Abstract
The Diabetes Prevention Program (DPP) was a randomized clinical trial of prevention of type 2 diabetes in high-risk people. Troglitazone, an insulin-sensitizing agent, was used initially but was discontinued during the trial. Troglitazone therapy was compared with other DPP interventions, considering both the short-term “in-trial” results and the longer-term results after troglitazone were discontinued. From 1996 to 1998, participants were randomly assigned to treatment with metformin (n = 587), troglitazone (n = 585), double placebo (n = 582), or intensive lifestyle intervention (ILS) (n = 589). Because of concern regarding its liver toxicity, the troglitazone arm was discontinued in June 1998, after which follow-up of all participants continued. During the mean 0.9 year (range 0.5–1.5 years) of troglitazone treatment, the diabetes incidence rate was 3.0 cases/100 person-years, compared with 12.0, 6.7, and 5.1 cases/100 person-years in the placebo, metformin, and ILS participants (P < 0.001, troglitazone vs. placebo; P = 0.02, troglitazone vs. metformin; P = 0.18, troglitazone vs. ILS). This effect of troglitazone was in part due to improved insulin sensitivity with maintenance of insulin secretion. During the 3 years after troglitazone withdrawal, the diabetes incidence rate was almost identical to that of the placebo group. Troglitazone, therefore, markedly reduced the incidence of diabetes during its limited period of use, but this action did not persist. Whether other thiazolidinedione drugs used for longer periods can safely prevent diabetes remains to be determined.
The Diabetes Prevention Program (DPP) was a randomized clinical trial of prevention of type 2 diabetes in people at high risk of diabetes (1). Enrollment began in 1996 with randomization to four treatment groups. Three treatments were standard healthy lifestyle recommendations plus placebo, metformin, or troglitazone. The fourth treatment was intensive lifestyle (ILS), which consisted of no drugs and the same lifestyle recommendations given to the pharmacologic groups, but the lifestyle advice was given with much more behavioral support. The details of these treatments and the results of three of the interventions have been described (1–3), and the protocol is available at http://www.bsc.gwu.edu/dpp/protocol.htmlvdoc.
In planning the DPP (from 1994 to 1996), the research group considered testing several drugs affecting insulin secretion and sensitivity. Metformin and troglitazone, two drugs of different classes with different actions, were chosen. Metformin had been used worldwide in treating type 2 diabetes for several decades but was not approved in the U.S. until 1994. Troglitazone, an insulin-sensitizing thiazolidinedione, showed promise in improving insulin sensitivity and glucose tolerance (4). Its use was started in the DPP in 1996 as an investigational drug. It was then approved in the U.S. for diabetes treatment in 1997 but was withdrawn from the DPP in 1998 and from the U.S. market in 2000 because of liver toxicity. In this study, we report the results of troglitazone treatment both before and after its discontinuation during the DPP.
RESEARCH DESIGN AND METHODS
Previous publications describe the DPP study design (1–3), recruitment (5), measurement methods (6), characteristics of the randomized participants (6), and main outcomes of the three main treatment arms: placebo, metformin, and ILS (1). Eligibility criteria, described in detail previously (2), included age of ≥25 years, fasting plasma glucose (FPG) level of 5.6–7.7 mmol/l before June 1997 and 5.3–6.9 mmol/l after that date, 2-h plasma glucose level of 7.8–11.0 mmol/l, and BMI of ≥24 kg/m2 (≥22 kg/m2 in Asian Americans). The development of diabetes was assessed by 6-month measurements of FPG and annual oral glucose tolerance tests (OGTTs) following American Diabetes Association 1997 criteria (7). The diagnosis was confirmed by a second test (2).
Insulin secretion and sensitivity were estimated from the OGTT (8–9). Insulin secretion was estimated by two methods: 1) the corrected insulin response (CIR) = 30 min insulin/[30 min glucose × (30 min glucose − 3.89 mmol/l)] (10), and 2) the insulin-to-glucose ratio (IGR) = (30 min insulin − fasting insulin)/(30 min glucose − fasting glucose) (11). IGR and CIR were highly correlated at baseline (Spearman r = 0.95). Insulin sensitivity was estimated by 1/fasting insulin or by the insulin sensitivity index (ISI), which is 1/(fasting insulin × fasting glucose) (12). An index proportional to the reciprocal of ISI, i.e., the product of fasting insulin and fasting glucose, has also been named HOMA-IR for homeostasis model assessment of insulin resistance (13). ISI and 1/fasting insulin were, as expected, highly correlated at baseline (Spearman r = 0.99). The glucose and insulin measurements and the indexes derived from them are expressed in SI units, although some indexes were originally defined in other units. Some have been multiplied by constants (shown in Table 3) to give results on a convenient scale. These indexes and the serum insulin concentrations were not normally distributed and included outliers that might influence relationships. Furthermore, the IGR could not be logarithmically transformed because of negative values. Therefore, medians, rather than means, are presented, and changes over time and group differences were analyzed nonparametrically.
TABLE 3.
Baseline and follow-up median values of serum insulin–related variables by treatment over time
| Variable | Time (years) | Placebo | Metformin | Troglitazone | ILS | P difference among four treatments (Kruskal-Wallis) | Placebo vs. troglitazone | Metformin vs. troglitazone | ILS vs. troglitazone |
|---|---|---|---|---|---|---|---|---|---|
| n | 172 | 164 | 187 | 183 | |||||
| Fasting insulin (pmol/l) | 0 | 144 | 138 | 132 | 132 | 0.58 | — | — | — |
| 1 | 132 | 111 | 90 | 102 | <0.01 | † | † | — | |
| Δ | — | * | * | * | <0.01 | † | — | — | |
| 30-min insulin (pmol/l) | 0 | 513 | 498 | 498 | 474 | 0.72 | — | — | — |
| 1 | 522 | 447 | 402 | 408 | <0.01 | † | † | — | |
| Δ | — | * | * | — | <0.01 | † | † | — | |
| Sensitivity [1/fasting insulin 1,000/(pmol/l)] | 0 | 6.94 | 7.25 | 7.58 | 7.58 | 0.58 | — | — | — |
| 1 | 7.58 | 9.02 | 11.11 | 9.80 | <0.01 | † | † | — | |
| Δ | — | * | * | * | <0.01 | † | — | — | |
| Sensitivity {ISI 10,000/[(pmol/l) × (mmol/l)]} | 0 | 11.8 | 12.0 | 12.3 | 12.5 | 0.64 | — | — | — |
| 1 | 13.2 | 15.4 | 19.9 | 17.0 | <0.01 | † | † | — | |
| Δ | — | * | * | * | <0.01 | † | † | — | |
| Secretion {CIR (pmol/l)/[(mmol/l)2]} | 0 | 10.1 | 9.05 | 9.58 | 9.06 | 0.19 | — | — | — |
| 1 | 10.3 | 8.47 | 10.15 | 8.34 | 0.05 | — | — | — | |
| Δ | — | — | — | — | 0.99 | — | — | — | |
| Secretion [IGR (pmol/l)/(mmol/l)] | 0 | 116.4 | 102.1 | 108.1 | 101.5 | 0.24 | — | — | — |
| 1 | 109.1 | 91.1 | 103.2 | 91.4 | 0.03 | — | — | — | |
| Δ | — | — | — | — | 0.43 | — | — | — |
P < 0.05 for difference from baseline to 1 year (signed-rank test).
P < 0.05 compared with troglitazone (Wilcoxon test, adjusted for multiple comparisons, i.e., unadjusted P < 0.0167). Dash indicates no significant difference; n, number of participants with both baseline and 1-year measurements; Δ, test for difference between baseline and 1 year. Conversion of units: for insulin, 1 μU/ml = 6.0 pmol/l; for glucose, 1 mg/dl = 0.05551 mmol/l.
Four treatments (placebo, 850 mg of metformin twice a day, 400 mg of troglitazone every day, and ILS) were included initially. By protocol, each participant in the pharmacologic intervention groups was to take two coded medications in a double-masked fashion daily, consisting of two coded metformin pills and one coded troglitazone pill. They were randomly assigned either to active metformin plus placebo troglitazone, active troglitazone plus placebo metformin, or placebo metformin plus placebo troglitazone. Standard lifestyle recommendations for all medication treatment group participants were provided in the form of written information and an annual 20- to 30-min individual session on healthy eating and increasing physical activity. Approximately one-fourth of those enrolled before 4 June 1998 were randomly assigned to troglitazone. Three of the four participating Native-American communities declined to include the troglitazone arm, so results from all participants in all intervention arms from these centers are excluded here. Native-American participants from the other centers are included.
Adverse events were ascertained from the participants at all scheduled follow-up visits. Potential adverse hepatic events of troglitazone were monitored in the three pharmacologic treatment arms by tests of alanine aminotransferase and aspartate aminotransferase. Initially, these liver enzymes were tested at baseline, 3 and 6 months after start of drug therapy, and every 6 months thereafter. As concerns regarding liver toxicity developed, increased monitoring, including monthly testing for the first 7 months of therapy, was initiated. The troglitazone arm was discontinued in June 1998 (2). All study participants were informed of the drug discontinuation. Participants assigned to troglitazone were unmasked to their drug assignment, instructed to stop taking the study drug, and followed for outcomes on the same schedule as the other DPP participants, i.e., semiannual measurement of FPG and annual OGTTs based on the original randomization date. The participants originally randomized to troglitazone were offered quarterly lifestyle group sessions starting in September 1998 that were designed to provide basic information about losing weight through healthy eating and physical activity. This intervention was less intensive than that provided to the ILS participants because individual counseling was not provided.
Follow-up experience reported in this article is divided into time before and after 4 June 1998, when troglitazone was discontinued, and is presented only for participants from all four intervention groups who had been randomly assigned by this date. Virtually all participants assigned to troglitazone were contacted within a few days of 4 June 1998 to implement the treatment changes. No additional unscheduled OGTT outcome visits were performed at the time of drug discontinuation. All outcome assessments before 4 June 1998 were performed under the original treatment assignments. After troglitazone was stopped on that date, follow-up FPG and OGTT measurements were continued at 6- and 12-month intervals from the date of randomization, not from 4 June 1998. Therefore, after discontinuation of troglitazone, outcome data in this group represent various durations of exposure to troglitazone and time off of the drug, depending on each participant’s date of randomization.
Analyses were done using SAS version 8.2 (SAS Institute, Cary, NC). Incidence rates of diabetes were computed as new cases per 100 person-years of follow-up. Both cases and person-time were stratified based on whether follow-up occurred before or after 4 June 1998. Cumulative incidence rates of diabetes were computed (14) over two periods: starting from randomization and among those remaining nondiabetic on 4 June 1998, starting from that date through 31 July 2001, when the initial masked phase of the DPP was completed. Time to outcome events was assessed by life-table methods (14). Modified product-limit cumulative incidence curves were compared using the log-rank test.
Normal-errors fixed-effects models (15) assessed differences among the three groups over time for body weight, plasma glucose, and waist circumference. P values for comparisons between any two treatment arms were adjusted for multiple comparisons (16). Nonparametric tests were used for insulin secretion and sensitivity estimates, because these variables were highly skewed. P values for comparisons at baseline and year 1 and for the change from baseline to year 1 were computed using the Kruskal-Wallis test across all four treatment arms; two-way comparisons between each treatment with troglitazone used the Wilcoxon test with Bonferroni-adjusted P values. The signed-rank test was used to compute P values for the significance of the changes within treatment arm from baseline to year 1.
RESULTS
Participants and adherence
Participants were randomly assigned to placebo (n = 582), metformin (n = 587), troglitazone (n = 585), and ILS (n = 589). The mean age was 51 years, the mean BMI was 33.9 kg/m2, 65% were women, and the racial/ethnic distribution was white (58%), African American (19%), Hispanic (17%), Asian American (5%), and Native American (1%). None of these baseline variables differed significantly by treatment group.
Among the three pharmacologic treatment arms, adherence to taking DPP pills was defined as the percentage of participants taking at least 80% of their prescribed dose as measured by pill count. Adherence was assessed quarterly and averaged over all visits before 4 June 1998, when troglitazone was stopped. Adherence, assessed for each medicine (metformin or troglitazone) or its placebo in each of the three pharmacologic arms (placebo, metformin, and troglitazone), is shown in Table 1. Adherence for all groups taking placebo ranged from 76 to 78%; it was lower for active metformin (72%) and higher for active troglitazone (83%).
TABLE 1.
Adherence to study medication
| Treatment | |
|---|---|
| Placebo group | |
| M-placebo | 76% |
| T-placebo | 77% |
| Metformin group | |
| M-active | 72% |
| T-placebo | 78% |
| Troglitazone group | |
| M-placebo | 77% |
| T-active | 83% |
Data are percentages of participants taking ≥80% of study pills averaged throughout the study. M-placebo, placebo pill for metformin; M-active, active metformin pill; T-placebo, placebo pill for troglitazone; T-active, active troglitazone pill.
Responses to treatment
Baseline and follow-up values of body size and glucose measurements are shown in Table 2 and Fig. 1. Weight, waist circumference, and fasting glucose were measured in all participants regardless of development of diabetes. The OGTT was not performed once a diagnosis of diabetes had been confirmed, but as this occurred rarely before the first annual visit, there were few missing observations by this time. The numbers of observations declined with increasing follow-up because of the variable randomization date together with a common closing date for the analysis. Body weight increased slightly in the placebo group and to a greater extent in the troglitazone group, and it declined in the metformin and ILS groups, most markedly in the latter. Waist circumference also increased slightly in the troglitazone group and decreased markedly in the ILS group. Fasting glucose increased, on average, in the placebo group and fell in the active treatment groups, whereas the average 2-h postload glucose concentrations declined in all groups, especially in the ILS and troglitazone groups.
TABLE 2.
Body weight, waist circumference, and FPG and 2-h postload plasma glucose concentrations
| Placebo
|
Metformin
|
Troglitazone
|
ILS
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Time (years) | n | Mean | n | Mean | n | Mean | n | Mean |
| Body weight (kg) | 0.0 | 582 | 94.6 | 587 | 94.5 | 585 | 93.3 | 589 | 95.5 |
| 0.5 | 384 | 94.5 | 389 | 92.8 | 378 | 95.2 | 386 | 88.1 | |
| 1.0 | 182 | 95.3 | 172 | 93.3 | 196 | 97.0 | 191 | 89.7 | |
| 1.5 | 58 | 97.6 | 59 | 94.3 | 72 | 98.0 | 53 | 91.1 | |
| Waist circumference (cm) | 0.0 | 582 | 105.6 | 586 | 105.0 | 585 | 104.3 | 588 | 106.1 |
| 1.0 | 180 | 105.1 | 172 | 104.4 | 197 | 105.7 | 191 | 100.4 | |
| FPG (mmol/l) | 0.0 | 582 | 5.96 | 587 | 5.96 | 585 | 5.96 | 589 | 5.98 |
| 0.5 | 388 | 5.97 | 394 | 5.72 | 383 | 5.63 | 388 | 5.71 | |
| 1.0 | 182 | 6.00 | 172 | 5.80 | 197 | 5.66 | 190 | 5.75 | |
| 1.5 | 58 | 6.27 | 61 | 5.88 | 72 | 5.74 | 54 | 6.00 | |
| 2-h plasma glucose (mmol/l) | 0.0 | 582 | 9.22 | 587 | 9.19 | 585 | 9.18 | 589 | 9.19 |
| 1.0 | 174 | 8.79 | 172 | 8.81 | 196 | 8.03 | 188 | 7.86 | |
None of the variables differed significantly between treatment groups at baseline (time = 0). By repeated-measures ANOVA, the troglitazone group differed significantly from each of the others (P < 0.05 adjusted for three comparisons) for body weight and FPG. It differed significantly from the ILS group for waist circumference and from the placebo and metformin groups in 2-h plasma glucose. n, number of participants with the measurement at each time point. Conversion of units for glucose: 1 mg/dl = 0.05551 mmol/l.
FIG. 1.
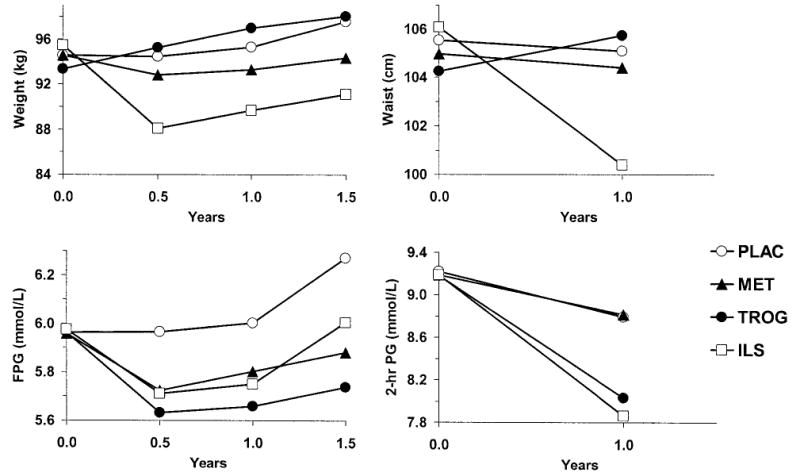
Mean values of body weight, waist circumference, and FPG and 2-h postload glucose concentrations in response to treatment. Sample sizes are shown in Table 2.
Treatment effects on insulin secretion and sensitivity were assessed by changes in the median values among the 706 participants who had all the OGTT and insulin measurements at baseline and at the first annual follow-up examination (Table 3). Fasting insulin concentrations decreased significantly from baseline in all but the placebo group. The improvement in insulin sensitivity (estimated by 1/fasting insulin or ISI) was significantly greater with troglitazone than with placebo or metformin but not significantly greater than in the ILS group. Despite the improvement in both estimates of insulin sensitivity in all three active treatment groups, insulin secretion in response to oral glucose (Table 3, CIR and IGR) did not change significantly during the year of treatment in any group. Furthermore, the 1-year changes in CIR and IGR did not differ significantly among treatment groups.
Diabetes incidence by 4 June 1998
Figure 2A shows the cumulative incidence of diabetes in 1.5 years during the period when troglitazone was administered. Because recruitment took place from June 1996 up to the time troglitazone was discontinued, fewer people are represented at each follow-up time point, as shown by the sample sizes in the figure. Diabetes developed in 10 of the 387 troglitazone participants (with 330 person-years of follow-up) who had a follow-up diabetes assessment visit during the mean 0.9 years (range 0.5–1.5 years) of troglitazone treatment. During the same time period, 21 of 397 participants (313 person-years) assigned to metformin, 16 of 393 (317 person-years) assigned to ILS, and 37 of 391 (309 person-years) assigned to placebo developed diabetes. Diabetes incidence rates were lower in all three active treatment groups than in the placebo group (Fig. 2B). Similar to the 2.8-year three-group study results reported previously (1), in this subset, diabetes incidence rates were 12.0 cases/100 person-years in the placebo group, 6.7/100 person-years with metformin, and 5.1/100 person-years in the ILS group. The rate was lowest in the troglitazone group (3.0 cases/100 person-years), a 75% reduction from the placebo rate. Overall, there was a highly significant difference among the four groups (P < 0.001). In pairwise group comparisons, the rate in the troglitazone group was significantly lower than those in the placebo (P < 0.001) and metformin (P = 0.02) groups, but it did not differ significantly from that in the ILS group (P = 0.18).
FIG. 2.
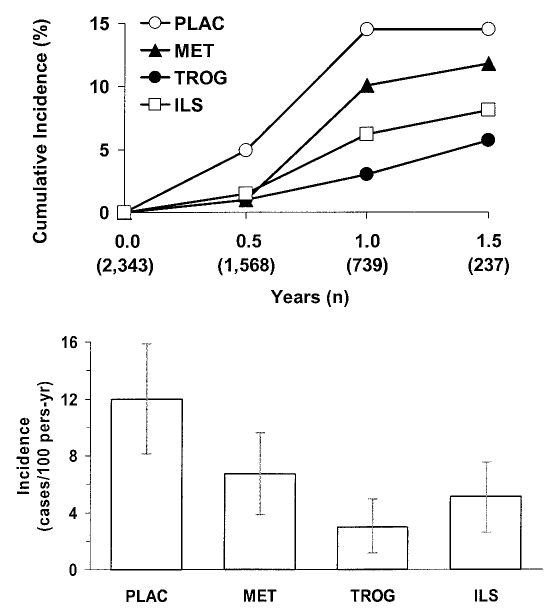
A: Cumulative incidence of diabetes during the time when troglitazone was used, according to treatment assignment. B: Diabetes incidence rates (cases/100 person-years) with 95% CIs during the time when troglitazone was used, according to treatment assignment.
Diabetes incidence before and after discontinuation of troglitazone
Figure 3A shows the cumulative incidence of diabetes in the placebo and troglitazone groups as a function of time since randomization until 31 July 2001, immediately preceding the announcement of study results. This includes the time before and after troglitazone was discontinued on 4 June 1998. There was a large separation of these curves in the 1st year or so, reflecting the time of troglitazone use. After that, the curves were nearly parallel.
FIG. 3.
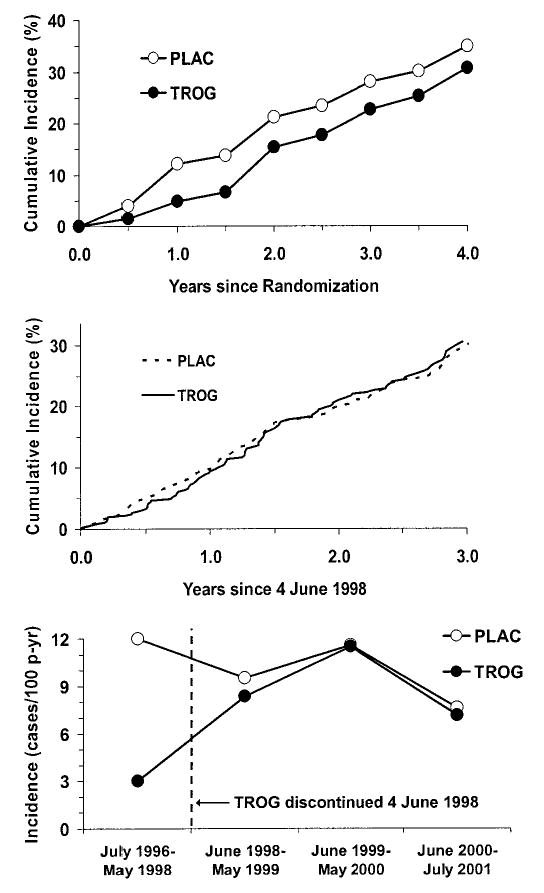
A: Cumulative incidence of diabetes (%) from date of randomization in participants assigned to placebo or troglitazone. B: Cumulative incidence of diabetes (%) from date of discontinuation of troglitazone (4 June 1998) in participants assigned to placebo or troglitazone. C: Diabetes incidence rates (cases/100 person-years) from date of randomization, showing the date of discontinuation of troglitazone (4 June 1998), in participants assigned to placebo or troglitazone.
Figure 3B shows the cumulative incidence of diabetes after 4 June 1998, when the troglitazone treatment was discontinued, among individuals who had not developed diabetes by this date. There was virtually no difference between the two groups from this date onward.
Figure 3C shows the diabetes incidence rates, in new cases per 100 person-years, as a function of calendar time so that results before and after the discontinuation of troglitazone can be easily compared. Before 4 June 1998, the troglitazone group had a 75% lower diabetes incidence rate than the placebo group, as shown in Fig. 2. In the year after troglitazone discontinuation, the incidence rate in this group had risen to nearly the placebo rate, and the rates in the two groups were virtually identical in subsequent years.
Adverse events
During the active troglitazone period, the numbers (and percents) of participants for whom selected adverse events was reported are shown in Table 4. The numbers developing congestive heart failure, myocardial infarction, and anemia did not differ significantly among treatment groups, whereas edema was reported in fewer metformin participants (P < 0.05 adjusted for multiple comparisons). There were no significant differences among the three drug-treatment arms in elevations of the routinely monitored liver enzymes to at least 3.0 times the upper limit of normal. Comparable statistics are not available for the ILS group, because liver enzymes were not measured on the same schedule due to lack of a safety concern. Elevations to at least 10 times the upper limit of normal occurred in one (0.2%), zero, and seven (1.2%) individuals in these three treatment groups. When subjects with this degree of elevation were compared with all others, the difference was statistically significant (P < 0.01 by Fisher’s exact test). One death due to liver disease occurred in the troglitazone group, contributing to the decision to withdraw troglitazone from the DPP (2).
TABLE 4.
Adverse events by treatment assignment
| Event | Placebo | Metformin | Troglitazone | ILS |
|---|---|---|---|---|
| n | 582 | 587 | 585 | 589 |
| CHF | 0 (0.0) | 1 (0.2) | 0 (0.0) | 1 (0.2) |
| MI | 2 (0.3) | 0 (0.0) | 0 (0.0) | 2 (0.3) |
| Anemia | 7 (1.2) | 5 (0.9) | 7 (1.2) | 4 (0.7) |
| Edema | 31 (5.3) | 8 (1.4) | 27 (4.6) | 26 (4.4) |
| LFT ≥3 | 21 (3.6) | 18 (3.1) | 25 (4.3) | NA |
| LFT ≥10 | 1 (0.2) | 0 (0.0) | 7 (1.2) | NA |
Data are n (%) of individuals in each randomization group experiencing at least one adverse event of the type specified. Liver function tests were not measured on the same schedule in the ILS group. CHF, congestive heart failure; MI, myocardial infarction; LFT ≥3, liver function test (either alanine aminotransferase or aspartate aminotransferase) ≥3 times the upper limit of normal; LFT ≥10, liver function test (either alanine aminotransferase or aspartate aminotransferase) ≥10 times the upper limit of normal; NA, not applicable.
DISCUSSION
The DPP was designed to determine whether type 2 diabetes could be prevented or delayed through lifestyle or medication interventions applied in a high-risk population. Troglitazone was selected for the DPP before it was approved in the U.S. for the treatment of diabetes based on data demonstrating its ability to reduce insulin resistance (4), a major pathophysiologic abnormality leading to diabetes. Clinical data available at the time (from treatment of >3,000 diabetic and nondiabetic volunteers) supported the lack of side effects and safety of the drug, and troglitazone was approved for diabetes treatment in the U.S. ~1 year after the DPP was initiated. Its potential for rare but potentially fatal hepatotoxicity was recognized only after tens of thousands of patients were treated. On the basis of increasing concern regarding its safety and of a death in a troglitazone-treated DPP participant (2), the troglitazone arm of the DPP was stopped on 4 June 1998, ~2 years before its withdrawal from the market in the U.S.
Despite the mean exposure of only 0.9 years and the limited number of troglitazone-treated individuals with adequate follow-up to assess the incidence of diabetes, the DPP results suggest that troglitazone might have been the most effective of all of the DPP interventions. Compared with placebo, metformin, or ILS interventions, troglitazone had the greatest impact on the development of diabetes, reducing the development by 75% compared with placebo. ILS and metformin reduced the development of diabetes by 58 and 44%, respectively, during the same abbreviated period described in this article, similar to the 58 and 31% reductions compared with placebo after 2.8 years in the complete trial (1). There were insufficient data, with only 10 cases of diabetes developing during 330 person-years of follow-up in the troglitazone group, to examine the results according to age, ethnicity, BMI, etc., as reported in the complete trial (1). Whether longer-term experience with troglitazone would have resulted in similar or greater prevention of diabetes than the ILS intervention and metformin is not known. In addition, whether the currently available and less hepatotoxic thiazolidinediones, rosiglitazone and pioglitazone, would have a similar reduction in the development of diabetes remains to be determined. At least one such clinical trial with rosiglitazone, the Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication Trial, is in progress (H. Gerstein, personal communication).
Possible mechanisms of the preventive effects of the interventions include effects on insulin sensitivity and insulin secretion, because defects in each contribute to the development of type 2 diabetes in people with impaired glucose tolerance (17). No direct measures of insulin secretion or sensitivity were feasible in this large clinical trial, so we relied on indirect estimates from the fasting and 30-min glucose and insulin concentrations during the OGTT. We selected two estimates of secretion (CIR and IGR) that were highly correlated with each other and that, in other studies of nondiabetic individuals, were well correlated with a more direct measure of insulin secretion, the acute insulin response to intravenous glucose (8). The two estimates of insulin sensitivity (1/fasting insulin and ISI) were also highly correlated with each other and are associated with insulin sensitivity measured by the euglycemic clamp (8–9). We used two estimates of each parameter because neither is clearly superior and both strongly predicted the development of diabetes in the full-scale trial (DPP Research Group, unpublished observations). Not surprisingly, the conclusions were the same using either estimate of secretion or of sensitivity. After 1 year of treatment, there was no significant change in secretion or sensitivity in the placebo group, but sensitivity increased in the other groups with no significant change in secretion. A compensatory drop in insulin secretion might have been expected to accompany the increased sensitivity, because of the inverse (hyperbolic) relationship between insulin secretion and sensitivity (18). The apparent lack of change in insulin secretion in the active treatment groups may represent preservation of β-cell function or could simply result from the imprecision of these estimates of secretion (8) or the relatively short follow-up period.
We also determined the long-term incidence of diabetes after withdrawal of troglitazone. The DPP included 1,467 person-years of observation of the troglitazone group after the drug was discontinued and 1,529 person-years in the placebo group. During this time, diabetes developed in 140 and 137 subjects previously assigned over the same time period to placebo or troglitazone, respectively. Thus, we did not confirm a sustained effect of troglitazone to reduce diabetes incidence when the drug was no longer administered. This contrasts with the report from the TRIPOD study of diabetes prevention in high-risk Hispanic women with a history of gestational diabetes (19). In that study, 84 women were followed for <1 year after the drug was stopped, during which time seven new cases of diabetes developed: six in the former placebo group and one in the former troglitazone group. The investigators interpreted these results as a troglitazone effect persisting after drug discontinuation, an effect not seen in the DPP.
The postwithdrawal findings in the DPP, however, highlight an interesting long-term effect. Although the rate of development of diabetes did not differ between troglitazone and placebo groups after the drug was discontinued, the cumulative incidence remained different for the remainder of follow-up. Thus, the preventive effect persisted in that the subsequent cumulative incidence rate in the former troglitazone group did not “catch up” to that of the placebo group. This suggests that troglitazone did not simply “mask” diabetes. With “masking,” after drug discontinuation, the incidence rate might have rebounded to exceed the placebo rate; this did not occur. Instead, troglitazone actually delayed or prevented the development of diabetes in some individuals but was effective only during its active administration.
During the short period of treatment in the DPP, troglitazone was more effective than metformin in reducing the incidence of diabetes and both the fasting and postload glucose concentrations. This difference may be related, in part, to better adherence with troglitazone and to the different mechanisms of action. Adherence to active troglitazone was higher than that to active metformin, perhaps due to the well-known gastrointestinal side effects of metformin. Metformin primarily inhibits hepatic glucose production (20), whereas troglitazone, a peroxisome proliferator–activated receptor γ agonist, predominantly enhances insulin-mediated glucose uptake by improving insulin sensitivity (4). Because the postload plasma glucose depends on the ability of circulating insulin to mediate glucose uptake in the tissues and suppress hepatic glucose production, either increases in plasma insulin levels or improvements in insulin sensitivity or both can decrease hepatic glucose output, improve glucose disposal, and lower the postload glucose level.
In summary, troglitazone reduced the incidence of diabetes by 75% during its short period of use in the DPP. However, as soon as treatment with this medicine was discontinued, the rates of development of diabetes in the troglitazone group equaled that of the placebo group, compatible with little or no persistent effect on diabetes incidence. The main results previously reported for the DPP (1), as well as from other recent clinical trials (21–22), have also established that lifestyle interventions directed at weight reduction and increased physical activity are potent means of preventing or delaying the onset of diabetes. In the DPP and the STOP-NIDDM study (23), metformin and acarbose, respectively, were also effective, but less so than lifestyle intervention, in preventing the development of diabetes (1,21). Whether other drugs in the thiazolidinedione class are effective remains to be determined.
Acknowledgments
Support to the clinical centers and the coordinating center was provided by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) through a cooperative agreement. The Southwestern American Indian Centers were supported directly by the NIDDK and the Indian Health Service. The General Clinical Research Center Program, National Center for Research Resources supported many of the DPP clinical centers. Funding was also provided by the Office of Research on Minority Health, the Office of Women’s Health, the National Institute of Child Health and Human Development, the National Institute on Aging, the Centers for Disease Control and Prevention, the American Diabetes Association, Bristol-Myers Squibb, and Parke-Davis.
We gratefully acknowledge the commitment and dedication of the participants of the DPP. A list of all centers, investigators, and staff has been published (1).
Footnotes
CIR, corrected insulin response; DPP, Diabetes Prevention Program; FPG, fasting plasma glucose; IGR, insulin-to-glucose ratio; ILS, intensive lifestyle intervention; ISI, insulin sensitivity index; OGTT, oral glucose tolerance test.
References
- 1.The Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Diabetes Prevention Program Research Group. The Diabetes Prevention Program: design and methods for a clinical trial in the prevention of type 2 diabetes mellitus. Diabetes Care. 1999;22:623–634. doi: 10.2337/diacare.22.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Diabetes Prevention Program Research Group. The Diabetes Prevention Program (DPP): description of the lifestyle intervention. Diabetes Care. 2002;25:2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nolan JJ, Ludvik B, Beerdsen P, Joyce M, Olefsky J. Improvement in glucose tolerance and insulin resistance in obese subjects treated with troglitazone. N Engl J Med. 1994;331:1188–1193. doi: 10.1056/NEJM199411033311803. [DOI] [PubMed] [Google Scholar]
- 5.The Diabetes Prevention Program Research Group. The Diabetes Prevention Program: recruitment methods and results. Control Clin Trials. 2002;23:157–171. doi: 10.1016/s0197-2456(01)00184-2. [DOI] [PubMed] [Google Scholar]
- 6.The Diabetes Prevention Program Research Group. The Diabetes Prevention Program: baseline characteristics of the randomized cohort. Diabetes Care. 2000;23:1619–1629. doi: 10.2337/diacare.23.11.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 8.Hanson RL, Pratley RE, Bogardus C, Venkat Narayan KM, Roumain JML, Imperatore G, Fagot-Campagna A, Pettitt DJ, Bennett PH, Knowler WC. Evaluation of simple indices of insulin sensitivity and insulin secretion for use in epidemiologic studies. Am J Epidemiol. 2000;151:190–198. doi: 10.1093/oxfordjournals.aje.a010187. [DOI] [PubMed] [Google Scholar]
- 9.Hanley AJG, Williams K, Gonzalez C, D’Agostino RB, Jr, Wagenknecht LE, Stern MP, Haffner SM. Prediction of type 2 diabetes using simple measures of insulin resistance: combined results from the San Antonio Heart Study, the Mexico City Diabetes Study, and the Insulin Resistance Atherosclerosis Study. Diabetes. 2003;52:463–469. doi: 10.2337/diabetes.52.2.463. [DOI] [PubMed] [Google Scholar]
- 10.Sluiter WJ, Erkelens DW, Terpstra P, Reitsma WD, Doorenbos H. Glucose tolerance and insulin release, a mathematical approach. I Assay of the β-cell response after oral glucose loading. Diabetes. 1976;25:241–244. doi: 10.2337/diab.25.4.241. [DOI] [PubMed] [Google Scholar]
- 11.Phillips DI, Clark PM, Hales CN, Osmond D. Understanding oral glucose tolerance: comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and secretion. Diabet Med. 1994;11:286–292. doi: 10.1111/j.1464-5491.1994.tb00273.x. [DOI] [PubMed] [Google Scholar]
- 12.Sluiter WJ, Erkelens DW, Terpstra P, Reitsma WD, Doorenbos H. Glucose tolerance and insulin release, a mathematical approach. II Approximation of the peripheral insulin resistance after oral glucose loading. Diabetes. 1976;25:245–249. doi: 10.2337/diab.25.4.245. [DOI] [PubMed] [Google Scholar]
- 13.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 14.Lachin JM. Biostatistical Methods: The Assessment of Relative Risks. New York, Wiley-Interscience, 2000
- 15.Diggle PJ, Liang KY, Zeger SL. Analysis of Longitudinal Data. New York, Oxford University Press, 1994
- 16.Holm S. A simple sequentially rejective Bonferroni test procedure. Scand J Stat. 1979;6:5–70. [Google Scholar]
- 17.Lillioja S, Mott DM, Spraul M, Ferraro R, Foley JE, Ravussin E, Knowler WC, Bennett PH, Bogardus C. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus: prospective studies of Pima Indians. N Engl J Med. 1993;329:1988–1992. doi: 10.1056/NEJM199312303292703. [DOI] [PubMed] [Google Scholar]
- 18.Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, Neifing JL, Ward WK, Beard JC, Palmer JP, Porte D., Jr Quantification of the relationship between insulin sensitivity and B-cell function in human subjects: evidence for a hyperbolic function. Diabetes. 1993;42:1663–1672. doi: 10.2337/diab.42.11.1663. [DOI] [PubMed] [Google Scholar]
- 19.Buchanan TA, Xiang AH, Peters RK, Kjos LK, Marroquin A, Goico J, Ochoa C, Tan S, Berkowitz K, Hodis HN, Azen SP. Preservation of pancreatic β-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk Hispanic women. Diabetes. 2002;51:2796–2803. doi: 10.2337/diabetes.51.9.2796. [DOI] [PubMed] [Google Scholar]
- 20.DeFronzo RA. Pharmacologic therapy for type 2 diabetes mellitus. Ann Intern Med. 1999;131:281–303. doi: 10.7326/0003-4819-131-4-199908170-00008. [DOI] [PubMed] [Google Scholar]
- 21.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 22.Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, Hu ZX, Lin J, Xiao JZ, Cao HB, Liu PA, Jiang XG, Jiang YY, Wang JP, Zheng H, Zhang H, Bennett PH, Howard BV. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance: the Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 23.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M, STOP-NIDDM Trial Research Group Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomized trial. Lancet. 2002;359:2072–2077. doi: 10.1016/S0140-6736(02)08905-5. [DOI] [PubMed] [Google Scholar]


