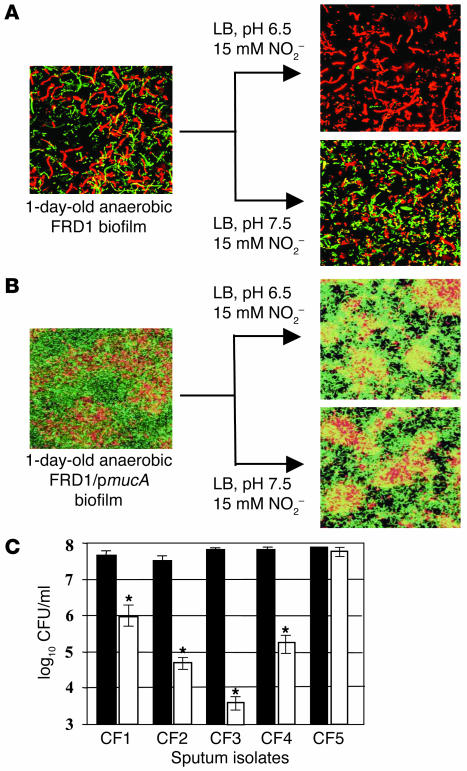
To explore the potential clinical application of HNO2 in the treatment of mucoid P. aeruginosa infections in chronic CF patients, we first tested the effect of NO2– on the viability of biofilm bacteria. Anaerobic biofilms of strains FRD1 and FRD1/pmucA were grown for 1 day in media containing NO3–, which supports anaerobic respiration. Since mucoid strain FRD1 lacks a flagellum (35), a surface appendage that is critical for P. aeruginosa biofilm initiation (36), strain FRD1 formed much weaker biofilms than those of flagellated FRD1/pmucA (Figure 6, compare A and B). When the FRD1 biofilm was treated with NO2– at pH 7.5 for 2 days, no difference in biofilm structure and cell viability was observed relative to control biofilms. In contrast, nearly complete death of biofilm organisms was observed after a 2-day incubation with NO2– at pH 6.5 (Figure 6A, top right). In biofilms of FRD1/pmucA, however, resistance to acidified NO2– was clearly evident (Figure 6B).
Figure 6.
Effect of HNO2 on killing P. aeruginosa in biofilms and fresh sputum isolates. (A) Confocal laser microscopic analysis of anaerobic FRD1 biofilms. Live cells are stained with syto-9 (green), and dead cells are stained with propidium iodide (red). Top (x-y plane) views are projected from a stack of 125 images taken at 0.4-μm intervals for a total of 50 μm. Before staining, 1-day-old anaerobic FRD1 biofilms were treated anaerobically with 15 mM NO2– at pH 6.5 and 7.5 for 2 days. (B) Biofilms were grown as in A using FRD1/pmucA. (C) Toxicity of 15 mM NO2– (pH 6.5) toward P. aeruginosa sputum isolates. Viable cells in the initial inoculum (black bars) and after a 24-hour anaerobic incubation (white bars) are presented in logarithmic scale. CF1–CF4 are mucoid mucA mutants, and CF5 is nonmuocid and possesses a wild-type mucA gene. Each strain was tested in triplicate and the mean ± SEM is presented. *P < 0.05.
We next monitored the effect of HNO2 on the killing of sputum isolates from CF patients. Sputum isolates CF1, -2, -3, and -4 harbored nearly 100% mucoid mucA mutant bacteria, while the CF5 isolate harbored only nonmucoid MucA-proficient P. aeruginosa (Figure 6C). To minimize loss of the properties acquired in vivo, these isolates were passed only once in L-broth and immediately assayed for anaerobic NO2– sensitivity. When these isolates were exposed to 15 mM NO2– at pH 6.5 under anaerobic conditions for 1 day, a sharp decrease in viability was observed in the mucoid mucA mutant isolates. Again, the inherent utility of these isolates was that they were freshly isolated from CF patients and were not from long-term frozen stocks derived from CF patients. These results clearly indicate that high levels of HNO2 selectively kill mucoid mucA mutant P. aeruginosa.
HNO2 kills mucoid P. aeruginosa in a sterile ultrasupernatant derived from explanted CF lungs and in mouse airways.
Next, we determined whether mucoid strain FRD1 could be killed by HNO2 in sterile ultrasupernatants (pH 6.24) of CF airway secretions derived from explanted CF lungs. This reagent arguably represents the best medium to investigate P. aeruginosa in the context of bacterial growth and the effects of HNO2 ex vivo. Figure 7A shows that mucoid bacteria were actually killed faster by HNO2 in the CF ultrasupernatants than in L-broth (Figure 2B).
Figure 7.
Applications of HNO2-mediated killing of mucoid P. aeruginosa to clinical specimens. (A) Killing of FRD1 by NO2– in sterile ultrasupernatants of CF airway secretions derived from explanted CF lungs. Bacteria were incubated anaerobically for 24 hours, and 15 mM NO2– was added. CFUs were determined (n = 3) and plotted as the X ± SEM versus time. *P < 0.01 versus CFU decrease before adding NO2–. (B) NO generation in CF ASL by 15 mM NO2–. Except for the media and pH, experimental conditions were identical to those used in Figure 4B. (C) Effects of NO2– on killing of FRD1 and FRD1/pmucA in mouse lungs. CD1 mice were infected with FRD1 or FRD1/pmucA as described in Methods. Infected mice were treated with buffer (black bars) and buffered NO2– (white bars) daily, and viable bacteria from the lung homogenates were enumerated. n = 8 per group. *P < 0.01 versus buffer alone. (D) Effects of long-term NO2– treatment on killing of FRD1 in mouse lungs. Another group of FRD1-infected mice were treated daily with buffer (–, 50 mM sodium phosphate, pH 6.5) or buffer with 15 mM NO2– (+) for 16 days (n = 8 per group). Organisms surviving treatment with buffer and NO2– were shown in logarithmic scale. *P < 0.01 versus buffer alone. (E) CI experiments with 106 FRD1 and FRD1/pmucA intratracheally instilled into CD1 mouse airways and incubated for 6 days prior to harvesting of mouse lungs and enumeration of CFUs after homogenization.
Next, we measured NO levels generated from HNO2 disproportionation in ASL collected from primary CF airway epithelia. Figure 7B indicates that NO was produced in CF ASL at levels even greater (562 nM) than those generated in LB at pH 6.5 (489 nM, Figure 4B). The higher levels of NO produced upon addition of NO2– in this milieu were likely due to the lower pH of the sample (6.43 versus 6.5).
We then determined the efficacy of HNO2 to kill strain FRD1 in a P. aeruginosa chronic lung infection model. Currently, there is no animal model for the anaerobic biofilm mode of CF airway disease or a CF animal that acquires spontaneous P. aeruginosa infections. However, CD1 mice inoculated with agarose beads impregnated with bacteria have been useful for studying chronic lung infection by P. aeruginosa (37). Consistent with our in vitro results, mucoid FRD1, but not nonmucoid FRD1/pmucA, were decreased more than 2 logs at pH 6.5 and more than 3 logs at pH 5.5 by HNO2– in vivo (Figure 7C). Because NO concentrations derived from acidified NO2– are 10-fold greater with a reduction of 1 pH unit, our results are consistent with classical NO2– reduction chemistry. Furthermore, organisms that were recovered from the mouse airways after NO2– exposure were still sensitive to NO2– in vitro (data not shown), although these results do not clearly indicate that NO itself within the bacterial cytoplasm is the toxic species.
We also addressed whether long-term treatment with HNO2 produced progressively decreasing airway titers of mucoid, mucA mutant bacteria. NO2– was instilled on a daily basis in mice infected with mucoid organisms for a period of 16 days. Figure 7D shows that there was no bacteria detected in mice treated for 16 days with HNO2, while buffer control mice still harbored nearly 104 mucoid organisms per lung.
To address the possibility that acidified HNO2 can kill mucoid mucA mutant bacteria in the presence of nonmucoid bacteria in vivo (similar to the results obtained in vitro in Figure 2D), competitive index (CI) experiments were performed. Figure 7E demonstrates that the CI was only approximately 0.2 for mucoid, mucA mutant strain FRD1 relative to its complemented strain, FRD1/pmucA, which was approximately 1.0.
NO2– does not elicit adverse effects on airway epithelia in vitro.
The clinical utility of NO2– as a treatment would be diminished if it exerted significant toxic or adverse effects on airway epithelia. Therefore, we tested the effect of NO2– on cell viability and function of cultured airway epithelia. Furthermore, since NO2– may elicit a proinflammatory response that would be undesirable in the CF airways, and NO has been reported to increase IL-8 gene transcription in a lung epithelial cell line (38), we also tested whether NO2– induces IL-8 release from cultured airway epithelia. Aerosolization, a potential therapeutic delivery route for NO2– to the CF airways, would deliver it in small volumes on the epithelial surface. To mimic this situation in vitro, we added a low volume (2 μl) of test solution containing various concentrations of NO2– to the apical surface of CF airway epithelia at pH 6.5. Exposure to concentrations as high as 20 times the dose required to kill mucoid mucA mutant P. aeruginosa exerted no cytotoxicity toward CF airway epithelia after 24 hours, as determined by lactate dehydrogenase release (Figure 8A). We performed such experiments because aerosol exposure of any effective agent, regardless of treatment, requires that significantly higher concentrations (about 25-fold) of stock solution be used so that appropriate doses are administered efficiently to the areas of interest.
Figure 8.
Effect of NO2– on function of cultured human airway epithelia. (A) Culture preparations of human CF airway epithelium (duplicate preparations, n = 3) were exposed apically to 2 μl of varying concentrations of NO2–. After 24 hours, lactate dehydrogenase activity in the basolateral media was measured to monitor cytotoxicity. (B) CF airway epithelial cultures were mounted in Ussing chambers and treated with 2 μl of liquid containing NO2– at varying concentrations. Transepithelial short circuit current (Isc, in μA/cm2) was measured to monitor change in bioelectric properties. (C) CF airway epithelial cultures (triplicate preparations, n = 4), were treated luminally with 100 μl Krebs bicarbonate Ringer buffer containing 2% blue dextran and supplemented with either 15 mM NaCl (control) or NO2– for 24 hours. Transepithelial water flux (Jv) was calculated by measuring blue dextran concentration optically after 24 hours in microaliquots of sampled luminal liquid. (D) IL-8 release assay using primary cultures of CF airway epithelia (n = 4) exposed to 15 mM NO2– compared with control cultures. (E) Determination of NO2– half life on the surface of airway epithelia. The 3 different concentrations of NO2– were applied to airway epithelial cell monolayers, and NO2– levels were assayed at the indicated intervals (n = 3 for each measurement). P = NS for all comparisons (ANOVA).
In CF culture preparations mounted in Ussing chambers, basal transepithelial short circuit current (Isc) was not affected by NO2– exposure (Figure 8B). Further, 15 mM NO2– failed to affect amiloride-sensitive Isc (control, 15.4 ± 1.4 μA/cm2; treated, 15.7 ± 1.7 μA/cm2; P = 0.92) and UTP-activated Isc (control, 17.7 ± 3.0 μA/cm2; treated, 18.1 ± 3.3 μA/cm2; P = 0.92). Consistent with these data, 15 mM NO2– did not alter transepithelial water flux (Jv) in the same cultures (Figure 8C) or trigger IL-8 release over 24 hours in CF epithelia (Figure 8D). Finally, we performed preliminary studies of the durability of NO2– on CF airway surfaces. We found that the half life of NO2– was approximately 5 hours (Figure 8E), indicating that NO2– is not immediately removed from the luminal side of CF airway epithelia.
Discussion
A hallmark of CF airway disease is the emergence of alginate-overproducing P. aeruginosa, bacteria that have genotypically and/or phenotypically changed from a nonmucoid to mucoid forms and are highly resistant to host defenses (8) and antimicrobial therapy (7). Clinically, the appearance of this organism is correlated with a marked reduction in lung function and nutritional status (39). One major mechanism for P. aeruginosa mucoid conversion in CF isolates involves mutations in the mucA gene (9). The only other known genes that are involved in mucoid conversion include mucB (algN) (27), mucD (25), and algW (25), but these mutations are not common in clinical isolates.
In this study, we believe that we have discovered the Achilles’ heel of the formidable mucoid form of P. aeruginosa, which could lead to improved treatment for CF airway disease. Under conditions that mimicked the CF airway mucus, HNO2 was transformed into toxic species that specifically and negatively affected viability of mucoid P. aeruginosa. Strikingly, mucA mutant bacteria were very sensitive to species derived from HNO2, which was due at least in part to the inherently low NIR and NOR activity in these strains. In fact, recent work by Firoved et al. (40) has shown that mucA mutant bacteria have a markedly reduced capacity to remove the NO generated even aerobically from S-nitrosoglutathione. This is consistent with our results, which indicated that NO levels accumulate and remain for greater than 24 hours. Since MucA is an inner membrane–spanning protein and catalytic activities of NIR and NOR reside in the periplasmic space, we speculate that the periplasmic portion of MucA plays a crucial role in orchestrating the biological function of these periplasmic enzymes; indeed, virtually all of the critical mutations in the mucA gene predict defects in the periplasmic localization of MucA. Studies to define a structural relationship between MucA and potential periplasmic proteins are currently underway. HNO2 derivatives can damage DNA (41) and modify protein micromoieties including Fe-S clusters (42), tyrosine residues (43), heme (44), and sulfhydryl groups (45). These mechanisms may be involved in the adverse effects on the overall biology of mucoid, mucA mutant organisms.
Our mucA sequencing data from 94 different strains from 5 CF clinics confirm the previous findings of Martin et al. (9) that mucoid conversion is mainly caused (87% frequency) by mucA mutations (Supplemental Table 1). However, variations exist in the mechanisms by which P. aeruginosa undergoes mucoid conversion (9, 12), and mucoid organisms that have intact mucA alleles are being detected, particularly in Europe. The data presented in this study indicate that only mucA mutant bacteria are susceptible to HNO2.
Airway epithelial expression of iNOS does not differ between young CF patients and normal children (23). However, as CF patients age, expression of iNOS is significantly reduced in CF patients (23). This reduced expression of iNOS in chronic CF is associated with the emergence of mucoid mucA mutant subpopulations. Currently, however, it is unclear whether conversion to the mucoid form, which has limited capacity for NO removal, is facilitated by the abnormally low NO or S-nitrosothiol levels in the airways of older CF subjects (15).
Our data suggest that 15 mM NO2– kills mucA mutant P. aeruginosa in CF airways at pH 6.5. The NO chemistry of bacteria and the CF airways is complex, and several downstream mechanisms could account for the effect of HNO2 on mucoid P aeruginosa. Our data suggest that NO itself, whether directly or as a precursor to iron-nitrosyl species, may be involved in the antimicrobial effect. Of note, there is evidence that airway acid stress characterizes a variety of pulmonary disorders (46), and our results are consistent with those of Hunt et al. (47), which suggest that delivery of concentrated NO2– to airway regions with low pH likely serves to generate NO and S-nitrosothiols. Indeed, NO generated in animal studies of inhaled NO2– may arise from HNO2 formation (18). Inhaled NO2– has appeal as a CF therapy because it may (a) provide sustained NO release, (b) exploit the low pH of the CF airway epithelial mucus layer, (c) selectively inhibit growth of mucoid, mucA mutant P. aeruginosa when protonated to form HNO2, and (d) provide a chemical feedback mechanism that maintains the desired NO levels in response to its consumption or removal (Figure 4B). Therefore, we hope that the data provided in this study stimulates further investigation. However, it is important to caution that (a) the airway pH may not be homogeneous in vivo as excessive regional acidity and high HNO2 levels could result in airway injury; (b) excessive NO production in the airway could inhibit platelet aggregation, potentially aggravating hemoptysis; (c) HNO2 produces carcinogenic nitrosoamines; (d) the nitrogen redox chemistry in the CF airway in vivo is exceptionally complex, and several unexpected (and untoward) reactions could result from formation of high airway levels of HNO2; (e) for denitrifying organisms that have intact NIR and NOR activity (including the Aspergillus species), inhaled NO2– could paradoxically promote growth as a nutrient; (f) there are no human data regarding the safety of inhaling near-molar quantities of NO2–, which could have adverse local and systemic effects; and (g) the precise mechanism by which HNO2 affects mucoid P. aeruginosa growth is not known. Still, despite these known pitfalls, mucoid P. aeruginosa organisms were previously considered impossible to eradicate from the airways of patients with chronic CF lung disease. We believe that our data offer hope that effective treatment strategies can be designed with the ultimate goal of eradicating this formidable foe in CF lung disease.
Methods
Bacterial culture and enzymatic assays.
P. aeruginosa strains used in this study included nonmucoid strain PAO1 (48), CF isolate FRD1 (49), and sputum isolates from CF patients at 5 different North American clinics, totaling 94 strains (Supplemental Table 1). All procedures using human patients were approved by the respective university’s Institutional Review Board with respect to informed consent issues. Complementation of FRD1 mucA was achieved by transformation with the plasmid ptacmucA (9). Aerobic starter cultures were grown in LB (10 g tryptone, 5 g NaCl, and 5 g yeast extract per liter) at 37°C. Anaerobic growth was achieved in a Coy anaerobic chamber (Coy Laboratory Products). To support anaerobic respiration, KNO3 and/or NaNO2 (Sigma-Aldrich) were added to the medium. The pH of the medium was adjusted with 100 mM sodium phosphate (for pH 6.5) or 100 mM sodium acetate (for pH 5.5). To enumerate viable bacteria, serial dilutions were spotted on LB agar plates. NO2– sensitivity using a filter paper disk assay was performed as previously described (31). NAR, NIR, and NOR activities were measured as described previously (21). Biofilm staining and image acquisition were accomplished as described previously (21).
NO experiments.
NO levels were measured polarimetrically using a NO electrode system (Model Apollo 400; World Precision Instruments Inc.) according to the manufacturer’s instructions. The NO gas exposure study was performed as described previously (32).
Measurement of pH in airway secretions.
In situ pH measurements of mucopurulent airway secretions from CF airways were made by inserting the tip of a pH microelectrode (MI-413; Microelectrodes Inc.) into mucopurulent secretions within lobar, segmental, and subsegmental bronchi of freshly explanted lungs from 9 CF patients. Lungs were removed at the time of organ transplantation. Duplicate readings at 3 different sites per patient were recorded, and the mean value from each measurement was used for analysis.
Infection of mouse airways.
CD1 mice (n = 8) were infected with approximately 106 FRD1 or FRD1/pmucA entrapped in agar beads intratracheally as previously described (33). Following 24 hours’ incubation, mouse lungs were instilled with 25 μl of 15 mM NO2– at pH 5.5 (in 0.1 M acetate buffer) or pH 6.5 (in 0.1 M phosphate buffer) intranasally twice daily. On the fifth day mice were sacrificed, and the viable bacteria from serially diluted lung homogenates were enumerated. One group of FRD1-infected mice was treated with 15 mM NO2– at pH 6.5 as described above for 16 days to examine whether bacteria develop resistance after prolonged exposure to NO2–. All animal studies were performed in accordance with the protocols approved by the Animal Care Committee of the University of Cincinnati College of Medicine.
In vivo competition assays.
In vivo competition assays between mucoid mucA mutant strain FRD1 and FRD1/pmucA embedded in agar beads were carried out by infecting CD1 mice (n = 6) intratracheally (2 × 105 bacteria) with a 1:1 ratio of each strain as previously described (50). Twenty-four hours after infection, mouse lungs were instilled with 25 μl of PBS at pH 6.5 or PBS containing 15 mM NaNO2 at pH 6.5 twice daily for 4 days. Infected lungs were recovered after 6 days for bacterial load and CI calculations. The CI was defined as the output ratio of mutant to wild-type bacteria divided by the input ratio of mutant to wild-type bacteria (51). Thus, if a mutant strain is as competitive as its isogenic wild-type parent, a value of 1 will be achieved. A CI of less than 1 implies that the mutant is not as competitive as the wild-type strain.
Biological properties of airway epithelia in response to NO2–.
Primary bronchial epithelial cultures, grown on Transwell Col (1.13 cm2 surface area) permeable supports (52), were mounted in Ussing chambers (ADInstruments Co.), and bioelectric properties were analyzed as previously described (53). IL-8 concentrations in basolateral media were measured using commercially available antibody pairs (R&D Systems) according to the manufacturer’s instructions. Cellular cytotoxicity was assessed by comparing release of lactate dehydrogenase into the basolateral media of cultured airway epithelial cell preparations treated apically with varying NO2– concentrations. Lactate dehydrogenase was measured using a commercially available spectrophotometric assay kit (BioVision Research Products). For experiments measuring transepithelial water flux, culture preparations were treated apically with 100 μl of Krebs bicarbonate Ringer solution containing 2% blue dextran (a cell-impermeable volume marker dye) supplemented with 15 mM NO2–. After 24 hours, microaliquots (2–5 μl) of apical liquid were collected and stored at –20°C until analyzed. Blue dextran concentrations were measured spectrophotometrically. To determine the half life of NO2– on the surface of cultured airway epithelia, NO2– levels were measured by the Griess reaction (54), and the percent rate of NO2– removal was calculated.
Preparation of sterile ultrasupernatants of CF airway secretions.
Purulent secretions were harvested from the airways of CF lungs that were removed at the time of transplantation. Purulent secretions were centrifuged (100,000 g for 1 hour) and passed through a sterile filter (0.22 μm, Costar 8110 mStar; Millipore).
Statistics.
Results are presented as mean ± SEM. Student’s t test (2-tailed, unequal variance, for Figures 1–6) and ANOVA (Figure 7) were used to analyze the significance of differences between experimental groups. A P value less than 0.05 was considered statistically significant. Kinetic modeling was carried out using the INTKIN computer program, developed at the Brookhaven National Laboratory by Harold A. Schwarz (55). The necessary rate data were obtained from the literature (56).
Supplementary Material
Acknowledgments
This work was support by NIH Public Health Service grants (AI-40541 and GM-69845) and Cystic Fibrosis Foundation grant HASSETT03P0 to D.J. Hassett. Research at Brookhaven National Laboratory was carried out under the auspices of the US Department of Energy under contract DE-AC02-98CH10886 from the Division of Chemical Sciences, Office of Basic Energy Sciences.
Footnotes
Sang Sun Yoon’s present address is: Department of Microbiology and Molecular Genetics, Harvard Medical School, Boston, Massachusetts, USA.
Nonstandard abbreviations used: ASL, airway surface liquid; carboxy-PTIO, 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide; CF, cystic fibrosis; CI, competitive index; LB, Luria-Bertani broth; NAR, nitrate reductase; NIR, nitrite reductase; NOR, NO reductase.
Conflict of interest: A patent has been filed on this technology by the University of Cincinnati naming D.J. Hassett as the sole inventor. He would share in royalties, if any, according to the university’s patent policy set forth in university rule no. 3361:10-19-01.
References
- 1.Boucher RC. An overview of the pathogenesis of cystic fibrosis lung disease. Adv. Drug Deliv. Rev. 2002;54:1359–1371. doi: 10.1016/s0169-409x(02)00144-8. [DOI] [PubMed] [Google Scholar]
- 2.Yoon SS, Hassett DJ. Chronic Pseudomonas aeruginosa infection in cystic fibrosis airway disease: metabolic changes that unravel novel drug targets. Expert Rev. Anti. Infect. Ther. 2004;2:89–101. doi: 10.1586/14787210.2.4.611. [DOI] [PubMed] [Google Scholar]
- 3.Coakley RD, et al. Abnormal surface liquid pH regulation by cultured cystic fibrosis bronchial epithelium. Proc. Natl. Acad. Sci. U. S. A. 2003;100:16083–16088. doi: 10.1073/pnas.2634339100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Worlitzsch D, et al. Reduced oxygen concentrations in airway mucus contribute to the early and late pathogenesis of Pseudomonas aeruginosa cystic fibrosis airway infection. J. Clin. Invest. 2002;109:317–325. doi:10.1172/JCI200213870. doi: 10.1172/JCI13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hassett DJ, et al. Anaerobic metabolism and quorum sensing by Pseudomonas aeruginosa biofilms in chronically infected cystic fibrosis airways: rethinking antibiotic treatment strategies and drug targets. Adv. Drug Deliv. Rev. 2002;54:1425–1443. doi: 10.1016/s0169-409x(02)00152-7. [DOI] [PubMed] [Google Scholar]
- 6.Govan JRW, Deretic V. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol. Rev. 1996;60:539–574. doi: 10.1128/mr.60.3.539-574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Govan JRW, Fyfe JAM. Mucoid Pseudomonas aeruginosa and cystic fibrosis: resistance of the mucoid form to carbenicillin, flucloxacillin and tobramycin and the isolation of mucoid variants in vitro. J. Antimicrob. Chemother. 1978;4:233–240. doi: 10.1093/jac/4.3.233. [DOI] [PubMed] [Google Scholar]
- 8.Cabral DA, Loh BA, Speert DP. Mucoid Pseudomonas aeruginosa resists nonopsonic phagocytosis by human neutrophils and macrophages. Pediatr. Res. 1987;22:429–431. doi: 10.1203/00006450-198710000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Martin DW, et al. Mechanism of conversion to mucoidy in Pseudomonas aeruginosa infecting cystic fibrosis patients. Proc. Natl. Acad. Sci. U. S. A. 1993;90:8377–8381. doi: 10.1073/pnas.90.18.8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathee K, et al. Mucoid conversion of Pseudomonas aeruginosa by hydrogen peroxide: a mechanism for virulence activation in the cystic fibrosis lung. Microbiology. 1999;145:1349–1357. doi: 10.1099/13500872-145-6-1349. [DOI] [PubMed] [Google Scholar]
- 11.Boucher JC, Yu H, Mudd MH, Deretic V. Analysis of mucA mutations in clinical isolates of Pseudomonas aeruginosa: frequency and types in patients with cystic fibrosis and analysis of clearance in a mouse aerosol infection model. Infect. Immun. 1997;65:3838–3846. doi: 10.1128/iai.65.9.3838-3846.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anthony M, et al. Genetic analysis of Pseudomonas aeruginosa isolates from the sputa of Australian adult cystic fibrosis patients. J. Clin. Microbiol. 2002;40:2772–2778. doi: 10.1128/JCM.40.8.2772-2778.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hassett DJ. Anaerobic production of alginate by Pseudomonas aeruginosa: alginate restricts diffusion of oxygen. J. Bacteriol. 1996;178:7322–7325. doi: 10.1128/jb.178.24.7322-7325.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wyckoff TJ, Thomas B, Hassett DJ, Wozniak DJ. Static growth of mucoid Pseudomonas aeruginosa selects for non-mucoid variants that have acquired flagellum-dependent motility. Microbiology. 2002;148:3423–3430. doi: 10.1099/00221287-148-11-3423. [DOI] [PubMed] [Google Scholar]
- 15.Grasemann H, Gaston B, Fang K, Paul K, Ratjen F. Decreased levels of nitrosothiols in the lower airways of patients with cystic fibrosis and normal pulmonary function. J. Pediatr. 1999;135:770–772. doi: 10.1016/s0022-3476(99)70101-0. [DOI] [PubMed] [Google Scholar]
- 16.Grasemann H, Ioannidis I, de Groot H, Ratjen F. Metabolites of nitric oxide in the lower respiratory tract of children. Eur. J. Pediatr. 1997;156:575–578. doi: 10.1007/s004310050667. [DOI] [PubMed] [Google Scholar]
- 17.Grasemann H, et al. Nitric oxide metabolites in cystic fibrosis lung disease. Arch. Dis. Child. 1998;78:49–53. doi: 10.1136/adc.78.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ratjen F, Gartig S, Wiesemann HG, Grasemann H. Effect of inhaled nitric oxide on pulmonary function in cystic fibrosis. Respir. Med. 1999;93:579–583. doi: 10.1016/s0954-6111(99)90158-0. [DOI] [PubMed] [Google Scholar]
- 19.Jones KL, et al. Elevation of nitrotyrosine and nitrate concentrations in cystic fibrosis sputum. Pediatr. Pulmonol. 2000;30:79–85. doi: 10.1002/1099-0496(200008)30:2<79::aid-ppul1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 20.Gaston B, et al. Nitrogen redox balance in the cystic fibrosis airway: effects of antipseudomonal therapy. Am. J. Respir. Crit. Care Med. 2002;165:387–390. doi: 10.1164/ajrccm.165.3.2106006. [DOI] [PubMed] [Google Scholar]
- 21.Yoon SS, et al. Pseudomonas aeruginosa anaerobic respiration in biofilms: relationships to cystic fibrosis pathogenesis. Dev. Cell. 2002;3:593–603. doi: 10.1016/s1534-5807(02)00295-2. [DOI] [PubMed] [Google Scholar]
- 22.Fierro IM, et al. Induction of NOS in rat blood PMN in vivo and in vitro: modulation by tyrosine kinase and involvement in bactericidal activity. J. Leukoc. Biol. 1999;65:508–514. doi: 10.1002/jlb.65.4.508. [DOI] [PubMed] [Google Scholar]
- 23.Wooldridge JL, et al. NO pathway in CF and non-CF children. Pediatr. Pulmonol. 2004;37:338–350. doi: 10.1002/ppul.10455. [DOI] [PubMed] [Google Scholar]
- 24.Goldberg JB, Ohman DE. Cloning and expression in Pseudomonas aeruginosa of a gene involved in the production of alginate. J. Bacteriol. 1984;158:1115–1121. doi: 10.1128/jb.158.3.1115-1121.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boucher JC, et al. Two distinct loci affecting conversion to mucoidy in Pseudomonas aeruginosa in cystic fibrosis encode homologs of the serine protease HtrA. J. Bacteriol. 1996;178:511–523. doi: 10.1128/jb.178.2.511-523.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boucher JC, Schurr MJ, Yu H, Rowen DW, Deretic V. Pseudomonas aeruginosa in cystic fibrosis: role of mucC in the regulation of alginate production and stress sensitivity. Microbiology. 1997;143:3473–3480. doi: 10.1099/00221287-143-11-3473. [DOI] [PubMed] [Google Scholar]
- 27.Goldberg JB, Gorman WL, Flynn JL, Ohman DE. A mutation in algN permits trans activation of alginate production by algT in Pseudomonas aeruginosa. J. Bacteriol. 1993;175:1303–1308. doi: 10.1128/jb.175.5.1303-1308.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hassett, D.J., et al. 2004. Anaerobic metabolism by Pseudomonas aeruginosa in cystic fibrosis airway biofilms: role of nitric oxide, quorum sensing and alginate production. In Strict and facultative anaerobes: medical and environmental aspects. Horizon Bioscience. Wymondham, United Kingdom. 87–108.
- 29.Olson JS, Phillips GN., Jr Kinetic pathways and barriers for ligand binding to myoglobin. J. Biol. Chem. 1996;271:17596. [PubMed] [Google Scholar]
- 30.Liu L, et al. A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature. 2001;410:490–494. doi: 10.1038/35068596. [DOI] [PubMed] [Google Scholar]
- 31.Lobysheva II, Stupakova MV, Mikoyan VD, Vasilieva SV, Vanin AF. Induction of the SOS DNA repair response in Escherichia coli by nitric oxide donating agents: dinitrosyl iron complexes with thiol-containing ligands and S-nitrosothiols. FEBS Lett. 1999;454:177–180. doi: 10.1016/s0014-5793(99)00777-2. [DOI] [PubMed] [Google Scholar]
- 32.Hurst JK, Lymar SV. Toxicity of peroxynitrite and related reactive nitrogen species toward Escherichia coli. Chem. Res. Toxicol. 1997;10:802–810. doi: 10.1021/tx970008v. [DOI] [PubMed] [Google Scholar]
- 33.Zhu L, Gunn C, Beckman JS. Bactericidal activity of peroxynitrite. Arch. Biochem. Biophys. 1992;298:452–457. doi: 10.1016/0003-9861(92)90434-x. [DOI] [PubMed] [Google Scholar]
- 34.Hassett DJ, Schweizer HP, Ohman DE. Pseudomonas aeruginosa sodA and sodB mutants defective in manganese- and iron-cofactored superoxide dismutase activity demonstrate the importance of the iron-cofactored form in aerobic metabolism. J. Bacteriol. 1995;177:6330–6337. doi: 10.1128/jb.177.22.6330-6337.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garrett ES, Perlegas D, Wozniak DJ. Negative control of flagellum synthesis in Pseudomonas aeruginosa is modulated by the alternative sigma factor AlgT (AlgU) J. Bacteriol. 1999;181:7401–7404. doi: 10.1128/jb.181.23.7401-7404.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Toole GA, Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 1998;30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- 37.Lau GW, Ran H, Kong F, Hassett DJ, Mavrodi D. Pseudomonas aeruginosa pyocyanin is critical for lung infection in mice. Infect. Immun. 2004;72:4275–4278. doi: 10.1128/IAI.72.7.4275-4278.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sparkman L, Boggaram V. Nitric oxide increases IL-8 gene transcription and mRNA stability to enhance IL-8 gene expression in lung epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2004;287:L764–L773. doi: 10.1152/ajplung.00165.2004. [DOI] [PubMed] [Google Scholar]
- 39.Pedersen SS, Hoiby N, Espersen F, Koch C. Role of alginate in infection with mucoid Pseudomonas aeruginosa in cystic fibrosis. Thorax. 1992;47:6–13. doi: 10.1136/thx.47.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Firoved AM, Wood SR, Ornatowski W, Deretic V, Timmins GS. Microarray analysis and functional characterization of the nitrosative stress response in nonmucoid and mucoid Pseudomonas aeruginosa. J. Bacteriol. 2004;186:4046–4050. doi: 10.1128/JB.186.12.4046-4050.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woodmansee AN, Imlay JA. A mechanism by which nitric oxide accelerates the rate of oxidative DNA damage in Escherichia coli. Mol. Microbiol. 2003;49:11–22. doi: 10.1046/j.1365-2958.2003.03530.x. [DOI] [PubMed] [Google Scholar]
- 42.Soum E, Drapier JC. Nitric oxide and peroxynitrite promote complete disruption of the [4Fe-4S] cluster of recombinant human iron regulatory protein 1. J. Biol. Inorg. Chem. 2003;8:226–232. doi: 10.1007/s00775-002-0412-9. [DOI] [PubMed] [Google Scholar]
- 43.Schopfer FJ, Baker PR, Freeman BA. NO-dependent protein nitration:a cell signaling event or an oxidative inflammatory response? Trends Biochem. Sci. 2003;28:646–654. doi: 10.1016/j.tibs.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 44.Mayburd AL, Kassner RJ. Mechanism and biological role of nitric oxide binding to cytochrome c’. Biochemistry. 2002;41:11582–11591. doi: 10.1021/bi020058l. [DOI] [PubMed] [Google Scholar]
- 45.Spallarossa A, et al. Inhibition of Azotobacter vinelandii rhodanese by NO-donors. Biochem. Biophys. Res. Commun. 2003;306:1002–1007. doi: 10.1016/s0006-291x(03)01067-2. [DOI] [PubMed] [Google Scholar]
- 46.Hunt JF, Gaston BJ, Ricciardolo F. Acid stress in the airway. J. Allergy Clin. Immunol. 2004;84:731–765. doi: 10.1016/j.jaci.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 47.Hunt JF, et al. Endogenous airway acidification. Implications for asthma pathophysiology. Am. J. Respir. Crit. Care Med. 2000;161:694–699. doi: 10.1164/ajrccm.161.3.9911005. [DOI] [PubMed] [Google Scholar]
- 48.Holloway BW. Genetics of Pseudomonas. Bacteriol. Rev. 1969;33:419–443. doi: 10.1128/br.33.3.419-443.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ohman DE, Chakrabarty AM. Genetic mapping of chromosomal determinants for the production of the exopolysaccharide alginate in a Pseudomonas aeruginosa cystic fibrosis isolate. Infect. Immun. 1981;33:142–148. doi: 10.1128/iai.33.1.142-148.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lau GW, Hassett DJ, Ran H, Kong F. The role of pyocyanin in Pseudomonas aeruginosa infection. Trends Mol. Med. 2004;10:599–606. doi: 10.1016/j.molmed.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 51.Lau GW, et al. A functional genomic analysis of type 3 Streptococcus pneumoniae virulence. Mol. Microbiol. 2001;40:555–571. doi: 10.1046/j.1365-2958.2001.02335.x. [DOI] [PubMed] [Google Scholar]
- 52.Matsui H, et al. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell. 1998;95:1005–1015. doi: 10.1016/s0092-8674(00)81724-9. [DOI] [PubMed] [Google Scholar]
- 53.Grubb BR, Paradiso AM, Boucher RC. Anomalies in ion transport in CF mouse tracheal epithelium. Am. J. Physiol. 1994;267:C293–C300. doi: 10.1152/ajpcell.1994.267.1.C293. [DOI] [PubMed] [Google Scholar]
- 54.Green LC, et al. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 55.Coddington JW, Hurst JK, Lymar SV. Hydroxyl radical formation during peroxynitrous acid decomposition. J. Am. Chem. Soc. 1999;121:2438–2443. [Google Scholar]
- 56.Konorev EA, Tarpey MM, Joseph J, Baker JE, Kalyanaraman B. Nitronyl nitroxides as probes to study the mechanism of vasodilatory action of nitrovasodilators, nitrone spin traps, and nitroxides:role of nitric oxide. Free Radic. Biol. Med. 1995;18:169–177. doi: 10.1016/0891-5849(94)00112-w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.