Abstract
A novel, non-CB1 cannabinoid receptor has been defined by the persistence of inhibition of glutamatergic EPSPs by the cannabinoid receptor agonist WIN55,212-2 in mice lacking the cloned CB1 receptor (CB1−/−) (Hajos et al., 2001). This novel receptor was also distinguished from CB1 by its sensitivity to the antagonist SR141716A and its insensitivity to the antagonist AM251 (Hajos & Freund, 2002). We have chosen to refer to this putative receptor as CBsc due to its identification on Schaffer collateral axon terminals in the hippocampus. We examined properties of CBsc receptors in Sprague Dawley (SD) rats and two strains of wild-type (WT) mice (C57BL/6J and CD1) used as backgrounds for two independent lines of CB1−/− mice (Ledent et al., 1999; Zimmer et al., 1999). The inhibition of synaptic glutamate release by WIN55,212-2 was observed in hippocampal slices from WT CD1 mice and SD rats but was absent in WT C57 mice. We also found that AM251 and SR141716A antagonized the effect of WIN55,212-2 in hippocampal slices from CD1 mice and SD rats demonstrating a lack of selectivity of these ligands for CB1 and CBsc receptors in these animals. The results indicate that the glutamate-modulating CBsc cannabinoid receptor is present in the hippocampi of CD1 mice and SD rats but not in C57BL/6J mice. Thus, we have identified animal models that may permit the study of cannabinoids independently of the novel CBsc receptor (C57CB1+/+), the CBsc receptor independently of the cloned CB1 receptor (CD1CB1−/−), or in the absence of both receptors (C57CB1−/−).
Keywords: Keywords: C57BL mouse, CB1 cannabinoid receptor, CD1 mouse, electrophysiology, knockout, Sprague Dawley rat
Abbreviations : fEPSP, field EPSP; IRP, Intramural Research Program; NIDA, National Institute on Drug Abuse; SD, Sprague Dawley; WT, wild-type
Introduction
The physiological effects of cannabinoid receptor agonists are amply described in the hippocampus, where they inhibit the release of the inhibitory neurotransmitter GABA and the excitatory neurotransmitter glutamate via receptors located on axon terminals (Ameri et al., 1999; Katona et al., 1999; Misner & Sullivan, 1999; Hoffman & Lupica, 2000). The inhibition of synaptic GABAA receptor-mediated responses occurs via activation of the cloned CB1 receptor (Matsuda et al., 1990) that is densely expressed on the axon terminals of cholecystokinin-immunoreactive GABA neurones (Katona et al., 1999; Hoffman & Lupica, 2000). The inhibition of GABA release results from the inhibitory modulation of N-type voltage-dependent calcium channels by G protein βγ subunits (Hoffman & Lupica, 2000; Wilson et al., 2001). Whereas the presynaptic inhibition of GABA release by CB1 receptors is well understood, the inhibition of glutamate release from Schaffer collateral/commissural (SC) axon terminals in the CA1 region of the hippocampus appears to occur through the activation of a novel non-CB1, non-CB2 cannabinoid receptor (Hajos et al., 2001; Hajos & Freund, 2002). This novel cannabinoid receptor (hereafter referred to as CBsc) has a pharmacological profile that is defined by its activation by the agonist WIN55,212-2 and by its antagonism by SR141716A (Rimonabant, Acomplia®). Further evidence for the existence of this receptor comes from the demonstration that WIN55,212-2 could inhibit EPSPs in hippocampal brain slices obtained from CB1−/− mice and that SR141716A blocked this effect (Hajos et al., 2001). Another study demonstrated in the Wistar rat hippocampus that the CBsc receptor was less sensitive to WIN55,212-2 than the cloned CB1 receptor and that it was antagonized by SR141716A but not by another commonly employed cannabinoid antagonist, AM251 (Hajos & Freund, 2002). Based on these data, it was suggested that these antagonists could be used to distinguish the CBsc cannabinoid receptor from CB1.
A more complete characterization of glutamate-modulating CBsc receptors is needed because of the role that they play in regulating the strength of excitatory synaptic transmission and in gating glutamate-dependent synaptic plasticity in the hippocampus. In addition, these receptors may be involved in the well-described ability of cannabinoid agonists, including a psychoactive component in marijuana, Δ9-tetrahydrocannabinol, to disrupt hippocampal-dependent memory (Heyser et al., 1993; Misner & Sullivan, 1999; Sullivan, 2000). Here we report that the inhibition of glutamate-mediated EPSPs by WIN55,212-2 in the CA1 region of the hippocampus was absent in C57 mice but was observed in CD1 mice and SD rats.
Materials and methods
Animals
Animal protocols were approved by the Animal Care and Use Committee of the National Institute on Drug Abuse (NIDA) Intramural Research Program (IRP) and were carried out according to National Institutes of Health guidelines. Male SD rats (2–6 weeks) and WT Swiss CD1 mice (4–12 weeks) were purchased from Charles River Laboratories (Raleigh, NC, USA). Male WT C57 mice (4–52 weeks) were purchased from Charles River Laboratories or obtained from the NIDA-IRP transgenic facility colony. The WT and CB1−/− C57 mice obtained from NIDA-IRP were descendants of three CB1+/− breeding pairs, generously donated by Dr Andreas Zimmer and the National Institute of Mental Health (Bethesda, MD, USA). Genotyping was performed by Charles River Laboratories.
Slice preparation
Hippocampal slices were prepared as described by Hoffman & Lupica (2000). Briefly, animals were decapitated, the brains rapidly removed and chilled in an ice-cold, oxygenated solution consisting of (in mm): NaCl, 87; KCl, 2.5; MgCl2, 7; CaCl2, 0.5; NaH2PO4, 1.25; glucose, 25; sucrose 75 and NaHCO3, 25. Transverse slices were cut at 300 μm using a vibrating tissue slicer (VT1000S, Leica Instruments, Germany). Brain slices were then incubated in a normal artificial cerebrospinal fluid consisting of (in mm): NaCl, 126; KCl, 3.0; MgCl2, 1.5; CaCl2, 2.4; NaH2PO4, 1.2; glucose, 11.0; NaHCO3, 26 and saturated with 95% O2/5% CO2 at room temperature (21–23 °C) for ≥90 min. Slices were placed in a recording chamber (~200 μL), immersed in flowing (2 mL/min) artificial cerebrospinal fluid and maintained at 30–32 °C. All drugs were delivered from concentrated stocks that were added to the flowing artificial cerebrospinal fluid using calibrated syringe pumps (Razel, Stamford, CT, USA).
Electrophysiology
Recordings were performed in the CA1 region of the hippocampus. Extracellular field EPSPs (fEPSPs) were obtained using 3 m NaCl-filled electrodes and an AC amplifier (Model 1800, A-M Systems, Carlsborg, WA, USA). Signals were amplified (1000×), filtered at 10 kHz and 10 Hz, digitized at 4 kHz on an A/D board (PCI 6024E, National Instruments, Austin, TX, USA or Digidata 1320A, Axon Instruments, Foster, CA, USA) and acquired to a PC hard-drive using Windows-based software (WinWCP, courtesy of Dr John Dempster, University of Strathclyde, Glasgow, UK; http://spider.science.strath.ac.uk/PhysPharm/). Responses were elicited by the activation of SC axons via electrical stimulation of the stratum radiatum with single pulses (0.1 ms) at 0.033 Hz using a bipolar stimulating electrode made with insulated Ni-chromium wire (180 μm diameter). Stimulus intensity was adjusted to produce fEPSPs with peaks of 0.5–1 mV (30–40% of the maximal response). At least 10 min of stable baseline was obtained prior to drug delivery. Peak fEPSP amplitude and the slope of its initial (1–2 ms) rising phase were measured off-line using the software.
Whole-cell recordings of evoked IPSCs and EPSCs in CA1 pyramidal neurones were performed using an Axopatch 200B amplifier (Axon Instruments) and electrodes pulled from borosilicate capillary glass (1.5 mm O.D., 0.86 mm I.D.). IPSCs were measured with electrodes containing (in mm): CsCl, 125.0; HEPES, 10.0; EGTA, 1.0; CaCl2, 0.1; Mg2+-ATP, 2.0; Na+-GTP, 0.2 and QX-314 (1 mg/mL) adjusted to pH 7.2–7.4. EPSCs were obtained using the same intrapipette solution, except for the substitution of equimolar K+-gluconate for CsCl. Series resistance was monitored using −10 mV voltage steps (200 ms) and only cells maintaining stable access (< 10% change) were used. IPSCs were measured in pyramidal neurones which were voltage clamped at −80 mV by stimulating near stratum pyramidale in the presence of the ionotropic glutamate receptor antagonists d-(−)-2-amino-5-phosphonopentanoic acid (APV, 40 μm) and 6,7-dinitroquinoxaline-2,3-dione (DNQX, 10 μm) to block EPSCs. EPSCs were measured at −60 mV in artificial cerebrospinal fluid containing picrotoxin (100 μm) to isolate these currents from GABAA receptor-mediated IPSCs.
Drugs
WIN55,212-2 and AM251 were purchased from Tocris-Cookson (Ballwin, MO, USA). DNQX, APV and picrotoxin were purchased from Sigma (St Louis, MO, USA) and SR141716A was obtained from the NIDA drug supply system (Bethesda, MD, USA). AM251, SR141716A and WIN55,212-2 were prepared as 10-mm stock solutions in dimethylsulphoxide (DMSO). The drugs were then diluted to 100× their final concentration in a solution of 1% Tween-80, 2% DMSO, 97% saline. Final concentrations of the vehicle in the tissue bath were never more than 0.01% Tween-80 and 0.02% DMSO and were found to not affect the physiological responses at these concentrations.
Statistics
Statistical tests were performed with a critical probability of P < 0.05 using Prism v4.0 (GraphPad Software, San Diego, CA, USA).
Results
The effects of the non-selective cannabinoid agonist WIN55,212-2 on glutamatergic fEPSP responses were measured in the stratum radiatum of area CA1 of hippocampal slices. Brain slices were prepared from C57 mice, WT Swiss CD1 mice and SD rats. The C57 and CD1 strains were chosen because they are used as background strains for two distinct lines of CB1−/− mice (Ledent et al., 1999; Zimmer et al., 1999), whereas the SD rats were chosen because they represent a common animal model used in cannabinoid research.
Field EPSPs recorded in hippocampal slices from CD1 mice (Fig. 1B) and SD rats (Fig. 1A and B) were inhibited by WIN55,212-2 (1 μm) by approximately 40% (Fig. 1B). In contrast, fEPSPs were not altered by WIN55,212-2 in brain slices obtained from WT C57 mice (Fig. 1A and C). These disparate results are further illustrated in Fig. 1A where the effects of WIN55,212-2 (1 μm) on fEPSPs are shown in single hippocampal slices obtained from an SD rat and a WT C57 mouse, recorded concurrently in the same brain slice chamber. A time-dependent inhibition of the fEPSP response is clearly observed in the rat hippocampal slice, whereas no effect of the agonist is observed in the WT C57 mouse hippocampus (Fig. 1A). Similarly, WIN55,212-2 had no effect on EPSCs recorded using whole-cell techniques in CA1 pyramidal neurones from the WT C57 mice (Fig. 1C and D). In contrast, GABAergic IPSCs recorded from CA1 pyramidal neurones were inhibited by WIN55,212-2 in hippocampal slices from WT C57 mice but not in C57CB1−/− mice (Fig. 1D), suggesting that, unlike putative CBsc receptors, CB1 receptors were indeed functional in these WT mice.
Fig. 1.
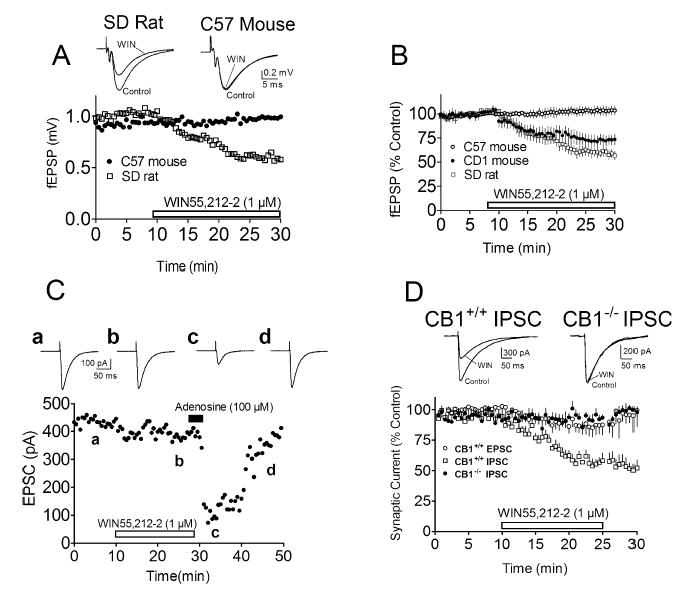
Effects of the agonist WIN55,212-2 on synaptic responses recorded in the Sprague Dawley (SD) rat, C57 mouse and CD1 mouse hippocampus. (A) Time course of the effect of WIN55,212-2 on fEPSPs recorded from single C57 mouse and SD rat hippocampal slices, recorded simultaneously in the same tissue bath. Note the decrease of the fEPSP recorded in the SD rat hippocampal slice and the absence of a response to WIN55,212-2 in the C57 mouse hippocampal slice. (B) Mean effect of WIN55,212-2 on the fEPSP responses in CD1 (n = 11) and C57 (n = 17) mouse and SD rat (n = 9) hippocampal slices. (C) Absence of an effect of WIN55,212-2 on EPSCs recorded using whole-cell techniques in a hippocampal slice from a C57 mouse. Note also that, despite the absence of an effect of WIN55,212-2, adenosine decreased the synaptic currents. (D) Mean inhibition of GABAergic IPSCs and EPSCs in C57CB1+/+ (n = 9) and C57CB1−/− (n = 7) hippocampal slices by WIN55,212-2. Note the lack of effect of WIN55,212-2 on IPSCs in the C57CB1−/− animals and on EPSCs in the C57CB1+/+ animals. In each panel, the duration of WIN55,212-2 application is indicated by the horizontal bar. Waveforms are averaged synaptic responses (≥5 responses) collected during the indicated periods.
As the WT C57 mice used in these experiments were obtained from our transgenic breeding colony, it was possible that the absence of fEPSP modulation by WIN55,212-2 might be unique to this particular population of animals. Therefore, the experiment was repeated using C57BL/6J mice obtained directly from a commercial breeder (Charles River Laboratories). However, as observed in the original experiments, fEPSPs recorded in hippocampal brain slices obtained from these C57 animals were also insensitive to WIN55,212-2. As the average age of the WT C57 mice available from the NIDA-IRP colony was greater than that of the WT CD1 mice and the SD rats obtained from the commercial supplier, we also examined the effect of WIN55,212-2 in younger C57 mice (2–4 weeks, n = 6). Once again, fEPSPs recorded in hippocampal slices obtained from these animals were insensitive to this agonist (data not shown).
The absence of inhibition of fEPSPs/EPSCs by WIN55,212-2 might result from increased basal endogenous cannabinoid levels in the brains of the C57 mice, as compared with the CD1 mice, or the SD rats. If this was the case, then these endogenous cannabinoids might occlude the effects of WIN55,212-2 by occupying the available CBsc receptors. To test this possibility we compared the effects of SR141716A on fEPSPs in hippocampal slices obtained from WT C57 mice and SD rats. As described previously in hippocampal slices (Hoffman & Lupica, 2000), SR141716A (500 nm) alone had no effect on these synaptic responses in either species (e.g. C57, 110 ± 5% of control, n = 4). This suggested that an increased basal level of endogenous cannabinoids in the C57 mice and the occupation of the CBsc receptor could not explain the observed differences. The lack of effect of WIN55,212-2 on fEPSPs in the WT C57 mouse hippocampus might alternatively reflect a general deficit in the presynaptic modulation of glutamate release by G protein-coupled receptors. To test this possibility we examined the effects of adenosine (50–100 μm) and baclofen (30 μm) on fEPSPs and EPSCs in these mice. These agonists activate adenosine A1 and GABAB receptors, respectively, and are expressed on SC axon terminals, where they decrease the probability of glutamate release (Lupica et al., 1992; Thompson & Gahwiler, 1992). Both adenosine (64 ± 11% of control, Fig. 1C) and baclofen (68 ± 7% of control) inhibited the fEPSPs and the EPSCs to a degree that was similar to these previous reports, suggesting that the lack of effect of WIN55,212-2 on fEPSPs in the C57 mouse hippocampus did not reflect a general deficit in presynaptic modulation.
A previous study showed that this novel CBsc receptor appeared to differ from CB1 in its affinity for WIN55,212-2 and the ability of SR141716A and AM251 to antagonize these agonist effects (Hajos & Freund, 2002). In agreement with this, we also found that fEPSPs were inhibited by WIN55,212-2 in a concentration-dependent manner (EC50 = 465 nm) in hippocampal slices obtained from SD rats (Fig. 2A). Furthermore, this EC50 value was approximately fourfold larger than that measured at confirmed CB1 receptors located on GABAergic axon terminals in the hippocampus in our laboratory (Hoffman & Lupica, 2000).
Fig. 2.
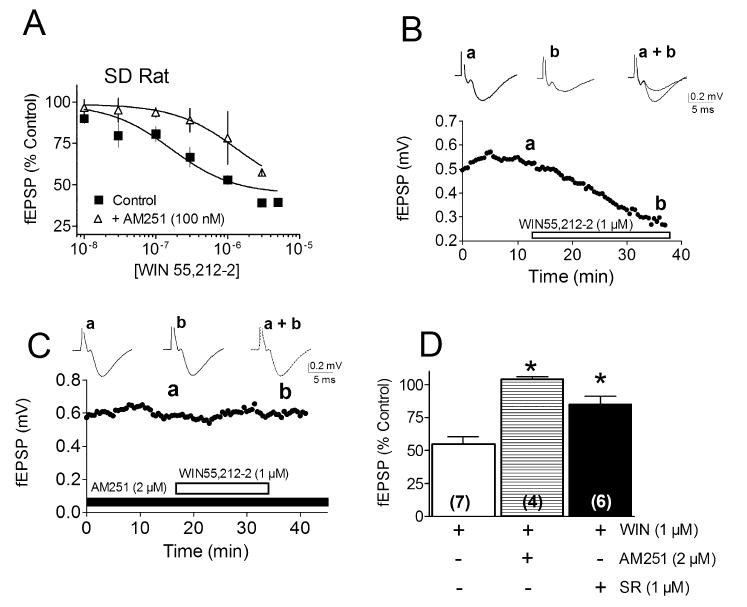
Pharmacological effects of cannabinoid receptor ligands in the Sprague Dawley (SD) rat hippocampus. (A) Concentration-dependent effects of WIN55,212-2 on fEPSPs recorded in the CA1 region of the hippocampus, in the absence and presence of the antagonist AM251 (100 nm). The apparent affinity of WIN55,212-2 at the receptor mediating the inhibition of fEPSPs (EC50, 465 nm) is lower than recorded previously at the CB1 receptor mediating the inhibition of GABA release in the hippocampus (EC50, 138 nm, Hoffman & Lupica, 2000). (B) Time course of the effect of WIN55,212-2 on the fEPSP recorded in a single hippocampal slice. (C) Time course of WIN55,212-2 effects in a single hippocampal slice after treatment with the cannabinoid receptor antagonist AM251. (D) Mean effects of WIN55,212-2 in the absence and presence of AM251 or SR141716A in SD rat hippocampal slices (*P < 0.001, t-test).
The study by Hajos & Freund (2002) also found that, whereas the cannabinoid receptor antagonist SR141716A could block the inhibitory effect of WIN55,212-2 on glutamatergic EPSPs in the Wistar rat hippocampus, the antagonist AM251 did not produce this effect (Hajos & Freund, 2002). The authors concluded that this apparent ability of AM251 to antagonize the effects of WIN55,212-2 at GABAergic synapses but not at glutamatergic synapses indicated that it was selective for CB1 vs. CBsc receptors, whereas SR141716A could not distinguish these binding sites (Hajos & Freund, 2002). However, in the present study we found that AM251 could block the inhibition of glutamatergic fEPSPs produced by WIN55,212-2 (1 μm, Fig. 2) in hippocampal slices obtained from both WT CD1 mice and SD rats. Furthermore, the maximum level of antagonism of the effects of WIN55,212-2 by AM251 was also similar to that seen with SR141716A in our laboratory (Fig. 2D).
Discussion
The C57 and Swiss CD1 strains of mice have been utilized as backgrounds for a variety of genetically modified pharmacological animal models. Of particular relevance to the present study, these strains of mice have been used to develop two independent CB1−/− receptor lines, one used primarily in the USA (Zimmer et al., 1999) and the other primarily in Europe (Ledent et al., 1999), respectively. The existence of a novel cannabinoid receptor in the CA1 region of the hippocampus had previously been inferred from the persistence of the inhibitory effect of WIN55,212-2 on glutamatergic EPSPs in CB1−/− animals from the CD1 background (Hajos et al., 2001) and by observations that this agonist is less potent at inhibiting these EPSPs when compared with GABAergic IPSCs in the hippocampus (Hajos & Freund, 2002; present study). These studies are supported by others reporting that antibodies directed against the CB1 receptor consistently fail to detect this protein in association with asymmetric glutamatergic axon terminals in the adult rat hippocampus (Katona et al., 1999; Hajos et al., 2000) but find it abundantly associated with symmetric GABAergic axon terminals (Katona et al., 1999, 2000). As the reduction of glutamate release by cannabinoids has been identified as a primary mechanism through which these molecules inhibit long-term potentiation in the hippocampus (Stella et al., 1997; Misner & Sullivan, 1999), it is likely that this occurs through the activation of the novel non-CB1 cannabinoid receptor that we have chosen to define as the CBsc receptor, due to its unique localization.
In the present study, we found that WIN55,212-2 did not affect glutamatergic fEPSPs in hippocampal slices obtained from WT C57 mice but did inhibit these responses in slices obtained from WT CD1 mice and from SD rats. Also, inhibition of fEPSPs was not observed in WT C57 mice obtained from our NIDA-IRP animal facility or from C57 mice purchased from a commercial supplier, suggesting that this difference was not unique to animals bred in our colony. In addition, the possibility that glutamate responses in the C57 mice might be insensitive to WIN55,212-2 because of an augmented level of endogenous cannabinoid in the brains of these animals was discounted because SR141716A alone had no effect on these responses and because the effect of WIN55,212-2 on IPSCs was similar to that observed previously in the SD rat hippocampus (Hoffman & Lupica, 2000).
In addition to electrophysiological studies that pharmacologically identified the novel CBsc receptor, Breivogel et al. 2001 demonstrated that [35S]GTPγS binding was stimulated by the endogenous cannabinoid anandamide and by WIN55,212-2 in a variety of brain areas in CB1−/− mice. However, these studies were conducted using brain homogenates from C57 CB1−/− mice (Breivogel et al., 2001) that, as we have shown, do not express the CBsc receptor in the hippocampus. Because of this, and the observation that the stimulation of [35S]GTPγS binding by WIN55,212-2 was insensitive to SR141716A, it seems unlikely that the receptor identified by Breivogel et al. (2001) is the same as the CBsc receptor that modulates glutamate release in the hippocampus (Hajos et al., 2001). Thus, on the basis of the above data, we propose that at least two distinct novel cannabinoid receptors may be found in the rodent brain, one mediating the inhibition of glutamate release and the other permitting the incorporation of [35S]GTPγS into brain tissue membranes of C57CB1−/− animals (Breivogel et al., 2001; Hajos et al., 2001). It is also noteworthy that this SR141716A-insensitive incorporation of [35S]GTPγS by WIN55,212-2 and anandamide has also been reported in cerebellar homogenates from CD1CB1−/− mice (Monory et al., 2002).
Another study that appears to be at odds with our observed lack of effect of WIN55,212-2 in the C57 mouse hippocampus demonstrated that WIN55,212-2 could inhibit glutamatergic EPSCs in primary cultures of hippocampal neurones obtained from immature (postnatal day 1–2) C57 WT mice and that this effect was eliminated in hippocampal cultures obtained from C57CB1−/− mice (Ohno-Shosaku et al., 2002). We believe that this disparity may be explained by the fact that our study utilized adult animals, whereas those used for preparation of the cell cultures were mouse pups 1–2 days after birth (Ohno-Shosaku et al., 2002). Taken together, these studies may indicate that CB1 receptors are transiently expressed on glutamate axon terminals at early developmental stages in the rodent hippocampus or that the cell culture conditions played some role in facilitating the expression of CB1 receptors on these terminals (Ohno-Shosaku et al., 2002).
The present study also demonstrated that the affinity of WIN55,212-2 for the CBsc receptor (EC50 = 465 nm) was lower than that described for this agonist at the CB1 receptor in the SD rat hippocampus in our laboratory (EC50 = 138 nm, Hoffman & Lupica, 2000). In relative terms, this agrees with the findings of Hajos & Freund (2002) in the Wistar rat hippocampus. However, we also found that, in contrast to their previous report (Hajos & Freund, 2002), the antagonist AM251 blocked the inhibition of glutamatergic fEPSPs by WIN55,212-2 in hippocampal slices from SD rats and CD1 mice. It is possible that AM251 may be an effective antagonist of this response in the SD rat hippocampus and is ineffective in the Wistar rat hippocampus, as demonstrated by Hajos & Freund (2002). However, it is also true that AM251 and SR141716A are close structural analogues, suggesting that they may indeed recognize the same binding sites in brain tissue (Palmer et al., 2002).
Collectively, the data from the present study suggest that, unlike CD1 mice and SD and Wistar (Hajos & Freund, 2002) rats, the C57 mouse strain does not possess the CBsc receptor that modulates glutamate release in the hippocampus. The absence of this receptor in the C57 mice further suggests that these WT animals may be utilized to study the CB1 receptor in isolation or, in the case of C57CB1−/− mice, to determine the consequences of the absence of both CB1 and CBsc receptors in this brain structure. Furthermore, the comparison of CD1CB1−/− and C57CB1−/− mice may represent a useful strategy to study the functional importance of the novel CBsc receptor in the CD1 mouse hippocampus in the absence of CB1.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health.
References
- Ameri A, Wilhelm A, Simmet T. Effects of the endogenous cannabinoid, anandamide, on neuronal activity in rat hippocampal slices. Br J Pharmacol. 1999;126:1831–1839. doi: 10.1038/sj.bjp.0702478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breivogel CS, Di Griffin GMV, Martin BR. Evidence for a new G protein-coupled cannabinoid receptor in mouse brain. Mol Pharmacol. 2001;60:155–163. [PubMed] [Google Scholar]
- Hajos N, Katona I, Nalem SS, Mackie K, Ledent C, Mody I, Freund TF. Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur J Neurosci. 2000;12:3239–3249. doi: 10.1046/j.1460-9568.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- Hajos N, Freund TF. Pharmacological separation of cannabinoid sensitive receptors on hippocampal excitatory and inhibitory fibers. Neuropharmacology. 2002;43:503–510. doi: 10.1016/s0028-3908(02)00157-0. [DOI] [PubMed] [Google Scholar]
- Hajos N, Ledent C, Freund TF. Novel cannabinoid-sensitive receptor mediates inhibition of glutamatergic synaptic transmission in the hippocampus. Neuroscience. 2001;106:1–4. doi: 10.1016/s0306-4522(01)00287-1. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Hampson RE, Deadwyler SA. Effects of delta-9-tetrahydrocannabinol on delayed match to sample performance in rats: alterations in short-term memory associated with changes in task specific firing of hippocampal cells. J Pharmacol Exp Ther. 1993;264:294–307. [PubMed] [Google Scholar]
- Hoffman AF, Lupica CR. Mechanisms of cannabinoid inhibition of GABA(A) synaptic transmission in the hippocampus. J Neurosci. 2000;20:2470–2479. doi: 10.1523/JNEUROSCI.20-07-02470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Magloczky Z, Santha E, Kofalvi A, Czirjak S, Mackie K, Vizi ES, Freund TF. GABAergic interneurons are the targets of cannabinoid actions in the human hippocampus. Neuroscience. 2000;100:797–804. doi: 10.1016/s0306-4522(00)00286-4. [DOI] [PubMed] [Google Scholar]
- Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, Bohme GA, Imperato A, Pedrazzini T, Roques BP, Vassart G, Fratta W, Parmentier M. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- Lupica CR, Proctor WR, Dunwiddie TV. Presynaptic inhibition of excitatory synaptic transmission by adenosine in rat hippocampus: Analysis of unitary EPSP variance measured by whole-cell recordings. J Neurosci. 1992;12:3753–3764. doi: 10.1523/JNEUROSCI.12-10-03753.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA [see comments] Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Misner DL, Sullivan JM. Mechanism of cannabinoid effects on long-term potentiation and depression in hippocampal CA1 neurons. J Neurosci. 1999;19:6795–6805. doi: 10.1523/JNEUROSCI.19-16-06795.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monory K, Tzavara ET, Lexime J, Ledent C, Parmentier M, Borsodi A, Hanoune J. Novel, not adenylyl cyclase-coupled cannabinoid binding site in cerebellum of mice. Biochem Biophys Res Commun. 2002;292:231–235. doi: 10.1006/bbrc.2002.6635. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Tsubokawa H, Mizushima I, Yoneda N, Zimmer A, Kano M. Presynaptic cannabinoid sensitivity is a major determinant of depolarization-induced retrograde suppression at hippocampal synapses. J Neurosci. 2002;22:3864–3872. doi: 10.1523/JNEUROSCI.22-10-03864.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer SL, Thakur GA, Makriyannis A. Cannabinergic ligands. Chem Phys Lipids. 2002;121:3–19. doi: 10.1016/s0009-3084(02)00143-3. [DOI] [PubMed] [Google Scholar]
- Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- Sullivan JM. Cellular and molecular mechanisms underlying learning and memory impairments produced by cannabinoids. Learn Mem. 2000;7:132–139. doi: 10.1101/lm.7.3.132. [DOI] [PubMed] [Google Scholar]
- Thompson SM, Gahwiler BH. Comparison of the actions of baclofen at pre-and postsynaptic receptors in the rat hippocampus. in vitro J Physiol. 1992;451:329–345. doi: 10.1113/jphysiol.1992.sp019167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Kunos G, Nicoll RA. Presynaptic specificity of endocannabinoid signaling in the hippocampus. Neuron. 2001;31:453–462. doi: 10.1016/s0896-6273(01)00372-5. [DOI] [PubMed] [Google Scholar]
- Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]


