Abstract
Background
Localized aggressive periodontitis (LAgP) is associated with neutrophil dysfunction including decreased chemotaxis and reduced calcium entry. It has been suggested that CD38 is involved in chemotaxis. Little is known, however, about the relationship of CD38 and LAgP patients. In this study, we focused on the level of CD38 expression between LAgP and normal subjects and examined the involvement of CD38 in abnormal neutrophil chemotaxis of LAgP patients.
Methods
Neutrophils from 12 normal subjects and 12 LAgP patients were isolated from peripheral venous blood. Membrane associated proteins were extracted from cells with or without N-formylmethionine leucyl-phenylalanine (fMLP) stimulation. CD38 expression was measured using Western blotting. Band density was measured using an imaging densitometer.
Results
There was no statistical difference between normal subjects and LAgP patients in resting CD38 expression (basal level). However, the fMLP-stimulated neutrophils exhibited a significant decrease of CD38 expression in LAgP subjects compared to normal subjects. The decrease of CD38 was positively correlated with the defect in chemotactic migration to fMLP.
Conclusion
These data suggest that the lower expression of CD38 in neutrophils may be related to altered neutrophil function in LAgP.
Keywords: Calcium, CD38, chemotaxis, neutrophil, periodontitis
It is well established that neutrophils play a crucial role in host defense, especially in infectious disease. Neutrophil chemotaxis to the site of infection is the first step of host defense and plays an important role against bacterial infection.1 N-formylmethionine leucyl-phenylalanine (fMLP) can elicit directed neutrophil chemotaxis to the inflammatory site. The cell surface receptors for fMLP are members of the seven transmembrane spanning group of G-protein-coupled membrane receptors and induce intracellular calcium release and extra-cellular calcium influx in neutrophils.2 It is known that two types of calcium receptors, inositol 1,4,5-triphosphate (IP3) and ryanodine receptors, regulate the release of calcium from intracellular stores.3 IP3 receptors are known to play an important role in neutrophil chemotaxis. Recently, a second pathway that acts through the ryanodine receptor and CD38 has been identified for fMLP mediated chemotaxis.4,5
CD38 is a 42- to 46-kDa type II trans-membrane glycoprotein expressed by neutrophils as well as other cell types. CD38 is an important immunoregulatory molecule on lymphocytes, enabling induction of B-T cell proliferation, protection of B cells from apoptosis, and inhibition of B lymphopoeisis.6 In addition, it has been shown to enhance antigen-presenting function in macrophages in vitro.6 Moreover, because CD38 is the only well-characterized mammalian adenosine 5′-diphosphate (ADP) ribosyl cyclase enzyme, it is believed that CD38 may be necessary for calcium sensitive biologic responses in a variety of biologic systems. CD38 is believed to be an exclusive ectoenzyme located on the plasma membrane, with its ADP ribosyl cyclase activity situated in the extracellular carboxyl domain. This cyclase activity is able to convert nicotinamide adenine dinucleotide (NAD+) into the metabolite known as cyclic ADP-ribose (cADPR), which is a potent calcium mobilizing agent that can act independently of IP37 The calcium mobilizing properties of cADPR have been implicated in neutrophil chemotaxis to formylpeptide receptor-like 1 (FPRL1) ligands via a calcium-induced calcium release mechanism.5 In a previous study, we reported that fMLP and IL-8 decreased CD38 protein without changing CD38 mRNA and increased CD38 in supernatants, suggesting that CD38-mediated chemotaxis to fMLP is characterized by proteolytic cleavage of CD38.8
Localized aggressive periodontitis (LAgP) is characterized by incisor and first molar bone loss with interproximal attachment loss9 and is associated with several abnormalities of neutrophil function. Based on recent findings, it has been suggested that the pathogenesis of LAgP is associated with excessive neutrophil function leading to neutrophil-mediated tissue damage.10 In other words, the neutrophil abnormalities in LAgP are the result of a chronic hyper-activated or primed state of the LAgP neutrophil. These findings are consistent with earlier reports of reduced chemotaxis by neutrophils in LAgP11,12 because primed neutrophils exhibit reduced motility but secrete greater quantities of superoxide and granule contents. To understand the mechanisms that are related to the onset and outcomes of these abnormal neutrophil functions in LAgP, previous and recent work from our group has demonstrated several signal transduction anomalies. Neutrophils from LAgP patients show reduced calcium entry,13 defective calcium influx factor,14 and abnormal protein kinase activity C,15 among other abnormalities.16–20
Taken together with prior evidence, we hypothesized that the decrease in CD38 expression in LAgP neutrophils may be involved in reduced chemotaxis of LAgP patients. To clearly understand the mechanism of reduced chemotaxis in LAgP neutrophils, we sought to investigate if CD38 is involved in reduced chemotaxis of LAgP patients. In this report, the CD38 reduction in neutrophils from patients with LAgP is described.
MATERIALS AND METHODS
Patient Population
Twelve patients with LAgP were recruited from the patient population of Boston University Goldman Dental School, Clinical Research Center, Boston, Massachusetts. The study protocol was approved by the Boston University Institutional Review Board (IRB). Subjects were enrolled between 2003 and 2004. Informed consent was obtained prior to drawing peripheral venous blood. The general health of subjects was good. The patients were diagnosed according to clinical and radiographic criteria, age of onset around circumpubertal period (< 13 years old), and alveolar bone loss localized around the first permanent molars and incisors. The 12 normal subjects were healthy individuals who had no radiographic evidence of bone loss or any sign of periodontal disease other than mild gingivitis.
Isolation of Neutrophils
Peripheral venous blood was collected into vacutainer tubes containing 25 U/ml heparin. Neutrophils were isolated by Ficoll-Hypaque density centrifugation as previously described.16 Peripheral blood (4.5 ml) was layered on the separating medium,‡ and the tubes were centrifuged at 500 × g for 30 minutes. After the neutrophil fraction was collected and contaminating erythrocytes were lysed, the isolated neutrophils were washed twice with Dulbecco’s phosphate-buffered saline without magnesium and calcium.§ Cell viability was continuously assessed and 99% of the cells were trypan blue negative during different stages of incubation and stimulation. The preparations were > 95% neutrophils.
CD38 Analysis by Western Blotting
Neutrophils were incubated with or without 10−8 M fMLP for 60 minutes at 37°C. After stimulation, cells were collected by centrifugation at 13,000 rpm, and cells were lysed in cold buffer (1% Triton X-100, 2 mM EDTA, leupeptin 2 μg/ml, pepstatin 1μg/ml, and phenylmethylsulphonyl fluoride [PMSF] 50 μg/ml). Protein content was measured by the Bradford method, and samples (50 μg) were resolved on a 10% sodium dodecyl sulfate polyacrylamide gel by electrophoresis (SDS-PAGE) under non-reducing conditions, and electrophoretically transferred onto polyvinylidene difluoride (PVDF) membranes (350 mA; 90 minutes). The membranes were blocked with 5% non-fat dry milk for 90 minutes, and incubated at room temperature for 3 hours with mouse monoclonal anti-human CD38 antibody (2 μg/ml).|| The membranes were incubated with horseradish peroxidase (HRP) conjugated goat anti-mouse immunoglobulin G (IgG) in Tris-buffered saline for 1 hour at room temperature. The band density was measured using an imaging densitometer.¶
Chemotaxis Assay
The chemotaxis assay was performed as previously described.20 Neutrophils were suspended in Gey’s balanced salt solution, supplemented with 2% bovine serum albumin at a concentration of 2.5 × 106 cells/ml. The cell suspension was placed in the upper compartment of a modified Boyden chamber separated by a 5-mm pore size micropore filter, whereas the lower compartment was loaded with either the buffer solution or a chemoattractant solution of fMLP at a concentration of 2 × 10−8 M. The cell migration response was evaluated by the enumeration of cells on the distal surface of the filter after a 2-hour incubation in a 37°C humidified air chamber. Three representative high-power microscopic fields (×400) were counted for each of the triplicate filters. When performed in the absence of fMLP in the lower compartment, the assay provided a measure of neutrophil-random migration. The chemotactic migration of neutrophils from LAgP patients was expressed as a calculated percentage of the chemotactic migration of neutrophils from individual age-, race-, and gender-matched healthy subjects. Statistical differences between LAgP patients and controls were determined by analysis of variance (ANOVA).
Statistical Analysis
Comparisons between groups were analyzed by ANOVA. When statistically significant differences were observed, the difference between the two groups was analyzed by pairwise comparisons using the Bonferroni method. Pearson correlation analysis was performed where appropriate. The difference was considered significant if the P value was < 0.05.
RESULTS
Twelve normal subjects and 12 LAgP patients participated in the study. Table 1 represents demographics and chemotaxis assay. Control subjects (five males and seven females) were 19 to 34 years of age (mean age, 23.3 ± 4.5 [± SD] years). LAgP patients (five males and seven females) were 14 to 35 years of age (mean age, 22.5 ± 6.3 [± SD] years). Neutrophil chemotaxis was analyzed by chemotactic migration to fMLP. The chemotactic migration values of LAgP patients are expressed as an average of the calculated percentages of the chemotactic migration of neutrophils from individual age-, race-, and gender-matched controls. The chemotaxis activity of LAgP neutrophils was statistically significantly reduced from that of controls. With a mean of 12, LAgP chemotaxis decreased 71.8%.
Table 1.
Demographics and Chemotaxis in LAgP Patients
| Sample | Age | Race | Gender | Chemotaxis (% control) |
|---|---|---|---|---|
| 1 | 22 | Black | Female | 166.6 |
| 2 | 17 | Hispanic | Female | 57.5 |
| 3 | 16 | Black | Male | 95.4 |
| 4 | 25 | Hispanic | Female | 48.0 |
| 5 | 27 | Black | Female | 41.7 |
| 6 | 14 | Black | Female | 37.3 |
| 7 | 25 | Black | Male | 63.2 |
| 8 | 26 | Black | Female | 95.0 |
| 9 | 29 | Asian | Male | 77.5 |
| 10 | 35 | White | Female | 12.7 |
| 11 | 19 | Black | Male | 118.3 |
| 12 | 16 | Hispanic | Male | 48.2 |
| Mean | 71.8 |
Protein was extracted from neutrophils stimulated with 10−8 M fMLP from normal subjects and LAgP patients, and CD38 was measured using Western blotting. CD38 expression was measured by the band density (OD mm). The CD38 expression from normal subjects in a resting condition ranged from 14.3 to 40.0, and CD38 expression from LAgP subjects in a resting condition ranged from 13.1 to 35.7. The CD38 expression in stimulated condition from normal subjects ranged from 9.8 to 28.5, and the CD38 in stimulated condition from LAgP patients ranged from 7.1 to 23 (Table 2).
Table 2.
Densitometry Readings (OD mm) From Western Blotting Analyses and the Ratio of Decrease on CD38 Expression in Control and LAgP Neutrophils
| Control Subjects
|
LAgP Patients
|
|||||
|---|---|---|---|---|---|---|
| Sample | Resting (A) | fMLP (B) | % Defect (B/A %) | Resting (C) | fMLP (D) | % Defect (D/C %) |
| 1 | 26.2 | 18.5 | 70.3 | 29.9 | 25.4 | 84.6 |
| 2 | 16.3 | 12.3 | 75.3 | 27.2 | 13.3 | 48.8 |
| 3 | 37.8 | 29.0 | 76.6 | 20.8 | 12.4 | 59.5 |
| 4 | 18.4 | 14.8 | 80.1 | 13.5 | 7.1 | 52.8 |
| 5 | 40.1 | 21.5 | 53.6 | 29.5 | 8.9 | 30.1 |
| 6 | 29.4 | 21.0 | 71.3 | 34.9 | 14.7 | 41.9 |
| 7 | 14.3 | 9.9 | 69.1 | 14.4 | 7.5 | 52.2 |
| 8 | 23.1 | 18.1 | 78.4 | 35.7 | 23.1 | 64.7 |
| 9 | 32.7 | 23.3 | 71.4 | 30.2 | 13.8 | 45.8 |
| 10 | 35.6 | 26.3 | 73.9 | 29.8 | 8.3 | 27.7 |
| 11 | 17.3 | 14.7 | 84.6 | 13.1 | 10.6 | 80.0 |
| 12 | 26.8 | 28.6 | 106.4 | 24.7 | 19.9 | 81.0 |
Figure 1 shows the expression levels of CD38 before and after stimulation by fMLP from control subjects and LAgP subjects. In resting cells, the level of CD38 expression (basal level) was not statistically different between normal subjects and LAgP patients. However, the decrease in CD38 levels was significantly more reduced in LAgP than in control neutrophils (Fig. 1).
Figure 1.
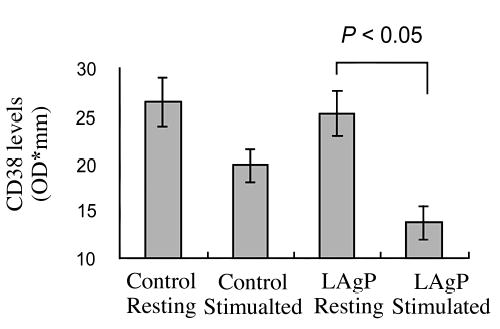
Neutrophils were incubated with or without 10−8 M fMLP for 60 minutes. Samples (50 μg protein per lane) were loaded and analyzed as described in Materials and Methods. Values are means ± SE of 12 subjects.
The correlation between the CD38 defect and chemotaxis defect of neutrophils from LAgP patients was then analyzed (Fig. 2). The CD38 decrease in neutrophils from patients with LAgP was positively correlated with the defect of chemotactic migration to fMLP (r2 = 0.8924).
Figure 2.
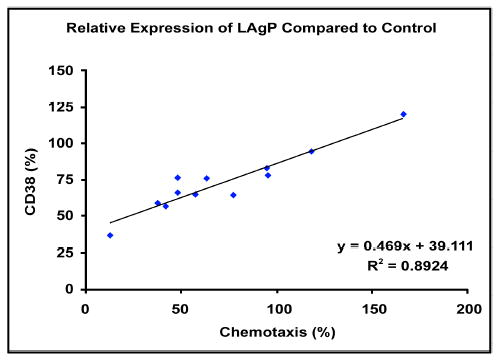
Correlation between chemotactic migration to fMLP and CD38 expression of neutrophils from patients with LAgP. The chemotactic migration and CD38 expression values of patients are represented by percentages of the responses obtained from the matching controls. The correlation coefficient is shown in the lower right-hand corner.
DISCUSSION
In this paper, we report a reduction in CD38 expression in chemotactically abnormal neutrophils from LAgP patients. The decreased levels of CD38 expression correlated well with chemotactic migration to fMLP in each patient in the LAgP population. Thus, the decrease of CD38 expression seems to relate to neutrophil chemotactic migration.
Reduced chemotaxis is one of the neutrophil abnormalities in LAgP patients.12,13 Various mechanisms have been suggested to explain these observations, including cytoskeleton dysfunction and abnormalities in signal transduction cascade. LAgP signaling abnormalities are known to be associated with increased intracellular diacylglycerol (DAG) levels16 and decreased PKC activity.15 Furthermore, Agarwal et al.21 demonstrated that intracellular Ca2+ increases in response to chemotactic factor do not occur in neutrophils from patients with LAgP. Daniel et al.13 further revealed that low levels of intracellular Ca2+ seemed to be caused by a dysfunction of the influx of extracellular Ca2+ in neutrophils from the patients. Therefore, calcium receptors seem to relate to abnormalities. Recently, the ryanodine receptor and CD38 has been identified for fMLP-mediated chemotaxis working through FPRL1.4,5 The involvement of CD38 cleavage in chemotaxis in human neutrophils has been reported.8 There is the possibility that the level of CD38 expression is related to the abnormality in LAgP patients. In this study, there is no statistical difference in basal level of CD38 expression between normal subjects and LAgP patients, but there is statistical significance in the reduction of CD38 after stimulation by fMLP. The reduction induced by fMLP in LAgP patients is > 1 in normal subjects. It has been reported that the decrease in CD38 expression correlated with a reduction in NADase activity,22 and the expression of CD38 increased intracellular calcium activity.23 Therefore, decreased levels of CD38 may coincide with cADPR production thereby regulating chemotaxis and suggesting that downregulation of CD38 levels induced by fMLP is an important step in the chemotaxis signaling cascade of neutrophils.
A previous report shows that total calcium-dependent protein kinase-C (PKC) activity of neutrophils from patients with LAgP and depressed chemotactic migration to fMLP was lower than that of neutrophils from healthy subjects.15 In addition, the calcium-dependent PKC activity in neutrophils from LAgP patients exhibited a positive correlation with chemotactic migration to fMLP.15 It is known that two types of PKC, calcium dependent PKC and calcium independent PKC, are involved in cellular signaling. Because CD38 is essential for calcium mobilization via the ryanodine receptor, calcium-dependent PKC may be involved in CD38 mediated chemotaxis. It may be possible that the more cleavage of CD38 in LAgP patients is related to lower PKC activity.
We have suggested that p38 mitogen-activated protein (MAP) kinase, but not p44/42 MAP kinase, is involved in CD38 mediated chemotaxis.8 However, in this study, we have not examined the levels of MAP kinase activity. Between LAgP and healthy subjects, there might be a difference of MAP kinase activity induced by fMLP, which causes the significance of CD38 levels. It would be useful to compare the levels of MAP kinase activity between normal and LAgP subjects. Further experiments to detect MAP kinase in normal and LAgP subjects might be useful in resolving this issue.
Acknowledgments
The authors thank Dr. Fran Lund, Trudeau Institute, and Dr. Eraldo L. Batista Jr., Department of Periodontology and Oral Biology, Boston University, for helpful suggestions. This work was supported in part by USPHS grant DE13499 and GCRC grant MO1 RR00533.
Footnotes
Histopaque 1199 and Histopaque 1077, Sigma, St. Louis, MO.
Sigma.
Santa Cruz Biotechnology, Santa Cruz, CA.
Bio Rad, Hercules, CA.
References
- 1.Malech HL, Gallin JI. Current concepts: Immunology. Neutrophils in human diseases. N Engl J Med. 1987;317:687–694. doi: 10.1056/NEJM198709103171107. [DOI] [PubMed] [Google Scholar]
- 2.Normark S, Normark BH, Hornef M. How neutrophils recognize bacteria and move toward infection. Nat Med. 2001;7:1182–1184. doi: 10.1038/nm1101-1182. [DOI] [PubMed] [Google Scholar]
- 3.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: Dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 4.Partida-Sanchez S, Cockayne DA, Monard S, et al. Cyclic ADP-ribose production by CD38 regulates intracellular calcium release, extracellular calcium influx and chemotaxis in neutrophils and is required for bacterial clearance in vivo. Nat Med. 2001;7:1209–1216. doi: 10.1038/nm1101-1209. [DOI] [PubMed] [Google Scholar]
- 5.Partida-Sanchez S, Iribarren P, Moreno-Garcia ME, et al. Chemotaxis and calcium responses of phagocytes to formyl peptide receptor ligands is differentially regulated by cyclic ADP ribose. J Immunol. 2004;172:1896–1906. doi: 10.4049/jimmunol.172.3.1896. [DOI] [PubMed] [Google Scholar]
- 6.Lund F, Solvason N, Grimaldi JC, Parkhouse RM, Howard M. Murine CD38: An immunoregulatory ectoenzyme. Immunol Today. 1995;16:469–473. doi: 10.1016/0167-5699(95)80029-8. [DOI] [PubMed] [Google Scholar]
- 7.Dargie PJ, Agre MC, Lee HC. Comparison of Ca2+ mobilizing activities of cyclic ADP-ribose and inositol trisphosphate. Cell Regul. 1990;1:279–290. doi: 10.1091/mbc.1.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujita T, Zawawi KH, Kurihara H, Van Dyke TE. CD38 cleavage in fMLP- and IL-8-induced chemotaxis is dependent on p38 MAP kinase but independent of p44/42 MAP kinase. Cell Signal. 2005;17:167–175. doi: 10.1016/j.cellsig.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 10.Kantarci A, Oyaizu K, Van Dyke TE. Neutrophil-mediated tissue injury in periodontal disease pathogenesis: Findings from localized aggressive periodontitis. J Periodontol. 2003;74:66–75. doi: 10.1902/jop.2003.74.1.66. [DOI] [PubMed] [Google Scholar]
- 11.Van Dyke TE, Horoszewicz HU, Cianciola LJ, Genco RJ. Neutrophil chemotaxis dysfunction in human periodontitis. Infect Immun. 1980;27:124–132. doi: 10.1128/iai.27.1.124-132.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Dyke TE, Schweinebraten M, Cianciola LJ, Offenbacher S, Genco RJ. Neutrophil chemotaxis in families with localized juvenile periodontitis. J Periodontal Res. 1985;20:503–514. doi: 10.1111/j.1600-0765.1985.tb00834.x. [DOI] [PubMed] [Google Scholar]
- 13.Daniel MA, McDonald G, Offenbacher S, Van Dyke TE. Defective chemotaxis and calcium response in localized juvenile periodontitis neutrophils. J Periodontol. 1993;64:617–621. doi: 10.1902/jop.1993.64.7.617. [DOI] [PubMed] [Google Scholar]
- 14.Shibata K, Warbington ML, Gordon BJ, Kurihara H, Van Dyke TE. Defective calcium influx factor activity in neutrophils from patients with localized juvenile periodontitis. J Periodontol. 2000;71:797–802. doi: 10.1902/jop.2000.71.5.797. [DOI] [PubMed] [Google Scholar]
- 15.Kurihara H, Murayama Y, Warbington ML, Champagne CM, Van Dyke TE. Calcium-dependent protein kinase C activity of neutrophils in localized juvenile periodontitis. Infect Immun. 1993;61:3137–3142. doi: 10.1128/iai.61.8.3137-3142.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tyagi SR, Uhlinger DJ, Lambeth JD, Champagne C, Van Dyke TE. Altered diacylglycerol level and metabolism in neutrophils from patients with localized juvenile periodontitis. Infect Immun. 1992;60:2481–2487. doi: 10.1128/iai.60.6.2481-2487.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Dyke TE, Warbington M, Gardner M, Offenbacher S. Neutrophil surface protein markers as indicators of defective chemotaxis in LJP. J Periodontol. 1990;61:180–184. doi: 10.1902/jop.1990.61.3.180. [DOI] [PubMed] [Google Scholar]
- 18.Van Dyke TE, Zinney W, Winkel K, Taufiq A, Offenbacher S, Arnold RR. Neutrophil function in localized juvenile periodontitis. Phagocytosis, superoxide production and specific granule release. J Periodontol. 1986;57:703–708. doi: 10.1902/jop.1986.57.11.703. [DOI] [PubMed] [Google Scholar]
- 19.Offenbacher S, Scott SS, Odle BM, Wilson-Burrows C, Van Dyke TE. Depressed leukotriene B4 chemo-tactic response of neutrophils from localized juvenile periodontitis patients. J Periodontol. 1987;58:602–606. doi: 10.1902/jop.1987.58.9.602. [DOI] [PubMed] [Google Scholar]
- 20.Shibata K, Warbington ML, Gordon BJ, Kurihara H, Van Dyke TE. Nitric oxide synthase activity in neutrophils from patients with localized aggressive periodontitis. J Periodontol. 2001;72:1052–1058. doi: 10.1902/jop.2001.72.8.1052. [DOI] [PubMed] [Google Scholar]
- 21.Agarwal S, Reynolds MA, Duckett LD, Suzuki JB. Altered free cytosolic calcium changes and neutrophil chemotaxis in patients with juvenile periodontitis. J Periodontal Res. 1989;24:149–154. doi: 10.1111/j.1600-0765.1989.tb00870.x. [DOI] [PubMed] [Google Scholar]
- 22.Pfister M, Ogilvie A, da Silva CP, Grahnert A, Guse AH. NAD degradation and regulation of CD38 expression by human monocytes/macrophages. Eur J Biochem. 2001;268:5601–5608. doi: 10.1046/j.1432-1033.2001.02495.x. [DOI] [PubMed] [Google Scholar]
- 23.Zocchi E, Daga A, Usai C, et al. Expression of CD38 increases intracellular calcium concentration and reduces doubling time in HeLa and 3T3 cells. J Biol Chem. 1998;273:8017–8024. doi: 10.1074/jbc.273.14.8017. [DOI] [PubMed] [Google Scholar]


