Abstract
In two experiments, the temporal dynamics of neural activity underlying perceptual priming of visual motion was examined using event-related potentials (ERPs) during directional judgments of the apparent motion of two-dimensional sine-wave gratings. Compared to perceptually ambiguous motion, unambiguous left- or rightward motion was associated with enhanced ERP activity about 300 ms after the onset of apparent motion. In the second experiment, ERPs were recorded to two successive motion jumps in which an unambiguous motion jump served as a prime for a subsequent target motion that was ambiguous. The prime-target time interval was varied between 200, 400, and 1000 ms. In a control (motion reversal) condition, the two motion jumps were both unambiguous but in opposite directions. Compared to the motion reversal condition, motion priming was associated with an enhancement of ERP amplitudes at 100 ms and 350 ms following target stimulus onset. ERP enhancement was greatest at a short prime-target interval of 200 ms, which was also associated behaviorally with the strongest priming. The ERP enhancement and behavioral priming were both eliminated at the long 1000 ms prime-target interval. Functional magnetic resonance imaging (fMRI) data from a subset of subjects supported the view that motion priming involves modulation of neural responses both in early visual cortex and in later stages of visual processing.
Keywords: Perceptual priming, Visual motion, Brain imaging, ERP, fMRI
INTRODUCTION
Perceptual priming is an important form of automatic learning (or implicit memory) that provides for efficient perception and action in a changing visual environment. The perception of a visual event can be biased, or primed, by prior exposure to the same or related visual information. For instance, people generally respond faster to an object they have seen previously than to a new object, even though they may not consciously remember having seen the object before [25]. Perceptual priming occurs for many features of objects, including object motion. Motion perception can be biased in favor of a particular direction by previous exposure to a preceding stimulus in that direction [2,6,15,20,21,23].
Although perceptual priming reflects automatic or implicit learning, it differs from conscious recollection. The neural basis of perceptual priming of stationary objects has been studied extensively using electrophysiological and neuroimaging techniques (e.g., [4,5,8,14,17]). For example, repetition priming of visual objects is typically associated with reduced neural activation (see [28] for a review). Other forms of priming, such as word-stem completion, have also been extensively studied using electrophysiological techniques. The typical finding from these studies is a greater electrical positivity at about 300–500 ms from occipitoparietal scalp sites for primed visual items (see [19] for a review). However, little is known of the neural mechanisms underlying perceptual priming of dynamic visual motion stimuli.
Many forms of perceptual priming are quite long lasting, from several seconds to minutes to days (e.g., [9]). Neuroimaging techniques that assess neural activity over several seconds or minutes are therefore appropriate for investigating the neural correlates of these types of priming. Motion priming, on the other hand, may last only hundreds of milliseconds. Examining the neural correlates of this form of priming thus requires a technique with high temporal resolution, such as event-related brain potentials (ERPs).
The millisecond resolution of ERPs allows for investigation of the temporal dynamics of neural activity underlying fast-acting motion priming. In the present study, we used ERPs to examine two-dimensional visual motion priming. We used a priming paradigm developed by Pinkus and Pantle [21] in which observers were shown sine-wave gratings that moved ambiguously to either the left or the right. When such a target stimulus is preceded by a prime that moves unambiguously in one direction, motion direction is disambiguated, and most observers report seeing the target move in the same direction as the prime. Such motion priming typically decays over a prime-target interval of about 800 ms [21].
In the present studies we used ERPs to examine the temporal characteristics of neural activity associated with motion priming of 2-D sine-wave gratings. The objective was to determine whether motion priming occurs at a relatively early stage of processing in visual cortex by modulating early-latency ERP components, at a later visual processing stage, or both. We also took advantage of the high spatial resolution of functional magnetic resonance imaging (fMRI) to localize the early- and late-stage visual cortical areas involved in this motion priming task.
EXPERIMENT 1. SINGLE MOTION JUMPS: AMBIGUOUS VS. UNAMBIGUOUS MOVING GRATINGS
Before examining motion priming, i.e., the neural signals associated with the influence of unambiguous motion jumps on the subsequent perception of an ambiguous motion jump, we first examined the ERPs associated with perceiving single motion jumps. The aim of Experiment 1 was to compare the ERP components associated with perception of unambiguous (90° phase shift) and ambiguous (180° counter-phase shift) motion. This would then allow subsequent examination of ERPs associated with the effects of unambiguous motion on ambiguous motion.
Materials and Methods
Subjects.
Seventeen young adults (mean age 22) participated in two experiments in which ERPs were recorded. They were college students from the Catholic University of America. All participants had corrected vision of at least 20/40 in Snellen and Rosenbaum eye examination tests.
Visual stimuli and display.
The apparent motion stimuli used in the current study were image sequences of vertical sine-wave gratings. They were constructed as in the 2-D motion priming experiments reported previously [21]. The motion jumps were viewed through a circular hole of a cardboard of 16 cm in diameter, at viewing distance of 104 cm (8.8° of visual angle). The average luminance of the display was 14 cd/m2. The circular viewing aperture was stationary while the gratings moved horizontally (Fig. 1A).
FIG. 1.

Visual stimuli for single apparent motion steps, corresponding perception of motion direction, from Experiment 1. (A) In the actual experiment, the stimuli were presented through a circular window on a computer screen. (B) Three types of apparent motion stimuli. The figure shows the whole frame to illustrate the phase shift between frames. The abrupt 90° phase shift is associated with perception of an unambigous single motion step to the right or to the left. A 0–180° counterphase shift between frames results in perception of movement to either the left or right (ambiguous or bistable), even though the physical stimuli remain the same.
In Experiment 1, there were three types of one-step single motion sequences. An abrupt 90° or −90° phase shift of a sine-wave grating is associated with perception of an unambiguous, single motion step to the right or to the left. A 0°–180° counter-phase shift between frames, on the other hand, results in perception of movement to either the left or right (ambiguous or bistable), even though the physical stimuli remain the same. When shown rapidly one after another, the sequence produced a vivid impression of leftward or rightward motion. The schematic diagram of the sinusoidal gratings of apparent motion sequences for leftward, rightward, and ambiguous motion jumps is shown in Fig. 1B. Thus, the three types of single movement steps were: (1) unambiguous left; (2) unambiguous right; and (3) ambiguous (left or right). The order of the presentation of the three types of single motion jumps was counterbalanced.
Task and data analysis.
For each trial, an observer viewed an apparent motion sequence. The participants were instructed to look globally towards the center of the computer screen and not to track a particular grating. After viewing each trial, the observer was required to press the left or right button on the response box to indicate their perceived direction of each motion jump (right or left). There were 70 trials for each of the three conditions in Experiment 1. Participants could not predict on any given trial whether an ambiguous or an unambiguous jump would be presented.
The electroencephalogram (EEG) was recorded from 14 scalp electrodes at Fz, Cz, Pz, Oz, C3, C4, P3, P4, T5, T6, O1, O2, OL, and OR. The EEG was amplified with a bandpass of 0.1–100 Hz and continuously sampled (250 Hz/channel) and digitized for off-line analysis. ERPs were selectively averaged to the last apparent motion frame (target). Each ERP component was measured relative to a 200 ms baseline preceding the onset of the stimuli. Peak amplitudes and latencies were computed for the P1 (50–150 ms), N1 (100–200 ms), and P3 (250–550 ms) components.
Results and Discussion
Behavioral results for ambiguous and unambiguous single motion jumps.
Single leftward motion was consistently perceived 99% of the time within and across subjects in the 90° (leftward) phase shift condition. The corresponding proportion in the −90° (rightward) condition was also 99%. Thus in these conditions, motion direction was perceptually unambiguous. In the 180° counter-phase shift condition, however, leftward motion was reported 56% of the time within and across subjects. This counter-phase apparent motion was therefore perceptually ambiguous. These psychophysical results are consistent with Pinkus and Pantle’s definition of ambiguous and unambiguous motion stimuli [21].
ERPs to ambiguous and unambiguous single motion jumps.
Figure 2 shows the ERPs associated with the different single motion jump conditions. There were no significant differences in ERP component amplitudes or latencies between the unambiguous leftward (Fig. 2 in light grey) and rightward (Fig. 2 in dark grey) motion directions. ANOVA (3 conditions × 14 sites) revealed no significant difference among leftward, rightward, and ambiguous conditions for the P1 component [F(2,32) = 1.509, p > 0.2]. There was also no difference among leftward, rightward, and ambiguous conditions for the N1 component [F(2,32) = 1.294, p > 0.2]. However, the amplitude of the P3 component elicited by ambiguous motion was significantly smaller than that evoked by unambiguous motion, [F(2,32) = 8.248, p < 0.005] (Fig. 2 in black).
FIG. 2.
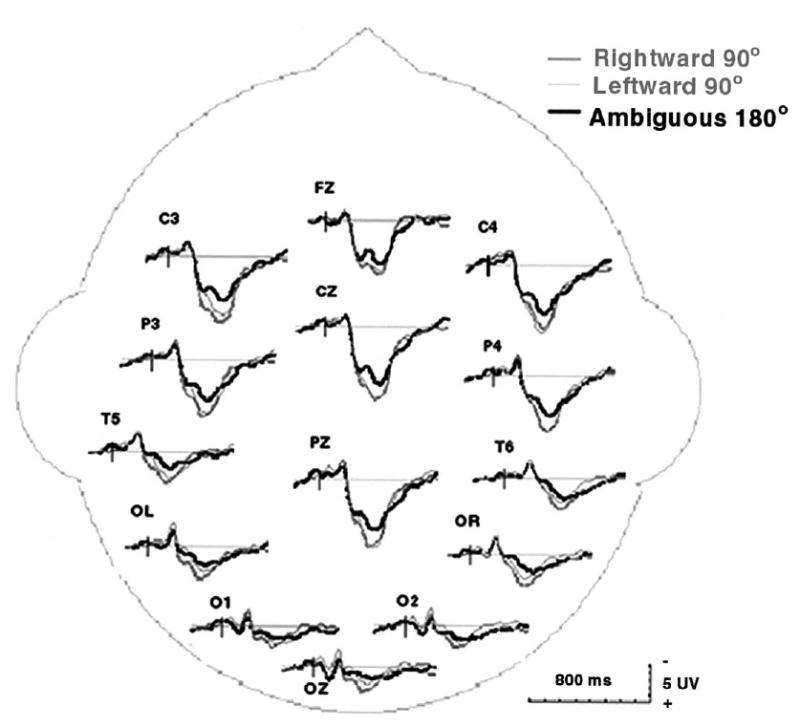
Averaged ERP waveforms for each of three conditions of perceiving motion direction at 14 posterior electrode sites, averaged across 17 subjects. Unambiguous leftward and rightward motion (in grey) elicited more late positive activity than ambiguous motion direction (in black), especially in the anterior sites.
Previous ERP studies of motion direction judgments have found that an electrophysiological correlate of visual motion processing can be found in the N2, a transient negativity triggered by the onset of a coherent motion [3,7,16,18]. The visual ERPs in the present experiment also generated negativity 160–200 ms following the onset of coherent movement (Fig. 2). However, this early component associated with motion perception was identical for ambiguous and unambiguous motion.
The main difference between ambiguous vs. unambiguous motion was in the late P3 component. Previous perceptual studies [1,22,24,27] suggest that visual motion perception is mediated by neural mechanisms that correspond to mutual suppressive populations of neurons that are able to detect motion in opposite directions. Using fMRI, Heeger et al. [12] reported that the suppressive motion mechanism was found at a late visual motion processing stage—known as the human MT complex, but not in the early visual processing stage of primary visual cortex (V1). Our results suggest that the decision concerning motion direction is made at a later stage of visual information processing at about 300 ms. This finding is consistent with Heeger et al.’s results [12].
EXPERIMENT 2: VISUAL MOTION PRIMING AND MOTION REVERSAL
In the second experiment, observers were asked to judge two successive motion jumps. In the motion priming condition, an unambiguous jump (left or right) was followed by an ambiguous jump. If priming occurred, observers would report both jumps in the same direction (both left or both right). A motion reversal condition was used as a control condition. In this condition, two unambiguous jumps were presented in succession, but in opposite directions (e.g., left-right or right-left). In both the motion priming and motion reversal conditions, therefore, the first motion jump was always unambiguous. However, the second target motion was ambiguous in the priming condition and unambiguously in the opposite direction to the first in the motion reversal condition. We included this control condition to test whether observers are sensitive to motion reversals. If so, any motion priming results could not be attributed simply to a bias or tendency for perceiving two motion jumps in the same direction.
The time interval between jumps in the priming condition was varied and ERPs were recorded to examine the temporal dynamics of the neural mechanisms underlying visual motion priming.
Materials and Methods
Experimental design.
The stimuli for double motion steps were presented on a computer screen as in Experiment 1. The corresponding perception was two moving steps. As shown in Fig. 3A, the first motion step was always an unambiguous motion step as used in Experiment 1. The second motion step was either ambiguous motion in the case of the motion priming condition, or unambiguous motion in the motion reversal condition. The phase shifts between successive sine-wave gratings in the motion reversal trials were in the sequence 0° → 90° → 0°, which was perceived consistently as two opposite motion directions (right then left; or left then right). This is because, as indicated before, 90° phase shifts, whether negative (0° → −90° → 0°) or positive (0° → 90° → 0°) are unambiguous. The phase shifts between successive frames in the priming condition were in the sequence of 0° → 90° → 270° or 0° → −90 → −270°. This resulted in an unambiguous movement to the right or left (90° shift), followed by ambiguous movement (180° shift). There were three time delays or SOA (200, 400, and 1000 ms) between the first and the second motion steps. There were 80 trials in each of the six conditions in Experiment 2. The reversal and priming conditions were presented in a random order. The six conditions in Experiment 2 were counterbalanced to avoid response bias. Observers were asked to press the left button if two successive motion jumps were perceived to be in the same direction, and the right button if motion jumps were in opposite directions. The instructions to press the left and right buttons were switched for half the subjects.
FIG. 3.
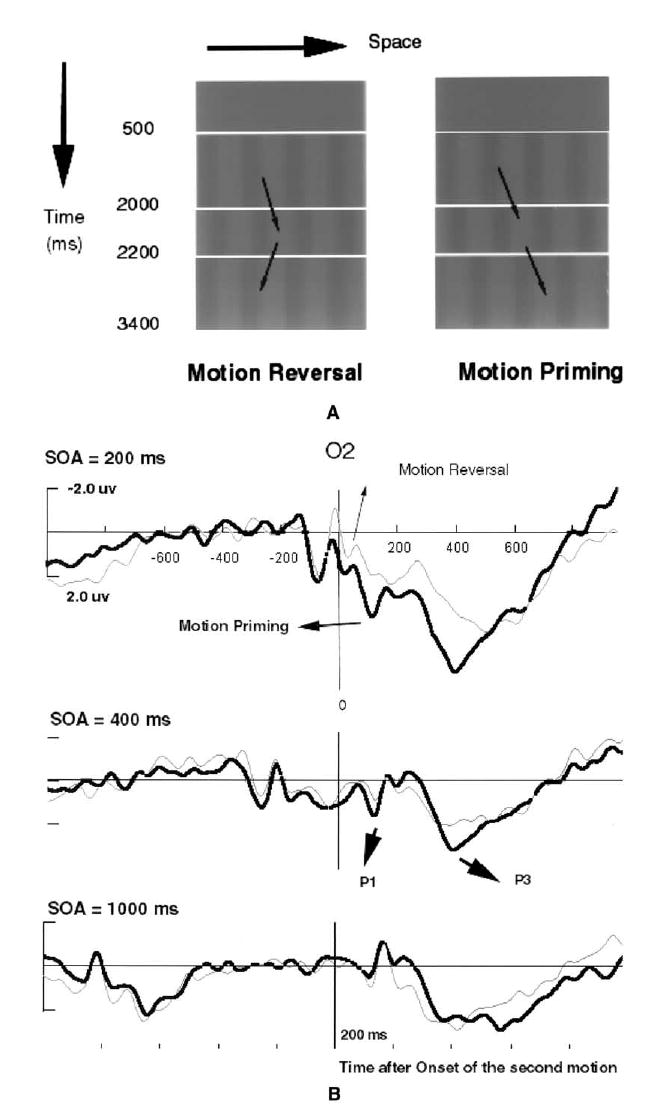
(A) Visual stimuli for double apparent motion steps, corresponding perception of direction of two successive motion jumps. In each of the three time delays (200 ms, 400 ms, and 800 ms) between first and second motion steps, there were two types of motion stimuli: motion priming and motion reversal conditions. The phase shifts between successive frames in reversal motion were 0°–90°–0°, which were perceived consistently as two opposite motion directions (right then left or right then left); the phase shifts between successive frames in the priming condition were 0°–90°–270°. That is, an ambiguous 180° step followed a perceptually unambiguous 90° left- or rightward step. The first unambiguous motion step primes the second counter-phase motion step to appear to move in the rightward direction. (B) ERP measures at O2 for the two types of motion perception at time delay (SOA) of 200 ms, 400 ms, and 1000 ms. The vertical line (time 0) indicates the onset of the fourth frame as in Fig. 3A, i.e., the time of onset of the second motion stimulus. The P1 and P3 components of the ERP were significantly different between the motion priming (in black) and reversal conditions (in grey) with a delay of 200 ms, less different with time delay of 400 ms, and not statistically different at prime-target delay of 1000 ms.
Results and Discussion
The effect of time delay and early and late components.
The three-way ANOVA (3 Time delays × 2 conditions × 14 sites) revealed a very significant main effect of time delay (p < 0.0001). The degree of priming was reduced as the prime-target intervals increased from 200 to 1000 ms. For the 200 ms interval, relative to the reversal condition, motion priming was associated with significantly more positive-going ERP activity at both early (P1: 100 ms, F(1,16) = 5.898, p < .05) and late (P3: 350 ms, F(1,16) = 11.373, p < .005) stages of processing following the onset of the target motion. When the delay interval was increased to 400 ms, the late P3 positivity in the priming condition was significantly greater than that in the reversal condition, [F(1,16) = 14.408, p < .001], the earlier P1 components of the two conditions approaching significant difference at the p = 0.5 level [F(1,16) = 3.698]. At a prime-target interval of 1000 ms, there were no significant differences between the priming and reversal conditions for any ERP component. As illustrated in Fig. 3B, ERP measures at O2 for the two types of motion perception at time interval of 200, 400, and 1000 ms. The P1 and P3 components of the ERP were significantly different between the motion priming and reversal conditions with a delay of 200 ms, less different with SOA of 400 ms, and not statistically different at prime-target delay of 1000 ms.
Behavioral results and corresponding ERPs in motion priming and motion reversal conditions.
Motion priming was measured by computing the percentage of same-direction motion judgments. These showed that priming decayed over the prime-target interval: target motion was perceived to move in the same direction as the prime on 96%, 80%, and 54% of trials at the 200, 400, and 1000 ms prime-target intervals, respectively (Fig. 4A). A 100% response proportion of same-direction responses represents perfect priming, whereas 50% corresponds to a chance level of same-direction responses, or lack of priming. These results therefore justify labeling of the three prime-target intervals as leading to “strong priming” (200 ms interval), “weak priming” (400 ms), and “no priming” (1000 ms), respectively. In general these findings agree with the original psychophysical findings of Pinkus and Pantle [21].
FIG. 4.
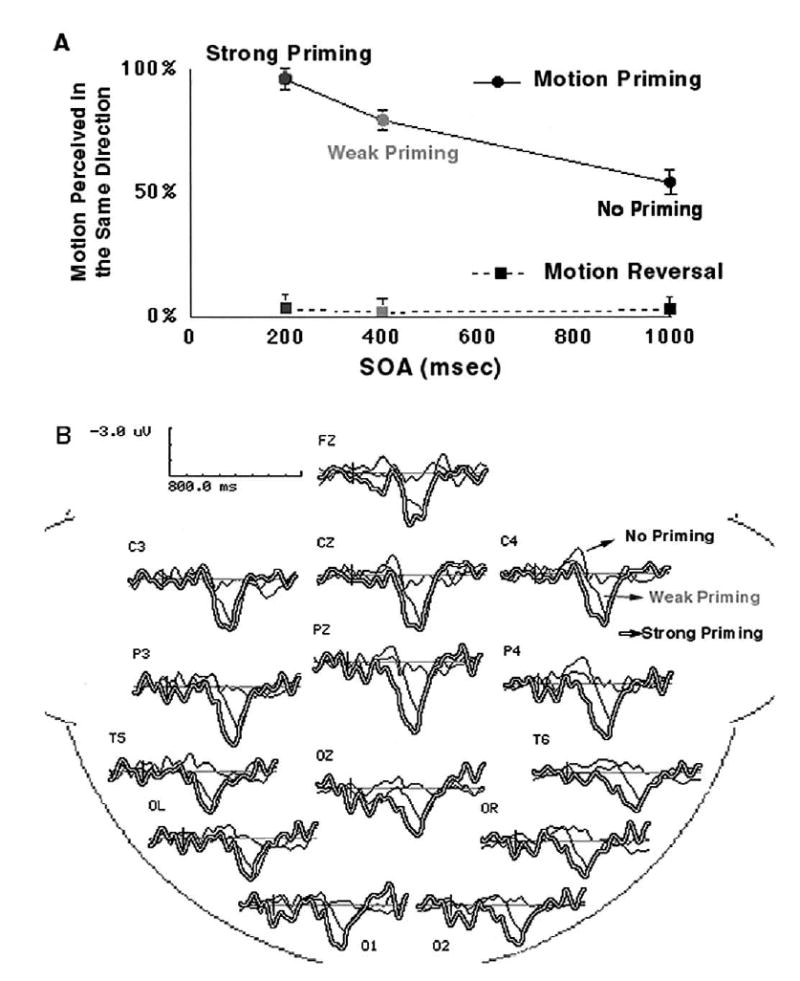
Behavioral measures of performance and difference waveforms for the motion priming and reversal conditions. (A) Percentage of perceived motion jumps in the same direction. Motion priming was strong at the 200 ms SOA, less so at 400 ms, and at chance level at the 1000 ms interval (no priming). Motion reversals were always at the floor, which confirms that the reversal motion was indeed perceived in opposite directions. (B) Difference waveforms between priming and reversals conditions at all 14 sites. Such difference between priming and reversal conditions decreased as a function of time interval, in terms of amplitude of the ERP components. The difference waves of strong priming (200 ms SOA) is in double lines, weak priming is in grey (400 ms delay), and no priming is in black (1000 ms delay).
Because the priming and reversal conditions had identical unambiguous first jumps, we were able to compare ERPs to the motion priming condition to those in the reversal motion condition so that neural activity specific to motion priming would be revealed. The ERP difference waveforms between the motion priming and motion reversal conditions at the three prime-target intervals. The difference waveform represents neural activity evoked by the target stimulus specifically associated with motion priming. Both the early 80–130 ms (p < 0.05) and late 250–500 ms (p < 0.001) phases of ERP activity were positively enhanced in the strong priming condition (200 ms prime-target interval) compared to the weak (400 ms) and no priming (1000 ms) conditions (Fig. 4B).
We also tested for possible motion priming effects for the N1, P2, and N2 components, which are associated with motion perception, However, no significant effects (p > 0.5) of priming condition were found for N1 [F(1,16) = 0.002], P2 [F(1,16) = 0.404], and N2 [F(1,16) = 0.002]. The interaction between time delay and priming condition was also not significant for any component (p > 0.3).
The topographic maps of the difference ERP waveforms (priming – reversal), partitioned by different time epochs of ERP activity are shown in Fig. 5A. At 98 ms following the target motion (in the P1 component latency range), there was significant priming-related enhancement in activity at posterior scalp electrode sites. At 386 ms following target motion (in the P3 component latency range), the difference appeared more extensively at posterior sites, but included some anterior areas as well. Because of the limited spatial resolution of scalp ERPs, no strong claims can be made for the intracortical localization of these neural effects. However, the P1 component of the visual ERP has been localized to extrastriate cortex [13]. Thus the observed regional scalp distribution of priming-related ERP activity suggests that motion priming has its first effect in extrastriate cortical areas associated with relatively early stages of visual processing.
FIG. 5.

Two dimensional plots of the ERP difference waveforms and fMRI measure of perception of the moving sine-wave gratings. (A) Plots of difference in ERP activity between the motion priming and reversal conditions at three levels of priming. (B) Cortical regions involved in the perception of apparent motion jumps for one subject. High-resolution fMRI revealed the specific cortical regions that activated by perceptual judgment of the motion direction, compared with perception of the stationary sine-wave gratings. These areas, in ventral occipital, human motion processing area MT/V5, superior temporal, and intraparietal cortices, could have contributed to the source of the ERP responses associated with perceiving motion jumps.
fMRI Localization of Motion Priming-Related Cortical Areas
We used fMRI to localize the cortical areas involved in visual perception of moving sine-wave gratings. Inside of the MR scanner, subjects repeated the same motion perception tasks as they performed in both ERP experiments, i.e., judging single motion jumps or successive jumps involving motion priming. The priming condition in the fMRI experiment only included double jumps with a 200 ms prime-target interval. In between these conditions, subjects viewed stationary sine-wave gratings as a baseline condition.
MR data acquisition and analysis.
Two of the 17 subjects who participated in the ERP testing were scanned. There were eight functional imaging runs (each run lasted about 5 min) for each subject. A GE 1.5 Tesla magnet was used to obtain T2*-weighted gradient echo-planar images with blood oxygen level dependent (BOLD) signals. Twenty-two 5-mm whole brain volumes of axial slices were acquired for each subject (repetition time 3 s, echo time 40 ms, flip angle 90°) and analyzed using multiple regression [10,11]. Brain regions showing significant signal enhancement to motion perception were defined as brain regions that activated significantly during perceiving both single motion jump and motion priming (Z > 3.09; corrected).
fMRI results.
We compared MR signals associated with perceptual judgments of motion direction with the perception of stationary sine-wave gratings. Significant (p < .001) voxels of MR responses associated with the sine-wave motion directions were found constrained to small cortical brain regions in the human brain. Significant activation was found in ventral occipital area, inferior temporal cortex known as motion processing area MT, superior temporal cortex, and intraparietal cortices. Figure 5B shows the resulting activation patterns of a single subject overlaid on a high-resolution structural MRI of the subject’s brain.
GENERAL DISCUSSION AND CONCLUSIONS
The results of the two experiments reveal the temporal dynamics of neural activity related to visual motion priming. This is a form of perceptual priming in which the ambiguous motion direction (left or right) of a 2-D sine wave grating (target) can be primed by previous presentation of motion that is perceived unambiguously in one direction only. The priming effect is fast acting, lasting about 800 ms. The results of the first experiment showed that for single motion jumps, neural activity for ambiguous motion differs from unambiguous motion at about 300 ms after motion onset. The second experiment showed that when the SOA, or the interval between prime and target motion was as short as 200 ms, priming of ambiguous motion direction was associated with enhanced neural responses at posterior scalp sites at both early (100 ms) and late (350 ms) stages of processing, following the onset of target motion. This enhancement was greatest at a short prime-target interval of 200 ms, which was also associated behaviorally with the strongest priming. The positive enhancement associated with motion priming was maintained significantly for late ERP activity (P3), and approached significance for the P1 component at the intermediate prime-target interval (400 ms). Both the early and late ERP priming effects were eliminated at a longer SOA of 1000 ms.
The use of the motion reversal condition confirmed that these ERP amplitude changes were associated specifically with motion priming and were not simply due to an observer tendency to report any two motion jumps as occurring in the same direction. In contrast, when two unambiguous jumps in opposite directions were presented, observers consistently reported them to move in a different, not same direction, and no ERP enhancement was obtained. The ERP signature of motion priming was quite well correlated with the behavioral reports, showing greatest enhancement at a short SOA, reduced enhancement at the medium SOA at which weak behavioral priming was observed, and no enhancement at the long SOA at which priming was eliminated.
The similar time course (over SOA) of neural activity and behavioral reports suggests that perceptual decisions of motion direction result from the summation of direction-selective neural responses. Previous psychophysical studies have proposed such a temporal interaction view of motion priming based on motion energy models [21,26]. In this view, temporal interactions between the neural responses of successive stimuli produce motion priming effects that decay as the interval between stimuli increases. In the priming condition, the neural response to the priming motion favoring one particular motion direction is combined with a weaker neural response associated with ambiguous motion. Such a summation of neural signals is reduced as the time interval between the two motion stimuli increases, consistent with our finding of reduced ERP amplitudes with increasing time delay between prime and target motion steps (Fig. 4B). In contrast, in the reversal condition opposite direction motion signals result in a net subtraction of neural responses, as reflected in smaller ERP amplitudes.
In contrast to conscious recollection, perceptual priming is an automatic process that reflects prior exposure to a related stimulus. Electrophysiological correlates of visual word-form priming typically are found for latencies between 300 and 500 ms, whereas conscious recollection is usually associated with modulated of neural responses in the 600–800 ms latency range [19]. Perceptual priming tasks, such as word-form or repetition priming of a stationary visual stimulus, are longer lasting than motion priming (minutes or hours vs. 1 s). Neuroimaging evidence using PET or fMRI have associated perceptual priming of static stimuli with occipitotemporal and other brain regions in late visual processing stages. The present results suggest that the neural mechanisms underlying visual motion priming differ from other forms of perceptual priming. Visual motion priming involves both early and late stages of visual processing. Combined with our fMRI data, the results of the present study indicate that motion priming involves modulation of neural responses beginning as early as 100 ms in early visual occipital cortical areas, as well as later processing areas in inferior temporal (MT), superior temporal and intraparietal cortices.
Acknowledgments
Authors Y. J. and Y.J.L. made equal contributions to the paper. We thank two anonymous reviewers, L. Chao, P. Grossenberg, A. Meyer-Lindenberg, and X.H. Peng, for their helpful comments, and the NMR center of the National Institutes of Health for assistance in MR imaging. Supported by NIH grant AG07569 to R.P., AG00986 to Y.J., and NSF (China) grant 30070262 and Chinese Academy of Science grant KJCX1-07, both to Y.J.L.
References
- 1.Adelson EH, Bergen JR. Spatial temporal energy models for the perception of motion. J Opt Soc Am A. 1985;2:284–299. doi: 10.1364/josaa.2.000284. [DOI] [PubMed] [Google Scholar]
- 2.Anstis S, Ramachandran V. Visual inertia in apparent motion. Vision Res. 1987;27:755–764. doi: 10.1016/0042-6989(87)90073-3. [DOI] [PubMed] [Google Scholar]
- 3.Bach M, Ullrich D. Motion adaption governs the shape of motion-evoked cortical potentials. Vision Res. 1994;34:1541–1547. doi: 10.1016/0042-6989(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 4.Begleiter H, Projesz B, Wang W. Event-related brain potentials differentiate priming and recognition to familiar and unfamiliar faces. Electroencephalogr Clin Neurophysiol. 1995;94:41–49. doi: 10.1016/0013-4694(94)00240-l. [DOI] [PubMed] [Google Scholar]
- 5.Bentin S, McCarthy G. The effects of immediate stimulus repetition on reaction time and event-related potentials in tasks of different complexity. J Exp Psychol Learn Mem Cogn. 1994;20:130–149. [Google Scholar]
- 6.Blake R. What can be “perceived” in the absence of visual awareness. Curr Dir Psychol Sci. 1998;6:157–162. [Google Scholar]
- 7.Buchner H, Gobbele R, Wager M, Fuchs M, Waberski TD, Bechmann R. Fast visual evoked potential input into human area V5. Neuroreport. 1997;8:2419–2422. doi: 10.1097/00001756-199707280-00002. [DOI] [PubMed] [Google Scholar]
- 8.Buckner RL, Goodman J, Burock M, Rotte M, Koustaal W, Schacter D, Rosen B, Dale A. Functional anatomic correlates of object priming in humans revealed by rapid presentation event related fMRI. Neuron. 1998;20:285–296. doi: 10.1016/s0896-6273(00)80456-0. [DOI] [PubMed] [Google Scholar]
- 9.Cave CB. Very long-lasting priming in picture naming. Psychol Sci. 1997;8:322–325. [Google Scholar]
- 10.Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, Turner R. Analysis of fMRI time-series revisited. Neuroimage. 1995;2:45–53. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- 11.Haxby, J. V.; Maisog, J. M.; Courtney, S. M. Multiple regression analysis of effects of interest in fMRI time series. In: Lancaster, J.; Fox, P.; Friston, K., eds. Mapping and modeling the human brain. New York: Wiley; in press.
- 12.Heeger D, Boynton GM, Demb JB, Seidemann E, Newsome WT. Motion opponency in visual cortex. J Neurosci. 1999;19:7182–7174. doi: 10.1523/JNEUROSCI.19-16-07162.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hillyard SA. Electrical and magnetic brain recordings: Contributions to cognitive neuroscience. Curr Opin Neurobiol. 1993;3:217–224. doi: 10.1016/0959-4388(93)90213-i. [DOI] [PubMed] [Google Scholar]
- 14.Jiang Y, Haxby JV, Martin A, Ungerleider LG, Parasuraman R. Complementary neural mechanisms for tracking familiar items in human working memory. Science. 2000;287:643–646. doi: 10.1126/science.287.5453.643. [DOI] [PubMed] [Google Scholar]
- 15.Jiang Y, Pantle AJ, Mark LS. Visual inertia of rotating 3-D objects. Percept Psychophys. 1998;60:275–286. doi: 10.3758/bf03206036. [DOI] [PubMed] [Google Scholar]
- 16.Kuba M, Tonyonaga N, Kubova Z. Motion-reversal visual evoked responses. Physiol Res. 1992;41:369–373. [PubMed] [Google Scholar]
- 17.Miller EK, Desimone R. Parallel neuronal mechanisms for short-term memory. Science. 1994;263:520. doi: 10.1126/science.8290960. [DOI] [PubMed] [Google Scholar]
- 18.Niedeggen M, Wist ER. Characteristics of visual evoked potentials generated by motion coherence onset. Brain Res Cogn Brain Res. 1999;8:95–105. doi: 10.1016/s0926-6410(99)00009-9. [DOI] [PubMed] [Google Scholar]
- 19.Paller KA. Neural measures of conscious and unconscious memory. Behav Neurosci. 2000;12:127–141. doi: 10.1155/2000/865250. [DOI] [PubMed] [Google Scholar]
- 20.Pantle AJ, Gallogly DP, Piehler OC. Direction biasing by brief apparent motion stimuli. Vision Res. 2000;40:1979–1991. doi: 10.1016/s0042-6989(00)00071-7. [DOI] [PubMed] [Google Scholar]
- 21.Pinkus A, Pantle A. Probing visual motion signals with a priming paradigm. Vision Res. 1997;37:541–552. doi: 10.1016/s0042-6989(96)00162-9. [DOI] [PubMed] [Google Scholar]
- 22.Qian N, Andersen RA, Adelsen EH. Transparent motion perception as detection of unbalanced motion signals: III. Modeling. J Neurosci. 1994;14:7381–7392. doi: 10.1523/JNEUROSCI.14-12-07381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramachandran V, Anstis S. Extrapolation of motion path in human visual perception. Vision Res. 1983;23:83– 85. doi: 10.1016/0042-6989(83)90044-5. [DOI] [PubMed] [Google Scholar]
- 24.Raymond J, Braddick O. Responses to opposed directions of motion: Continuum or independent mechanisms? Vision Res. 1996;36:1931–1937. doi: 10.1016/0042-6989(95)00241-3. [DOI] [PubMed] [Google Scholar]
- 25.Schacter DL, Buckner RL. Priming and the brain. Neuron. 1998;20:185–195. doi: 10.1016/s0896-6273(00)80448-1. [DOI] [PubMed] [Google Scholar]
- 26.Strout JJ, Pantle A, Mills SL. An energy model of interframe interval effects in single-step apparent motion. Vision Res. 1994;33:3223–3240. doi: 10.1016/0042-6989(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 27.Van Santen J, Sperling G. Temporal covariance model of human motion perception. J Opt Soc Am A. 1984;1:451–473. doi: 10.1364/josaa.1.000451. [DOI] [PubMed] [Google Scholar]
- 28.Wiggs CL, Martin A. Properties and mechanisms for perceptual priming. Curr Opin Neurobiol. 1998;8:227–233. doi: 10.1016/s0959-4388(98)80144-x. [DOI] [PubMed] [Google Scholar]


