Abstract
The Pyrococcus furiosus fbpA gene was cloned and expressed in Escherichia coli, and the fructose-1,6-bisphosphatase produced was subsequently purified and characterized. The dimeric enzyme showed a preference for fructose-1,6-bisphosphate, with a Km of 0.32 mM and a Vmax of 12.2 U/mg. The P. furiosus fructose-1,6-bisphosphatase was strongly inhibited by Li+ (50% inhibitory concentration, 1 mM). Based on the presence of conserved sequence motifs and the substrate specificity of the P. furiosus fructose-1,6-bisphosphatase, we propose that this enzyme belongs to a new family, class IV fructose-1,6-bisphosphatase.
The hyperthermophilic archaeon Pyrococcus furiosus is capable of metabolizing sugar via an Embden-Meyerhof-like pathway. A combination of physiological, biochemical, and genetic studies has revealed that pyrococcal glycolysis differs from the regular Embden-Meyerhof pathway by its incorporation of new conversions, its novel enzymes, and its unique control (5). Compelling evidence of the deviation of pyrococcal glycolysis from the canonical glycolysis includes the recruitment of two unique ADP-dependent sugar kinases (10, 21) and the presence of a structurally distinct phosphoglucose isomerase (23) and a glyceraldehyde-3-phosphate ferredoxin oxidoreductase (22) in P. furiosus. In addition, the genes encoding the homologous and distantly related fructose-1,6-bisphosphate aldolase and phosphoglycerate mutase were recently predicted, and their functions were subsequently confirmed experimentally (19; J. van der Oost, unpublished data). The remaining glycolytic and gluconeogenic enzymes were easily identified in the genome sequence of P. furiosus. However, no gene coding for a homolog of the gluconeogenic fructose-1,6-bisphosphatase (EC 3.1.3.11) (FBPase) could be identified. This observation also holds true for other archaea, with the exception of Halobacterium sp. strain NRC1, which contains a classical FBPase (13).
FBPase is an essential regulatory enzyme in the gluconeogenic pathway. It converts d-fructose-1,6-bisphosphate to d-fructose-6-phosphate, an important precursor in biosynthetic pathways. Generally, a divalent metal ion such as Mg2+, Mn2+, Co2+, or Zn2+ is required for catalytic activity. The three-dimensional structures of several FBPases have been elucidated (9, 24, 26), and all exhibit a typical sugar phosphatase fold (http://scop.mrc-lmb.cam.ac.uk/scop).
It has recently been reported that the inositol monophosphatase (I-1-Pase) (EC 3.1.3.25) from Methanococcus jannaschii (MJ0109) exhibits FBPase activity, and it has been suggested that this enzyme might be the missing FBPase in archaea (20). In addition, MJ0109 orthologs in Archaeoglobus fulgidus and Thermotoga maritima show FBPase activity (20). In an attempt to complete the set of determined glycolytic and gluconeogenic enzymes in P. furiosus, we cloned and expressed the MJ0109 ortholog from P. furiosus (48% identity on the amino acid level) in Escherichia coli and investigated its ability to function as a thermoactive FBPase.
Transcript analysis and cloning of fbpA.
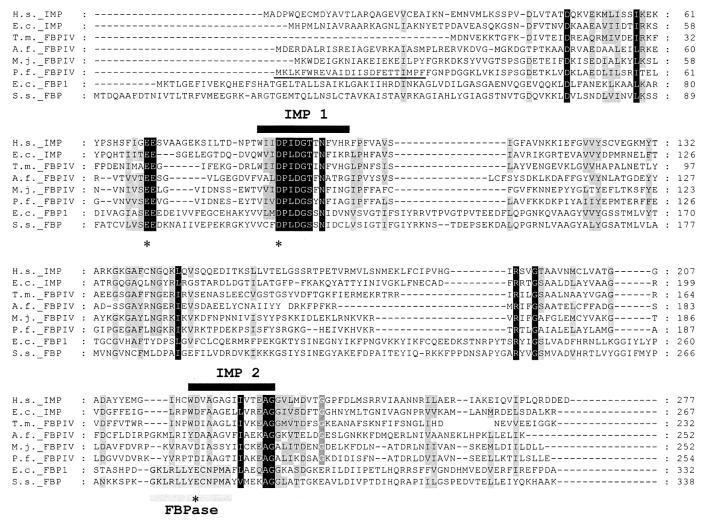
An ortholog (fbpA) of MJ0109 (2) was identified in the P. furiosus genome database (http://www.genome.utah.edu). This ortholog was originally designated as an extragenic suppressor, suhB. The start of the fbpA gene was predicted based on the presence and proper spacing of a potential Shine-Dalgarno sequence and on multiple alignment of the deduced amino acid sequence with those of related enzymes (Fig. 1). To test whether the fbpA gene was transcribed in P. furiosus, total RNA was isolated from a pyruvate-grown P. furiosus culture (40 mM) as described previously (25). The presence of the fbpA transcript was confirmed (data not shown) by using a reverse transcription-PCR system according to the instructions of the manufacturer (Promega) with 1 μg of P. furiosus RNA and the primers BG977 and BG978 (see below). Moreover, a recent genome-based microarray analysis of P. furiosus also revealed the expression of fbpA (designated suhB) (18).
FIG. 1.
Multiple sequence alignment of the deduced amino acid sequence of the P. furiosus FBPase with the sequences of its FBPase IV homologs and of I-1-Pases and FBPases from eukarya and bacteria. Abbreviations (accession numbers are given in parentheses): H.s. IMP, Homo sapiens I-1-Pase 1 (P29218); E.c. IMP, E. coli SuhB I-1-Pase (P22783); T.m. FBPIV, T. maritima TM1415 FBPase (O33832); A.f. FBPIV; A. fulgidus AF2372 FBPase (NP_071195); M.j. FBPIV, M. jannaschii MJ0109 FBPase (Q57573); P.f. FBPIV, P. furiosus FBPase (AF453319); E.c. FBP1, E. coli FBPase (P09200); S.s. FBP, Sus scrofa FBPase (P00636). Gaps introduced by the alignment are indicated by dashes. Completely conserved regions are indicated by black boxes. Highly conserved regions are shaded gray. The IMP motifs are indicated with horizontal black bars above the alignments. The FBPase motif is indicated with a horizontal gray bar under the alignment. IMP 1 motif, [FWV]-x (0, 1)-[LIVM]-D-P-[LIVM]-D-[SG]-[ST]-x (2)-[FY]-x-[HKRNSTY]; inositol monophosphatase family signature 1 (PS00629). IMP 2 motif, [WV]-D-x-[AC]-[GSA]-[GSAPV]-x-[LIVACP]-[LIV]-[LIVAC]-x (3)-[GH]-[GA]; inositol monophosphatase family signature 2 (PS00630). FBPase motif, [AG]-[RK]-[LI]-x (1, 2)-[LIV]-[FY]-E-x (2)-P-[LIVM]-[GSA] (PS00124) (http://www.expasy.ch/prosite). Asterisks denote residues involved in the Li+ binding site (24). The determined N-terminal amino acid sequence from the purified P. furiosus FBPase described in the text is underlined.
The fbpA gene (765 bp) was PCR amplified from chromosomal DNA of P. furiosus as described previously (21) with the primers BG977 (5′-GCGCGTCATGAAGCTTAAGTTCTGGAGGG [with the BspHI restriction site underlined], sense) and BG978 (5′-GCGCGGATCCCTACTCCAGTAAGCTTAAAATTGTTTT [with the BamHI restriction site underlined], antisense). The PCR product was digested with BspHI/BamHI and cloned into E. coli XL1-Blue by use of an NcoI/BamHI-digested pET24d vector according to established procedures and with 50 μg of kanamycin/ml for selection. Subsequently, the resulting plasmid, pLUW558, was transformed into E. coli BL21(DE3).
Overexpression and purification of FBPase.
An overnight culture of E. coli BL21(DE3) harboring pLUW558 was used as a 1% inoculum in 0.5 liter of Luria-Bertani medium with 50 μg of kanamycin/ml. Gene expression was induced by adding 0.1 mM isopropyl-1-thio-β-d-galactopyranoside (IPTG) at an optical density at 600 nm of 0.5. Growth was allowed to continue for 10 h at 37°C, and cells were harvested by centrifugation (2,200 × g for 20 min at 4°C) and resuspended in 10 ml of 50 mM Tris-HCl buffer, pH 8.0. The cells were disrupted by French press treatment (100 MPa), and cell debris was removed by centrifugation (10,000 × g for 20 min at 4°C). The resulting cell extract was heat treated for 30 min at 80°C, and the precipitated proteins were removed by centrifugation (10,000 × g for 30 min at 4°C). The heat-stable cell extract was filtered through a 0.45-μm-pore-size filter and applied to a MonoQ HR 5/5 column (1 ml; Amersham Pharmacia Biotech) equilibrated with 50 mM Tris-HCl buffer, pH 8.0. The FBPase activity eluted at 0.37 M NaCl in a linear gradient of 0.0 to 1.0 M NaCl. Active fractions were pooled and concentrated 20-fold to a final volume of 100 μl by use of a filter with a 10-kDa cutoff (Microsep; Pall Filtron). The concentrated pool was loaded on a Superdex 200 HR 10/30 gel filtration column (24 ml; Amersham Pharmacia Biotech) equilibrated with 50 mM Tris-HCl buffer, pH 7.8, containing 100 mM NaCl. The elution pattern (not shown) suggested that the active configuration was a dimer (66.8 kDa) of two identical 33-kDa subunits, which is in good agreement with the results of a sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis (data not shown). The calculated size of the subunit was slightly smaller, i.e., 27.9 kDa. The purified enzyme was desalted in 50 mM Tris-HCl buffer, pH 8.0, by use of a filter with a 10-kDa cutoff (Microsep; Pall Filtron). From 2.7 g of cell paste consisting of E. coli BL21(DE3) containing pLUW558, a total of 27.7 mg of FBPase was purified to 95% purity, as judged by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (data not shown). To ensure that the detected activity corresponded to the P. furiosus FBPase, the N-terminal sequence of the purified enzyme was determined by the Edman degradation method to be Met-Lys-Leu-Lys-Phe-Trp-Arg-Glu-Val-Ala-Ile-Asp-Ile-Ile-Ser-Asp-Phe-Glu-Thr-Thr-Ile-Met-Pro-Phe, revealing that the obtained amino acid sequence exactly matched the N-terminal sequence of the translated fbpA from P. furiosus (Fig. 1). This indicates that the P. furiosus FBPase had been produced and purified successfully.
Temperature dependence of FBPase.
For the determination of the temperature optimum, an appropriate amount of purified FBPase (6 to 30 ng) was incubated in 1-ml crimp-sealed vials containing 100 mM MOPS (morpholinepropanesulfonic acid) buffer, pH 7.4, and 10 mM MgCl2. The vials were submerged in an oil bath at temperatures varying from 20 to 120°C and preheated for 2 min, and the enzyme reaction was initiated by the injection of 15 mM fructose-1,6-bisphosphate. At different times up to 15 min, the reaction was stopped by transferring the vials to a mixture of ice and ethanol. Aliquots were taken, and the amount of fructose-6-phosphate formed was determined spectrophotometrically by measuring the reduction of NADP+ (340 nm) at room temperature in an assay with glucose-6-phosphate isomerase (EC 5.3.1.9) and glucose-6-phosphate dehydrogenase (EC 1.1.1.49), both from Saccharomyces cerevisiae. A linear increase in fructose-6-phosphate production over time was observed, indicating that no P. furiosus FBPase was inactivated during incubation. The P. furiosus FBPase showed maximal activity at approximately 100°C (data not shown).
In accordance with first-order inactivation kinetics (data not shown), the enzyme (18 μg/ml) lost 50% of its activity after incubation for 2 h at 100°C in 50 mM Tris-HCl buffer, pH 8.0. For the determination of the melting temperature, P. furiosus FBPase was dialyzed extensively against a 100 mM sodium phosphate buffer, pH 8.0, and diluted to 0.3 mg/ml in dialysis buffer. After being degassed for 10 min, the samples were analyzed against the dialysis buffer in a differential scanning microcalorimeter (VP-DSC; MicroCal) between 50 and 125°C at 0.5°C/min. Enzyme scans were corrected with a buffer-buffer baseline, and data were analyzed with the MicroCal Origin 5.0 SR2 software package. For FBPase, an apparent melting temperature of 107.5°C was determined (data not shown), which is in good agreement with the inactivation kinetics.
Catalytic properties.
The kinetic parameters of the P. furiosus FBPase were determined discontinuously at 85°C by varying the concentration of fructose-1,6-bisphosphate (between 0.005 and 5 mM) and by measuring the release of inorganic phosphate at room temperature as described previously (8). The 0.2-ml assay mixture contained 50 mM Tris-HCl buffer (pH 8.0; room temperature), 10 mM MgCl2, and 0.4 μg of purified FBPase. At this temperature, the Km and Vmax of the P. furiosus FBPase with fructose-1,6-bisphosphate were 0.32 ± 0.03 mM and 12.2 ± 0.1 U/mg, respectively, resulting in a catalytic efficiency (kcat/Km) of 17.7 s−1 mM−1. The determined affinity of the purified FBPase for fructose-1,6-bisphosphate is in good agreement with the previously determined Km of 0.5 mM (75°C) in a P. furiosus extract (16). The kinetic parameters of the purified FBPase determined at 50°C were as follows: a Km of 0.31 ± 0.06 mM, a Vmax of 0.72 ± 0.04 U/mg, and a catalytic efficiency of 1.12 s−1 mM−1. Thus, the P. furiosus FBPase clearly is a thermoactive enzyme with affinities for fructose-1,6-bisphosphate at 50 and 85°C that are similar.
The specific activities of the P. furiosus FBPase for fructose-1,6-bisphosphate and related substrates were determined at 85°C in the standard assay that measures the release of inorganic phosphate. The 1-ml assay mixture contained 50 mM Tris-HCl buffer (pH 8.0; room temperature), 2.5 mM substrate, 10 mM MgCl2, and 0.02 mg of purified FBPase. At fructose-1,6-bisphosphate concentrations above 10 mM, the enzyme was subjected to substrate inhibition (data not shown). The highest activity was obtained with fructose-1,6-bisphosphate (12.2 U/mg). In addition, myo-inositol-1-phosphate, glucose-1-phosphate, and glycerol-2-phosphate could also be dephosphorylated by the enzyme, although the activities for these substrates were relatively low (1.7 to 7.5%) (Table 1). The recently described I-1-Pase/FBPase from M. jannaschii (MJ0109) also dephosphorylates these substrates but with a higher relative activity (42 to 61%) (20) (Table 1). The P. furiosus FBPase appeared to be a rather specific phosphatase, since fructose-1-phosphate, fructose-6-phosphate, glucose-6-phosphate, phosphoenolpyruvate (PEP), 5′-AMP, 5′-ADP, and 5′-ATP could not be used as substrates under the tested conditions.
TABLE 1.
Substrate specificity of P. furiosus FBPase compared to that of M. jannaschii MJ0109a
| Substrate | Relative activity (%)
|
|
|---|---|---|
| P. furiosus FBPase | M. jannaschii MJ0109 | |
| Fructose-1,6-bisphosphate | 100 | 100 |
| Inositol-1-phosphate | 7.5 | 61 |
| Glycerol-2-phosphate | 1.7 | 49 |
| Glucose-1-phosphate | 2.8 | 42 |
Assays for P. furiosus FBPase were performed at 85°C as described in the text. The data from assays of MJ0109 performed at 85°C were obtained from Stec et al. (20). An activity of 100% corresponds to 12.2 and 15.2 U/mg for P. furiosus FBPase and MJ0109, respectively. Fructose-1-phosphate, fructose-6-phosphate, glucose-6-phosphate, PEP, 5′-AMP, 5′-ADP, and 5′-ATP could not be used as substrates by the P. furiosus FBPase.
The low I-1-Pase activity of the P. furiosus FBPase might be explained as follows. In thermophilic archaea and bacteria, several intracellular solutes are accumulated in response to osmotic and temperature stresses (15). One of these compatible solutes is di-myo-inositol phosphate (DIP), a solute that accumulates at supraoptimal growth temperatures in some thermophilic species (15, 17). In P. furiosus, temperatures above the growth optimum also lead to a significant increase of this compound (11). Two different routes for DIP synthesis are known: (i) in Methanococcus igneus (closely related to M. jannaschii), I-1-Pase activity is required to form myo-inositol, which acts as a precursor in DIP biosynthesis (4), and (ii) in Pyrococcus woesei, DIP is synthesized in a different way, without the myo-inositol-forming step (17). This latter pathway includes the coupling of two myo-inositol-1-phosphates without a preceding I-1-Pase-mediated dephosphorylation of one of the myo-inositol-1-phosphate moieties. Since P. furiosus is closely related to P. woesei, it is most likely that in P. furiosus, I-1-Pase activity is not required for DIP synthesis either, which would be in good agreement with the observed low activity of the P. furiosus FBPase on myo-inositol-1-phosphate.
Effectors of FBPase.
The effect of inhibitors on the activity of the P. furiosus FBPase was investigated by adding cations and metabolites (0 to 100 mM) to the standard enzyme assay mixture (85°C) (Table 2). The enzyme has an absolute requirement for Mg2+ (data not shown). The inhibition characteristics of the P. furiosus FBPase clearly differ from those of characterized eukaryal and bacterial FBPases as well as from those of the other presently characterized archaeal I-1-Pase/FBPase homologs. FBPase I from E. coli is very sensitive to AMP and PEP (1). FBPase II from E. coli is strongly inhibited by ATP and ADP, whereas AMP has no effect on the enzyme activity. Furthermore, FBPase II activity is enhanced in the presence of PEP (6). PEP also affects FBPase III activity, i.e., inhibition by AMP is reduced when PEP is present (7). The P. furiosus FBPase was inhibited by ADP and ATP (and to some extent by AMP), but PEP (up to 100 mM) did not influence FBPase activity at all. Therefore, PEP presumably is not an important metabolite in the regulation of FBPase in P. furiosus. In addition, glucose-6-phosphate significantly reduced P. furiosus FBPase activity in vitro (Table 2).
TABLE 2.
Inhibitors of P. furiosus FBPase activitya
| Effector | IC50 (mM) |
|---|---|
| Li+ | 1 |
| Ca2+ | 5 |
| AMP | 30 |
| ADP | 3 |
| ATP | 4 |
| Glucose-6-phosphate | 4 |
| Fructose-6-phosphate | 25 |
| Pyruvate | 60 |
Enzyme assays were performed at 85°C as described in the text (10 mM fructose-1,6-bisphosphate). IC50, the concentration of the effector when activity of the P. furiosus FBPase was reduced by 50%. The addition of up to 100 mM Na+, K+, glucose, or PEP to the assay mixture had no effect on FBPase activity.
Li+ is generally a strong inhibitor of FBPase activity (Ki ∼ 0.3 mM) (24). Under the test conditions we used, Li+ significantly reduced the P. furiosus FBPase activity (50% inhibitory concentration [IC50], 1 mM) (Table 2), whereas the addition of Na+ and K+ produced no effect. Previously, it was shown that I-1-Pases are also strongly inhibited by Li+ (IC50 ∼ 0.3 mM) (12). These enzymes have a structural fold similar to that of FBPases (27), and both are members of the sugar phosphatase superfamily (http://scop.mrc-lmb.cam.ac.uk/scop). Inhibition of mammalian I-1-Pase by Li+ is of particular interest, since this enzyme is expressed in brain tissue and forms the main target in medical treatment of manic-depression (14). The mechanisms of the Li+ inhibition of FBPases and IMPases are believed to be essentially the same: Li+ binds at one of the metal binding sites, thereby retarding turnover or phosphate release (9, 24). The residues that constitute this metal binding site are conserved in lithium-sensitive I-1-Pase and in FBPase (Fig. 1). Remarkably, Li+ does not have such a strong effect on the T. maritima (TM1415) and M. jannaschii (MJ0109) enzymes (IC50s, 100 mM for TM1415 and >250 mM for MJ0109), although residues constituting the Li+ binding site are conserved (Fig. 1) (9). Minor variations will probably distinguish the inhibitory effect of Li+ on the I-1-Pase from that on FBPase (9).
Classification of FBPases.
Recently, a new classification of bacterial FBPases into three groups (FBPase I, FBPase II, and FBPase III) has been proposed (6). Eukaryal FBPases are orthologous to the bacterial FBPase I enzymes, since both contain typical FBPase domains (http://www.expasy.ch) and display no I-1-Pase activity (20). The typical FBPase domain is absent in the bacterial FBPase II and FBPase III enzymes (Table 3), suggesting that they are phylogenetically unrelated to FBPase I enzymes. Remarkably, typical I-1-Pase domains (IMP 1) are also present in the eukaryal FBPase and the bacterial FBPase I enzymes (http://www.expasy.ch). Bacterial and eukaryal I-1-Pases contain two specific domains (IMP 1 and IMP 2) and, together with the eukaryal FBPase and bacterial FBPase I enzymes, belong to the sugar phosphatase superfamily (http://scop.mrc-lmb.cam.ac.uk/scop). Comparison of the primary structure of the P. furiosus FBPase with the FBPase and IMP family signatures revealed that this enzyme contains both I-1-Pase domains (IMP 1 and IMP 2). No obvious FBPase domain could be detected in the P. furiosus sequence (Table 3) (Fig. 1). The P. furiosus FBPase is homologous to M. jannaschii MJ0109, A. fulgidus AF2372, and T. maritima TM1415, with all three enzymes having IMP 1 and IMP 2 domains present in their primary structures (Fig. 1) and possessing dual activity (i.e., FBPase and I-1-Pase activity) (20). Since these FBPases display limited sequence identity with both eukaryal and mesophilic bacterial FBPases (12 to 16% identity with FBPase I and 11 to 15% identities with FBPase II and FBPase III) but seem to be significantly related to the I-1-Pases (16 to 35% identity), we propose on the basis of sequence identity and substrate specificity that the P. furiosus FBPase and its homologs constitute a new FBPase family, type IV FBPases (FBPase IV), which is present in euryarchaeal and hyperthermophilic bacterial species and is potentially involved in gluconeogenesis. The presence of conserved domains (IMP 1) in type I and type IV FBPases and I-1-Pases, as well as the similar structural folds of these enzymes (20, 27), suggests that these enzymes share the same phylogenetic origin, as has been suggested previously (20, 27). It is tempting to speculate that the FBPase IV enzymes originally belonged to the I-1-Pase family and subsequently evolved to efficiently convert fructose-1,6-bisphosphate for function in gluconeogenesis.
TABLE 3.
Classification of phosphatases
| Phosphatase class | Characteristic
|
||||
|---|---|---|---|---|---|
| Taxonomic range | Subunit size (kDa) | Oligomerization | Fold type | Sequence motif(s) | |
| FBPase I | Eukarya, bacteria | ∼38 | Tetramer | Sugar phosphatase | FBPase, IMP 1 |
| FBPase II | Bacteria | ∼36 | Dimer | Unknown | None |
| FBPase III | Bacteria | ∼76 | Tetramer | Unknown | None |
| FBPase IVa | Archaea, HT bacteria | ∼28 | Dimerb | Sugar phosphatase | IMP 1, IMP 2 |
| I-1-Pasec | Eukarya, bacteria | ∼30 | Dimer | Sugar phosphatase | IMP 1, IMP 2 |
FBPase IV enzymes are at least present in the euryarchaea P. furiosus (AF453319), Pyrococcus horikoshii (PH1897), Pyrococcus abyssi (PAB0189), Methanococcus jannaschii (MJ0109), Archaeoglobus fulgidus (AF2372), Methanosarcina barkeri (MB1918), and Methanobacterium thermoautotrophicum (MTH871) and the hyperthermophilic (HT) bacteria Thermotoga maritima (TM1415) and Aquifex aeolicus (AQ1983).
The T. maritima enzyme is an exception, having a tetrameric structure.
Nucleotide sequence accession number. The P. furiosus fbpA nucleotide and amino acid sequence data reported in this study have been submitted to GenBank under accession no. AF453319.
Acknowledgments
We thank L. Kluskens (Wageningen University) for assistance during the differential scanning calorimetry measurements and Stefan Wolff (Essen University, Essen, Germany) for providing myo-inositol-1-phosphate.
This work was supported by the Earth and Life Sciences foundation (ALW), which is subsidized by The Netherlands Organization for Scientific Research (NWO).
REFERENCES
- 1.Babul, J., and V. Guixe. 1983. Fructose bisphosphatase from Escherichia coli. Purification and characterization. Arch. Biochem. Biophys. 225:944-949. [DOI] [PubMed] [Google Scholar]
- 2.Bult, C. J., O. White, G. J. Olsen, L. Zhou, R. D. Fleischmann, G. G. Sutton, J. A. Blake, L. M. FitzGerald, R. A. Clayton, J. D. Gocayne, A. R. Kerlavage, B. A. Dougherty, J. F. Tomb, M. D. Adams, C. I. Reich, R. Overbeek, E. F. Kirkness, K. G. Weinstock, J. M. Merrick, A. Glodek, J. L. Scott, N. S. Geoghagen, and J. C. Venter. 1996. Complete genome sequence of the methanogenic archaeon, Methanococcus jannaschii. Science 273:1058-1073. [DOI] [PubMed] [Google Scholar]
- 3.Chen, L., and M. F. Roberts. 2000. Overexpression, purification, and analysis of complementation behavior of E. coli SuhB protein: comparison with bacterial and archaeal inositol monophosphatases. Biochemistry 39:4145-4153. [DOI] [PubMed] [Google Scholar]
- 4.Chen, L., E. T. Spiliotis, and M. F. Roberts. 1998. Biosynthesis of di-myo-inositol-1,1′-phosphate, a novel osmolyte in hyperthermophilic archaea. J. Bacteriol. 180:3785-3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Vos, W. M., S. W. M. Kengen, W. G. B. Voorhost, and J. van der Oost. 1998. Sugar utilization and its control in hyperthermophiles. Extremophiles 2:201-205. [DOI] [PubMed] [Google Scholar]
- 6.Donahue, J. L., J. L. Bownas, W. G. Niehaus, and T. J. Larson. 2000. Purification and characterization of glpX-encoded fructose 1,6-bisphosphatase, a new enzyme of the glycerol 3-phosphate regulon of Escherichia coli. J. Bacteriol. 182:5624-5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujita, Y., K. Yoshida, Y. Miwa, N. Yanai, E. Nagakawa, and Y. Kasahara. 1998. Identification and expression of the Bacillus subtilis fructose-1,6-bisphosphatase gene (fbp). J. Bacteriol. 180:4309-4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geladopoulos, T. P., T. G. Sotiroudis, and A. E. Evangelopoulos. 1991. A malachite green colorimetric assay for protein phosphatase activity. Anal. Biochem. 192:112-116. [DOI] [PubMed] [Google Scholar]
- 9.Johnson, K. A., L. Chen, H. Yang, M. F. Roberts, and B. Stec. 2001. Crystal structure and catalytic mechanism of the MJ0109 gene product: a bifunctional enzyme with inositol monophosphatase and fructose 1,6-bisphosphatase activities. Biochemistry 40:618-630. [DOI] [PubMed] [Google Scholar]
- 10.Kengen, S. W. M., J. E. Tuininga, F. A. M. de Bok, A. J. M. Stams, and W. M. de Vos. 1995. Purification and characterization of a novel ADP-dependent glucokinase from the hyperthermophilic archaeon Pyrococcus furiosus. J. Biol. Chem. 270:30453-30457. [DOI] [PubMed] [Google Scholar]
- 11.Martins, L. O., and H. Santos. 1995. Accumulation of mannosylglycerate and di-myo-inositol-phosphate by Pyrococcus furiosus in response to salinity and temperature. Appl. Environ. Microbiol. 61:3299-3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuhisa, A., N. Suzuki, T. Noda, and K. Shiba. 1995. Inositol monophosphatase activity from the Escherichia coli suhB gene product. J. Bacteriol. 177:200-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ng, W. V., S. P. Kennedy, G. G. Mahairas, B. Berquist, M. Pan, H. D. Shukla, S. R. Lasky, N. S. Baliga, V. Thorsson, J. Sbrogna, S. Swartzell, D. Weir, J. Hall, T. A. Dahl, R. Welti, Y. A. Goo, B. Leithauser, K. Keller, R. Cruz, M. J. Danson, D. W. Hough, D. G. Maddocks, P. E. Jablonski, M. P. Krebs, C. M. Angevine, H. Dale, T. A. Isenbarger, R. F. Peck, M. Pohlschroder, J. L. Spudich, K. W. Jung, M. Alam, T. Freitas, S. Hou, C. J. Daniels, P. P. Dennis, A. D. Omer, H. Ebhardt, T. M. Lowe, P. Liang, M. Riley, L. Hood, and S. DasSarma. 2000. Genome sequence of Halobacterium species NRC-1. Proc. Natl. Acad. Sci. USA 97:12176-12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pollack, S. J., J. R. Atack, M. R. Knowles, G. McAllister, C. I. Ragan, R. Baker, S. R. Fletcher, L. L. Iversen, and H. B. Broughton. 1994. Mechanism of inositol monophosphatase, the putative target of lithium therapy. Proc. Natl. Acad. Sci. USA 91:5766-5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santos, H., and M. S. da Costa. 2001. Organic solutes from thermophiles and hyperthermophiles. Methods Enzymol. 334:302-315. [DOI] [PubMed] [Google Scholar]
- 16.Schäfer, T., and P. Schönheit. 1993. Gluconeogenesis from pyruvate in the hyperthermophilic archaeon Pyrococcus furiosus: involvement of reactions of the Embden-Meyerhof pathway. Arch. Microbiol. 159:359-363. [Google Scholar]
- 17.Scholz, S., S. Wolff, and R. Hensel. 1998. The biosynthesis pathway of di-myo-inositol-1,1′-phosphate in Pyrococcus woesei. FEMS Microbiol. Lett. 168:37-42. [Google Scholar]
- 18.Schut, G. J., J. Zhou, and M. W. W. Adams. 2001. DNA microarray analysis of the hyperthermophilic archaeon Pyrococcus furiosus: evidence for a new type of sulfur-reducing enzyme complex. J. Bacteriol. 183:7027-7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siebers, B., H. Brinkmann, C. Dorr, B. Tjaden, H. Lilie, J. van der Oost, and C. H. Verhees. 2001. Archaeal fructose-1,6-bisphosphate aldolases constitute a new family of archaeal type class I aldolase. J. Biol. Chem. 276:28710-28718. [DOI] [PubMed] [Google Scholar]
- 20.Stec, B., H. Yang, K. A. Johnson, L. Chen, and M. F. Roberts. 2000. MJ0109 is an enzyme that is both an inositol monophosphatase and the ‘missing' archaeal fructose-1,6-bisphosphatase. Nat. Struct. Biol. 7:1046-1050. [DOI] [PubMed] [Google Scholar]
- 21.Tuininga, J. E., C. H. Verhees, J. van der Oost, S. W. M. Kengen, A. J. M. Stams, and W. M. de Vos. 1999. Molecular and biochemical characterization of the ADP-dependent phosphofructokinase from the hyperthermophilic archaeon Pyrococcus furiosus. J. Biol. Chem. 274:21023-21028. [DOI] [PubMed] [Google Scholar]
- 22.van der Oost, J., G. Schut, S. W. M. Kengen, W. R. Hagen, M. Thomm, and W. M. de Vos. 1998. The ferredoxin-dependent conversion of glyceraldehyde-3-phosphate in the hyperthermophilic archaeon Pyrococcus furiosus represents a novel site of glycolytic regulation. J. Biol. Chem. 273:28149-28154. [DOI] [PubMed] [Google Scholar]
- 23.Verhees, C. H., M. A. Huynen, D. E. Ward, E. Schiltz, W. M. de Vos, and J. van der Oost. 2001. The phosphoglucose isomerase from the hyperthermophilic archaeon Pyrococcus furiosus is a unique glycolytic enzyme that belongs to the cupin superfamily. J. Biol. Chem. 276:40926-40932. [DOI] [PubMed] [Google Scholar]
- 24.Villeret, V., S. Huang, Y. Zhang, Y. Xue, and W. N. Lipscomb. 1995. Crystal structure of spinach chloroplast fructose-1,6-bisphosphatase at 2.8 Å resolution. Biochemistry 34:4299-4306. [DOI] [PubMed] [Google Scholar]
- 25.Ward, D. E., S. W. M. Kengen, J. van der Oost, and W. M. de Vos. 2000. Purification and characterization of the alanine aminotransferase from the hyperthermophilic archaeon Pyrococcus furiosus and its role in alanine production. J. Bacteriol. 182:2559-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weeks, C. M., A. W. Roszak, M. Erman, R. Kaiser, H. Jörnvall, and D. Ghosh. 1999. Structure of rabbit liver fructose 1,6-bisphosphatase at 2.3 Å resolution. Acta Crystallogr. Sect. D 55:93-102. [DOI] [PubMed] [Google Scholar]
- 27.Zhang, Y., J. Y. Liang, and W. N. Lipscomb. 1993. Structural similarities between fructose-1,6-bisphosphatase and inositol monophosphatase. Biochem. Biophys. Res. Commun. 190:1080-1083. [DOI] [PubMed] [Google Scholar]