Abstract
It is believed that the efficacy of antigen-specific CD8+ cytotoxic T lymphocytes (CTLs) depends not only on the quantity of CTL generated, but perhaps, more importantly, on the avidity of the CTL. To date, however, no strategy has been demonstrated to preferentially induce higher avidity human CTL. In the present study, antigen-presenting cells (APC) generated from human peripheral blood mononuclear cells (PBMC) were infected with a recombinant avipox vector (rF-) containing the transgenes for a TRIad of COstimulatory Molecules (human B7-1, ICAM-1 and LFA-3, designated as rF-TRICOM) and then used to elicit peptide-specific CTL from autologous T cells. Compared with peptide-pulsed non-infected APC, or peptide-pulsed APC infected with wild-type vector, peptide-pulsed APC infected with rF-TRICOM induced not only more CTL, but also higher avidity CTL; this was demonstrated by tetramer staining, tetramer dissociation, IFN-γ production and cytolytic assays. Peptide-pulsed rF-TRICOM–infected dendritic cells (DC) were also shown to induce CTL with a greater than 10-fold higher avidity than CTL induced using CD40L-matured dendritic cells (DC); the use of peptide-pulsed CD40L-matured DC infected with rF-TRICOM as APC induced CTL of even greater avidity. To our knowledge, these studies are the first to demonstrate a methodology to induce higher avidity human CTL and have implications for the development of more efficient vaccines for a range of human cancers.
Keywords: Avidity, CTL, human, costimulation, tumor vaccine
Introduction
Increasing evidence has shown that high functional avidity CD8+ T cells can mediate effective immunity to viral infection (1) and against tumors (2–10). However, no vaccine strategy has been developed to effectively enhance the functional avidity of human cytotoxic T lymphocytes (CTL).
Several studies in cancer patients have demonstrated that peptide vaccination can induce a heterogeneous peptide-specific CD8+ T-cell response; some CD8+ T cells have been shown to poorly recognize tumor cells endogenously expressing TAAs (4, 9, 11, 12). However, the peptide-specific, tumor non-reactive CTL were rendered tumor reactive once tumor cells were loaded with cognate peptide, indicating that the tumor non-reactive CTL were of low avidity, and not lytic defective (4, 7, 11). In fact, clinical trials have shown that enhanced levels of CD8+ T-cell responses following peptide vaccination were not associated with improvement in clinical outcome (12, 13). Many of these studies thus suggest that the efficacy of immune responses may depend on the avidity (quality) of the T cells induced, as well as the magnitude (quantity) of T cells induced.
Two different strategies have been described to enrich higher avidity Ag-specific CTL populations in vitro. One approach is based on the structural affinity of T-cell receptors as determined by MHC-tetramer binding. Several studies have since reported that strong tetramer staining is correlated with enhanced tumor reactivity and the approach could be used to isolate “high-avidity” (stronger tetramer binding) CTL from tumor patients for adoptive transfer therapy (6, 9, 14). However, other studies demonstrated that some strong tetramer binding CTL have low or no tumor reactivity (15–19). Alternatively, other investigators (1–3) have demonstrated that higher functional avidity murine CTL can be enriched by using lower concentrations of peptide during in vitro stimulation (IVS) in a murine viral infection model and a murine tumor model. Although a lower dose of stimulating peptide has successfully expanded higher avidity CTL in mouse models (1–3), simply lowering the stimulating peptide dose did not elicit the enhanced avidity of human CTL, but only reduced the magnitude of CTL responses; this was shown in both peptide vaccinated melanoma patients (5) and normal donors (4). All of the above murine and human studies were conducted by qualitatively or quantitatively altering signal 1, i.e., antigen.
Previous murine preclinical studies demonstrated that vaccination of mice with recombinant poxviruses containing the transgenes for a TRIad of COstimulatory Molecules (B7-1, ICAM-1 and LFA-3, designated as TRICOM) and a TAA enhanced the level of antigen-specific CD8+ T cells generated (20). Oh et al. (21) has shown that vaccination of mice with peptide-pulsed murine B cells infected with a replication defective avipox (fowlpox) TRICOM vector can lead to the generation of higher avidity murine T cells. Hodge et al. have recently shown (22) that vaccination of mice s.c. with recombinant vaccinia viruses (rV-) containing transgenes for antigen (β-gal or CEA) and TRICOM (murine) led to the induction of higher avidity CTL than the use of recombinant vaccinia containing the transgenes for the antigens and one costimulatory molecule (B7.1), or just the antigens and no costimulatory molecules. For example, in carcinoembryonic antigen (CEA)–transgenic mice, where CEA is a “self antigen,” s.c. vaccination with rV-CEA-TRICOM led to the induction of only a 1.3-fold increase in antigen-specific splenic CTL than did vaccination with rV-CEA-B7.1, and 2.4 more CEA-specific CTL than the use of rV-CEA. The avidity of the T cells produced, however, was 20-fold greater when employing rV-CEA-TRICOM rather than rV-CEA-B7.1, and 100-fold greater than the use of rV-CEA as the immunogen (22).
We have previously shown (23) that infection of peptide-pulsed human B cells with recombinant avipox (fowlpox, rF-) expressing human B7.1, ICAM-1 and LFA-3 transgenes (designated rF-TRICOM) will enhance the quantity of antigen-specific human T cells as compared with the use of uninfected or wild-type vector-infected peptide-pulsed B cells. We have also shown that the use of peptide-pulsed human DC infected with rF-TRICOM vectors enhances the level of human T cells better than the use of peptide-pulsed uninfected DC, or control vector (FP-WT) infected DC (24). Neither of these studies (23, 24), however, addressed the avidity of the T cells generated. In the present study, we have investigated whether the use of peptide-pulsed human APC, infected with the replication defective rF-TRICOM vector, would induce higher avidity CTL as compared with uninfected or control vector–infected peptide-pulsed APC. We employed immature DC (generated with granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4) to simulate conditions when such recombinant vectors are used as i.d. or s.c. injected vaccines. We also employed DC matured with CD40L to determine whether similar results could be obtained with such APC populations. To our knowledge, these studies are the first to demonstrate a method to enhance the avidity of human T cells; they have implications for both the generation of more potent cancer vaccines and the in vitro generation of more effective T cells for adoptive transfer therapy regimens.
Materials and Methods
Cell line
The human melanoma lines SKMEL24 and DM13 (both HLA-A2+, CEA−), colon cancer cell lines SW1463 and SW480 (both HLA-A2+, CEA+), and the T2 cells (TAP-deficient, HLA-A2+ human lymphoblastoid cells) were used as targets in 51Cr release assay or stimulators for cytokine production. All cells were cultured in RPMI supplemented with 10% FCS (Invitrogen, Carlsbad, CA), antibiotics (penicillin 100 units/ml, streptomycin 100 μg/ml, amphotericin B 0.25 μg/ml, gentamicin 50 μg/ml), L-glutamine 450 μg/ml, and sodium bicarbonate 2.5 mg/ml in 75 cm2 T flasks (Costar, Cambridge, MA).
HLA typing
HLA-A*02 subtyping was performed by the Blood Bank of the National Institutes of Health with PCR using sequence-specific primers (PCR-SSP). The primers used were previously described by Bunce et al. (25) and Krausa et al. (26). All donors used in this study were HLA-A*0201 positive.
Recombinant viruses
The recombinant fowlpox virus rF-TRICOM containing the transgenes for the human costimulatory molecules B7-1 (CD80), ICAM-1 (CD54) and LFA-3 (CD58) has been described previously (27–29).
Peptides
The HLA-A*0201 binding CEA agonist peptide, CAP1-6D (YLSGADLNL), has been previously described in detail, and is designated here, unless otherwise specificed, as CEA peptide (30, 31). The Flu-M1 peptide (GILGFVFTL) was derived from influenza matrix protein (32). Both peptides were used to pulse DC or target cells as indicated. They were synthesized by SynPep (Dubin, CA), and their purity was greater than 95%.
Generation of DC from PBMC
DC were generated from PBMC as described by Romani et al. (33) with some modifications (34) by using GM-CSF (100 ng/ml, PeproTech, Rocky Hill, NJ) and IL-4 (20 ng/ml, PeproTech). On day 6, DC were either uninfected or infected with fowlpox-based vectors as previously described (24), and then loaded with peptide as APC to generate CTL. For some experiments, day 6 DC generated with GM-CSF/IL-4 were also matured by incubating with CD40L plus the cross linking antibody Enhancer (each at 1 μg/ml, Alexis, San Diego, CA) for 24 hours; the DC were then left uninfected or infected with fowlpox-based vectors as described (24).
Antibody, tetramer staining and flow cytometry assay
FITC-labeled anti-human CD8, CD58, CD80, CD83, HLA-A2(BB7.2), HLA-DR, PE-labeled CD11c, CD54, and Cy-labeled CD8 were used for staining cell surface molecules. All of the antibodies were purchased from BD Pharmingen (San Diego, CA). FITC-labeled COL-1 (anti-human CEA) was prepared in the laboratory. PE-labeled HLA-A2 CAP1-6D tetramer, designated here as CEA tetramer, was provided by the National Institutes of Health Tetramer Core Facility (Atlanta, GA) and HLA-A2 Flu tetramer was purchased from BechmanCoulter (San Diego, CA). For flow cytometric analysis of cell surface, 2–5 x 105 cells were incubated on ice with the appropriate Abs for 30–45 minutes, washed twice and analyzed on a FACS Calibur (BD Biosciences, Mountain View, CA). Background staining was assessed using isotype control Abs. For tetramer staining, cells were stained with FITC- or Cy-labeled anti-CD8 and PE-labeled tetramer for 60 minutes on ice. Data were analyzed using CellQuest.
CTL generation
CTL were generated using autologous DC as previously described (4). In brief, Pan T cells isolated using Pan-T kits (Miltenyi Biotech, Bergisch Gladbach, Germany) were stimulated with autologous DC pulsed with CEA peptide (20 μg/ml) at T:DC ratio of 20–30:1 for 3–4 cycles of IVS at 7–10 days intervals. IL-2 (20 IU/ml) was added 3 days after each IVS, except the first IVS. CTL activity was screened using T2 cells pulsed with native CEA peptide CAP1 (YLSGANLNL) in 51Cr release assay 7 days after three cycles of IVS.
Purification of tetramer positive CTL
Bulk CTL cultures were stained with PE-labeled CEA tetramer for 1 hour at 4°C 7 days following 3–4 cycles of IVS. Cells were washed twice and incubated with anti-PE labeled beads for 15 minutes at 4–8°C, and tetramer positive CTL were isolated with autoMACS (Miltenyi Biotech) according to the instructions provided by the manufacturer.
Cytotoxicity assays
Cultured CTL were tested for cytotoxicity in a standard 4-h 51Cr-release assay (4). Tumor cells (1–2 x 106/ml) were labeled with 100 μCi sodium-51Chromate for 1 hour at 37°C. Peptide-pulsed targets (1 x 106/ml in the presence of peptide) were labeled with 100 μCi sodium-51Chromate for 2 hours at 37°C. Target cells (5000 targets/well) were added to wells containing effector CTL. The percent specific 51Cr release was calculated as previously described (4).
Cytokine induction and detection
T cells were co-cultured with T2 cells pulsed with or without various concentrations of peptide for 24 hours. The T cells to T2 cells ratio was 10:1. Supernatants were collected at the end of culture and cytokine production was detected using FACS-based Cytometric Bead Array (CBA) (Human Th1/Th2 Cytokine CBA Kit was purchased from BD Pharmingen).
Cytokine/chemokine detection by Luminex
Supernatants from T cell cultures were collected 24 hours after stimulation with APC-pulsed peptide. A panel of 22 cytokines/chemokines was detected using a Human Cytokine/Chemokine Multiplex Immunoassay Kit (Cat# HCYTO-60K-PMX22) from LINCO Research (St. Charles, MO) on a Luminex100TM machine (Luminex Corporation, Austin, TX), according to the instructions provided by the manufacturer. Data were analyzed using software MasterPlex QT2.0.
Avidity titration
Avidity of peptide-specific CTL was titrated by cytolytic activity and IFN-γ production against T2 cells pulsed with various concentrations of peptide. Briefly, T2 cells (1x106/ml) were pulsed with various concentrations of peptide as indicated at 37°C for 2 hours with (for lytic activity) or without (for IFN-γ production) 51Cr. T2 cells were washed twice before being used for lytic activity and IFN-γ production assay as described above. Avidity, expressed as MC50 in mole (M), was defined as the concentration of peptide required to achieve 50% of maximal response, and calculated using Microsoft Excel.
Statistical analysis
To assess the statistical significance of different treatment, the ANOVA test was performed. The difference between two means was determined by Student t test. To assess the statistical differences between two curves, a two-tailed t test was performed using the asymptotic standard error estimate of the difference between the two offset parameters of the curves. The P values were corrected for multiple comparisons by the method of Sidak (35).
Results
rF-TRICOM–infected immature DC induce a greater magnitude of peptide-specific CTL
Human DC, unlike murine DC, usually express low levels of CD80. The present and previous studies (24, 36, 37) have shown that DC generated from PBMC, following GM-CSF and IL-4 treatment, were generally <20% positive for expression of CD80, with the majority of them being <10% CD80+. In the present study, adherent monocytes of PBMC exposed to GM-CSF/IL-4 for 7 days gave rise to a cell population containing approximately 90% of CD11c and HLA-class II double positive, CD14 and CD19 negative cells, indicating typical phenotypes of DC most likely encountered by vector-based vaccines when administered to humans i.d. and/or s.c. As shown in Figure 1, infection of human DC with the rF-TRICOM vector resulted in upregulation of CD80, CD54 and CD58 in terms of mean fluorescence intensity (MFI) and percent cells positive. Control fowlpox virus infection of DC had no significant effect on surface molecule expression of HLA class I and II, CD11c, CD54, CD58 and CD80 as compared with uninfected DC. No change was observed on DC maturation following either FP-WT or rF-TRICOM infection in terms of CD83 expression.
Fig. 1.

Phenotypic analysis of DC infected with rF-TRICOM. DC were generated from PBMC of a representative normal blood donor in the presence of GM-CSF and IL-4. On day 6, DC were either left uninfected, or infected with FP-WT or rF-TRICOM at an MOI of 40:1 for 24 hours in the presence of GM-CSF/IL-4. DC were then stained with fluorochrome-labeled antibodies as described in Materials and Methods. Percentage of specific molecule positive cells, with MFI in parentheses, is shown in each panel. Surface molecule expression was monitored by FACS and analyzed by CellQuest. Isotype control antibody (thin curves) was overlaid with specific antibody staining in histogram (thick curves). The experiments were repeated over six times with similar results.
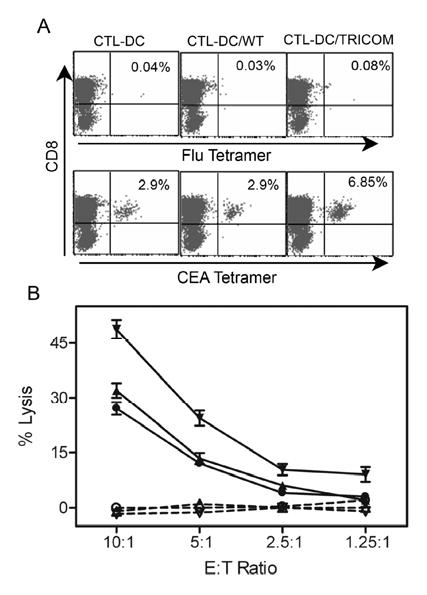
To investigate whether peptide-pulsed DC/TRICOM vs peptide-pulsed DC expanded CTL to a greater number, DC generated from PBMC of apparently healthy individuals were used either uninfected, or infected with either fowlpox wild-type (FP-WT) or recombinant rF-TRICOM, and then pulsed with the CEA peptide. In initial experiments, CEA peptide-pulsed DC/TRICOM, peptide-pulsed DC/WT, or peptide-pulsed DC were used as APC to stimulate autologous T cells from normal donors for the first cycle of stimulation (priming). The T cells were then further stimulated for two IVS using peptide-pulsed DC or peptide-pulsed DC/WT. T cells generated using DC/TRICOM as priming stimulation produced slightly higher levels of IFN-γ than T cells induced using DC/WT as priming stimulation (263 pg/ml vs 161 pg/ml using 1 μg/ml peptide loaded T2). There was, however, no difference in the avidity of T cells induced using the two methods of generating T cells. Thus, in subsequent studies, T cells were stimulated for all three or four IVS with peptide-pulsed DC/TRICOM vs DC/WT. After such three cycles of IVS, the bulk CTL were stained with MHC-tetramer. As seen in Figure 2A, the percentage of CEA tetramer positive CD8+ T cells in CTL elicited by DC/TRICOM was 6.83%, while that of tetramer positive T cells in both DC and DC/WT groups was 2.9%. As a control, CTL from all three groups were reacted with Flu-M1 tetramer and were negative (upper panel of Fig. 2A).
Fig. 2.

Tetramer staining and cytolytic activity of antigen-specific CTL induced peptide-pulsed DC. DC were generated with GM-CSF + IL-4 with or without infection with either FP-WT or rF-TRICOM. Pan T cells isolated from an apparently healthy donor were stimulated with CEA peptide-pulsed autologous DC as indicated for three cycles of in vitro stimulation (IVS) at 7-10 day intervals. IL-2 (20 IU/ml) was added to the cultures 3 days after each IVS. Ten days after the third IVS, T cells were stained with Cy-CD8 and PE-CEA tetramer (lower 3 panels of A), or control tetramer (Flu-M1 tetramer, upper 3 panels of A). Tetramer positive cells were monitored by FACS and analyzed by CellQuest (A). (B) CTL activity was determined by standard 4-h 51Cr release assay toward T2 cells without peptide (dotted lines and open symbols), or T2 cells pulsed with CEA peptide (5 μg/ml) as targets (solid lines and closed symbols). Circles: CTL/DC; triangles: CTL/DC/WT; inverted triangles: CTL/DC/TRICOM. n=3, mean ± SD of triplicates. The results are representative of three experiments.
To test the efficacy of the CTL induced with peptide-pulsed DC to lyse target cells, lytic activity of CTL was measured. As shown in Figure 2B, CTL induced by both uninfected DC and DC/WT demonstrated specific cytotoxic activity toward T2 cells pulsed with CEA peptide. However, CTL elicited by DC/TRICOM displayed a greater ability to lyse T2 cells pulsed with CEA peptide at all effector to target (E:T) ratios compared with DC (P=0.0216, ANOVA) and DC/WT (P=0.0386, ANOVA) groups. None of the CTL could lyse T2 cells without peptide (Fig. 2B).
CTL generated by rF-TRICOM–infected immature DC produce a higher level of cytokines and chemokines following peptide stimulation
The capacity of the different CTL, induced by DC with or without rF-TRICOM infection, to produce cytokine following stimulation with peptide was compared. After 24 hours of stimulation with T2 pulsed with CEA peptide, CTL induced by DC/WT released a low, but detectable (<100 pg/ml), amount of IFN-γ (see insert of Fig. 3A). However, these CTL did not produce detectable levels of IL-2 (<10 pg/ml; Fig. 3B). In contrast, CTL induced by peptide-pulsed DC/TRICOM produced markedly increased levels of IFN-γ as compared with CTL induced by peptide-pulsed DC/WT (P<0.0001, two-tailed t test; Fig. 3A) and IL-2 (P<0.0001, two-tailed t test; Fig. 3B) in a dose-dependent manner following peptide stimulation. No detectable IL-4 and IL-10 (<5 pg/ml) was seen in the supernatants from CTL-elicited DC/TRICOM or DC/WT following peptide stimulation.
Fig. 3.
CTL induced by peptide-pulsed DC infected with rF-TRICOM vector demonstrate a higher amount of cytokine production following Ag stimulation. CTL were generated as described in the legend of Fig. 2. IFN-γ (A) and IL-2 (B) production following peptide stimulation are shown. IFN-γ production by CTL generated with peptide-pulsed DC/WT is graphed as an insert of Fig. 3A (note different scale) to display the dose-effect response curve. The results are representative of five experiments using PBMC from five different donors.
To further investigate cytokine and chemokine production following TRICOM co-stimulation, CTL generated by peptide-pulsed autologous DC infected with FP-WT or rF-TRICOM further stimulated one more time in vitro with the corresponding DC pulsed with or without the CEA peptide. When stimulated with peptide-pulsed DC infected with rF-TRICOM, T cells dramatically increased production of IL-2, IL-4, IL-13, TNFα, IFNγ , GM-CSF, MIP-1α and RANTES as compared with peptide-pulsed DC infected with FP-WT control vector or other control groups (Table 1). Other cytokines/chemokines, such as IL-1α, 1β, 5, 6, 7, 8, 10, 12, 15, 17, G-CSF, Eotaxin and IP-10, were marginally increased or no change following TRICOM stimulation (data not shown).
Table 1.
CTL stimulated with TRICOM-infected peptide-pulsed dendritic cells produce higher levels of cytokines and chemokines1
| Donor 6 (pg/ml produced by CTL) |
||||||
|---|---|---|---|---|---|---|
| A | B | C | D | |||
| Cytokine/chemokine | DC infected with: | FP-WT | rF-TRICOM | FP-WT | rF-TRICOM | Fold increase |
| Peptide pulsed: | None | none | CEA | CEA | (D/C): | |
| IL-2 | <30 | <30 | 52 | 2,597 | 49.9x | |
| IL-4 | 75 | 100 | 119 | 1,169 | 9.8x | |
| IFN-γ | 439 | 962 | 4,148 | 96,414 | 23.2x | |
| IL-13 | 1,084 | 829 | 1,335 | 4,077 | 3.1x | |
| TNFα | 144 | 129 | 170 | 2,157 | 12.7x | |
| GM-CSF | 156 | 291 | 379 | 3,038 | 8.1x | |
| MIP-1α | 57 | 1,235 | 939 | 7,176 | 7.6x | |
| RANTES | 133 | 760 | 519 | 3,472 | 6.7x | |
| Donor 7 (pg/ml produced by CTL) |
||||||
| A | B | C | D | |||
| Cytokine/chemokine | DC infected with: | FP-WT | rF-TRICOM | FP-WT | rF-TRICOM | Fold increase |
| Peptide pulsed: | None | none | CEA | CEA | (D/C): | |
| IL-2 | <30 | <30 | <30 | 117 | >3.9x | |
| IL-4 | <30 | 53 | 36 | 384 | 10.7x | |
| IFN-γ | <30 | 202 | 215 | 7,429 | 34.6x | |
| IL-13 | <30 | 380 | 156 | 3,340 | 21.4x | |
| TNFα | <30 | <30 | 509 | 1,501 | 2.9x | |
| GM-CSF | <30 | 55 | 62 | 633 | 10.2x | |
| MIP-1α | <30 | 70 | 998 | 20,203 | 20.2x | |
| RANTES | 78 | 128 | 989 | 1,781 | 1.8x | |
CTL were generated by in vitro stimulation of T cells isolated from PBMC of normal donors with autologous DC infected with either FP-WT or rF-TRICOM pulsed with the CEA CAP1-6D peptide. CTL then were stimulated with the corresponding virus-infected DC pulsed with or without peptide for 24 hours. The supernatants were collected for cytokine/chemokine production using Luminex, as described in Materials and Methods. Data shown here are the average of duplicate wells.
TRICOM stimulation facilitates the induction of high functional avidity CTL: cytolytic assay and IFN-γ assay
Studies were then undertaken to determine whether TRICOM had any effect on the induction of higher avidity CD8+ T cells. CTL generated by uninfected peptide-pulsed DC, peptide-pulsed DC/WT or DC/TRICOM were titrated for their ability to mediate lysis using different densities of peptide-MHC complexes on T2 target cells. As seen in Figure 4A, CTL induced by uninfected DC and peptide-pulsed DC/WT displayed the same lytic abilities to various peptide-MHC complex densities on T2 cells. However, CTL induced by DC/TRICOM were more efficient in the lysis of T2 cells with lower peptide density as compared with CTL-DC (P=0.02, ANOVA) and CTL-DC/WT (P=0.009, ANOVA), as demonstrated by a shift of dose-response curve to the right (Fig. 4A). As previously demonstrated in murine systems (21) using different CTL generated by altering signal 1, this is more telling when the data are normalized to percentage of maximal cytolytic responses, as shown in Figure 4B. The avidity (21) of CTL-DC/TRICOM was 1.27 x 10−9 M, while the avidities for CTL-DC and CTL-DC/WT were 3.21 and 3.32 x 10−8 M, respectively.
Fig. 4.
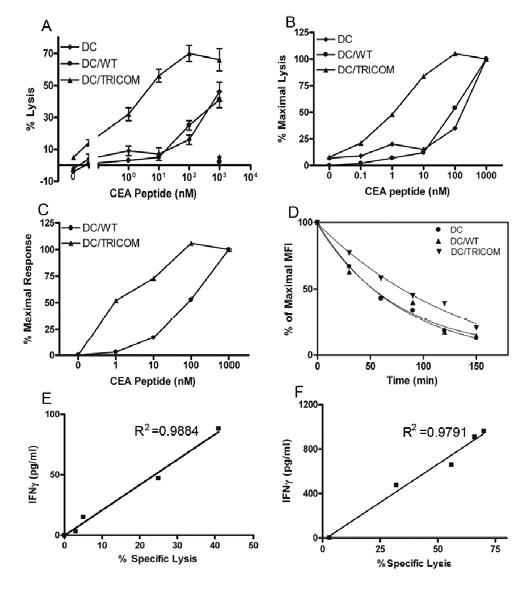
CTL generated by peptide-pulsed DC infected with TRICOM vector demonstrate higher functional and structural avidity. CTL elicited from PBMC of a representative normal donor following stimulation with CEA peptide-pulsed DC, or peptide-pulsed DC infected with FP-WT or rF-TRICOM were titrated for functional avidity using a cytolytic assay (E:T = 10:1), IFN-γ production assay, and structural avidity. A, Titration of functional avidity of CTL using 51Cr release assay. Lysis of control peptide (Flu-M1, 1,000 ng/mL) pulsed target cells was <5% for all three cell lines (panel A, bottom right symbols). n=3, mean ± SD of triplicates. B, Normalization of cytolytic data shown in Fig. 4A is expressed as percentage of maximal lysis of target cells. C, Normalization of IFN-γ production data shown in Fig. 3A is expressed as percentage of maximal response of IFN-γ production. D, Tetramer dissociation assay of CTL. CTL were stained with CD8-FITC and PE-tetramer on ice for 90 minutes and then washed twice. Cells were resuspended in staining buffer containing 5-fold unlabeled MHC-CEA tetramer and incubated at room temperature. An aliquot was taken at indicated time points for FACS analysis. Mean fluorescence intensity (MFI) vs time was plotted for calculation of a half time of fluorescence decay. CTL were generated by uninfected peptide-pulsed DC or peptide-pulsed DC infected with FP-WT or rF-TRICOM. E and F, Correlation of IFN-γ production vs specific lysis of target cells by CTL induced by DC/WT (E) and DC/TRICOM (F). IFN-γ production by CTL following various concentrations of peptide stimulation was plotted against specific lysis of T2 cells pulsed with corresponding concentrations of peptide by the CTL. Correlation analysis between IFN-γ production and cytolytic activity was performed using Microsoft Excel. These experiments were done four times with similar results.
In addition, avidity of the same CTL lines was also determined using IFN-γ production. The avidity of CTL-DC/TRICOM calculated by IFN-γ production was 1.32 x 10−9 M, while that for CTL-DC/WT was 4.97 x 10−8 M (Fig. 4C); these results are very similar to those determined by the cytolytic method for the corresponding CTL (Fig. 4B).
The stability of tetramer binding to TCR has been shown to correlate with functional avidity (17, 18). To test whether tetramer-TCR complexes on higher functional avidity CTL elicited by DC/TRICOM were more stable, a tetramer dissociation assay was performed as described (18). As seen in Figure 4D, CTL generated by both uninfected peptide-pulsed DC and peptide-pulsed DC/WT displayed very similar off-rates of tetramer with a half-time of dissociation close to 50 minutes. However, CTL induced by peptide-pulsed DC/TRICOM demonstrated a slower off-rate of 101.5 minutes, which is a 48% increase as compared with that of CTL-DC and CTL-DC/WT (P=0.021, two-tailed t test).
Although the cytolytic method is considered the gold standard for titration of functional avidity of CTL (1, 3, 4, 6, 8, 15, 38), IFN-γ production has also been widely used (2, 3, 5, 38–41); this method also requires fewer cells and does not require radioactive materials. To test whether there was any difference in the avidity of CTL as determined by the cytolytic method vs IFN-γ production, correlation analysis of lysis vs IFN-γ production employing various concentrations of peptide was performed. As shown in Figure 4E and F, respectively, IFN-γ release was directly correlated with cytolytic activity for CTL induced by DC/WT (R2=0.988), and for CTL induced by DC/TRICOM (R2=0.979).
We then compared the functional avidity of CTL induced by peptide-pulsed DC/WT and peptide-pulsed DC/TRICOM from four additional donors using the IFN-γ production method. Table 2 shows that functional avidity of CTL induced by peptide-pulsed DC/TRICOM was at least 10-fold higher for four of the five donors than that of CTL elicited by peptide-pulsed DC/WT from the same donors.
Table 2.
Functional avidity of CTL induced by peptide-pulsed uninfected DC, DC infected with FP-WT, or rF-TRICOM from five apparently healthy donors1
| Functional Avidity |
|||||
|---|---|---|---|---|---|
| Donor 1 | Donor 2 | Donor 3 | Donor 4 | Donor 5 | |
| CTL-DC/TRICOM | 1.32 x 10−9 | 4. 2 x 10−9 | 3.5 x 10−10 | 1.0 x 10−7 | 2.1 x 10−10 |
| CTL-DC/WT | 4.97 x 10−8 | 2.08 x 10−7 | 9.3 x 10−9 | 1.88 x 10−7 | 2.4 x 10−8 |
| CTL-DC | ND3 | 1.93 x 10−7 | 1.87 x 10−8 | ND | 2.03 x 10−8 |
| ΔAvidity (TRICOM vs WT)2 | 37.7 | 49.5 | 26.6 | 1.9 | 114 |
| ΔAvidity (TRICOM vs uninfected)2 | ND | 45.9 | 53.4 | ND | 97 |
Functional avidity, defined as the concentration of peptide required to achieve 50% of maximal response (MC50, expressed in mole), was determined by the IFN-γ production method as described in Materials and Methods. DC were generated with GM-CSF + IL-4 as described in Materials and Methods with or without infection with either FP-WT or rF-TRICOM. CTL were induced from T cells of normal blood donors following stimulation with CEA peptide-pulsed autologous DC. FN-γ production in 24-hour supernatants from CTL stimulated with various concentrations of peptide was detected by CBA.
ΔAvidity was expressed as fold difference in functional avidity of CTL induced by peptide-pulsed DC/TRICOM vs CTL induced by either peptide-pulsed DC or DC/WT and was calculated as a ratio of the MC50 of CTL-DC or CTL-DC/WT to the MC50 of the corresponding CTL-DC/TRICOM.
ND = Not determined.
Comparison of the avidity of CTL generated by CD40L-matured DC and rF-TRICOM–infected DC
Some studies have demonstrated that mature DC are more potent to induce antigen-specific CTL than immature DC (42–44). It was thus of interest to compare the avidity of CTL induced by TRICOM-infected DC and by CD40L-matured DC. To do this, day 6 DC generated by GM-CSF/IL-4 were matured with CD40L as described in Materials and Methods, with or without infection with rF-TRICOM, and were then used to induce peptide-specific CTL. As seen in Figure 5A, exposure of DC to CD40L greatly increased the expression of CD83 and slightly increased the expression of the costimulatory molecules CD54, CD58 and CD80. Infection of CD40L-treated DC with either FP-WT or rF-TRICOM did not change the maturation status of DC in terms of CD83 expression (Fig. 5A). Expression of CD54, CD58 and CD80 on CD40L-treated DC following infection with rF-TRICOM, however, was greatly upregulated as compared with CD40L-matured DC in terms of both percentage of cells expressing each of the three costimulatory molecules in TRICOM as well as their MFI (Fig. 5A).
Fig. 5.
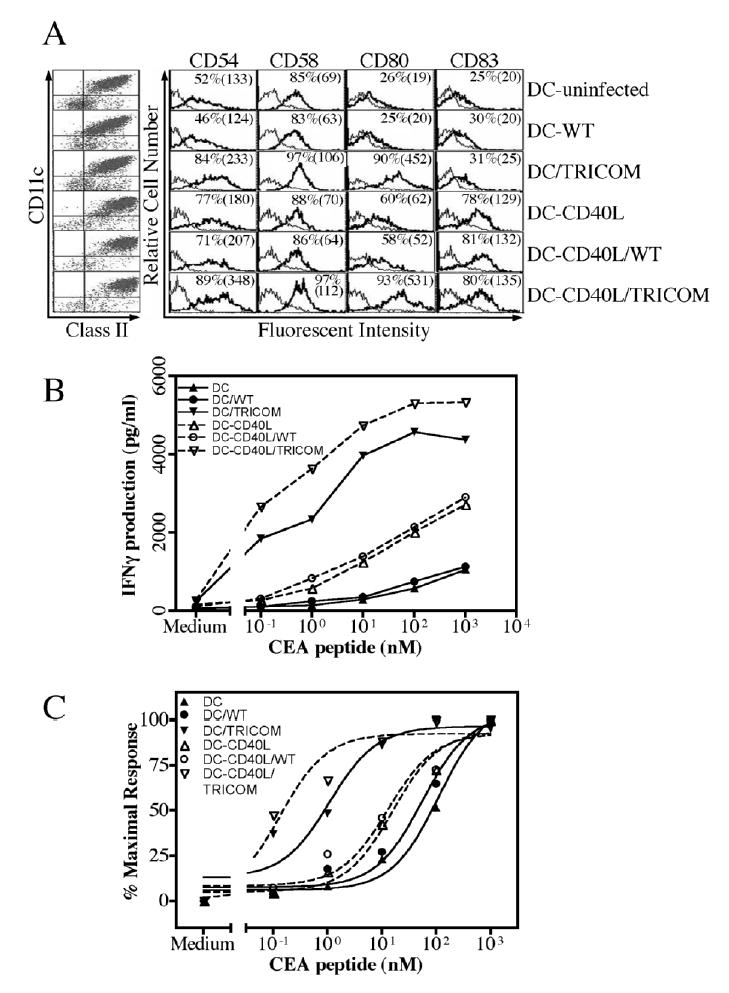
Comparison of avidity of CTL generated by peptide-pulsed immature or CD40L-matured DC with or without infection of rF-TRICOM or FP-WT. A, DC were generated using GM-CSF/IL-4 as described in Figure 1. On day 6, DC were either untreated or treated with CD40L (1 μg/ml) + Enhancer (1μg/ml) for 24 hours. They were then either left uninfected, or infected with FP-WT or rF-TRICOM at an MOI of 40:1 for 24 hours in the presence of GM-CSF/IL-4 with or without CD40L/Enhancer. DC were then stained with fluorochrome-labeled antibodies as described in Materials and Methods. The dot plot graph on the left side of Panel A depicts the percentage of CD11c+/Class II+ cells in each DC population. From top to bottom, the percentage of CD11c+/Class II+ cells was 89, 91, 93, 97, 98, and 99. The percentage of CD54, CD58, CD80 and CD83 positive cells, with mean fluorescence intensity (MFI) in parentheses, is shown in each panel. Surface molecule expression was monitored by FACS and analyzed by CellQuest. Isotype control antibody (thin curves) was overlaid with specific antibody staining in histogram (thick curves). B and C, CTL were generated with CEA peptide-pulsed autologous DC as described in Materials and Methods. After three IVS, IFN-γ production by the CTL following 24-hour stimulation with various concentrations of the peptide was determined by cytometric bead array. All the experiments were conducted at the same time. To display the differences, dose-response of IFN-γ production by CTL generated by immature DC and CD40L-matured DC, with or without infection of FP-WT or rF-TRICOM, is shown in (B). Normalization of dose-response of IFN-γ production is shown in (C). Avidity of CTL is given in Table 3.
The DC described above were pulsed with CEA peptide and used as APC to induce peptide-specific CTL from autologous human T cells. Avidity was titrated using the IFN-γ production method. As can be seen from Fig. 5B, there is approximately a 2.6–4.3 fold increase in IFN-γ production following various doses of peptide stimulation by CTL induced by CD40L-matured DC vs non-matured DC (P<0.0001, two-tailed t test). IFN-γ production by CTL elicited by DC/TRICOM was much higher than that by CTL elicited by DC (P<0.0001, two-tailed t test); IFN-γ production by CTL-DC-CD40L/TRICOM was much higher than that by CTL-DC-CD40L (P<0.0001, two-tailed t test). The capacity of IFN-γ production by CTL elicited by DC-CD40L/TRICOM was slightly superior to that by CTL induced by DC/TRICOM (P<0.001, two-tailed t test) (Fig. 5B). In terms of vector controls, there was no significant difference between CTL-DC and CTL-DC/WT (P>0.70, two-tailed t test) or between CTL-DC-CD40L and CTL-DC-CD40L/WT (P>0.70, two-tailed t test) in IFN-γ production following peptide stimulation (Fig. 5B).
To compare the avidity of T cells, IFN-γ production was expressed as a percentage of maximal response (Fig. 5C). Avidity of CTL induced by TRICOM-infected DC was 65-fold higher than CTL induced by DC without TRICOM, and 28-fold higher than CTL induced by DC matured with CD40L. Avidity of CTL, induced using DC matured with CD40L, was over 2.3-fold higher than CTL induced using DC treated only with IL-4 and GM-CSF. Finally, CTL induced using DC matured with CD40L and infected with rF-TRICOM had an avidity over 100 times greater than that of CTL induced by DC matured with CD40L (Fig. 5C and Table 3).
Table 3.
Infection of DC (immature and matured with CD40L) with rF-TRICOM enhances avidity of CTL
| Treatment of DC | Avidity (M) of CTL | Avidity vs DC (fold)* | Avidity vs CD40L-matured DC (fold)** |
|---|---|---|---|
| DC | 2.29 x 10−8 | 1.0 | −2.3 |
| DC infected with FP-WT | 1.31 x 10−8 | 1.7 | −1.3 |
| DC infected with rF-TRICOM | 3.5 x 10−10 | 65.4 | 28.0 |
| DC matured with CD40L | 9.81 x 10−9 | 2.3 | 1.0 |
| DC matured with CD40L and infected with FP-WT | 8.12 x 10−9 | 2.8 | 1.2 |
| DC matured with CD40L and infected with rF-TRICOM | 7.0 x 10−11 | 327.0 | 140.0 |
DC were generated with GM-CSF and IL-4 with or without maturation with CD40L and/or infected with rF-TRICOM or wild-type vector FP-WT, as described in Materials and Methods.
Numbers are the fold increase in avidity of activated T cells, as compared with those activated with DC generated with GM-CSF and IL-4.
Numbers are the fold increase or decrease in avidity of activated T cells, as compared with those activated with DC generated with GM-CSF and IL-4 and matured with CD40L.
CTL generated by TRICOM lyse tumor cells more effectively
The functional difference of high- and low-avidity CTL is that high-avidity CTL should lyse tumor cells or targets with lower epitope density more efficiently. To compare the cytolytic activity of T cells toward tumor cells endogenously expressing CEA, CTL induced by CD40L-matured DC infected with either FP-WT or rF-TRICOM, and pulsed with CEA peptide, were tested for their lytic activity against human colon carcinoma cells.
Bulk cultures of CTL derived from a representative donor are shown in Figure 6; 3.55% of cells induced by CD40L-matured DC were CD8+/tetramer+, while 11.56% of CTL induced by CD40L-matured DC/TRICOM were CD8+/tetramer+ (Fig. 6A). The avidity of the two CTL lines was titrated using a cytolytic assay (Fig. 6B) and normalized as percentage of maximal lysis (Fig. 6C) to calculate the avidity. As seen in Figure 6B, CTL generated by CD40L-matured DC/TRICOM lysed T2 targets with lower peptide density more efficiently as compared with CTL elicited by CD40L-matured DC (P<0.001, two-tailed t test); avidity of CTL-DC was 4.6 x 10−8M and that of CTL-DC/TRICOM was 2.5 x 10−9 M (Fig. 6C). The CTL lines were then used to define their efficacy to kill colon carcinomas endogenously expressing CEA. As shown in Figure 6D, CTL elicited by CD40L-matured DC demonstrated marginal cytolytic activity toward colon carcinoma SW1463, which expresses both HLA-A2 and CEA. In contrast, CTL induced by CD40L-matured DC/TRICOM showed more potent cytolytic effect to SW1463 at E:T ratios of 40:1 and 13:1 (P<0.01 and <0.05, respectively, student t test). As a control, both cell lines did not kill a melanoma cell line SKMEL24 that is HLA-A2 positive and CEA negative (Fig. 6D).
Fig. 6.
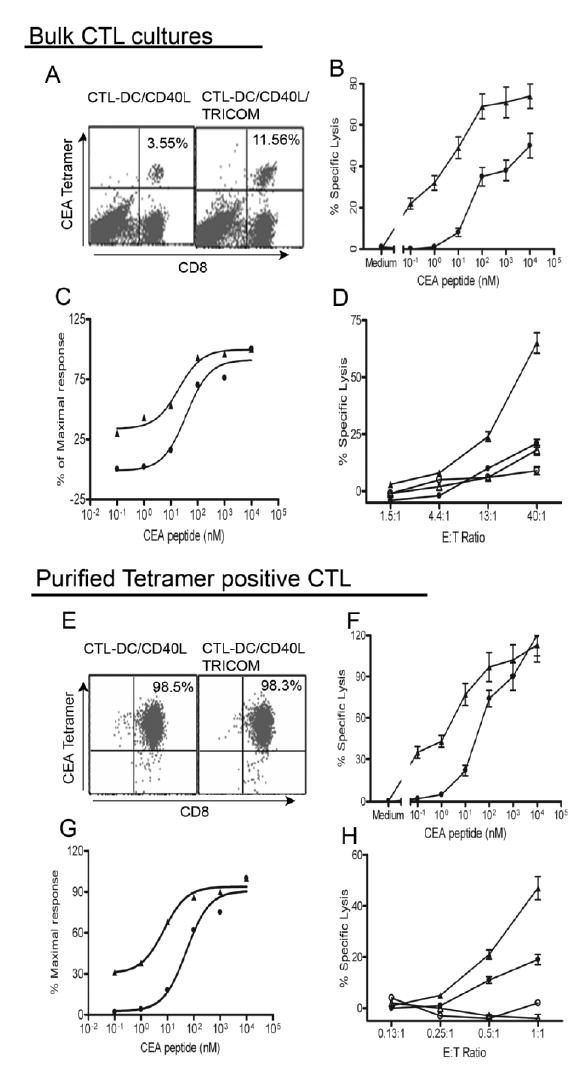
CTL elicited by peptide-pulsed, CD40L-matured DC infected with rF-TRICOM more efficiently lyse tumor cells presenting endogenously processed CEA antigen. DC were generated with GM-CSF/IL-4 for 6 days and matured with CD40L for 24 hours. CD40L-matured DC were infected with either FP-WT (closed circles) or rF-TRICOM (closed triangles) and then loaded with CEA peptide for induction of peptide-specific CTL from autologous Pan T cells. After three cycles of in vitro stimulation (IVS), bulk cultures were analyzed for tetramer staining, avidity titration and tumor recognition. A. Bulk CTL cultures stained with CEA tetramer. B. Titration of CTL avidity using cytolytic assay of peptide-pulsed T2 cells at E:T = 10. CTL-DC/CD40L, closed circles; CTL-DC/CD40L/TRICOM, closed triangles. C. Normalization of data shown in B as expressed as percentage of maximal lysis to calculate avidity. D. CTL ability to lyse tumor cells expressing CEA using a standard 4-hour 51Cr release assay. CTL derived from DC/CD40L, lysis of HLA-A2+, CEA+ SW1463 colon cancer cells (closed circles) and HLA2+, CEA− SKMEL24 melanoma cells (open circles); CTL derived from DC/CD40L/TRICOM, lysis of SW1463 (closed triangles) and SKMEL24 (open triangles). E, Tetramer-purified CTL. Ten days after four IVS, tetramer positive CTL were isolated as described in Materials and Methods. Purity of the isolated T cells was analyzed by FACS. Avidity titration at E:T = 0.5. (F), normalization (G) and tumor cell killing assay (H) were performed as above for bulk CTL cultures. n=3, mean ± SD of triplicates. The legends are the same as in bulk CTL cultures. The results are representative of two experiments with two different donors.
Because peptide-specific CTL, as determined by tetramer staining (Fig. 6A), in CTL-DC/TRICOM were approximately 3-fold that in CTL-DC, one may argue that the failure of CTL-DC to kill SW1463 may be due to a lower number of peptide-specific CTL. Therefore, tetramer positive CTL were isolated as described in Materials and Methods. As shown in Figure 6E, purity of CD8+/tetramer+-CTL in both cell lines was over 98%. The avidity of purified tetramer positive CTL was titrated and calculated as above for bulk CTL cultures. Purified tetramer positive CTL induced by CD40L-matured DC/TRICOM were again more efficient at lower peptide densities on targets as compared with those induced by CD40L-matured DC (Fig. 6F); avidity of purified tetramer positive CTL induced by DC and DC/TRICOM was 6.1 x 10−8 M and 2.2 x 10−9 M, respectively, which was very similar to those of bulk CTL cultures (Fig. 6C and G). Cytolytic activity toward tumor cells by purified tetramer CTL induced by CD40L-matured DC and DC/TRICOM was performed at equal tetramer to target ratios. As shown in Figure 6H, purified tetramer positive CTL elicited by CD40L-matured DC demonstrated significant lysis toward SW1463. However, CTL induced by CD40L-matured DC/TRICOM were still more efficient in the recognition of the endogenously presented antigenic epitope (P<0.001 at E:T ratio of 1:1, student t test) compared with CD40L-matured DC CTL. In contrast, both purified antigen-specific CTL did not lyse the HLA-A2+, CEA negative melanoma SKMEL24 (Fig. 6H). In addition, the two CTL lines demonstrated a positive lysis of another colon carcinoma SW480 (HLA-A2+/CEA+) vs no lysis of another melanoma cell line DM13 (HLA-A2+/CEA−) (data not shown). Similar results were also obtained from PBMC from another donor regarding percentage of tetramer positive cells in bulk cultures (0.28% in CD40L-matured DC CTL vs 0.62% in CD40L-matured DC/TRICOM-CTL), avidity of purified tetramer positive T cells (1.03 x 10−7M vs 9.9 x 10−9M) and cytolytic activity of purified tetramer positive T cells toward colon carcinoma SW1463 (28±4.2% vs 51±6.1% at E:T = 1:1, P<0.01, student t test).
Discussion
Recent studies in both animal models and in clinical studies have demonstrated that functional avidity of CTL can be a major determinant for T-cell–mediated tumor immunity (2–8, 10). Therefore, one strategy is to develop vaccines that can selectively induce and expand higher avidity CD8+ T cells. In this study, we demonstrate for the first time that enhancing costimulatory signals on human APC facilitates the induction of higher avidity human T cells.
Recent clinical trials have shown that the level of immune responses following TAA peptide vaccination is not always consistent with clinical outcome (12, 13). Many factors, e.g., loss of HLA and/or TAA by the tumor, or inefficient infiltration of T cells into tumor masses, may be responsible for the failure of clinical response. The relatively low avidity of induced T cells may be another important factor. Several studies have demonstrated that TAA-specific CTL populations in melanoma and carcinoma patients, and normal donors, appear to be very heterogeneous (4–6, 10, 14, 17, 39, 45, 46). Some peptide-specific CTL generated from both cancer patients and normal donors could not recognize tumor cells that endogenously present Ag, although these T cells could efficiently mediate lysis of peptide-pulsed targets (4–8). Studies (4, 7, 10, 11) suggest that the majority of TAA peptide-specific T cells are of low avidity and that only a fraction of these cells are relatively higher avidity T cells, which mediate effective lysis of tumor cells.
It has been assumed that the intensity of tetramer staining would directly correlate with functional avidity of CTL. Initial attempts to apply this concept to sort higher tetramer-binding CTL from heterogeneous populations have met with some success (9, 14). However, discrepancy between tetramer binding and functional avidity has also been observed (15–19). In addition, the relative efficiency of staining with the corresponding fluorescent MHC class I/peptide tetramer complexes can vary considerably with staining conditions and does not necessarily correlate with the avidity of Ag recognition (10, 15, 18, 19). It has also been reported that the activation status of CTL can also affect tetramer binding (39).
Higher functional avidity CTL have been successfully generated from spleen cells of vaccinated mice by using lower doses of stimulating peptide, using both virus- and TAA-derived peptides (1, 2). However, reduction of peptide concentration to activate human T cells resulted in the decreased magnitude of peptide-specific CTL response in bulk cultures without a significant change in functional avidity from PBMC of both peptide vaccinated melanoma patients (5) and normal donors (4). These results underscore the differences that can be observed in the activation of murine vs human T cells. While Oh et al. (21) and Hodge et al. (22) demonstrated in animal studies that TRICOM-based vaccines increased the avidity of antigen-specific CTL, it was still unclear if human CTL of enhanced avidity could be generated in vitro. The studies reported here show for the first time that enhanced costimulation using vectors containing a triad of T-cell costimulatory molecules has the capacity to preferentially induce and expand higher avidity CTL from human PBMC. In the present study, we investigated the avidity of CTL generated by TRICOM-infected DC from seven normal donors (Table 2 and Figures 5 and 6); six of seven donors demonstrated substantial increases in CTL avidity following DC/TRICOM stimulation and one donor (Table 2, donor #4) showed a slight increase in CTL avidity. In addition, these studies demonstrate that human T cells stimulated with immature DC infected with rF-TRICOM also produce higher levels of certain important chemokines and cytokines. For example, GM-CSF, MIP-1α and RANTES were dramatically increased following TRICOM and peptide stimulation. GM-CSF is a well-known DC activation factor and MIP-1α and RANTES are important mediators of acute and chronic inflammation. Increased production of these chemokines will attract more DC, macrophages and monocytes to the immunization sites and thus potentially enhance antigen presentation and T-cell activation and expansion. The potential consequence of enhanced secretion of IL-13 by T cells as a consequence of DC/TRICOM stimulation is not known as this time. IL-13 is a pleomorphic cytokine produced mainly by T cells. Evidence exists that IL-13, among other activities, is an anti-inflammatory cytokine that can enhance monocyte survival and MHC Class II and CD23 expression (47) and can generate an “alternatively activated” phenotype in macrophages (48). It has also been indicated that immunosurveillance may be negatively regulated via CD4+ NKT cells possibly mediated via IL-13 (49).
The infection of human professional APC with TRICOM vectors may seem counterintuitive at first. In previous publications using murine DC (50) and human DC (24), we have demonstrated that when DC that are already expressing CD54, CD58 and CD80 (the three T-cell costimulatory molecules in TRICOM) are infected with TRICOM vectors, they then express more of these molecules on their cell surface. This, in turn, has been shown to correlate with their ability to enhance the quantity of activated T cells. In the studies reported here, we have shown that DC treated with CD40L only moderately upregulate CD54, CD58 and CD80, while infection with rF-TRICOM substantially upregulates each of these three molecules (Fig. 5). CD40L, on the other hand, is shown to upregulate CD83, while rF-TRICOM does not. We show here that the combination of CD40L and rF-TRICOM infection further upregulates CD54, CD58, CD80 and CD83 (Fig. 5), and results not only in the generation of more CTL but also in the generation of higher avidity CTL (Figs. 5 and 6). The purpose of the studies reported here is to provide a rationale that immature human DC, which are likely to be encountered by vectors when patients are injected i.d./s.c. with vector-based vaccines, will better facilitate the generation of higher avidity CTL. The studies reported here thus complement those recently completed in vivo in mice (22).
The studies reported here show that the higher avidity CTL elicited by peptide-pulsed DC infected with rF-TRICOM demonstrated more stable TCR-MHC-tetramer complex as determined by the tetramer dissociation assay (Fig. 4). Previous studies (17, 18, 24) suggest that increased stability of MHC-peptide and TCR complexes may be a general indicator, or a parameter, of high functional avidity of CTL, though some exceptions may exist. In other words, CTL with slower off-rates of MHC-peptide complexes from a TCR are not necessarily higher functional avidity CTL and vice versa.
CTL induced by TRICOM vector–infected DC also displayed higher recognition efficacy for tumors endogenously expressing TAA. This was demonstrated by tumor lysis not only by bulk CTL cultures but also by purified tetramer positive antigen-specific CTL, which further indicated that killer cells elicited by TRICOM were of higher avidity (Fig. 6). In addition, CTL avidity calculated in bulk CTL cultures and in purified tetramer positive T cells was very similar, indicating that the purity of antigen-specific CTL does not significantly affect titration of CTL avidity and the enhanced avidity in bulk CTL induced by TRICOM is not simply due to the larger number of antigen-specific CTL in the bulk cultures.
It has been shown that more mature DC enhance the level of induction of peptide- specific CTL in vitro. The studies reported here demonstrate that DC infected with rF-TRICOM (with or without CD40L maturation) enhanced CTL avidity. The present study is not consistent with some previous reports, which have shown that “mature” DC are required to induce peptide-specific CTL (42–44). However, the induction of CTL reactivity via DC peptide presentation in different laboratories shows a highly variable requirement for DC maturation or CD83 expression (51–54). For example, Kuniyoshi et al. (55) showed that an enhanced CTL response was observed after a single IVS with CD40L-treated DC compared with non-CD40L–treated DC, but after an additional IVS CD40L-treated and non-treated DC induced comparable peptide-specific CTL reactivity. In contrast, Wurtzen et al. (54) demonstrated that immature DC were slightly more efficient in inducing peptide-specific CTL than CD40L-treated DC following a single IVS; however, after a second IVS, both types of DC induced equal levels of CTL responses. Another study, reported by Terheyden et al. (52), demonstrated that although CD40-ligation did not change the phenotype of DC (including CD83), CD40-activated DC were superior to non-treated DC in inducing peptide-specific CD8+ T cells. Moreover, Zarling et al. (53) demonstrated that the induction of peptide-specific CTL was primarily donor- and peptide-dependent and did not reflect the maturation status of the DC. Taken together, the discrepancy among these studies on the relationship between the maturation stage of DC and CTL response is probably due to differences among individual donors, the different peptides used, culture conditions and timing for CTL assay.
There may be more than one mechanism underlining the enhanced avidity of CTL by increased costimulation. Membrane compartmentalization between rafts and non-rafts is required for efficient T-cell activation (56). It was reported that CD28 costimulation induced recruitment of Lck and lipid rafts as well as their accumulation at the immunological synapse (57, 58). Cawthon et al. (59) found that high avidity CTL colocalized substantially more TCR with CD8 compared with low avidity CTL. The ability of high avidity CTL to respond functionally to fewer TCR engagement events than low avidity CTL is directly related to integrating lipid rafts on their surface. In addition, our previous study demonstrated that enhanced expression of costimulatory molecules on target cells infected with rF-TRICOM led to the formation of stable and a greater number of conjugates/synapses between targets and T cells (60). The enhanced interaction between T cells and rF-TRICOM infected targets also led to enhanced signaling through Lck, ZAP70 and STAT-1 in CD8 T cells (60). Taken together, the results suggest that clustering of membrane and intracellular kinase-rich lipid rafts at the site of TCR engagements and enhanced synapses between T cells and targets/APC induced by enhanced co-stimulation may be attributed to the enhanced avidity of CTL observed in the present study.
In summary, the present study demonstrates for the first time a method in which one can preferentially induce higher avidity human CTL from heterogeneous populations, as judged by cytolytic activity, IFN-γ production, tetramer-TCR complex stability and recognition efficacy of tumors endogenously expressing TAA. These results also suggest that vectors expressing multiple costimulatory molecules may be used toward the development of more efficient anti-tumor vaccines, and in vitro to expand higher functional avidity human CTL for adoptive transfer therapy.
Acknowledgments
We thank Drs. James Hodge, Helen Sabzevari and Douglas Grosenbach, Laboratory of Tumor Immunology and Biology, Center for Cancer Research, NCI, for critical discussions, Drs. Seth Steinberg and David Venzon, Biostatistics and Data Management Section, NCI, for assistance in statistical analysis, and Debra Weingarten for her editorial assistance in the preparation of the manuscript.
References
- 1.Alexander-Miller MA, Leggatt GR, Berzofsky JA. Selective expansion of high- or low-avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proc Natl Acad Sci U S A. 1996;93:4102–7. doi: 10.1073/pnas.93.9.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeh HJ, 3rd, Perry-Lalley D, Dudley ME, Rosenberg SA, Yang JC. High avidity CTLs for two self-antigens demonstrate superior in vitro and in vivo antitumor efficacy. J Immunol. 1999;162:989–94. [PubMed] [Google Scholar]
- 3.Bullock TN, Mullins DW, Colella TA, Engelhard VH. Manipulation of avidity to improve effectiveness of adoptively transferred CD8(+) T cells for melanoma immunotherapy in human MHC class I-transgenic mice. J Immunol. 2001;167:5824–31. doi: 10.4049/jimmunol.167.10.5824. [DOI] [PubMed] [Google Scholar]
- 4.Yang S, Linette GP, Longerich S, Haluska FG. Antimelanoma activity of CTL generated from peripheral blood mononuclear cells after stimulation with autologous dendritic cells pulsed with melanoma gp100 peptide G209-2M is correlated to TCR avidity. J Immunol. 2002;169:531–9. doi: 10.4049/jimmunol.169.1.531. [DOI] [PubMed] [Google Scholar]
- 5.Dudley ME, Nishimura MI, Holt AK, Rosenberg SA. Antitumor immunization with a minimal peptide epitope (G9-209-2M) leads to a functionally heterogeneous CTL response. J Immunother. 1999;22:288–98. doi: 10.1097/00002371-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Dutoit V, Rubio-Godoy V, Dietrich PY, et al. Heterogeneous T-cell response to MAGE-A10(254–262): high avidity-specific cytolytic T lymphocytes show superior antitumor activity. Cancer Res. 2001;61:5850–6. [PubMed] [Google Scholar]
- 7.Sun Y, Song M, Stevanovic S, et al. Identification of a new HLA-A(*)0201-restricted T-cell epitope from the tyrosinase-related protein 2 (TRP2) melanoma antigen. Int J Cancer. 2000;87:399–404. doi: 10.1002/1097-0215(20000801)87:3<399::aid-ijc14>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 8.Valmori D, Dutoit V, Schnuriger V, et al. Vaccination with a Melan-A peptide selects an oligoclonal T cell population with increased functional avidity and tumor reactivity. J Immunol. 2002;168:4231–40. doi: 10.4049/jimmunol.168.8.4231. [DOI] [PubMed] [Google Scholar]
- 9.Yee C, Savage PA, Lee PP, Davis MM, Greenberg PD. Isolation of high avidity melanoma-reactive CTL from heterogeneous populations using peptide-MHC tetramers. J Immunol. 1999;162:2227–34. [PubMed] [Google Scholar]
- 10.Rubio V, Stuge TB, Singh N, et al. Ex vivo identification, isolation and analysis of tumor-cytolytic T cells. Nat Med. 2003;9:1377–82. doi: 10.1038/nm942. [DOI] [PubMed] [Google Scholar]
- 11.Clay TM, Custer MC, McKee MD, et al. Changes in the fine specificity of gp100(209–217)-reactive T cells in patients following vaccination with a peptide modified at an HLA-A2.1 anchor residue. J Immunol. 1999;162:1749–55. [PubMed] [Google Scholar]
- 12.Lee KH, Wang E, Nielsen MB, et al. Increased vaccine-specific T cell frequency after peptide-based vaccination correlates with increased susceptibility to in vitro stimulation but does not lead to tumor regression. J Immunol. 1999;163:6292–300. [PubMed] [Google Scholar]
- 13.Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–7. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsang KY, Zhu M, Even J, et al. The infection of human dendritic cells with recombinant avipox vectors expressing a costimulatory molecule transgene (CD80) to enhance the activation of antigen-specific cytolytic T cells. Cancer Res. 2001;61:7568–76. [PubMed] [Google Scholar]
- 15.Derby MA, Wang J, Margulies DH, Berzofsky JA. Two intermediate-avidity cytotoxic T lymphocyte clones with a disparity between functional avidity and MHC tetramer staining. Int Immunol. 2001;13:817–24. doi: 10.1093/intimm/13.6.817. [DOI] [PubMed] [Google Scholar]
- 16.Palermo B, Campanelli R, Mantovani S, et al. Diverse expansion potential and heterogeneous avidity in tumor-associated antigen-specific T lymphocytes from primary melanoma patients. Eur J Immunol. 2001;31:412–20. doi: 10.1002/1521-4141(200102)31:2<412::aid-immu412>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 17.Rubio-Godoy V, Dutoit V, Rimoldi D, et al. Discrepancy between ELISPOT IFN-gamma secretion and binding of A2/peptide multimers to TCR reveals interclonal dissociation of CTL effector function from TCR-peptide/MHC complexes half-life. Proc Natl Acad Sci U S A. 2001;98:10302–7. doi: 10.1073/pnas.181348898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dutoit V, Rubio-Godoy V, Doucey MA, et al. Functional avidity of tumor antigen-specific CTL recognition directly correlates with the stability of MHC/peptide multimer binding to TCR. J Immunol. 2002;168:1167–71. doi: 10.4049/jimmunol.168.3.1167. [DOI] [PubMed] [Google Scholar]
- 19.Echchakir H, Dorothee G, Vergnon I, et al. Cytotoxic T lymphocytes directed against a tumor-specific mutated antigen display similar HLA tetramer binding but distinct functional avidity and tissue distribution. Proc Natl Acad Sci U S A. 2002;99:9358–63. doi: 10.1073/pnas.142308199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aarts WM, Schlom J, Hodge JW. Vector-based vaccine/cytokine combination therapy to enhance induction of immune responses to a self-antigen and antitumor activity. Cancer Res. 2002;62:5770–7. [PubMed] [Google Scholar]
- 21.Oh S, Hodge JW, Ahlers JD, et al. Selective induction of high avidity CTL by altering the balance of signals from APC. J Immunol. 2003;170:2523–30. doi: 10.4049/jimmunol.170.5.2523. [DOI] [PubMed] [Google Scholar]
- 22.Hodge JW, Chakraborty M, Kudo-Saito C, Garnett CT, and Schlom J. Multiple costimulatory modalities enhance CTL avidity. J Immunol 2005. [DOI] [PMC free article] [PubMed]
- 23.Palena C, Zhu M, Schlom J, Tsang KY. Human B cells that hyperexpress a triad of costimulatory molecules via avipox-vector infection: an alternative source of efficient antigen-presenting cells. Blood. 2004;104:192–9. doi: 10.1182/blood-2003-09-3211. [DOI] [PubMed] [Google Scholar]
- 24.Zhu M, Terasawa H, Gulley J, et al. Enhanced activation of human T cells via avipox vector-mediated hyperexpression of a triad of costimulatory molecules in human dendritic cells. Cancer Res. 2001;61:3725–34. [PubMed] [Google Scholar]
- 25.Bunce M, O’Neill CM, Barnardo MC, et al. Phototyping: comprehensive DNA typing for HLA-A, B, C, DRB1, DRB3, DRB4, DRB5 & DQB1 by PCR with 144 primer mixes utilizing sequence-specific primers (PCR-SSP) Tissue Antigens. 1995;46:355–67. doi: 10.1111/j.1399-0039.1995.tb03127.x. [DOI] [PubMed] [Google Scholar]
- 26.Krausa P, Browning MJ. A comprehensive PCR-SSP typing system for identification of HLA-A locus alleles. Tissue Antigens. 1996;47:237–44. doi: 10.1111/j.1399-0039.1996.tb02547.x. [DOI] [PubMed] [Google Scholar]
- 27.Hodge JW, Sabzevari H, Yafal AG, et al. A triad of costimulatory molecules synergize to amplify T-cell activation. Cancer Res. 1999;59:5800–7. [PubMed] [Google Scholar]
- 28.Hodge JW, Rad AN, Grosenbach DW, et al. Enhanced activation of T cells by dendritic cells engineered to hyperexpress a triad of costimulatory molecules. J Natl Cancer Inst. 2000;92:1228–39. doi: 10.1093/jnci/92.15.1228. [DOI] [PubMed] [Google Scholar]
- 29.Grosenbach DW, Barrientos JC, Schlom J, Hodge JW. Synergy of vaccine strategies to amplify antigen-specific immune responses and antitumor effects. Cancer Res. 2001;61:4497–505. [PubMed] [Google Scholar]
- 30.Zaremba S, Barzaga E, Zhu M, et al. Identification of an enhancer agonist cytotoxic T lymphocyte peptide from human carcinoembryonic antigen. Cancer Res. 1997;57:4570–7. [PubMed] [Google Scholar]
- 31.Salazar E, Zaremba S, Arlen PM, Tsang KY, Schlom J. Agonist peptide from a cytotoxic t-lymphocyte epitope of human carcinoembryonic antigen stimulates production of tc1-type cytokines and increases tyrosine phosphorylation more efficiently than cognate peptide. Int J Cancer. 2000;85:829–38. doi: 10.1002/(sici)1097-0215(20000315)85:6<829::aid-ijc16>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 32.Falk K, Rotzschke O, Stevanovic S, Jung G, Rammensee HG. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991;351:290–6. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- 33.Romani N, Gruner S, Brang D, et al. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linette GP, Shankara S, Longerich S, et al. In vitro priming with adenovirus/gp100 antigen-transduced dendritic cells reveals the epitope specificity of HLA-A*0201-restricted CD8+ T cells in patients with melanoma. J Immunol. 2000;164:3402–12. doi: 10.4049/jimmunol.164.6.3402. [DOI] [PubMed] [Google Scholar]
- 35.Sidak Z. Rectangular confidence regions for the means of multivariate normal distributions. J Am Stat Assoc. 1967;62:626–633. [Google Scholar]
- 36.Morse MA, Deng Y, Coleman D, et al. A Phase I study of active immunotherapy with carcinoembryonic antigen peptide (CAP-1)-pulsed, autologous human cultured dendritic cells in patients with metastatic malignancies expressing carcinoembryonic antigen. Clin Cancer Res. 1999;5:1331–8. [PubMed] [Google Scholar]
- 37.Palucka K, Banchereau J. Dendritic cells: a link between innate and adaptive immunity. J Clin Immunol. 1999;19:12–25. doi: 10.1023/a:1020558317162. [DOI] [PubMed] [Google Scholar]
- 38.Lawson TM, Man S, Wang EC, et al. Functional differences between influenza A-specific cytotoxic T lymphocyte clones expressing dominant and subdominant TCR. Int Immunol. 2001;13:1383–90. doi: 10.1093/intimm/13.11.1383. [DOI] [PubMed] [Google Scholar]
- 39.Nielsen MB, Monsurro V, Migueles SA, et al. Status of activation of circulating vaccine-elicited CD8+ T cells. J Immunol. 2000;165:2287–96. doi: 10.4049/jimmunol.165.4.2287. [DOI] [PubMed] [Google Scholar]
- 40.Slifka MK, Whitton JL. Functional avidity maturation of CD8(+) T cells without selection of higher affinity TCR. Nat Immunol. 2001;2:711–7. doi: 10.1038/90650. [DOI] [PubMed] [Google Scholar]
- 41.Cordaro TA, de Visser KE, Tirion FH, Schumacher TN, Kruisbeek AM. Can the low-avidity self-specific T cell repertoire be exploited for tumor rejection? J Immunol. 2002;168:651–60. doi: 10.4049/jimmunol.168.2.651. [DOI] [PubMed] [Google Scholar]
- 42.Morel Y, Truneh A, Sweet RW, Olive D, Costello RT. The TNF superfamily members LIGHT and CD154 (CD40 ligand) costimulate induction of dendritic cell maturation and elicit specific CTL activity. J Immunol. 2001;167:2479–86. doi: 10.4049/jimmunol.167.5.2479. [DOI] [PubMed] [Google Scholar]
- 43.Mosca PJ, Hobeika AC, Clay TM, et al. A subset of human monocyte-derived dendritic cells expresses high levels of interleukin-12 in response to combined CD40 ligand and interferon-gamma treatment. Blood. 2000;96:3499–504. [PubMed] [Google Scholar]
- 44.Larsson M, Messmer D, Somersan S, et al. Requirement of mature dendritic cells for efficient activation of influenza A-specific memory CD8+ T cells. J Immunol. 2000;165:1182–90. doi: 10.4049/jimmunol.165.3.1182. [DOI] [PubMed] [Google Scholar]
- 45.Monsurro V, Nagorsen D, Wang E, et al. Functional heterogeneity of vaccine-induced CD8(+) T cells. J Immunol. 2002;168:5933–42. doi: 10.4049/jimmunol.168.11.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valmori D, Gervois N, Rimoldi D, et al. Diversity of the fine specificity displayed by HLA-A*0201-restricted CTL specific for the immunodominant Melan-A/MART-1 antigenic peptide. J Immunol. 1998;161:6956–62. [PubMed] [Google Scholar]
- 47.Collighan N, Giannoudis PV, Kourgeraki O, et al. Interleukin 13 and inflammatory markers in human sepsis. Br J Surg. 2004;91:762–8. doi: 10.1002/bjs.4521. [DOI] [PubMed] [Google Scholar]
- 48.Scotton CJ, Martinez FO, Smelt MJ, et al. Transcriptional profiling reveals complex regulation of the monocyte IL-1 beta system by IL-13. J Immunol. 2005;174:834–45. doi: 10.4049/jimmunol.174.2.834. [DOI] [PubMed] [Google Scholar]
- 49.Park JM, Terabe M, van den Broeke LT, Donaldson DD, Berzofsky JA. Unmasking immunosurveillance against a syngeneic colon cancer by elimination of CD4+ NKT regulatory cells and IL-13. Int J Cancer. 2005;114:80–7. doi: 10.1002/ijc.20669. [DOI] [PubMed] [Google Scholar]
- 50.Rad AN, Schlom J, Hodge JW. Vector-driven hyperexpression of a triad of costimulatory molecules confers enhanced T-cell stimulatory capacity to DC precursors. Crit Rev Oncol Hematol. 2001;39:43–57. doi: 10.1016/s1040-8428(01)00123-8. [DOI] [PubMed] [Google Scholar]
- 51.Pietschmann P, Stockl J, Draxler S, Majdic O, Knapp W. Functional and phenotypic characteristics of dendritic cells generated in human plasma supplemented medium. Scand J Immunol. 2000;51:377–83. doi: 10.1046/j.1365-3083.2000.00690.x. [DOI] [PubMed] [Google Scholar]
- 52.Terheyden P, Straten P, Brocker EB, Kampgen E, Becker JC. CD40-ligated dendritic cells effectively expand melanoma-specific CD8+ CTLs and CD4+ IFN-gamma-producing T cells from tumor-infiltrating lymphocytes. J Immunol. 2000;164:6633–9. doi: 10.4049/jimmunol.164.12.6633. [DOI] [PubMed] [Google Scholar]
- 53.Zarling AL, Johnson JG, Hoffman RW, Lee DR. Induction of primary human CD8+ T lymphocyte responses in vitro using dendritic cells. J Immunol. 1999;162:5197–204. [PubMed] [Google Scholar]
- 54.Wurtzen PA, Nissen MH, Claesson MH. Maturation of dendritic cells by recombinant human CD40L-trimer leads to a homogeneous cell population with enhanced surface marker expression and increased cytokine production. Scand J Immunol. 2001;53:579–87. doi: 10.1046/j.1365-3083.2001.00910.x. [DOI] [PubMed] [Google Scholar]
- 55.Kuniyoshi JS, Kuniyoshi CJ, Lim AM, et al. Dendritic cell secretion of IL-15 is induced by recombinant huCD40LT and augments the stimulation of antigen-specific cytolytic T cells. Cell Immunol. 1999;193:48–58. doi: 10.1006/cimm.1999.1469. [DOI] [PubMed] [Google Scholar]
- 56.Xavier R, Brennan T, Li Q, McCormack C, Seed B. Membrane compartmentation is required for efficient T cell activation. Immunity. 1998;8:723–32. doi: 10.1016/s1074-7613(00)80577-4. [DOI] [PubMed] [Google Scholar]
- 57.Viola A, Schroeder S, Sakakibara Y, Lanzavecchia A. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science. 1999;283:680–2. doi: 10.1126/science.283.5402.680. [DOI] [PubMed] [Google Scholar]
- 58.Tavano R, Gri G, Molon B, et al. CD28 and lipid rafts coordinate recruitment of Lck to the immunological synapse of human T lymphocytes. J Immunol. 2004;173:5392–7. doi: 10.4049/jimmunol.173.9.5392. [DOI] [PubMed] [Google Scholar]
- 59.Cawthon AG, Alexander-Miller MA. Optimal colocalization of TCR and CD8 as a novel mechanism for the control of functional avidity. J Immunol. 2002;169:3492–8. doi: 10.4049/jimmunol.169.7.3492. [DOI] [PubMed] [Google Scholar]
- 60.Slavin-Chiorini DC, Catalfamo M, Kudo-Saito C, et al. Amplification of the lytic potential of effector/memory CD8+ cells by vector-based enhancement of ICAM-1 (CD54) in target cells: implications for intratumoral vaccine therapy. Cancer Gene Ther. 2004;11:665–80. doi: 10.1038/sj.cgt.7700741. [DOI] [PubMed] [Google Scholar]


