Abstract
Ω4514 is the site of a Tn5 lac insertion in the Myxococcus xanthus genome that fuses lacZ expression to a developmentally regulated promoter. DNA upstream of the insertion site was cloned, and the promoter was localized. The promoter resembles vegetative promoters in sequence, and σA RNA polymerase, the major form of RNA polymerase in growing M. xanthus, initiated transcription from this promoter in vitro. Two complete open reading frames were identified downstream of the promoter and before the Ω4514 insertion. The first gene product (ORF1) has a putative helix-turn-helix DNA-binding motif and shows sequence similarity to transcriptional regulators. ORF2 is most similar to subunit A of glutaconate coenzyme A (CoA) transferase, which is involved in glutamate fermentation. Tn5 lac Ω4514 is inserted in the third codon of ORF3, which is similar to subunit B of glutaconate CoA-transferase. An orf1 disruption mutant exhibited a mild sporulation defect, whereas neither a disruption of orf2 nor insertion Ω4514 in orf3 caused a defect. Based on DNA sequence analysis, the three genes are likely to be cotranscribed with a fourth gene whose product is similar to alcohol dehydrogenases. ORF1 delays and reduces expression of the operon during development, but relief from this negative autoregulation does not fully explain the regulation of the operon, because expression from a small promoter-containing fragment is strongly induced during development of an orf1 mutant. Also, multiple upstream DNA elements are necessary for full developmental expression. These results suggest that transcriptional activation also regulates the operon. Ω4514 is the first example of a developmentally regulated M. xanthus operon that is transcribed by the major vegetative RNA polymerase, and its regulation appears to involve both negative autoregulation by ORF1 and positive regulation by one or more transcriptional activators.
Myxococcus xanthus is a gram-negative soil bacterium that undergoes multicellular development (11, 12). When starved at high cell density on a solid surface, cells move in a coordinated fashion into aggregation centers, where they form mound-shaped fruiting bodies that each contain approximately 105 cells. Within the fruiting bodies, some of the rod-shaped cells differentiate into dormant, ovoid spores that are heat and desiccation resistant.
Cell-cell interactions play a critical role in this multicellular developmental process (47). At least five extracellular signals, known as the A, B, C, D, and E signals, appear to be involved (10, 19). Mutants defective in the production of any one of these signals are arrested in development at a particular stage but are rescued by codevelopment with wild-type cells or cells that are defective in the production of a different signal.
To study the role of cell-cell interactions in controlling gene expression during M. xanthus development, Tn5 lac, a transposon containing a promoterless Escherichia coli lacZ gene, has been used to identify developmentally regulated genes (32). Transposition of Tn5 lac into the M. xanthus chromosome can generate a transcriptional fusion between lacZ and an M. xanthus promoter. Among 2,374 Tn5 lac insertions, 29 of them were activated during M. xanthus development (34). The dependence of developmental gene expression on cell-cell interactions was examined by monitoring β-galactosidase expression of the lacZ fusions in cell interaction mutants. A and B signaling are required for normal developmental gene expression at the onset of development (17, 33, 35), D and E signaling are required at 3 to 5 h into development (7, 10), and C signaling is required at about 6 h into development (33, 38).
To begin to understand the mechanism by which C signaling regulates developmental gene expression, the promoter regions of transcriptional units identified by Tn5 lac insertions Ω4403, Ω4400, and Ω4499 have been characterized (5, 14, 15). The promoters are not similar in the −10 and −35 regions and do not resemble promoters that are transcribed by E. coli σ70 or σ54 RNA polymerase (RNAP). However, both Ω4403 and Ω4400 have the sequence 5′-CATCCCT-3′ centered at −49 bp. Similar sequences are also found in the Ω4499 promoter region and other C-signal-dependent promoters at positions near −50 bp (14). Hence, a sequence with the consensus 5′-CAYYCCY-3′ (known as the C box) has been proposed to be important for C-signal-dependent gene expression.
Here, we report studies on the regulation of Ω4514, a Tn5 lac insertion that does not depend upon C signaling for expression and yet is expressed with timing during development similar to that of promoters that do depend upon C signaling. The Ω4514 promoter is different from other M. xanthus development-specific promoters that have been characterized in that it resembles promoters transcribed by E. coli σ70 and it can be transcribed by σA RNAP isolated from vegetative M. xanthus cells. No C-box sequence is present near −50 bp. Regulation of the Ω4514 operon is complex, involving negative autoregulation by the product of the first gene of the operon and positive regulation mediated by upstream DNA elements. These studies provide the first example of a developmentally regulated M. xanthus gene that is transcribed by the major vegetative RNAP and establish a foundation for biochemical approaches toward identifying proteins that regulate Ω4514 expression.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Strains and plasmids used in this work are listed in Table 1.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α | φ80 lacZΔM15 ΔlacU169 recA1 endA1 hsdR17 supE44 thi-1 gyrA relA1 | 21 |
| JM83 | ara Δlac-pro strA thi φ80 lacZΔM15 | 42 |
| BL21(DE3) | F−ompT hsdSB (rB− mB−) gal dcm with DE3, a λ prophage carrying the T7 RNAP gene | Novagen |
| M. xanthus | ||
| DK1622 | Wild type | 29 |
| DK4514 | Tn5 lac (Kmr) Ω4514 | 34 |
| MMF1727 | attB::pREG1727 | 15 |
| MTH1-3 | attB::pTH1-6 | This study |
| MTH4-1 | attB::pTH4-1 | This study |
| MTH4-2 | attB::pTH4-2 | This study |
| MTH4-3 | attB::pTH4-3 | This study |
| MTH5-3 | attB::pTH5-3 | This study |
| MTH5-4 | attB::pTH5-4 | This study |
| MTH5-5 | attB::pTH5-5 | This study |
| MTH10-3 | attB::pTH10-3 | This study |
| MTH11-1 | attB::pTH10-3 orf2::pTH11-1 | This study |
| MTH11-2 | orf2::pTH11-1 | This study |
| MGV01 | orf1::pBRE47Sp4514 | This study |
| MGV02 | attB::pTH10-3 orf1::pBRE47Sp4514 | This study |
| MTH8-1 | Tn5 lac (Tcr) Ω4514 | This study |
| MTH9-1 | MTH8-1::pTH9-1, Kmr Tcr | This study |
| MTH9-2 | MTH8-1::pTH9-2, Kmr Tcr | This study |
| MTH9-4 | MTH8-1::pTH9-4, Kmr Tcr | This study |
| DK5208 | csgA::Tn5-132 (Tcr) Ω205 | 48 |
| MTH7-1 | csgA::Tn5-132 (Tcr) Ω205 attB::pTH1-6 | This study |
| MTH7-2 | csgA::Tn5-132 (Tcr) Ω205 attB::pTH4-3 | This study |
| MTH7-3 | csgA::Tn5-132 (Tcr) Ω205 attB::pTH4-1 | This study |
| JPB03 | csgA::Tn5-132 (Tcr) Ω205 attB::pREG1727 | 5 |
| DK5225 | csgA::Tn5-132 (Tcr) Ω205 Tn5 lac (Kmr) Ω4514 | 33 |
| MGV03 | DK1622::p1175SmSl4514 | This study |
| Plasmids | ||
| pUC19 | Aprlacα | 54 |
| pTH4-4 | Apr (pUC19)a; 1.6-kb SmaI-BamHI fragment from pTH1-3 | This study |
| pTH4-6 | Apr (pUC19); 0.7-kb SacI-BamHI fragment from pTH4-4 | This study |
| pTH4-7 | Apr (pUC19); 1.3-kb SphI-BamHI fragment from pTH4-4 | This study |
| pTH7-1 | Apr (pUC19); 2.2-kb SalI-BamHI fragment from pTH1-3 | This study |
| pTH7-2 | Apr (pUC19); 4.3-kb XhoI-BamHI fragment from pTH1-3 | This study |
| pTH10-1 | Apr (pUC19); 120-bp SmaI-Eco47III fragment from pTH4-4 | This study |
| pGEM-7Zf | Aprlacα | Promega |
| pTH1-3 | Apr Kmr (pGEM-7Zf); 13.3-kb XhoI fragment from DK4514 | This study |
| pTH5-1 | Apr (pGEM-7Zf); 0.7-kb EcoRI-BamHI fragment from pTH4-6 | This study |
| pTH5-2 | Apr (pGEM-7Zf); 1.3-kb NruI-BamHI fragment from pTH4-4 | This study |
| pGEME47Sp4514 | Apr (pGEM-7Zf); 242-bp Eco47III-SphI fragment from pTH4-4 | This study |
| pTH9-3 | Apr (pGEM-7Zf); 4.3-kb HindIII-BamHI fragment from pTH7-2 | This study |
| pBR322 | Apr Tcr | 3 |
| pBRE47Sp4514 | Tcr (pBR322); 250-bp NsiI-AatII fragment from pGEME47Sp4514 | This study |
| pTH11-1 | Tcr (pBR322); 323-bp fragment of orf2 generated by PCR of pTH4-4 | This study |
| pET21a | Apr | Novagen |
| pTH191 | Apr (pET21a); complete orf1 sequence generated by PCR of pTH4-4 with primers LK190 and LK191 | This study |
| pTH192 | Apr (pET21a); complete orf1 sequence followed by a stop codon generated by PCR of pTH4-4 with primers LK190 and LK192 | This study |
| pET16b | Apr | Novagen |
| pTH193 | Apr (pET16b); complete orf1 sequence generated by PCR of pTH4-4 with primers LK190 and LK193 | This study |
| pREG1727 | Apr Kmr P1-inc attP ′lacZ | 15 |
| pTH1-6 | Apr Kmr (pREG1727); 4.3-kb XhoI-BamHI fragment from pTH1-3 | This study |
| pTH4-1 | Apr Kmr (pREG1727); 2.2-kb SalI-BamHI fragment from pTH1-3 | This study |
| pTH4-2 | Apr Kmr (pREG1727); 2.1-kb XhoI-SalI fragment from pTH1-3 | This study |
| pTH4-3 | Apr Kmr (pREG1727); 1.6-kb SmaI-BamHI fragment from pTH1-3 | This study |
| pTH5-3 | Apr Kmr (pREG1727); 0.7-kb XhoI-BamHI fragment from pTH5-1 | This study |
| pTH5-4 | Apr Kmr (pREG1727); 1.3-kb XhoI-BamHI fragment from pTH5-2 | This study |
| pTH5-5 | Apr Kmr (pREG1727); 1.3-kb HindIII-BamHI fragment from pTH4-7 | This study |
| pTH10-3 | Apr Kmr (pREG1727); 120-bp HindIII-BamHI fragment from pTH10-1 | This study |
| pREG429 | Apr Kmr P1-inc | 18 |
| pTH9-1 | Apr Kmr (pREG429); 1.6-kb EcoRI-BamHI fragment from pTH4-4 | This study |
| pTH9-2 | Apr Kmr (pREG429); 2.2-kb AccI-BamHI fragment from pTH7-1 | This study |
| pTH9-4 | Apr Kmr (pREG429); 4.3-kb EcoRI-BamHI fragment from pTH9-3 | This study |
| pREG1175 | Apr Kmr P1-inc ′lacZ | 16 |
| p1175SmSl4514 | Apr Kmr (pREG1175); 2.7-kb XhoI-SmaI fragment from pTH7-2 | This study |
The vector is indicated in parentheses.
Growth and development.
E. coli cells were grown at 37°C in Luria-Bertani (LB) medium (44) containing 50 μg of ampicillin, 25 μg of kanamycin, or 10 μg of tetracycline per ml, as necessary. M. xanthus was grown at 32°C in CTT medium (26) in liquid culture or on agar (1.5%) plates with 40 μg of kanamycin or 12.5 μg of oxytetracycline per ml when required. Fruiting body development was performed on TPM (10 mM Tris-HCl [pH 8.0], 1 mM KH2PO4, 8 mM MgSO4, final pH 7.6) agar (1.5%) plates as described previously (34).
Molecular cloning.
Recombinant DNA work was performed by standard techniques (44). Plasmid DNA was prepared from E. coli DH5α or JM83.
To clone the DNA upstream of Tn5 lac Ω4514, chromosomal DNA was prepared (36) from M. xanthus DK4514 and digested with XhoI, the fragments were ligated to XhoI-digested pGEM-7Zf, and the mixture was transformed into E. coli DH5α, selecting for both ampicillin resistance (Apr) of the vector and kanamycin resistance (Kmr) of the desired insert. One transformant with a plasmid bearing an insert of the expected size was characterized further. Restriction fragments of M. xanthus DNA from this plasmid, pTH1-3, were gel purified and ligated into vectors as indicated in Table 1. In these and the subsequent subcloning steps described for Table 1, vectors were digested with the same restriction enzymes used to produce the fragments, except as indicated below.
To construct pTH5-2, the 0.9-kb NruI-BamHI fragment from pTH4-4 was gel purified and ligated into pGEM-7Zf that had been digested with SmaI and BamHI.
Plasmid pTH9-2 was constructed as follows. The 2.2-kb AccI-BamHI fragment from pTH7-1 was gel purified and ligated into pREG429, which had been digested with ClaI and BamHI.
To subclone the 120-bp SmaI-Eco47III fragment from the region upstream of Tn5 lac Ω4514 into pREG1727, the fragment was gel purified after digestion of pTH4-4 and ligated into SmaI-digested pUC19. A plasmid with the fragment in the correct orientation for further cloning into pREG1727 (i.e., the SmaI end of the fragment close to the HindIII site in pUC19) was identified and named pTH10-1. A HindIII-BamHI fragment of pTH10-1 that contains the 120-bp SmaI-Eco47III fragment of Ω4514 was obtained and cloned into HindIII-BamHI-linearized pREG1727, resulting in pTH10-3, in which the 120-bp SmaI-Eco47III fragment was positioned before the promoterless lacZ.
Plasmid pGEME47Sp4514 was constructed by digestion of pTH4-4 with Eco47III and SphI, gel purification of the resulting 242-bp fragment, and ligation into pGEM-7Zf, which had been linearized with SmaI and SphI. This plasmid was digested with NsiI and AatII to obtain a 250-bp fragment, which was then ligated into AatII-PstI-digested pBR322 to construct pBRE47Sp4514.
Plasmid pTH11-1 was constructed by ligating part of the orf2 sequence into pBR322. The partial orf2 sequence was generated by PCR using pTH4-4 as a template. The upstream primer was 5′-AAACTGCAGTCCGGATGGCGCGTCG-3′, which has an underlined PstI site and anneals to the sequence between positions 814 and 829, and the downstream primer was 5′-AAGAATTCGGGATGAAGGGCAGCC-3′, which has an underlined EcoRI site and anneals to the sequence between positions 1137 and 1122. The amplified fragment was digested with PstI and EcoRI, gel purified, and ligated to PstI-EcoRI-digested pBR322 to construct pTH11-1. The insert was sequenced to ensure that no error occurred during the PCR.
Plasmids pTH191, pTH192, and pTH193 were constructed as follows. The insert for each plasmid was generated by PCR with the same upstream primer, 5′-AGAAGGGACATATGACGAACACCGGAGGA-3′, which has an NdeI site (underlined) and the first 18 bases of the orf1 sequence. The downstream primer (LK191) for generating the insert of pTH191 was 5′-CTCCTCGAGTGCGTCCTCCGAATCCGT-3′, which has a XhoI site (underlined) and the last 18 bases of the orf1 sequence without a stop codon. The downstream primer (LK192) for generating the insert of pTH192 was 5′-CTCCTCGAGTCATGCGTCCTCCGAATC-3′, which has the stop codon TGA adjacent to the XhoI site. The downstream primer (LK193) for generating the insert of pTH193 was like LK192, except that it had a BamHI site instead of the XhoI site. The amplified fragments were digested with appropriate restriction enzymes, gel purified, and ligated into NdeI-XhoI-digested pET21a to construct pTH191 and pTH192 or into NdeI-BamHI-digested pET16b to construct pTH193. Plasmids pET21a and pET16b are designed for high-level protein expression in E. coli under the control of T7 RNAP. The orf1 DNA was upstream of an in-frame sequence encoding a six-His tag followed by a stop codon in pTH191 (i.e., able to produce ORF1-6xHis), while in pTH192, the orf1 DNA was followed immediately by a stop codon (to produce native ORF1). In pTH193, the orf1 DNA was downstream of an in-frame sequence encoding a six-His tag (to produce 6xHis-ORF1). The inserts and junctions were sequenced to ensure that no error occurred during the PCR.
To construct p1175SmSl4514, the 2.7-kb XhoI-SmaI fragment from pTH4-4 was gel purified and ligated into pREG1175 that had been digested with SalI and SmaI.
DNA sequencing.
Plasmid pTH4-4 was used as the template in sequencing reactions performed by the method of Sanger et al. (45) with a Sequenase kit (United States Biochemical). Ambiguities arising from premature termination were resolved by the protocol of Fawcett and Bartlett (13). Briefly, 1 μl of a reaction mixture containing terminal deoxynucleotide transferase (1 μM [each] deoxynucleoside triphosphate [pH 7.0], 2 U of terminal deoxynucleotide transferase per μl, 1× Sequenase reaction buffer) was added to each of the termination reaction mixtures (16 μl) and incubated at 37°C for 30 min. The reaction was terminated by adding 4 μl of stop buffer (United States Biochemical). 7-Deaza-dGTP reaction mixtures were used to resolve regions of compression. DNA, and protein sequence analyses were done with the University of Wisconsin Genetics Computer Group software package. The Michigan State University Macromolecular Structure Facility synthesized oligonucleotide primers as needed to sequence both strands of the 1.9-kb Ω4514 upstream DNA.
Construction of M. xanthus strains.
Strains containing pREG1727 derivatives integrated at Mx8 attB were constructed by P1 specialized transduction from the rec+ E. coli strain JM83 (18) or by electroporation (30) of the wild-type M. xanthus strain DK1622 or the csgA mutant strain DK5208. Except where noted otherwise, at least three derivatives, each containing a single copy of integrated plasmid, were identified by Southern blot analysis (15, 44), and β-galactosidase production was measured under developmental conditions as described previously (34).
Strain MTH8-1 was constructed by transducing bacteriophage P1::Tn5 lac (Tcr) (a gift from R. Gill) into DK4514 with selection for oxytetracycline resistance. Screening for kanamycin-sensitive transductants identified MTH8-1, in which the Kmr gene was replaced by the Tcr gene, as verified by Southern blot analysis (5). MTH9-1, MTH9-2, and MTH9-4 are Kmr Tcr strains resulting from P1 specialized transduction of pTH9-1, pTH9-2, and pTH9-4, respectively, from E. coli JM83 into M. xanthus MTH8-1.
MGV01 and MTH11-1 were constructed by electroporating pBR322 derivatives pBRE47Sp4514 and pTH11-1 into DK1622 and MTH10-3, respectively. Three transformants containing a single copy of either plasmid integrated by homologous recombination at orf1 or orf2 were identified by Southern blot analysis and PCR (data not shown). Likewise, MGV03 was constructed by electroporating p1175SmSl4514 into DK1622.
Preparation of M. xanthus genomic DNA for PCR.
Diagnostic PCR was used to identify M. xanthus integrants by preparing genomic DNA as described by Magrini et al. (41) with the following modifications. Cells were grown in CTT medium with appropriate antibiotics, collected by centrifugation, and resuspended in 500 μl of Tris-EDTA with 100 μg of RNase A/ml. The cell suspension was incubated first at room temperature for 15 min and then at 85°C for 15 min, followed by extraction with 1 volume of phenol and then two extractions with 1 volume of chloroform. The aqueous supernatant after microcentrifugation was used for the PCR.
RNA analysis.
RNA was prepared as described previously (15) from M. xanthus DK1622. An S1 nuclease protection assay was performed with a probe from pTH7-2 digested with NruI. The 2.6-kb fragment was gel purified, phosphatase treated, and 5′ end labeled with [γ-32P]ATP and T4 polynucleotide kinase. RNA (100 μg) was precipitated with the labeled probe. The pellet was resuspended in 30 μl of hybridization buffer (80% formamide, 20 μM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)] [pH 6.4], 0.4 μM NaCl) and incubated for 10 min at 85°C followed by 3 h at 52°C. S1 nuclease buffer (0.03 M NaOAc [pH 4.6], 0.05 M NaCl, 1 mM ZnSO4, 5% glycerol) and 500 U of S1 nuclease (Boehringer Mannheim) were then added, giving a final volume of 300 μl. After 30 min at 37°C, the reaction mixtures were extracted with 300 μl of phenol-chloroform, precipitated with ethanol, and resuspended in formamide loading buffer (80% formamide, 10 mM EDTA, 0.01% xylene cyanol, 0.01% bromophenol blue). The protected products were resolved on a 5% polyacrylamide-8 M urea gel and visualized by autoradiography.
Primer extension analysis was performed as described previously (44), using the oligonucleotide 5′-GATCTCCTGCATCGACGTGCCCTC-3′, which corresponds to a sequence located about 140 bp downstream of the mRNA 5′ end mapped by S1 nuclease protection. The primer was end labeled with T4 polynucleotide kinase and [γ-32P]ATP and purified with a QIAquick Nucleotide Removal kit (Qiagen). The reaction products were analyzed on a 5% polyacrylamide-8 M urea sequencing gel next to dideoxy sequencing reactions that utilized the same primer.
In vitro transcription.
Reactions were performed with partially purified M. xanthus σA RNAP or reconstituted σA RNAP holoenzyme as described previously (2).
Western blot analysis.
To prepare an extract of E. coli containing ORF1, BL21(λDE3) containing pTH192 was grown in LB medium containing ampicillin and harvested by centrifugation 3 h after induction with 0.4 mM IPTG (isopropyl-β-d-thiogalactopyranoside). The cell pellet was resuspended in 1× sample buffer (0.125 M Tris-HCl [pH 6.8], 5% β-mercaptoethanol, 2% sodium dodecyl sulfate [SDS], 10% glycerol, 0.1% bromophenol blue) and boiled for 5 min. To prepare whole-cell extracts containing M. xanthus proteins, DK1622 and orf1 mutant (MGV01) cells were collected at the indicated times, resuspended in sample buffer, and boiled for 5 min. Equal amounts of M. xanthus protein, as determined by measuring total protein in sonic lysates of the same samples by the Bradford method (4), were subjected to Western blot analysis. Proteins were separated by SDS-10 or 12% polyacrylamide gel electrophoresis (PAGE) and electrotransferred to a nitrocellulose or polyvinylidene difluoride membrane. The membrane was incubated in TBST (20 mM Tris-HCl [pH 7.5], 0.5 M NaCl, 0.1% Tween 20) containing 2% nonfat dry milk for 4 h at room temperature with shaking in order to block nonspecific interaction between the primary antibodies and the membrane. The membrane was then probed for 4 h with shaking at room temperature with either a polyclonal antiserum to Bacillus subtilis σ43 (a gift from B.-Y. Chang and R. Doi) or affinity-purified anti-ORF1-6xHis antibody diluted 1:500 in TBST-2% nonfat dry milk. Immunodetection with goat anti-rabbit immunoglobulin G-horseradish peroxidase (Bio-Rad) and chemiluminescence (ECL; Amersham Pharmacia Biotech) was performed according to the manufacturer's instructions.
Production of ORF1-6xHis.
pTH191 was transformed into E. coli strain BL21(λDE3), which contains the gene for T7 RNAP under the control of an IPTG-inducible lacUV5 promoter. Cells containing pTH191 were inoculated into LB medium containing ampicillin and grown at 37°C until the optical density at 600 nm reached about 0.6, and then the culture was induced with 0.4 mM IPTG. Cells were collected 3 h after the induction by centrifugation (5,000 × g, 5 min), and the cell pellet was stored at −70°C. Since the initial experiments suggested that ORF1-6xHis was located primarily in inclusion bodies (data not shown), cells were grown at 30°C and induced with IPTG as described above to maximize the proportion of ORF1-6xHis in the soluble fraction. To prepare a whole-cell extract, the cell pellet was resuspended in buffer containing 5 mM imidazole, 0.5 M NaCl, 20 mM Tris-Cl (pH 7.9), and 1 mM phenylmethylsulfonyl fluoride. Cells were lysed by sonication (Sonicator W-225; microtip maximum power setting at 6; Heat System-Ultrasonics, Inc.) while the mixture was kept cold on ice. The lysate was centrifuged at 39,000 × g for 20 min at 4°C. The ORF1-6xHis protein in the supernatant was isolated under nondenaturing conditions by nickel chelate affinity chromatography as follows. A well-packed column was washed with 5 volumes of column binding buffer (5 mM imidazole, 0.5 M NaCl, 20 mM Tris-Cl [pH 7.9]) and allowed to drain until the buffer reached the top of the column bed. After the whole-cell extract was loaded onto the column, it was washed with 10 volumes of column binding buffer, followed by 6 volumes of wash buffer (60 mM imidazole, 0.5 M NaCl, 20 mM Tris-Cl [pH 7.9]). Proteins were then eluted with 6 volumes of 1 M imidazole-0.5 M NaCl-20 mM Tris-Cl (pH 7.9). The eluate was dialyzed against gel shift binding buffer (12% glycerol, 20 mM HEPES [pH 7.9], 50 mM KCl, 1 mM EDTA, 1 mM dithiothreitol, 5 mM MgCl2) for 16 h at 4°C. The protein solution was then concentrated 20-fold by centrifugation in a Millipore 15 filter device for 3 h at 4°C according to the manufacturer's specifications and stored at 4°C.
Mobility shift experiments.
The SmaI-Eco47III fragment containing DNA from −54 bp to +65 bp relative to the transcriptional start site was used as a probe in mobility shift experiments. This fragment from pTH4-4 digested with SmaI and Eco47III was gel purified, phosphatase treated, and 5′ end labeled with [γ-32P]ATP and T4 polynucleotide kinase. The labeled DNA was purified with a QIAquick Nucleotide Removal kit (Qiagen). Various amounts of affinity-purified ORF1-6xHis protein or crude extracts made from cells overexpressing ORF1-6xHis, ORF1, or 6xHis-ORF1 were incubated with 4.5 ng (57 fmol) of probe and 1 μg of poly(dI-dC) at 30°C for 15 min to allow DNA binding. These 20-μl binding reaction mixtures also contained 12% glycerol, 20 mM HEPES (pH 7.9), 50 mM KCl, 1 mM EDTA, 1 mM dithiothreitol, and 5 mM MgCl2. Samples were then subjected to electrophoresis on a 5 or 8% polyacrylamide gel in low-ionic-strength buffer at 4°C (8). After electrophoresis, the gels were dried and exposed to film.
Preparation and purification of antibodies.
Purified ORF1-6xHis protein (500 μg) was mixed with Freund's complete adjuvant (Gibco BRL) and injected subcutaneously into a rabbit. Four weeks later, a booster injection (300 μg of ORF1-6xHis mixed with Freund's incomplete adjuvant) was performed. The rabbit was bled 1 week after the boost, and the serum was prepared as described previously (22).
Affinity purification of the antibodies was performed as follows. Purified ORF1-6xHis protein (100 μg) was electrophoresed on a 12% polyacrylamide-SDS gel, and the protein was electrotransferred to a polyvinylidene difluoride membrane (Millipore). The region of the membrane with bound ORF1-6xHis was excised and incubated with TBST (20 mM Tris-HCl [pH 7.5], 0.5 M NaCl, 0.05% Tween 20) containing 5% nonfat dry milk for 2 h to block nonspecific binding of the antibodies. Anti-ORF1-6xHis antiserum (2 ml) was incubated with the membrane overnight at 4°C with agitation. The membrane was then washed twice (5 min each) with TBST and twice with TBS (20 mM Tris-HCl [pH 7.5], 0.5 mM NaCl). To elute antibodies bound to the membrane, 1 ml of 100 mM glycine (pH 2.5) was added and incubated for 10 s with shaking. The solution was collected and immediately neutralized to pH 7.0 by adding approximately 100 μl of 1 M Tris-Cl (pH 8.0). The elution steps were repeated twice, and the neutralized solutions were combined and stored at 4°C.
Sporulation efficiency.
Cells were plated for development on TPM agar and harvested after 3 days at 32°C, then the samples were subjected to heat and sonication treatment to kill rod-shaped cells, and dilutions were plated to quantitate the number of spores able to germinate and form colonies, as described previously (33).
Nucleotide sequence accession number.
The DNA sequence of 1,914 bp of M. xanthus DNA immediately upstream of the Ω4514 insertion site has been deposited in the GenBank database under accession no. AF432227.
RESULTS
Cloning DNA upstream of Ω4514 and testing it for promoter activity.
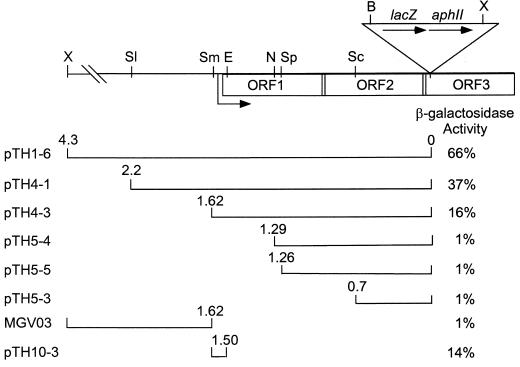
To clone the DNA upstream of the developmentally regulated Tn5 lac insertion Ω4514, we took advantage of a XhoI restriction site approximately 4.3 kb upstream of the Ω4514 insertion in M. xanthus DK4514 (34) and a XhoI site in Tn5 lac (32) approximately 9 kb from the left end (Fig. 1). Digestion of chromosomal DNA from DK4514 with XhoI was expected to yield a 13.3-kb fragment that includes the aphII gene encoding aminoglycoside phosphotransferase which confers Kmr when cloned in E. coli. The 13.3-kb fragment was cloned as described in Materials and Methods. Restriction mapping of the resulting plasmid, pTH1-3 (Table 1), showed the patterns expected on the basis of restriction sites in DNA upstream of Ω4514 (34) and restriction sites in Tn5 lac and the vector. Figure 1 shows the restriction map of DNA upstream of Ω4514.
FIG. 1.
Physical map of the Ω4514 insertion region and summary of upstream segments tested for promoter activity. The upper schematic depicts the restriction sites in Tn5 lac and the upstream M. xanthus chromosome that were used in this study. B, BamHI; E, Eco47III; N, NruI; Sc, SacI; Sl, SalI; Sm, SmaI; Sp, SphI; X, XhoI. Also shown are the transcription start site (right-angled arrow), and three ORFs deduced from the DNA sequence. The lower diagram depicts the segments of Ω4514 upstream DNA fused to the promoterless lacZ gene in pREG1727 or in pREG1175, in the case of strain MGV03, to test for promoter activity. The plasmid or strain designation is indicated on the left (Table 1). The numbers above the ends of the segments indicate the distances in kilobases from the Tn5 lac Ω4514 insertion. Derivatives of wild-type M. xanthus DK1622 containing a single copy of each pREG1727 derivative integrated at Mx8 attB or a single copy of the pREG1175 derivative integrated by homologous recombination were measured for β-galactosidase activity. The maximum β-galactosidase specific activity during a 72-h developmental time course is given as a percentage of the maximum activity observed for Tn5 lac Ω4514-containing strain DK4514. In each case, the activity of at least three independent transductants was measured at least once as described previously (34), and the average is given.
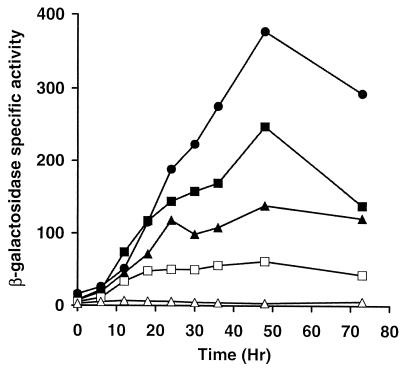
To test Ω4514 upstream DNA for promoter activity, the XhoI-BamHI restriction fragment from pTH1-3, which contains 4.3 kb of M. xanthus DNA and approximately 60 bp of the left end of Tn5 lac (Fig. 1), was subcloned into XhoI-BamHI-digested pREG1727 to construct pTH1-6 (Table 1). Since the BamHI site of pREG1727 is located immediately upstream of the same lacZ-containing fragment found in Tn5 lac (15), pTH1-6 contains Ω4514 upstream DNA fused to a promoterless lacZ gene in the same manner as Ω4514-containing M. xanthus DK4514. pTH1-6 was transduced from E. coli JM83 into the wild-type M. xanthus strain DK1622 by bacteriophage P1 specialized transduction (18). Due to the presence of the attP segment from myxophage Mx8, pTH1-6 integrated efficiently into the M. xanthus chromosome at attB (50, 51). Transductants containing a single copy of pTH1-6 integrated at Mx8 attB were identified by Southern blot hybridization (data not shown). Several of these transductants were assayed for β-galactosidase activity during development and showed, on average, 66% of the maximal level observed in DK4514 (Fig. 1). The timing of β-galactosidase production from pTH1-6 was similar to that in DK4514 (Fig. 2). The results indicate that the 4.3-kb Ω4514 upstream DNA segment contains a promoter that is able to direct development-specific expression.
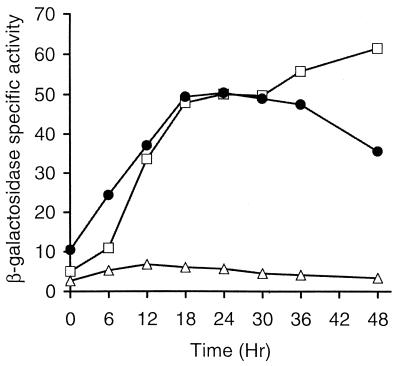
FIG. 2.
Developmental expression of lacZ under the control of the Ω4514 promoter. Developmental β-galactosidase specific activity was measured as described previously (34) for Tn5 lac-containing strain DK4514 (•) and for at least three independent transductants of DK1622 containing a single copy of the 4.3-kb (▪), 2.2-kb (▴), or 1.62-kb (□) Ω4514 upstream DNA fused to promoterless lacZ within pREG1727 and integrated at Mx8 attB. The β-galactosidase specific activity of DK1622 containing the pREG1727 vector alone with no insert integrated at Mx8 attB (▵) is also shown. The average β-galactosidase specific activity from three determinations for DK4514 and from at least one determination for each of three independent transductants is plotted. β-Galactosidase specific activity is expressed in nanomoles of o-nitrophenyl phosphate per minute per milligram of protein.
To further localize the developmental promoter within this region, additional deletions of the Ω4514 upstream DNA were generated and tested for promoter activity as described above. The 2.2- and 1.62-kb Ω4514 upstream DNA segments directed lacZ expression with similar developmental timing as the Ω4514-containing strain but reached about 37 and 16% of the maximum level, respectively (Fig. 1 and 2). Transductants containing the 1.29-, 1.26-, or 0.7-kb Ω4514 upstream DNA segments failed to express lacZ above a low background level (Fig. 1). These results indicate that part of a development-specific promoter driving the expression of Tn5 lac Ω4514 lies between the SmaI site at 1.62 kb upstream and the NruI site at 1.29 kb upstream and that DNA farther upstream also contributes significantly to promoter activity.
To test for an additional promoter upstream of the SmaI site, the 2.7-kb XhoI-SmaI fragment was inserted into pREG1175 (16), resulting in p1175SmSl4514, in which the XhoI-SmaI fragment is fused to the same promoterless lacZ-containing fragment found in Tn5 lac. Since pREG1175 does not contain the attP segment of Mx8, when p1175SmSl4514 was transduced into wild-type DK1622, homologous recombination between the plasmid M. xanthus DNA segment and the M. xanthus chromosome resulted in MGV03, in which the lacZ reporter is positioned immediately downstream of the SmaI site in the chromosome. No developmental promoter activity was observed in three such independent transductants (Fig. 1), providing no evidence for a promoter upstream of the SmaI site.
Localization of an mRNA 5′ end upstream of Ω4514.
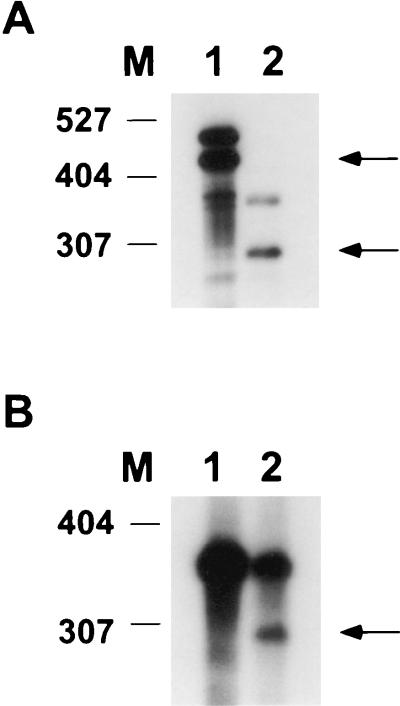
To test whether an mRNA 5′ end maps upstream of the Ω4514 insertion, an S1 nuclease protection assay was performed with a probe labeled at the NruI site located 1.29 kb upstream of Ω4514 (Fig. 1) and including about 2.6 kb of M. xanthus DNA farther upstream. The probe was hybridized to RNA from M. xanthus DK1622 and subjected to S1 nuclease digestion. The probe protected a development-specific RNA species as shown in Fig. 3. RNA from developing cells harvested at 12 or 18 h after starvation protected a fragment of about 270 bases in length. No protection was observed with RNA from growing cells, and very weak protection was observed with RNA from 24-h developing cells. These results indicate that a development-specific mRNA is transcribed from the region upstream of the Ω4514 Tn5 lac insertion and that the 5′ end of this mRNA species is located approximately 1.56 kb upstream of the insertion site. Together with the deletion analysis (Fig. 1 and 2), the results suggest that a promoter lies 1.56 kb upstream of Ω4514 and that the mRNA 5′ end reflects the transcriptional start site.
FIG. 3.
Localization of an mRNA 5′ end within the Ω4514 upstream region. Low-resolution S1 nuclease mapping of the Ω4514-associated transcript was performed by using RNA prepared from DK1622 cells growing vegetatively (lane 1) or cells that had undergone 12, 18, or 24 h of development in lanes 2 to 4, respectively. The numbers on the left indicate the length (in bases) of end-labeled MspI-digested pBR322 marker fragments.
DNA sequence of the Ω4514 region.
The nucleotide sequence of both strands of 1.9 kb of DNA immediately upstream of the Ω4514 insertion site was determined and deposited in GenBank. Two complete open reading frames (ORFs) and one partial ORF interrupted by Ω4514 were inferred from the sequence downstream of the Ω4514 transcriptional start site. The two ORFs exhibit a codon preference typical of M. xanthus genes, including a strong bias toward usage of guanine or cytosine at the third codon position (46). The first ORF (ORF1), beginning with GTG at position 434 and ending with a TGA stop codon at position 1118, is predicted to encode a 228-amino-acid polypeptide. The second ORF (ORF2), beginning at position 1117 and ending at position 1909, is predicted to encode a 264-amino-acid polypeptide. A putative third ORF, beginning at position 1908, is interrupted by the Ω4514 insertion after the first two amino acids. Each ORF is preceded by a sequence that might serve as a ribosomal binding site (AGAAGGGAG, 5 bp upstream of ORF1; GGAGG, 4 bp upstream of ORF2; and GGAGGG, 4 bp upstream of ORF3), since it is complementary to the sequence near the 3′ end of M. xanthus 16S rRNA (43). The positions of the start points of ORF2 and ORF3 suggest that these three ORFs might be translationally coupled.
The deduced amino acid sequence of ORF1 was analyzed by the Motif program (Wisconsin Genetics Computer Group sequence analysis software) and appeared to contain a helix-turn-helix motif near the N terminus (positions 539 to 598), suggesting that the product of ORF1 is a DNA-binding protein (6, 23). Analysis of the deduced amino acid sequence by the BLAST program (1) supported this observation by revealing that the ORF1 product shared significant similarity to several transcriptional regulatory proteins. Among these is a putative transcription factor of the TetR family identified in the Deinococcus radiodurans genome sequencing project, to which ORF1 exhibits 31% identity and 46% similarity over a 182-amino-acid stretch (53). ORF1 also shows significant similarity to other TetR family members. TetR is a repressor that regulates tetracycline resistance of many gram-negative bacteria (25). These analyses suggest that ORF1 might function as a transcriptional regulator.
A BLAST search (1) with ORF2 revealed significant similarity to several glutaconate coenzyme A (CoA) transferases. The deduced amino acid sequence of ORF2 shows 29% identity and 44% similarity over its entire length to glutaconate CoA-transferase subunit A of Acidaminococcus fermentans (27, 40). Glutaconate CoA-transferase is involved in glutamate fermentation. A search of M. xanthus sequences in the Cereon Microbial Genome Database indicated that Ω4514 is inserted in the third codon of a 246-amino-acid ORF (ORF3), which shows 28% identity and 49% similarity over its entire length to the probable subunit B of glutaconate CoA-transferase of Pseudomonas aeruginosa. Downstream of ORF3 is a fourth ORF (ORF4) of at least 264 amino acids that extends to the end of the sequence contig in the Cereon Database. ORF4 begins 47 bp downstream of the ORF3 stop codon. Since this short intergenic segment contains no apparent transcriptional terminator or promoter sequences, ORF4 may be cotranscribed with the preceding three ORFs as part of the Ω4514 operon. Both ORF3 and ORF4 exhibit a codon preference typical of M. xanthus genes (46). Also, like the other three ORFs, ORF4 is preceded by a sequence that might serve as a ribosomal binding site (GGAGG, 7 bp upstream) (43). A BLAST search (1) with the partial ORF4 sequence revealed significant similarity to several alcohol dehydrogenases. The best match was to Adh4 of Halobacterium sp. strain NRC-1, showing 38% identity and 52% similarity over the available sequence. Taken together, the analysis of DNA sequence in the Ω4514 region suggests that this developmentally regulated operon encodes a transcription regulator (ORF1) and components of an alternative metabolic pathway(s) including glutaconate CoA-transferase (ORF2 and ORF3) and an alcohol dehydrogenase (ORF4).
Precise localization of the Ω4514 mRNA 5′ end.
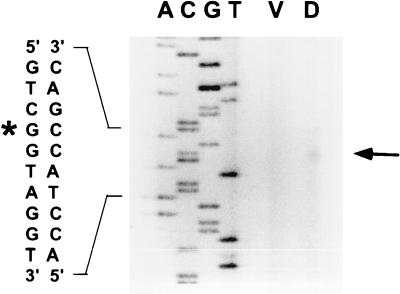
The DNA sequence of the Ω4514 upstream region and the S1 nuclease protection assay results facilitated the design of a primer for precise mapping of the location of the Ω4514 mRNA 5′ end by primer extension analysis. The primer was complementary to positions 527 to 550 in the sequence. When primer extension analysis was performed using RNA from M. xanthus wild-type DK1622 cells that had undergone 24 h of development, a single primer extension product was identified (Fig. 4). This localizes the Ω4514 mRNA 5′ end to a guanine nucleotide at position 409 in the sequence (Fig. 4). The mRNA 5′ end is 272 bp upstream of the NruI site that was labeled in the S1 nuclease protection experiment (Fig. 3), and so the results of the two methods are in good agreement. No primer extension product was generated when mRNA prepared from growing M. xanthus cells was subjected to primer extension analysis (Fig. 4).
FIG. 4.
Primer extension analysis of Ω4514 mRNA. RNA was isolated from wild-type DK1622 cells grown vegetatively (lane V) or cells that had undergone 24 h of development (lane D). The same primer was used to sequence Ω4514 upstream DNA. A portion of the DNA sequence is indicated at the left. The arrow indicates the extension product that was observed with RNA isolated from 24-h developing cells but not with RNA from vegetatively growing cells. The asterisk marks the position of the mRNA 5′ end in the sequence as inferred from the size of the primer extension product.
Further deletion analysis of the Ω4514 upstream region.
The 1.62-kb DNA segment upstream of Tn5 lac Ω4514 extends to a SmaI site (Fig. 1) located 54 bp upstream of the apparent transcriptional start site at position 409 in the sequence, and this segment exhibits developmental promoter activity (Fig. 1 and 2). A 3′ deletion was constructed to further define the sequence required for developmental promoter activity. An Eco47III site (Fig. 1) allowed construction of a 3′ deletion to 65 bp downstream of the proposed transcriptional start site. The SmaI-Eco47III fragment was inserted into pREG1727, and M. xanthus strains with a single copy of the plasmid integrated at the chromosomal attB site were tested for developmental promoter activity. Figure 5 shows that β-galactosidase activity reached a similar maximal level as that for strains containing the 1.62-kb Ω4514 upstream segment (with its 5′ end also at −54 but its 3′ end downstream of orf2) fused to lacZ, although there was a reproducible difference in lacZ expression at 0 and 6 h of development (see Discussion). These results demonstrate that a developmental promoter exists between −54 bp and +65 bp relative to the location in the DNA of the Ω4514 mRNA 5′ end, further supporting the idea that the mRNA 5′ end reflects the transcriptional start site.
FIG. 5.
Developmental expression of lacZ from a small segment containing the Ω4514 promoter. Developmental β-galactosidase specific activity was measured as described previously (34) for at least three independent transductants of DK1622 containing a single copy of the 1.62-kb Ω4514 upstream segment (□) or the SmaI-Eco47III fragment from −54 to +65 (•) fused to promoterless lacZ within pREG1727 and integrated at Mx8 attB. The β-galactosidase specific activity of DK1622 containing the pREG1727 vector alone with no insert integrated at Mx8 attB (▵) is also shown. β-Galactosidase specific activity is expressed in nanomoles of o-nitrophenyl phosphate per minute per milligram of protein.
Transcription of Ω4514 in vitro.
Inspection of the DNA sequence upstream of the Ω4514 transcriptional start site revealed a perfect match in the −35 region to the E. coli σ70 consensus (TTGACA), and a two-out-of-six match to the −10 region consensus (TATAAT) (39). To test whether RNAP isolated from growing M. xanthus cells can transcribe from the Ω4514 promoter, in vitro transcription experiments were performed. Two different DNA segments containing the Ω4514 promoter were used, and runoff transcription products of the expected sizes were observed (Fig. 6A). M. xanthus core RNAP alone did not transcribe from the Ω4514 promoter (Fig. 6B, lane 1). Only when the core RNAP was mixed with σA, the major vegetative σ factor of M. xanthus (2), was transcription observed (Fig. 6B, lane 2). Primer extension analysis of RNA produced in vitro using reconstituted σA RNAP mapped the 5′ end to the same position (data not shown) as Ω4514 mRNA produced in vivo (Fig. 4). Together, these results indicate that Ω4514 can be transcribed by the major vegetative RNAP of M. xanthus, suggesting that σA RNAP may be responsible for Ω4514 transcription in vivo.
FIG. 6.
In vitro transcription from the Ω4514 promoter. DNA fragments containing the Ω4514 promoter were transcribed in vitro with M. xanthus σA RNAP, and the reaction mixtures were subjected to 5% polyacrylamide-8 M urea gel electrophoresis. (A) Two different linear DNA templates containing the Ω4514 promoter region (SmaI-StuI and SmaI-SphI fragments in lanes 1 and 2, respectively) were transcribed with partially purified σA RNAP (2). Runoff transcription products of 458 (lane 1) and 303 (lane 2) bases are expected based on the Ω4514 mRNA 5′ end mapped in Fig. 4. Arrows mark the expected runoff transcription products. The upper band in each lane is the end-to-end transcript of the template. The numbers on the left indicate lengths (in bases) of end-labeled MspI-digested pBR322 marker fragments. (B) Transcription from the SmaI-SphI Ω4514 upstream fragment by M. xanthus core RNAP alone (lane 1) or core RNAP plus σA (lane 2). The arrow marks the expected 303-base runoff transcription product.
σA level during development.
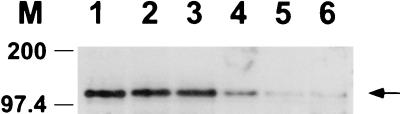
Since Ω4514 is transcribed primarily, if not exclusively, during development (Fig. 2 to 5), we asked whether σA is present during development. Antiserum against B. subtilis σ43 was used to detect σA in growing and developing M. xanthus cells (Fig. 7) by Western blot analysis as described previously (2). The level of σA remained fairly constant until 12 h into development and then decreased markedly by 15 h. Thus, σA is present early in development when the level of Ω4514 mRNA rises (Fig. 3).
FIG. 7.
Western blot analysis of σA in growing and developing M. xanthus cells. DK1622 cells were collected at 0, 9, 12, 15, 18, and 24 h after the onset of development (lanes 1 to 6), resuspended in sample buffer, and boiled for 5 min. Equal amounts of M. xanthus protein, as judged by measuring total protein in sonic lysates of the same samples, were separated by SDS-10% PAGE and electrotransferred to a nitrocellulose filter, which was incubated with a polyclonal antiserum to B. subtilis σ43 and visualized with goat anti-rabbit immunoglobulin G-horseradish peroxidase. The σA is indicated by an arrow, and protein standards are marked in kilodaltons.
Effect of an insertional mutation in ORF1 on Ω4514 expression.
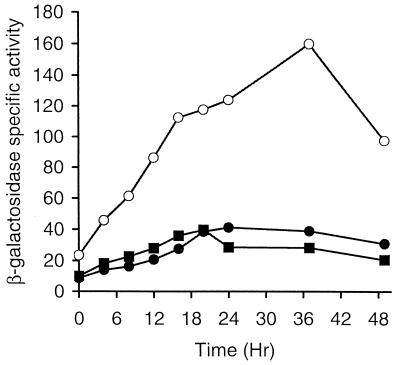
Since the Ω4514 promoter can be transcribed by σA RNAP (Fig. 6) and since σA is present in growing cells (Fig. 7), we reasoned that Ω4514 transcription might be negatively regulated during growth, because Ω4514 mRNA was not detected in growing cells (Fig. 3 and 4) and lacZ expression from Tn5 lac Ω4514 was at a low level (Fig. 2). ORF1 is predicted to encode a transcriptional regulator similar to TetR, which represses its own synthesis as well as that of TetA when tetracycline is absent (25). Therefore, we hypothesized that ORF1 functions as a repressor of Ω4514 transcription during growth. To test this hypothesis, an insertional disruption mutation of orf1 was constructed. An Eco47III-SphI restriction fragment (Fig. 1) from positions 473 to 713, which contains the putative helix-turn-helix motif, was subcloned into an integrational vector and transformed into wild-type DK1622. Transformants containing the plasmid integrated by homologous recombination were identified by Southern blot hybridization (data not shown). The effect of this orf1 disruption mutation on Ω4514 expression was tested by transforming the mutant (MGV01) with the plasmid (pTH10-3) containing the Ω4514 promoter region from −54 bp to +65 bp fused to lacZ. Transformants containing a single copy of pTH10-3 integrated at Mx8 attB were identified by Southern blot hybridization (data not shown). At least three of these transformants (MGV02) were assayed for β-galactosidase activity during growth and development along with MTH10-3, the strain with a single copy of pTH10-3 at attB in an otherwise wild-type background. Figure 8 shows that significantly higher lacZ expression was observed from the Ω4514 promoter in the orf1 mutant than in the wild-type background, both during growth and during development.
FIG. 8.
Effect of orf1 and orf2 mutations on developmental lacZ expression under the control of the Ω4514 promoter. Developmental β-galactosidase specific activity from pTH10-3 containing the Ω4514 promoter region from −54 bp to +65 bp fused to lacZ and integrated at attB was measured as described previously (34) for at least three independent isolates of M. xanthus containing wild-type orf1 and orf2 (•), the orf1 mutation (○), or the orf2 mutation (▪). β-Galactosidase specific activity is expressed in nanomoles of o-nitrophenyl phosphate per minute per milligram of protein.
To determine whether the higher lacZ expression observed in the orf1 mutant was due to disruption of orf1 or to a polar effect on expression of orf2, an orf2 insertional disruption mutant was constructed. A DNA segment containing part of orf2 from positions 1164 to 1487 was amplified by PCR, verified by DNA sequencing, and cloned into pBR322 to construct pTH11-1. This plasmid was transformed into MTH10-3, the strain containing the Ω4514 promoter fused to lacZ at the attB site in the chromosome. Transformants (MTH11-1) in which pTH11-1 had integrated by homologous recombination, disrupting orf2, were identified by diagnostic PCR (data not shown). As shown in Fig. 8, the orf2 mutation did not affect lacZ expression from the Ω4514 promoter. We conclude that disruption of orf1 increases Ω4514 expression during growth and development.
Testing ORF1 for binding to the Ω4514 promoter.
Since disruption of orf1 increased Ω4514 expression during growth and development (Fig. 8), ORF1 might be a repressor that binds in the Ω4514 promoter region. To test this hypothesis, E. coli cells were engineered to overproduce ORF1 or ORF1 chimeras tagged with six histidines at the N or C terminus as described in Materials and Methods. Extracts of all three strains contained a polypeptide of the expected size (approximately 26 kDa) but failed to shift the mobility of an Ω4514 promoter DNA fragment in an electrophoretic shift assay (8). The Ω4514 fragment from −54 to +65 bp was used because expression from this segment was elevated in the orf1 mutant (Fig. 8). Likewise, no shifted complex was observed with any of the three proteins purified from SDS gels after complete denaturation in guanidine and attempted refolding upon dilution (20). Also, ORF1-6xHis (tagged at the C terminus) was purified under nondenaturing conditions with a nickel affinity column, and yet it failed to shift the mobility of Ω4514 promoter DNA after incubation under a variety of conditions, including incubation with an extract of growing M. xanthus cells in the hope that the extract might contain a factor that would permit ORF1 binding. Therefore, we obtained no evidence to support the hypothesis that ORF1 is a direct repressor of Ω4514 transcription.
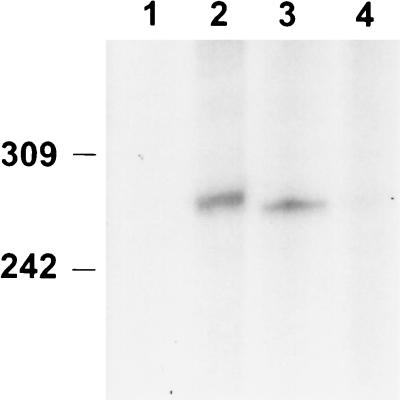
ORF1 level during M. xanthus growth and development.
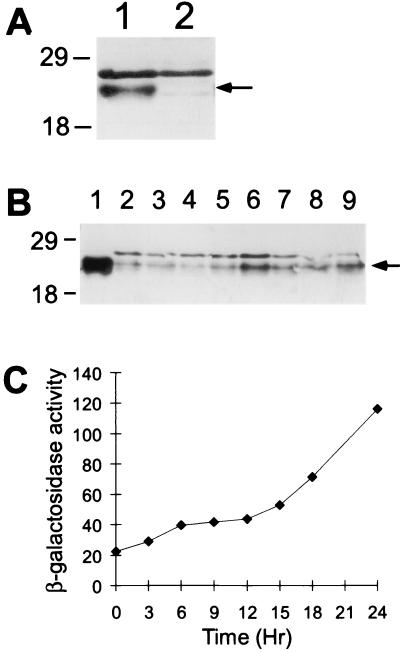
The ORF1-6xHis fusion protein purified with a nickel column was used to raise polyclonal antibodies in a rabbit, and these antibodies were affinity purified as described in Materials and Methods. Figure 9A shows that these antibodies detected a polypeptide in an extract of growing wild-type M. xanthus that was not present in an extract of the orf1 mutant, although the orf1 mutant does have a faint cross-reacting species that nearly comigrates with ORF1. This demonstrates that ORF1 is present in growing cells, as expected from the finding that ORF1 negatively autoregulates Ω4514 expression during growth (Fig. 8). Apparently, the Western blot assay to detect ORF1 is more sensitive than the assays that failed to detect Ω4514 RNA in growing cells (Fig. 3 and 4). Figure 9B shows that ORF1 detected in M. xanthus comigrated with ORF1 overproduced in E. coli and shows the level of ORF1 in extracts of M. xanthus cells harvested during growth or at various times of development. For this experiment, strain DK4514 containing Tn5 lac Ω4514 was used to allow comparison of the ORF1 protein level and Ω4514 expression. The ORF1 level decreased slightly at 3 to 6 h and then rose later in development to a level higher than that in growing cells (Fig. 9B). In extracts of the same samples, β-galactosidase activity from Tn5 lac Ω4514 increased slightly at 3 to 6 h, remained about the same at 9 to 12 h, and then rose later in development (Fig. 9C). Since ORF1 negatively regulates Ω4514 expression directly or indirectly (Fig. 8), it is possible that the slight decrease in the ORF1 level early in development (Fig. 9B) allows the initial slight increase in Ω4514 expression (Fig. 9C). This increase in Ω4514 expression may cause the ORF1 level to rise at 9 to 12 h, and Ω4514 expression is unchanged during this period. Later in development, Ω4514 expression rises as the ORF1 level decreases slightly at 15 to 18 h and then increases by 24 h. These results show that ORF1 is present during the first 24 h of development, in agreement with the finding that ORF1 negatively autoregulates Ω4514 expression during development (Fig. 8), but the level of ORF1 does not rise dramatically after 12 h, as does β-galactosidase activity from Tn5 lac Ω4514 (see Discussion).
FIG. 9.
Level of ORF1 protein and β-galactosidase specific activity during growth and development. (A) Western blot analysis of proteins in extracts of growing M. xanthus wild-type DK1622 (lane 1) and orf1 mutant MGV01 (lane 2) was performed with affinity-purified anti-ORF1-6xHis antibodies as described in Materials and Methods. (B) Western blot analysis of proteins in extracts of M. xanthus DK4514 collected during growth (lane 2) and at 3, 6, 9, 12, 15, 18, and 24 h after the onset of development (lanes 3 to 9, respectively). ORF1 protein overproduced in E. coli was detected in lane 1. For panels A and B, the arrow indicates ORF1 protein, and the positions of protein markers are indicated in kilodaltons. (C) β-Galactosidase specific activity of DK4514 harvested in the same experiment.
Sporulation efficiency of Ω4514 mutants.
A previous study showed that Tn5 lac Ω4514 did not cause a developmental defect, as judged qualitatively by the normal appearance of fruiting bodies (34). To be more quantitative and to test whether the orf1 or orf2 insertional mutants exhibit development defects, the sporulation efficiency was measured. The Tn5 lac Ω4514-containing strain DK4514 and the orf2 mutant MTH11-2 had a similar sporulation efficiency as did wild-type DK1622 (data not shown), indicating that interruption of orf3 or orf2 did not affect sporulation. The orf1 mutant MGV01 appeared to aggregate like wild type but exhibited about fourfold-reduced sporulation efficiency (data not shown), indicating that ORF1 plays a small but measurable role in development.
DISCUSSION
We have cloned the DNA upstream of Tn5 lac Ω4514 and identified a promoter that is induced during M. xanthus development. Promoter activity was lost in a graded fashion in a 5′-deletion series, suggesting that multiple upstream elements contribute to activity, but a deletion to −54 bp still permitted a low level of developmental induction. Interestingly, the promoter shares sequence similarity with promoters of genes that are transcribed during growth, and σA RNAP, the major form of RNAP in growing M. xanthus, initiated transcription from this promoter in vitro. Our in vivo results (Fig. 8) show that the Ω4514 promoter is negatively regulated by ORF1, the product of a downstream gene. This negative autoregulation keeps Ω4514 expression low during growth and early in development. Full induction of the Ω4514 operon during development involves positive regulation acting through DNA elements both near the promoter and far upstream.
Our deletion analysis of the Ω4514 promoter region indicates that multiple DNA elements spanning at least 650 bp upstream of the transcriptional start site contribute to developmental promoter activity (Fig. 1 and 2). Deletion of DNA between XhoI and SalI sites located approximately 2.7 kb and 650 bp, respectively, upstream of the start site reduced developmental lacZ expression by about 30% (Fig. 1 and 2). Therefore, DNA more than 650 bp upstream of the start site is necessary for full Ω4514 promoter activity. Likewise, deletion of DNA between the SalI site located approximately 650 bp upstream of the start site and the SmaI site located at −54 bp reduced developmental lacZ expression by an additional 20%, indicating the importance of this region (Fig. 1 and 2). The graded loss of promoter activity for these deletions was not specific to placement at the Mx8 attB site. Similar results were observed when plasmids were integrated by homologous recombination upstream of Tn5 lac Ω4514 to create the corresponding 5′ deletions fused to lacZ at the native chromosomal location (date not shown). The Ω4514 promoter is not unique among developmentally regulated M. xanthus genes in requiring multiple upstream elements (9). The tps, csgA, and Ω4499 promoters also exhibit large upstream regulatory regions (14, 31, 37). In the case of tps, multiple elements appear to act over distances of several kilobases from the transcriptional start site (31). In the cases of csgA and Ω4499, upstream regions spanning more than 500 bp were required for full expression, and in both cases expression was lost in a graded fashion as 5′-deletion endpoints approached the transcriptional start site (14, 37). It was proposed that multiple transcription factors bind to these upstream regions and regulate expression in response to different developmental cues. Similarly, expression of the Ω4514 promoter may involve several transcription factors that respond to developmental cues by binding to upstream DNA elements and activating transcription.
One cue that the Ω4514 promoter does not respond to is C signaling. Intercellular C signaling is mediated by the product of csgA (48, 49) and is required for the expression of many M. xanthus genes that begin to be expressed after 6 h into development (33). Introduction of a csgA mutation into cells containing Tn5 lac Ω4514 delayed developmental lacZ expression slightly in a previous study (33). However, when we repeated this experiment, we found no significant difference between the wild type and a csgA mutant when we compared developmental expression from Tn5 lac Ω4514 (data not shown). Also, expression of 5′ deletions (i.e., pTH1-6, pTH4-1, and pTH4-3 [Fig. 1]) was tested at Mx8 attB in a csgA mutant and was indistinguishable from that in wild-type cells (data not shown). Moreover, when extracellular C signal was provided (by codevelopment with wild-type cells) to the csgA mutants harboring the 5′ deletions, no increase in expression was observed (data not shown), in contrast to the results observed for C-signal-dependent promoters (5, 14, 15). Promoters that depend on C signaling for expression exhibit one or more sequences (centered near −50 bp) matching the consensus CAYYCCY (Y means pyrimidine), which has been called the C box (14). It has been speculated that C-box sequences are cis-acting regulatory elements important for the expression of C-signal-dependent genes. The Ω4514 promoter does not have a sequence matching the C-box consensus near −50 bp.
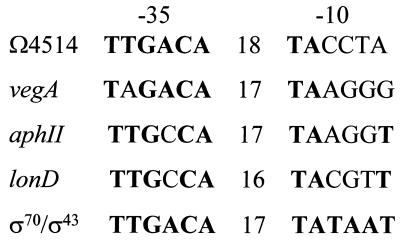
The Ω4514 promoter is the first example of a developmentally regulated M. xanthus promoter that appears to be recognized by σA RNAP. Previously, two genes expressed in growing M. xanthus cells, vegA and aphII, were shown to be transcribed by σA RNAP in vitro (2). Recently, it has been shown that the M. xanthus lonD gene can be transcribed by σA RNAP in vitro (52). All four of these promoters match the consensus −35 region of promoters recognized by E. coli σ70 (39) or B. subtilis σ43 (24) RNAP in at least five out of six positions, but these promoters match the σ70-σ43 consensus −10 region poorly and are more GC rich in this region (Fig. 10). Hence, the findings that Ω4514 (Fig. 6) and lonD (52) can be transcribed by M. xanthus σA RNAP support our previous proposal that σA interacts better with a more GC-rich −10 sequence than do the primary sigma factors of E. coli and B. subtilis (2).
FIG. 10.
Sequences of promoters transcribed in vitro by M. xanthus σA RNAP. The sequences of the −35 and −10 regions of the Ω4514 promoter and the distance in base pairs between these regions (numbers in the middle) are compared with those of other M. xanthus promoters shown previously to be transcribed by σA RNAP. Matches to the E. coli σ70-B. subtilis σ43 consensus are indicated by boldface letters.
We showed that σA persists at a high level at least until 12 h into development (Fig. 7), when the Ω4514 mRNA level is also high (Fig. 3). By 24 h into development, σA and Ω4514 mRNA were barely detectable, and yet β-galactosidase activity from Tn5 lac Ω4514 increased until 48 h of development (Fig. 2). The continued rise in lacZ expression after 18 h of development required upstream DNA beyond the SmaI site located at −54 (Fig. 2 and 5). There does not appear to be a second promoter located upstream of the SmaI site, because a single mRNA 5′ end was detected (Fig. 3 and 4) and because placing lacZ immediately downstream of the SmaI site (strain MGV03 in Fig. 1) did not give rise to β-galactosidase activity during development. To explain the rise in β-galactosidase activity from Tn5 lac Ω4514 between 18 and 48 h of development, we speculate that the Ω4514-lacZ fusion mRNA persists at a low level and continues to be translated or that another form of RNAP transcribes Ω4514 late in development. The latter possibility predicts that the Ω4514 mRNA level might rise late in development, which remains to be tested.
The product of the first ORF downstream of the Ω4514 promoter negatively regulates expression of the Ω4514 operon during both growth and development (Fig. 8). Although ORF1 has a helix-turn-helix DNA-binding motif and is most similar in sequence to a family of repressors, we were unable to demonstrate specific binding of ORF1 to the Ω4514 promoter region. It is possible that ORF1 regulates another gene(s) whose product(s) directly represses Ω4514 transcription. Alternatively, ORF1 may be a direct repressor of Ω4514 transcription, but it may not fold properly when expressed in E. coli or it may require an additional factor that was not present under the conditions that we tested. One such factor could be posttranslational modification of ORF1. However, we observed no significant difference in migration on SDS-PAGE between ORF1 produced in E. coli and ORF1 produced during M. xanthus growth and development (Fig. 9B), providing no evidence for posttranslational modification of ORF1. Perhaps ORF1 requires binding of a small molecule in order to bind specifically to the Ω4514 promoter, analogous to the case of tryptophan and the trp repressor (28).
Developmental induction of Ω4514 expression can occur in the absence of ORF1 and in the absence of upstream DNA beyond −54 (Fig. 8). This suggests that σA RNAP transcribes from the Ω4514 promoter more efficiently during development than during growth, because the level of σA remains about the same during growth and early in development (Fig. 7). If many σA-dependent genes are repressed as cells enter development, perhaps σA RNAP becomes available to transcribe the Ω4514 promoter. Another possibility is that a transcriptional activator is induced upon starvation and binds between −54 and +65 in the Ω4514 promoter region.
Insight into the regulation of Ω4514 expression can be gained from comparison of the levels of developmental lacZ expression observed for different promoter constructs. β-Galactosidase activity from the promoter region between −54 and +65 reached 160 U in the absence of ORF1 (Fig. 8). However, when additional upstream DNA was present, as in the case of the 4.3-kb XhoI-BamHI fragment, β-galactosidase activity reached 250 U, even though ORF1 was present (Fig. 2). Clearly, upstream DNA elements enhance Ω4514 promoter activity. It will be interesting to test the activity of the Ω4514 promoter with upstream elements in the absence of ORF1. We might observe a higher maximum level of expression, as suggested by the results shown for the Ω4514 promoter from −54 to +65 in the presence or absence of orf1 (Fig. 8).
ORF1 influences not only the level of Ω4514 expression but also its timing during development. Figure 8 shows that lacZ expression increased significantly by 4 h of development for strains with lacZ fused at +65. This is most obvious for the strain completely lacking ORF1 due to a disruption at the native site (Fig. 8, open circles). The other strains examined in this experiment had orf1 intact at the native site and therefore exhibited a lower level of expression due to ORF1 negative regulation in trans (since the lacZ fusion is located at the Mx8 attB site), but like the strain completely lacking orf1, these strains showed no obvious lag in lacZ expression early in development (Fig. 8). One of these strains was also used in the experiment shown in Fig. 5 (circles), and it is clear that expression increased by 6 h of development. In contrast, lacZ expression appeared to be delayed, increasing dramatically only after 6 h of development, for strains with lacZ fused downstream of orf2 (Fig. 2 and 5, squares). In these strains orf1 is expressed from the Ω4514 promoter at Mx8 attB as well as from the native site. We infer that ORF1 can accumulate sufficiently to delay Ω4514 expression only if each copy of the Ω4514 promoter drives expression of a functional copy of orf1. Our data do not exclude the possibility that orf1 must be expressed in cis in order to delay transcription from the Ω4514 promoter, but we think this is unlikely because it is clear that ORF1 produced in trans can reduce Ω4514 expression (Fig. 8).
If ORF1 is responsible for delaying Ω4514 expression early in development, how is this negative regulation overcome? There was a small decrease in the level of ORF1 at 3 to 6 h of development that correlated with a small increase in expression from Tn5 lac Ω4514, but the major rise in lacZ expression after 12 h of development did not correlate with much change in the level of ORF1 (Fig. 9). One might have expected the ORF1 level to rise due to increased Ω4514 expression. Perhaps a posttranscriptional mechanism (e.g., ORF1 turnover) prevents ORF1 from accumulating and exerting too strong a negative effect on Ω4514 expression.
Disruption of orf1 caused a mild sporulation defect. This is not due to a polar effect on expression of downstream genes, since neither disruption of orf2 nor insertion of Tn5 lac Ω4514 in orf3 caused such a defect. We speculate that ORF1 may regulate other genes in addition to the Ω4514 operon and that the products of these genes contribute to the sporulation process. Although questions remain about the function and regulation of the Ω4514 operon, it is the first example of a developmentally regulated M. xanthus operon that is transcribed by the major vegetative RNAP, and it is subject to complex control, including negative autoregulation by the product of the first gene in the operon and positive regulation by upstream DNA elements.
Acknowledgments
We thank Y. Cheng and D. Kaiser for providing bacterial strains, R. Gill for providing plasmids, and B.-Y. Chang and R. Doi for providing antibodies used in this study. We are grateful to the Monsanto Company and its subsidiary Cereon Genomics for providing access to their Microbial Sequence Database.
This research was supported by NIH grant GM47293, by NSF grant MCB-0090478, and by the Michigan Agricultural Experiment Station.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Biran, D., and L. Kroos. 1997. In vitro transcription of Myxococcus xanthus genes with RNA polymerase containing σA, the major sigma factor in growing cells. Mol. Microbiol. 25:463-472. [DOI] [PubMed] [Google Scholar]
- 3.Bolivar, F., R. L. Rodriguez, P. J. Greene, M. C. Betlach, H. L. Heyneker, and H. W. Boyer. 1977. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene 2:95-113. [PubMed] [Google Scholar]
- 4.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 5.Brandner, J. P., and L. Kroos. 1998. Identification of the Ω4400 regulatory region, a developmental promoter of Myxococcus xanthus. J. Bacteriol. 180:1995-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brennan, R. G., and B. W. Matthews. 1989. The helix-turn-helix DNA binding motif. J. Biol. Chem. 264:1903-1906. [PubMed] [Google Scholar]
- 7.Cheng, Y., and D. Kaiser. 1989. dsg, a gene required for cell-cell interaction early in Myxococcus development. J. Bacteriol. 171:3719-3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chodosh, L. A. 1988. Mobility shift DNA-binding assay using gel electrophoresis, p. 12.2.1-12.2.10. In F. M. Ausubel et al. (ed.), Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y. [DOI] [PubMed]
- 9.Downard, J., and L. Kroos. 1993. Transcriptional regulation of developmental gene expression in Myxococcus xanthus, p. 183-199. In M. Dworkin and D. Kaiser (ed.), Myxobacteria II. American Society for Microbiology, Washington, D.C.
- 10.Downard, J., S. V. Ramaswamy, and K. Kil. 1993. Identification of esg, a genetic locus involved in cell-cell signaling during Myxococcus xanthus development. J. Bacteriol. 175:7762-7770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dworkin, M. 1996. Recent advances in the social and developmental biology of the myxobacteria. Microbiol. Rev. 60:70-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dworkin, M., and D. Kaiser (ed.). 1993. Myxobacteria II. American Society for Microbiology, Washington, D.C.
- 13.Fawcett, T., and S. Bartlett. 1990. An effective method for eliminating “artifact banding” when sequencing double-stranded DNA templates. BioTechniques 9:46-48. [PubMed] [Google Scholar]
- 14.Fisseha, M., D. Biran, and L. Kroos. 1999. Identification of the Ω4499 regulatory region controlling developmental expression of a Myxococcus xanthus cytochrome P-450 system. J. Bacteriol. 181:5467-5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisseha, M., M. Gloudemans, R. Gill, and L. Kroos. 1996. Characterization of the regulatory region of a cell interaction-dependent gene in Myxococcus xanthus. J. Bacteriol. 178:2539-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gill, R. E., and M. C. Bornemann. 1988. Identification and characterization of the Myxococcus xanthus bsgA gene product. J. Bacteriol. 170:5289-5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gill, R. E., and M. G. Cull. 1986. Control of developmental gene expression by cell-to-cell interactions in Myxococcus xanthus. J. Bacteriol. 168:341-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gill, R. E., M. G. Cull, and S. Fly. 1988. Genetic identification and cloning of a gene required for developmental cell interactions in Myxococcus xanthus. J. Bacteriol. 170:5279-5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagen, D. C., A. P. Bretscher, and D. Kaiser. 1978. Synergism between morphogenetic mutants of Myxococcus xanthus. Dev. Biol. 64:284-296. [DOI] [PubMed] [Google Scholar]
- 20.Hager, D. A., and R. R. Burgess. 1980. Elution of proteins from sodium dodecyl sulfate-polyacrylamide gels, removal of sodium dodecyl sulfate, and renaturation of enzymatic activity: results with sigma subunit of Escherichia coli RNA polymerase, wheat germ DNA topoisomerase, and other enzymes. Anal. Biochem. 109:76-86. [DOI] [PubMed] [Google Scholar]
- 21.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557.. [DOI] [PubMed] [Google Scholar]
- 22.Harlow, E., and D. Lane. 1988. Antibodies. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 23.Harrison, S. C., and A. K. Aggarwal. 1990. DNA recognition by proteins with the helix-turn-helix motif. Annu. Rev. Biochem. 59:933-969. [DOI] [PubMed] [Google Scholar]
- 24.Helmann, J. 1995. Compilation and analysis of Bacillus subtilis σA-dependent promoter sequences: evidence for extended contact between RNA polymerase and upstream promoter DNA. Nucleic Acids Res. 23:2351-2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hillen, W., and C. Berens. 1994. Mechanisms underlying expression of Tn10 encoded tetracycline resistance. Annu. Rev. Microbiol. 48:345-369. [DOI] [PubMed] [Google Scholar]
- 26.Hodgkin, J., and D. Kaiser. 1977. Cell-to-cell stimulation of motility in nonmotile mutants of Myxococcus. Proc. Natl. Acad. Sci. USA 74:2938-2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacob, U., M. Mack, T. Clausen, R. Huber, W. Buckel, and A. Messerschmidt. 1997. Glutaconate CoA-transferase from Acidaminococcus fermentans: the crystal structure reveals homology with other CoA-transferases. Structure 5:415-426. [DOI] [PubMed] [Google Scholar]
- 28.Joachimiak, A., R. L. Kelley, R. P. Gunsalus, C. Yanofsky, and P. B. Sigler. 1983. Purification and characterization of trp aporepressor. Proc. Natl. Acad. Sci. USA 80:668-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaiser, D. 1979. Social gliding is correlated with the presence of pili in Myxococcus xanthus. Proc. Natl. Acad. Sci. USA 76:5952-5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kashefi, K., and P. Hartzell. 1995. Genetic suppression and phenotypic masking of a Myxococcus xanthux frzF− defect. Mol. Microbiol. 15:483-494. [DOI] [PubMed] [Google Scholar]
- 31.Kil, K.-S., G. Brown, and J. Downard. 1990. A segment of Myxococcus xanthus ops DNA functions as an upstream activation site for tsp gene transcription. J. Bacteriol. 172:3081-3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kroos, L., and D. Kaiser. 1984. Construction of Tn5 lac, a transposon that fuses lacZ expression to exogenous promoters, and its introduction into Myxococcus xanthus. Proc. Natl. Acad. Sci. USA 81:5816-5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kroos, L., and D. Kaiser. 1987. Expression of many developmentally regulated genes in Myxococcus depends on a sequence of cell interactions. Genes Dev. 1:840-854. [DOI] [PubMed] [Google Scholar]
- 34.Kroos, L., A. Kuspa, and D. Kaiser. 1986. A global analysis of developmentally regulated genes in Myxococcus xanthus. Dev. Biol. 117:252-266. [DOI] [PubMed] [Google Scholar]
- 35.Kuspa, A., L. Kroos, and D. Kaiser. 1986. Intercellular signaling is required for developmental gene expression in Myxococcus xanthus. Dev. Biol. 117:267-276. [DOI] [PubMed] [Google Scholar]
- 36.Laue, B. E., and R. Gill. 1994. Use of a phase variation-specific promoter of Myxococcus xanthus in a strategy for isolating a phase-locked mutant. J. Bacteriol. 176:5341-5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li, S.-F., B. Lee, and L. J. Shimkets. 1992. csgA expression entrains Myxococcus xanthus development. Genes Dev. 6:401-410. [DOI] [PubMed] [Google Scholar]
- 38.Li, S.-F., and L. J. Shimkets. 1993. Effect of dsp mutations on the cell-to-cell transmission of CsgA in Myxococcus xanthus. J. Bacteriol. 175:3648-3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lisser, S., and H. Margalit. 1993. Compilation of E. coli mRNA promoter sequences. Nucleic Acids Res. 21:1507-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mack, M., K. Bendrat, O. Zelder, E. Eckel, D. Linder, and W. Buckel. 1994. Location of the two genes encoding glutaconate coenzyme A-transferase at the beginning of the hydroxyglutarate operon in Acidaminococcus fermentans. Eur. J. Biochem. 226:41-51. [DOI] [PubMed] [Google Scholar]
- 41.Magrini, V., C. Creighton, and P. Youderian. 1999. Site-specific recombination of temperate Myxococcus xanthus phage Mx8: genetic elements required for integration. J. Bacteriol. 181:4050-4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Messing, J. 1979. A multipurpose cloning system based on the single-stranded DNA bacteriophage M13. Recombinant DNA bulletin. Publication no. 71-99, p. 43-48. National Institutes of Health, Bethesda, Md.
- 43.Oyaizu, H., and C. Woese. 1985. Phylogenetic relationships among the sulfate respiring bacteria, myxobacteria and purple bacteria. Syst. Appl. Microbiol. 6:257-263. [Google Scholar]
- 44.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 45.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shimkets, L. 1993. The myxobacterial genome, p. 85-107. In M. Dworkin and D. Kaiser (ed.), Myxobacteria II. American Society for Microbiology, Washington, D.C.
- 47.Shimkets, L. J. 1999. Intercellular signaling during fruiting-body development of Myxococcus xanthus. Annu. Rev. Microbiol. 53:525-549. [DOI] [PubMed] [Google Scholar]
- 48.Shimkets, L. J., and S. J. Asher. 1988. Use of recombination techniques to examine the structure of the csg locus of Myxococcus xanthus. Mol. Gen. Genet. 211:63-71. [DOI] [PubMed] [Google Scholar]
- 49.Shimkets, L. J., R. E. Gill, and D. Kaiser. 1983. Developmental cell interactions in Myxococcus xanthus and the spoC locus. Proc. Natl. Acad. Sci. USA 80:1406-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stellwag, E., J. M. Fink, and J. Zissler. 1985. Physical characterization of the genome of the Myxococcus xanthus bacteriophage MX-8. Mol. Gen. Genet. 199:123-132. [DOI] [PubMed] [Google Scholar]
- 51.Stephens, K., and D. Kaiser. 1987. Genetics of gliding motility in Myxococcus xanthus: molecular cloning of the mgl locus. Mol. Gen. Genet. 207:256-266. [Google Scholar]
- 52.Ueki, T., and S. Inouye. 2002. Transcriptional activation of a heat-shock gene, lonD, of Myxococcus xanthus by a two-component histidine-aspartate phosphorelay system. J. Biol. Chem. 277:6170-6177. [DOI] [PubMed] [Google Scholar]
- 53.White, O., et al. 1999. Genome sequence of the radioresistant bacterium Deinococcus radiodurans R1. Science 286:1571-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]