Abstract
Study Objective
To determine whether variation in sleep duration reflects variation in sleep need or self-imposed sleep restriction.
Design
After habitual bedrest duration of participants was assessed during a 2-week outpatient protocol, volunteers were scheduled to sleep according to this schedule for 1 week prior to and the first night after admission to a general clinical research center. The inpatient protocol included multiple sleep latency testing on the second day and sleep recordings during a bedrest extension protocol that included 16 hours of sleep opportunity (12 hours at night and 4 hours at midday) for 3 consecutive days.
Setting
Outpatient monitoring followed by inpatient assessment of sleep.
Participants
Seventeen healthy volunteers (10 women) aged 18–32 years without clinical sleep disorders.
Interventions
Extension of sleep opportunity.
Measurements and Results
The habitual bedrest duration varied from 6.1 to 10.3 hours. Individuals with shorter habitual bedrest duration fell asleep more quickly and frequently during the multiple sleep latency test than did those with longer habitual bedrest duration. On the first day of extended sleep opportunity, the total sleep time of all individuals was greater than their habitual bedrest duration; the average increase in total sleep time was 4.9 hours (P = 0.001). The increase in total sleep time declined across the 3 day bedrest-extension protocol (P = 0.003 for trend). During the third day of increased sleep opportunity, the total sleep time was negatively associated with habitual bedrest duration (P = 0.005); individuals with shorter habitual bedrest duration continued to sleep more than those with longer habitual bedrest duration.
Conclusion
Those individuals with shorter habitual sleep durations carry a higher sleep debt than do those with longer habitual sleep duration. Interindividual variation in sleep duration may primarily reflect variation in self-selected sleep restriction or wake extension.
Keywords: Sleep, homeostasis, recovery, sleep duration
INTRODUCTION
SELF-REPORTED SLEEP DURATION VARIES CONSIDERABLY BETWEEN INDIVIDUALS. RECENT SURVEY DATA OF THE US POPULATION INDICATE THAT CURRENTLY the average sleep duration is 6.9 hours, that 15% of the population sleeps less than 6 hours, and that 30% sleep more than 8 hours.1 Similar data have been obtained in a survey of sleep in Great Britain. 2 Recent epidemiological studies have shown that variation in self-reported sleep duration is associated with mortality, such that both short and long sleepers have increased mortality.3–5 Experimentally induced sleep debt (3–6 hours in bed each night for one or two weeks) is associated with abnormalities in endocrine function, including impaired glucose tolerance and GH and leptin secretion, and decrements in performance and alertness.6–9 The mechanisms underlying these associations are unknown.
The timing, duration and structure of sleep are regulated by processes related to both sleep homeostasis and circadian (~24 h) rhythmicity. Sleep homeostasis implies that sleep serves a recovery function, although the timing of recovery is modulated by the biological circadian clock.10–12 Variation in sleep duration may reflect differences in either the sleep homeostatic process or the circadian process. More specifically, these variations may reflect differences in the rate of build-up of sleep “need” during wakefulness, in the rate of dissipation of sleep “need” during sleep, in the amount of sleep people select to take, or modulation by circadian, environmental, behavioral or social factors. There is no consensus within the sleep research community as to which interpretation is correct. In fact, the significance and interpretation of variations in sleep duration and whether or not our society is chronically sleep-deprived is the subject of lively debate.3,13–15
The continuation of this debate is in part related to the multiple descriptive terms, variables and protocols used by the sleep research community. For example, frequently used terms such as sleep homeostasis, sleep need, sleep pressure, sleep intensity, recovery sleep, and sleep debt, some of which have not always been well defined, have been used. Sleep latency, sleep efficiency, Slow Wave Sleep (SWS), EEG Slow Wave Activity (SWA, delta frequency power in the EEG per epoch of NREM sleep), and total sleep time (TST) have all been used to quantify the sleep process and its response to sleep loss. Since the functions of sleep are not known, but probably include processes evident during wake, the relationships of these sleep homeostatic measures with daytime function and performance is of interest. However, for most of these variables, unequivocal associations with daytime function and performance have not been established.
The variety and limitations of protocols used to quantify the response to sleep loss have also contributed to confusion in the field. For example, only 20% of sleep time lost during total sleep deprivation is recovered during subsequent sleep at habitual time and duration16, while SWS and EEG SWA are disproportionately enhanced. This observation led to the hypothesis that lost sleep time can be recovered by increased sleep intensity.17, 18 However, it appears that this lack of substantial recovery of lost sleep time may partially be an artifact of the limited duration of the sleep opportunity following sleep deprivation and/or of a “ceiling” effect on the amount of sleep during a single nocturnal sleep episode. Indeed, when this limitation was realized, experiments in which volunteers were given sleep opportunities of 24 hours showed that up to 85% of lost sleep was recovered.19
Other experiments have also probed sleep homeostasis using bedrest extension protocols. In one study, extension of bedrest from habitual bedrest duration (HBD) to 10 hours nocturnal time in bed (TIB) was associated with an increase in Total Sleep Time (TST), daily sleep latency and a reduction in sleep efficiency in some individuals. Roehrs and colleagues reported that with an extension of TIB to 10 hours, baseline “sleepy” (MSLT latencies < 6 minutes) individuals had increased TST and/or decreased sleep efficiency relative to “alert” (MSLT latencies > 16 minutes) individuals.20,21
A question that is central to the debate about sleep need and sleep homeostasis is: does variation among individuals in self-reported HBD reflect differences in sleep need, or are these variations in HBD at least partially associated with differences in sleep debt?
Some reports have partially addressed this question. Wehr and colleagues reported that when volunteers were scheduled to 14-hr sleep opportunity for several weeks, there was an initial increase in TST to ~10.7 hours which then decreased until TST reached an asymptotic level of 8.2 hours.22 Although this report clearly demonstrated that the volunteers carried a sleep debt when they entered the protocol, it did not investigate whether this sleep debt was associated with inter-individual variation in HBD. In a study of HBD and sleepiness, individuals with HBD <6 hours had significantly shorter MSLT latencies than those with HBD > 9 hours. The two groups also differed in the relationship between MSLT latencies and subjective sleepiness: the MSLT latencies of individuals with HBD < 6 hours were not correlated with their subjective ratings on the Epworth Sleepiness Scale, while the latencies of those with HBD > 9 hours were inversely correlated with subjective ratings on the Epworth Sleepiness Scale.23 This suggests that individuals with short HBD may not accurately self-assess their sleepiness. However, there exist individuals with short sleep latencies but no other evidence of sleepiness.24,25
Aeschbach and colleagues reported that habitual short sleepers (< 6 hour HBD) live under higher sleep ”pressure” (defined by activity in the theta frequency range in the waking EEG) than habitual long sleepers (>10 hour HBD). They also reported that short and long sleepers have the same kinetics of sleep-wake dependent (i.e. homeostatic) variation in sleep pressure as based on an analysis of EEG SWA during sleep.26,27 They concluded that habitual short sleepers tolerate a high sleep pressure better than habitual long sleepers. Data on sleep duration under prolonged extended sleep opportunity conditions in these two groups were not collected; thus it is not known whether the two groups carried a differential sleep debt, as assayed by an increase in TST when given additional sleep opportunity.
In another experiment, the response to a 36-hr sleep deprivation was studied in Long Sleepers (HBD ~9.5 hours), Regular Sleepers (HBD ~8 hours) and Short Sleepers (HBD ~6 hours). After a 36-hr sleep deprivation ending at approximately habitual bedtime, the Short Sleepers increased their TST by 25%, the Regular Sleepers increased it by 20% and the Long Sleepers not at all.28 These results suggest that the Short, Regular and Long Sleepers had different prior levels of sleep debt or different responses to sleep deprivation.
Several studies have reported increased bedrest duration when individuals do not have a scheduled wake time, suggesting that HBD is shorter than optimal. In a large epidemiological study, Breslau et al. found that average nocturnal bedrest duration on weekdays was 6.7 hours, average nocturnal bedrest duration was longer on weekends, and subjective daytime sleepiness was inversely related to hours of sleep and positively related to the ease of falling asleep at night.29 Similar data on weekday versus weekend HBD were reported in the a National Sleep Foundation Poll.1 Other studies of ad lib vs. controlled amounts of sleep (with alarm clock or in laboratory conditions) have shown that individuals sleep 1–2 hours more when not scheduled to awaken, similar to their behavior on weekends.30–32 However, the relationship to HBD was not documented.
To clarify the significance of inter-individual variation in HBD, we used an extended sleep opportunity protocol. The design of this protocol is based on the following considerations. (1) Because HBD may not be reliably assessed by retrospective self-report, we quantified HBD by daily diaries, time-stamped call-ins, and actigraphy for three weeks prior to the assessment of sleep debt. (2) The circadian pacemaker and environmental factors such as knowledge of clock time and light exposure interfere with the homeostatic regulation of sleep at some circadian phases. Therefore, extended 12-hr nocturnal and 4-hr midday sleep opportunities in darkness and without knowledge of clock time were provided to maximize the opportunity for the expression of homeostatic sleep need. (3) Variations in sleep duration during extended sleep opportunities can be interpreted as either variations in optional sleep taken or variations in sleep debt. To distinguish between these two interpretations, it is necessary to analyze the time course of sleep variables over several days. If individuals carry a sleep debt, there would be an initial increase in TST, which would decrease during subsequent days. In contrast, the optional sleep hypothesis, in which an individual’s HBD accurately reflects their sleep need, predicts that excess sleep is identical during all extended sleep opportunities. We also chosen TST as our primary outcome variable because TST and HBD are the most frequently assessed sleep parameters in surveys that quantify associations between discussions of sleep and health, and there is no evidence that SWS and SWA are related to sleep duration. We report here that shorter HBD is associated with a larger sleep debt.
METHODS
Seventeen volunteers (7 males, 10 females) ages 18–32 (21.8 ± 3.7 (s.d.)) years were studied. These individuals were healthy by history and physical examination, laboratory tests of urine and blood, ECG, and psychological screening. All underwent a clinical polysomnogram to verify the absence of the clinical sleep disorders sleep apnea and nocturnal myoclonus. None were using any prescription or non-prescription medication. None had worked night or rotating shifts in the past three years nor crossed more than one time zone in the previous three months. Volunteers were recruited by print advertisements. There was no recruitment targeted to HBD.
The inpatient portion of the study was conducted in the Brigham and Women’s Hospital General Clinical Research Center. Conditions included an environment free of external time cues such as windows, clocks, radio, TV, or non-staff visitors. The protocol was approved by the Partners Healthcare Institutional Review Board.
For three weeks prior to admission, study volunteers were instructed to abstain from the use of all medication, health food supplements, caffeine, tobacco and alcohol. Volunteers were tested for the presence of these prohibited substances at least once during screening and again at inpatient admission. Volunteers were not instructed to change their sleep-wake habits. For two weeks, all self-selected sleep-wake times were reported in a daily diary and telephone call-in times to a time-stamped machine at every sleep time and at every wake time. Very few naps were reported, and they were included in further calculations. These self-selected sleep-wake times were averaged to produce the schedule for the following week, which was also the week before admission. This last-week schedule included only nocturnal sleep episodes. The actual sleep and wake times, as determined by self-report, telephone call-in times, and wrist actigraphy, during this last week were used to compute habitual bedrest duration (HBD) and also to schedule the timing and duration of the first nocturnal sleep episode after admission. To be eligible for the inpatient portion of the study, volunteers needed to maintain the scheduled sleep time and wake time within 30 minutes; volunteers were allowed a maximum of two minor deviations (out of a total of 14 sleep and wake times) from this schedule, and no naps.
All inpatient events were scheduled relative to the volunteer’s habitual sleep and wake time. All volunteers received a sleep opportunity at the same time and of a duration equal to their HBD for their first inpatient night, except for the one individual with HBD of 6.8 hours who, due to an error, was allowed 7.4 hours of sleep opportunity on the first inpatient night instead of 6.8 hours. On the second inpatient day, volunteers underwent a Multiple Sleep Latency Test (MSLT) protocol beginning two hours after awakening. During each of five tests scheduled two hours apart, a volunteer is instructed to lie quietly with eyes closed and try to fall asleep: latency to any stage of sleep is reported. The volunteer is awakened when specific sleep criteria are met. If the volunteer is still awake after 20 minutes, the test is terminated. Conduct and scoring of the MSLT was performed according to published guidelines.33 For the next three 24-hr days, the volunteers were placed on a bedrest extension protocol with 16 hours of sleep opportunity: 12 hours centered at mid-habitual nocturnal sleep episode and 4 hours centered 12 hours opposite this. Beginning with the second inpatient day, volunteers were exposed to 15 lux of light when awake and <0.01 lux when asleep.
All polysomnographic signals were recorded on Vitaport digital recorders (Temec, The Netherlands). EEGs were filtered with a high pass filter (TC=0.0680 sec) and low pass filter (70.1 Hz.) and then digitized with a sampling rate of 256 Hz and stored at 128 Hz. Sleep was scored in 30-second epochs using standard criteria34 into sleep stages Rapid Eye Movement (REM) sleep, non-REM (NREM) sleep stages 1–4 (of which NREM sleep stages 3 and 4 comprise SWS) and Wake. Sleep efficiency was defined as (REM sleep + NREM sleep)/TIB. Sleep latency was the length of time in minutes between Lights Out and the first epoch of any stage of sleep.
While they were awake, volunteers were asked to rate their subjective mood using a 100 mm non-numeric linear scale35,36 several times per day. Two subjective mood measures were Alert-Sleepy and Fresh-Tired. These measures were scored between 100 mm (Alert or Fresh) and 0 mm (Sleepy or Tired).
Repeated-measures mixed-model ANOVA was performed using SAS for Windows (SAS™ version 6.12). The large number of statistical comparisons should be taken into account when interpreting the p-value; specifics are included in the relevant portions of the text.
RESULTS
Reported HBD ranged from 6.1 to 10.3 hours with a mean (± s.d.) of 8.5 (± 1.1) hours. Habitual Sleep Onset ranged from 22:54 to 4:24 with a mean of 0:18 (± 1:00). Habitual Wake Onset ranged from 7:12 to 12:36 with a mean of 8:48 (± 1:24).
Data from the first sleep episode, the duration of which was equal to each individual’s HBD, are shown in Figure 1. Linear regression with HBD as the independent measure were significant for latency to NREM sleep stage 1 (slope = 3.5 min/hr HBD, p=0.004, R2=0.44), Sleep Efficiency (slope=−2.51%/hr, p=0.031, R2=0.25), Wake (slope = 0.29 hr/hr, p=0.015, R2=0.33), TST (slope=0.62 hr/hr, p=0.001, R2=0.70), and REM sleep (slope=0.29 hr/hr, p=0.019, R2=0.31). There was no significant relationship between SWS and HBD. For the amount of Wake relative to HBD, the relationship appears biphasic, with a constant lower level for all individuals with HBD less than ~9 hours and some individuals with HBD greater than ~9 hours, and a second higher level of Wake for some individuals with HBD greater than ~9 hours.
Figure 1.
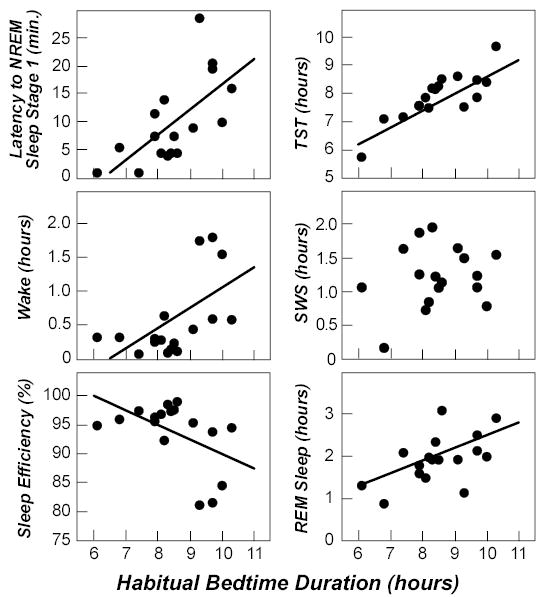
First inpatient sleep episode. Association between habitual bedtime duration (HBD) and sleep parameters during the first inpatient sleep episode (for which time in bed was equal to HBD). Each point represents a different volunteer. Regression lines are shown if the slope is significantly different from 0. NREM refers to non-rapid eye movement; TST, total sleep time; SWS, slow-wave sleep; REM, rapid eye movement.
All volunteers whose HBD was less than 9.3 hours fell asleep on all five sleep opportunities within the MSLT (Figure 2). 14 of the 17 volunteers had at least one sleep latency (out of five possible opportunities) less than 5 minutes; the three who did not have at least one sleep latency less than 5 minutes were one of the two volunteers with HBD of 9.7 hours and both volunteers with HBD longer than 9.7 hour. There were significant negative regression relationships between HBD and the number of times a volunteer fell asleep (p=0.02, R2= 0.31) and between HBD and the number of times a volunteer fell asleep in less than 5 minutes (p=0.0004, R2=0.58). There was a significant positive regression relationship between HBD and the median sleep latency of the five sleep opportunities within the MSLT (p=0.04, R2= 0.24).
Figure 2.
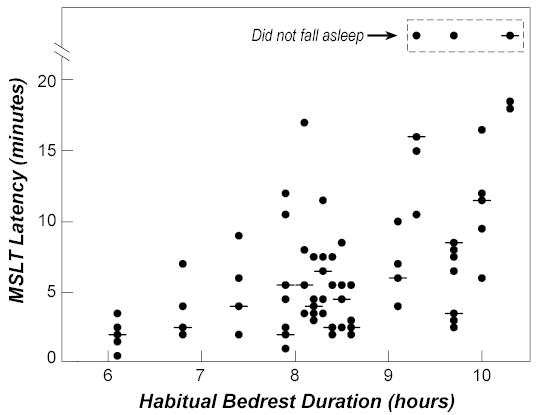
Habitual bedtime duration and Multiple Sleep Latency Test results. Association between habitual bedtime duration (HBD) and daytime sleep propensity as assessed by the Multiple Sleep Latency Test (MSLT). For each volunteer, the sleep latency for each of the 5 sleep opportunities (circles) and median (horizontal line) during the MSLT are shown. If the latency was the same on 2 or more of the sleep opportunities, only 1 circle is plotted.
TST, NREM sleep stages 2–4, SWS, and REM sleep during the first, second, and third day of the bedrest extension protocol changed both relative to the first inpatient sleep episode (with length equal to the volunteers’ HBD) and across these three days of the protocol. On the first day of bedrest extension, the average (across all volunteers) measures of sleep were higher than for the first sleep episode: TST increased 4.9 hours (p=0.001), REM increased 1.2 hours (p=0.001), and SWS increased 0.5 hours (p=0.001). The TST of every volunteer was higher than their HBD during the first day of bedrest extension. Note that HBD would be greater than TST during the week before admission (if sleep efficiency is less than 100%), so that TST increased from below to above HBD. The average amounts of TST decreased across the first three days of the bedrest extension protocol (Table 1, Figure 3).
Table 1.
Sleep During Bedrest Extension
| First 24 hours | Second 24 hours | Third 24 hours | P value for trend across days | P value for HBD | |
|---|---|---|---|---|---|
| Sleep efficiency, % | 80 (8) | 70 (10) | 64 (10)* | .0001 | .002 |
| Nighttime | 89 (7) | 77 (9) | 71 (9) | .0001 | NS |
| Daytime | 53 (22)* | 48 (26) | 39 (24)* | NS | .003 |
| Wake, h | 3.1 (1.4 ) | 4.8 (1.6) | 5.7 (1.6)* | .0001 | .002 |
| Nighttime | 1.3 (0.9) | 2.7 (1.1) | 3.5 (1.0)* | .0001 | NS |
| Daytime | 1.9 (0.9)* | 2.1 (1.1) | 2.4 (1.0)* | NS | .003 |
| NREM sleep, h | 8.3 (1.2) | 7.2 (1.1) | 6.6 (1.3) | .0001 | .001 |
| Nighttime | 6.8 (0.7) | 5.9 (0.8) | 5.4 (0.9) | .0001 | .004 |
| Daytime | 1.5 (0.7) | 1.3 (0.7) | 1.0 (0.7)* | NS | .004 |
| Slow-wave sleep, h | 1.8 (0.7) | 1.2 (0.6 ) | 1.2 (0.4) | .009 | NS |
| Nighttime | 1.4 (0.5) | 0.9 (0.4) | 1.0 (0.3) | .009 | NS |
| Daytime | 0.3 (0.2) | 0.3 (0.3) | 0.2 (0.1) | NS | NS |
| REM sleep, h | 3.0 (0.5) | 2.6 (0.7) | 2.3 (0.4) | .002 | NS |
| Nighttime | 2.7 (0.5) | 2.3 (0.7) | 2.0 (0.3) | .002 | NS |
| Daytime | 0.3 (0.3) | 0.3 (0.2) | 0.2 (0.2) | NS | NS |
| Total sleep time, h | 12.8 (1.3) | 11.1 (1.5) | 10.2 (1.6)* | .0001 | .003 |
| Nighttime | 10.6 (0.8) | 9.3 (1.1) | 8.5 (1.1) | .001 | NS |
| Daytime | 2.1 (0.9)* | 1.9 (1.1) | 1.5 (1.0)* | NS | .003 |
| Alert | 72.6 (15.5) | 73.2 (15.2) | 70.1 (16.0) | NS | NS |
| Fresh | 76.2 (11.4) | 73.6 (10.5) | 69.2 (13.3) | NS | NS |
Measures of sleep - mean (± SD) - in the first, second and third 24 hours of the bedrest extension portion of the protocol and in the nighttime (12- hours) and daytime (4-hours) bedrest episodes within each of these 24 hours for some of these measures. Because of the multiple comparisons, an alpha of 0.05/3 =0.017 is recommended. An average with an asterisk indicates statistically significant slope (P<0.017) for a linear regression with HBD for the first three columns (First, Second and Third 24 hours). The P value for the trend across days and HBD (last two columns) are from a mixed model analysis with HBD and day number as independent variables. NREM refers to non-rapid eye movement sleep stages 2, 3, and 4; REM refers to rapid eye movement.
Figure 3.
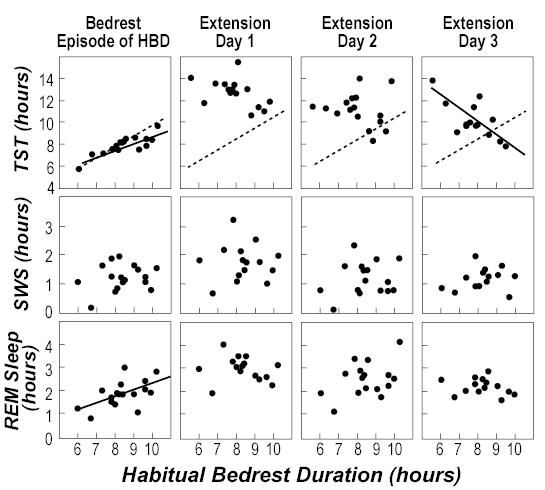
Sleep during first the first inpatient bedrest episode and the following three days of bedrest extension relative to habitual bedtime duration. The solid lines indicate regression lines for which the slope was statistically significant. The dotted lines for total sleep time (TST) (top 4 panels) are the lines at which TST equals habitual bedtime duration (HBD), which is what would be expected if HBD reflected steady-state TST at 100% sleep efficiency. The left column contains data for TST, slow-wave sleep (SWS), and rapid eye movement (REM) sleep during the first inpatient bedrest episode (time in bed equal to HBD for each volunteer); these data are repeated from Figure 1 for comparison of changes across the days of the protocol. The other 3 columns contain data from the 3 days of bedrest extension during which total time in bed was 16 hours for all volunteers.
Analysis of the individual responses to this bedrest extension protocol revealed marked differences. The individual values were not related to HBD for the first two days; only during the third day of bedrest extension were there significant regressions between HBD and Sleep Efficiency (p=0.004), TST (p=0.005) and Wake (p=0.004). Linear regression analyses between HBD and NREM sleep stages 2–4, SWS or REM sleep were not significant (Table 1, Figure 3). The negative slope of the relationship of TST with HBD during the 3rd day of bedrest extension indicates that those with the shortest HBD had increased TST (and decreased Wake and increased Sleep Efficiency) relative to those with a longer HBD. During this 3rd day of bedrest extension, volunteers with HBD less than 9 hours continued to have TST more than their HBD, whereas those volunteers with HBD greater than ~9.5 hours had TST less than their HBD. Four of the volunteers with HBD < 9 hours had TST of over 11 hours on the 3rd day of bedrest extension.
When the 12-hr nocturnal and 4-hr midday bedrest opportunities were examined individually (Table 1), substantial amounts of TST, SWS and REM sleep were seen during the diurnal bedrest opportunity. Significant regression relationships between TST, Sleep Efficiency and Wake with HBD were seen in the first diurnal sleep opportunity.
Volunteers reported feeling significantly more Alert and Fresh on all three days of bedrest extension compared with baseline. Between the first wake episode (following a sleep episode of HBD) and the wake episodes during the first day of bedrest extension, Alert increased 11.6 mm (p=0.01) and Fresh increased 14.2 mm (p=0.0004). There were no significant differences by HBD or by number of days of bedrest extension in these subjective measures.
DISCUSSION
HBD appears to be an inaccurate reflection of sleep need in young adults. Individuals with shorter HBD fell asleep more quickly and frequently during the MSLT than those with longer HBD. All of the volunteers with HBD <9.7 hours fell asleep at least once in less than 5 minutes during the MSLT. All individuals slept more than HBD during the first day of increased sleep opportunity (12-hr nocturnal, 4-hr midday), and a majority of volunteers did sleep more than HBD so on the second and third days. TST increased significantly during both nocturnal and diurnal opportunities. During the third day of increased opportunity, TST was negatively associated with HBD, indicating that individuals with shorter HBD continued to sleep longer than those with longer HBD. The lack of this relationship on the first and second bedrest extension days may reflect a “ceiling” effect of the amount of sleep in 24 hours. The gradual decline in TST and increase in Wake during the three days of increased sleep opportunity demonstrates that a sleep debt was being recovered. Consistent with this is the observation that volunteers reported increased levels of Alert and Fresh on mood ratings during the bedrest extension days. In a recent survey, shorter bedrest durations were also associated with higher levels of subjective tiredness.37
Associated with this increased time asleep, were increases in sleep latency and Wake during the sleep episode and therefore decreased Sleep Efficiency. It has been argued that during an extension of sleep opportunity, an increased sleep latency and Wake after sleep onset indicate an increased capacity for sleep, not a compensatory response.38 However, we argue that the transient increased TST, even associated with increased sleep latency and increased Wake, that is followed by declining TST suggests a compensatory response to a relative sleep debt, even in the presence of decreased sleep efficiency.
Even after three days of extended sleep opportunities, TST was considerably longer than the HBD in many of the volunteers, with two volunteers with HBD less than 7 hours sleeping more than 11 hours on this third day. Since there is some wake within a sleep episode, TST would be expected to be less than HBD if there were no sleep debt. This finding further illustrates that shorter HBDs provide inadequate TST for most individuals in this age-group. Wehr et al. reported an asymptotic sleep duration of 8.2 hours22, whereas Rajaratnam et al, in a study in which volunteers were given consecutive 16-hr sleep opportunity, reported an asymptotic sleep duration of 8.7 hours.39 On average, volunteers slept 10.2 hours on the third day of bedrest extension in this protocol. These sleep durations are considerably longer than those reported by most healthy individuals and would still be shorter than the TIB or HBD variable usually reported in epidemiology studies. The extant sleep debt of incoming volunteers should be considered in sleep deprivation and other experiments concerning timing, quantity and quality of sleep.
In our protocol, some of the individuals with short HBD had not reached a steady state of sleep duration after three days (or 48 total extra hours) of sleep opportunity, although the rate of change in TST declined across days of bedrest extension. Therefore, we can not calculate the rate at which volunteers reach steady-state or whether this rate differs among individuals. The rate may also depend on whether sleep opportunities are available only at night or during the night and day, as presented here. The time course of the transition to this longer sleep duration may provide useful information about the processes involved. Whether all markers of sleep homeostasis (sleep latency, TST, SWS) have the same time course is also unknown. The time course of recovery from chronic sleep debt may be longer than previously anticipated: recovery, as indexed by TST in this study, appears to require several days and nights of sleep opportunity, consistent with the results of Wehr et al.22 and Rajaratnam et al.39 Recovery of performance from one week of sleep restriction also required more than three days of increased sleep opportunity.8 These time courses of recovery, as indexed by TST and performance, differ markedly from the time course of recovery of SWA which has a very short time constant.10,18 More experiments are required to quantify the time course of multiple variables. Such experiments can be used to develop optimal recovery schedules from chronic sleep restriction.
In choosing HBD as the independent measure in this report, we assume that average HBD would be a valid measure of sleep “need”. Though it is possible that daily variations in HBD are important in determining the build-up and recovery of sleep debt; further experiments are needed. There is no current evidence that day-to-day variability affects TST; indeed, there is evidence that TST has a longer time-constant.22 In this study, subjects were on an imposed sleep-wake schedule for the week before admission and for the first inpatient sleep episode; this resulted in very minor day-to-day variability before the bedrest extension portion of the protocol and is another reason why day-to-day variability was probably not significant in our use of HBD to reflect sleep “need”. Systematic under- or over-estimation and report bias, i.e. similar error in ‘long’ and short’ sleepers will not affect the results, including the trend over consecutive days. Only HBD-dependent bias could affect the results. The most likely HBD-dependent bias may be that both short and long sleepers ‘over-report’ their deviation from the mean, in other words those with short reported HBD in fact sleep longer, i.e. closer to the population mean and those with long reported HBD in fact sleep shorter, i.e. closer to the population mean. If that were the case to the extreme, than we would not expect any correlation between HBD and TST on day three for example. In fact, we observe a negative correlation which is very difficult to explain by any reporting bias except by assuming that short HBD volunteers sleep longer than long HBD volunteers. The most parsimonious explanation for our data is that those individuals with short HBD carry a larger sleep debt.
The much-debated concept of sleep “need” is related to the known homeostatic regulation of sleep. It is possible that there are multiple homeostatic factors (metabolic, immune, learning, etc.) regulated during sleep, only some of which are included in the regulation of sleep duration. In the current experiment, different markers of the homeostatic process appear to have different relationships with HBD. This is consistent with Borbély’s observation that sleep duration and intensity are regulated differently.18 These different sleep ‘needs” may have different markers and different time constants of build-up during wake and dissipation during sleep. An additional interesting finding is that individuals may be able to “pay back” some of their sleep need while they are awake, since the sleep-deprived waking EEG is characterized by increased alpha/theta activity (5.25–9.0 Hz range).26,40,41
These results suggest that both lifestyle factors and possible inter-individual differences in sleep need govern choice of sleep duration in younger adults. If HBD reflected only sleep need, then there would not be different levels of sleep debt in individuals with different HBD, as was observed in this study. Choice of sleep duration may also, in part, be governed by individual’s perceived ability and willingness to function under different levels of sleep debt, as suggested by Aeschbach et al.26,27 The lack of correlation between HBD and Alert and Fresh self-report supports this interpretation. One possibility is that the inter-individual variation in tolerance of the effects of chronic sleep restriction is reflected in subjective performance but not in sleep measures. Inter-individual differences in subjective and objective response to sleep deprivation have been reported.42,43 Whether the inter-individual variance is reflected in other measures and whether these findings apply to other age groups should be studied.
Although we do not know the cause (e.g., work schedules, family obligations) of the self-reported sleep duration in each volunteer, this experiment documents the effects of the chosen sleep duration. This is a relevant approach for study, since the association between reported sleep duration and health may partially be a consequence of sleep restriction and associated sleep debt.
These results have wide-ranging implications because of the documented adverse effects of sleep restriction. Sleep restriction is associated with detrimental effects on metabolic and immune function.44,51 Under conditions of sleep restriction, changes in subjective alertness do not mirror the continued worsening of objective measures of alertness and performance.8,9 If individuals do not accurately assess (using subjective alertness measures) their attention and performance deficits, they cannot take appropriate countermeasures (sleep, caffeine, other pharmacological agents, lifestyle changes) to correct those deficits and their potentially hazardous outcomes (e.g., accidents and other health problems).
Acknowledgments
Research supported by NIH grants P01-AG09775, K02-HD045459 (EBK) and NCRR-GCRC-M01-RR02635 to the BWH GCRC. DJD is supported by the Research Fund of the University of Surrey and grant BBS/B/08523 from the Biotechnology and Biological Sciences Research Council-UK. The funding agencies had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript
Footnotes
Disclosure Statement
This was not an industry supported study. Dr. Klerman receives research support from Vanda Pharmaceuticals, and has had a consulting relationship with Vanda Pharmaceuticals. Dr. Dijk receives/received research support from London Underground, Ltd, H. Lundbeck A/S, and is/was a consulting advisor to Philips, H. Lundbeck A/S, Cephalon Inc., and Unilever.
References
- 1.National Sleep Foundation. 2003 “Sleep in America” Poll. 2003.
- 2.Groeger JA, Zijlstra FRH, Dijk DJ. Sleep quantity, sleep difficulties and their perceived consequences in a representative sample of some two thousand British adults. J Sleep Res. 2004;13:359–371. doi: 10.1111/j.1365-2869.2004.00418.x. [DOI] [PubMed] [Google Scholar]
- 3.Kripke DF. Do we sleep too much? Sleep. 2004;27:13–14. [PubMed] [Google Scholar]
- 4.Tamakoshi A, Ohno Y. Self-reported sleep duration as a predictor of all-cause mortality: results from the JACC study, Japan. Sleep. 2004;27:51–54. [PubMed] [Google Scholar]
- 5.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler M. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–136. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 6.Carskadon MA, Dement WC. Cumulative effects of sleep restriction on daytime sleepiness. Psychophysiol. 1981;18:107–113. doi: 10.1111/j.1469-8986.1981.tb02921.x. [DOI] [PubMed] [Google Scholar]
- 7.Dinges DF, Pack F, Williams K, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep. 1997;20:267–277. [PubMed] [Google Scholar]
- 8.Belenky G, Wesensten NJ, Thorne DR, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res. 2003;12:1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- 9.van Dongen HPA, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 10.Daan S, Beersma DGM, Borbély AA. Timing of human sleep: Recovery process gated by a circadian pacemaker. Am J Physiol. 1984;246:R161–R183. doi: 10.1152/ajpregu.1984.246.2.R161. [DOI] [PubMed] [Google Scholar]
- 11.Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci. 1995;15:3526–3538. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dijk DJ, Duffy JF, Riel E, Shanahan TL, Czeisler CA. Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. J Physiol. 1999;516.2:611–627. doi: 10.1111/j.1469-7793.1999.0611v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buysse DJ, Ganguli M. Can sleep be bad for you? Can insomnia be good? Arch Gen Psychiatry. 2002;59:137–138. doi: 10.1001/archpsyc.59.2.137. [DOI] [PubMed] [Google Scholar]
- 14.Dinges DF. Sleep debt and scientific evidence. Sleep. 2004;27:1050–1052. [PubMed] [Google Scholar]
- 15.Horne J. Is there a sleep debt? Sleep. 2004;27:1047–1049. [PubMed] [Google Scholar]
- 16.Patrick GTW, Gilbert JA. Studies from the Psychological Laboratory of the University of Iowa. Psychol Rev. 1896;III:468–483. [Google Scholar]
- 17.Feinberg I. Changes in sleep cycle patterns with age. J Psychiatr Res. 1974;10:283–306. doi: 10.1016/0022-3956(74)90011-9. [DOI] [PubMed] [Google Scholar]
- 18.Borbély AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 19.Rosenthal L, Roehrs TA, Rosen A, Roth T. Level of sleepiness and total sleep time following various time in bed conditions. Sleep. 1993;16:226–232. doi: 10.1093/sleep/16.3.226. [DOI] [PubMed] [Google Scholar]
- 20.Roehrs T, Shore E, Papineau K, Rosenthal L, Roth T. A two-week sleep extension in sleepy normals. Sleep. 1996;19:576–582. [PubMed] [Google Scholar]
- 21.Roehrs T, Timms V, Zwyghuizen-Doorenbos A, Roth T. Sleep extension in sleepy and alert normals. Sleep. 1989;1:449–457. doi: 10.1093/sleep/12.5.449. [DOI] [PubMed] [Google Scholar]
- 22.Wehr TA, Moul DE, Barbato G, et al. Conservation of photoperiod-responsive mechanisms in humans. Am J Physiol. 1993;265:R846–R857. doi: 10.1152/ajpregu.1993.265.4.R846. [DOI] [PubMed] [Google Scholar]
- 23.Richardson GS, Drake CL, Roehrs TA, Roth T. Habitual sleep time predicts accuracy of self-reported alertness. Sleep. 2002;25:A145. [Google Scholar]
- 24.Harrison Y, Horne JA. Long-term extension to sleep -- Are we really chronically sleep deprived? Psychophysiol. 1996;33:22–30. doi: 10.1111/j.1469-8986.1996.tb02105.x. [DOI] [PubMed] [Google Scholar]
- 25.Harrison Y, Horne JA. “High sleepability without sleepiness”. ” The ability to fall asleep rapidly without other signs of sleepiness. Neurophysiol Clin. 1996;26:15–20. doi: 10.1016/0987-7053(96)81530-9. [DOI] [PubMed] [Google Scholar]
- 26.Aeschbach D, Postolache TT, Sher L, Matthews JR, Jackson MA, Wehr TA. Evidence from the waking electroencephalogram that short sleepers live under higher homeostatic sleep pressure than long sleepers. Neuroscience. 2001;102:493–502. doi: 10.1016/s0306-4522(00)00518-2. [DOI] [PubMed] [Google Scholar]
- 27.Aeschbach D, Cajochen C, Landolt H-P, Borbély AA. Homeostatic sleep regulation in habitual short sleepers and long sleepers. Am J Physiol. 1996;270:R41–R53. doi: 10.1152/ajpregu.1996.270.1.R41. [DOI] [PubMed] [Google Scholar]
- 28.Benoit O, Foret J, Bouard G, Merle B, Landau J, Marc ME. Habitual sleep length and patterns of recovery sleep after 24 hour and 36 hour sleep deprivation. Electroenceph Clin Neurophysiol. 1980;50:477–485. doi: 10.1016/0013-4694(80)90014-0. [DOI] [PubMed] [Google Scholar]
- 29.Breslau N, Roth T, Rosenthal L, Andreski P. Daytime sleepiness: an epidemiological study of young adults. Am J Public Health. 1997;87:1649–1653. doi: 10.2105/ajph.87.10.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Webb WB, Agnew HW., Jr Are we chronically sleep deprived? Bulletin of the Psychonomic Society. 1975;6:47–48. [Google Scholar]
- 31.Webb WB, Agnew HW., Jr Sleep and waking in a time-free environment. Aerospace Med. 1974;45:617–622. [Google Scholar]
- 32.Webb WB, Agnew HW., Jr Regularity in the control of the free-running sleep-wakefulness rhythm. Aerospace Med. 1974;45:701–704. [PubMed] [Google Scholar]
- 33.Carskadon MA, Dement WC, Mitler MM, Roth T, Westbrook PR, Keenan S. Guidelines for the multiple sleep latency test MSLT. : a standard measure of sleepiness. Sleep. 1986;9:519–524. doi: 10.1093/sleep/9.4.519. [DOI] [PubMed] [Google Scholar]
- 34.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human Subjects. Washington, D.C.: U.S. Government Printing Office, 1968.
- 35.Dijk DJ, Duffy JF, Czeisler CA. Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. J Sleep Res. 1992;1:112–117. doi: 10.1111/j.1365-2869.1992.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 36.Johnson MP, Duffy JF, Dijk DJ, Ronda JM, Dyal CM, Czeisler CA. Short-term memory, alertness and performance: A reappraisal of their relationship to body temperature. J Sleep Res. 1992;1:24–29. doi: 10.1111/j.1365-2869.1992.tb00004.x. [DOI] [PubMed] [Google Scholar]
- 37.Kahneman D, Krueger AB, Schkade DA, Schwarz N, Stone AA. A survey method for characterizing daily life experience: the day reconstruction method. Science. 2004;306:1776–1780. doi: 10.1126/science.1103572. [DOI] [PubMed] [Google Scholar]
- 38.Harrison Y, Horne JA. Should we be taking more sleep? Sleep. 1995;18:901–907. [PubMed] [Google Scholar]
- 39.Rajaratnam SM, Middleton B, Stone BM, Arendt J, Dijk DJ. Melatonin advances the circadian timing of EEG sleep and directly facilitates sleep without altering its duration in extended sleep opportunities in humans. J Physiol. 2004;561(Pt 1):339–351. doi: 10.1113/jphysiol.2004.073742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cajochen C, Brunner DP, Krauchi K, Graw P, Wirz-Justice A. Power density in theta/alpha frequencies of the waking EEG progressively increases during sustained wakefulness. Sleep. 1995;18:890–894. doi: 10.1093/sleep/18.10.890. [DOI] [PubMed] [Google Scholar]
- 41.Cajochen C, Knoblauch V, Krauchi K, Renz C, Wirz-Justice A. Dynamics of frontal EEG activity, sleepiness and body temperature under high and low sleep pressure. Neuroreport. 2001;12:2277–2281. doi: 10.1097/00001756-200107200-00046. [DOI] [PubMed] [Google Scholar]
- 42.Leproult R, Colecchia EF, Berardi AM, Stickgold R, Kosslyn SM, Van Cauter E. Individual differences in subjective and objective alertness during sleep deprivation are stable and unrelated. Am J Physiol Regul Integr Comp Physiol. 2003;284:R280–R290. doi: 10.1152/ajpregu.00197.2002. [DOI] [PubMed] [Google Scholar]
- 43.Van Dongen HP, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep. 2004;27:423–433. [PubMed] [Google Scholar]
- 44.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 45.Spiegel K, Leproult R, L’Hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89:5762–5771. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 46.Buxton OM, Spiegel K, Van Cauter E. Modulation of endocrine function and metabolism by sleep and sleep loss. In: Lee-Chiong M, Carskadon M, Sateia M, editors. Sleep Medicine. Philadelphia: Hanley & Belfus, Inc., 2002: 59–69.
- 47.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 48.Leproult R, Copinschi G, Buxton O, Van Cauter E. Sleep loss results in an elevation of cortisol levels the next evening. Sleep. 1997;20:865–870. [PubMed] [Google Scholar]
- 49.Vigg A, Vigg A, Vigg A. Sleep in Type 2 diabetes. J Assoc Physicians India. 2003;51:479–481. [PubMed] [Google Scholar]
- 50.Taheri S, Lin L, Austin D, Young T, Mignot E. Short Sleep Duration Is Associated with Reduced Leptin, Elevated Ghrelin, and Increased Body Mass Index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vgontzas AN, Zoumakis E, Bixler EO, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89:2119–2126. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]


