Abstract
Background
Adherent bone marrow stromal cells are inducible osteoprogenitors, giving rise to cells expressing osteoblast markers including alkaline phosphatase, osteopontin, osteocalcin, and bone sialoprotein. However, the potency of inducers varies in a species-specific manner. Glucocorticoids such as dexamethasone induce alkaline phosphatase activity in both human and rat mesenchymal stem cells, while mouse bone marrow stromal cells are refractory to dexamethasone-induced alkaline phosphatase activity. In contrast, BMP induces alkaline phosphatase activity in both mouse and rat bone marrow stromal cells, while BMP effects on human bone marrow stromal cells are poorly characterized.
Methods
Bone marrow samples were isolated from patients undergoing hip replacement. Mononuclear marrow cells were cultured and grown to confluence without or with 10−7M dexamethasone. Cells from each isolate were passaged into medium containing 100 μg/mL ascorbate phosphate and treated with dexamethasone, 100 ng/mL BMP, or no inducer. At day 6, alkaline phosphatase activity was assayed, and RNA was prepared for mRNA analyses by real-time polymerase chain reaction.
Results
Bone marrow stromal cells from twenty-four of twenty-six patients showed no significant osteogenic response to BMP-2, 4, or 7 as determined by alkaline phosphatase induction. However, BMPs induced elevated levels of other genes associated with osteogenesis such as bone sialoprotein and osteopontin as well as BMP-2 and noggin. If primary cultures of human bone marrow stromal cells were pretreated with dexamethasone, BMP-2 treatment of first-passage cells induced alkaline phosphatase in approximately half of the isolates, and significantly greater induction was seen in cells from males. Dexamethasone treatment, like BMP treatment, also increased expression of the BMP-binding protein noggin.
Conclusions
Most human femur bone marrow stromal cell samples appear incapable of expressing elevated alkaline phosphatase levels in response to BMPs. Since BMP treatment induced expression of several other BMP-regulated genes, the defect in alkaline phosphatase induction is presumably not due to impaired BMP signaling. We hypothesize that the mechanism by which BMPs modulate alkaline phosphatase expression is indirect, involving a BMP-regulated transcription factor for alkaline phosphatase expression that is controlled differently in humans and rodents.
Clinical Relevance
We suggest that the relative insensitivity of alkaline phosphatase to BMP induction in human bone marrow stromal cells may contribute to the variation in efficacy reported with BMP in clinical settings.
The success of postnatal skeletal renewal processes such as remodeling and fracture healing is dependent on the ability of the body to promote the differentiation of precursor cells such as mesenchymal stem cells into functional osteoblasts. Impairment of this process of osteogenesis can lead to osteoporosis as well as impaired fracture healing. Elucidating how osteogenesis is regulated is vital to the understanding of the pathogenesis of these important diseases, and, in turn, to the validation of proposed strategies for therapeutic intervention.
Osteogenesis is characterized by the sequential expression of a series of markers collectively unique to this phenotype1. Among these markers of osteogenesis, the production of high levels of the tissue non-specific isoform of alkaline phosphatase stands out as the hallmark distinguishing osteogenic cells in culture2. The importance of alkaline phosphatase in bone formation resides in its ability to regulate mineralization of bone matrix3. Therefore, individuals containing deleterious mutations of the alkaline phosphatase gene leading to hypophosphatasia manifest defects in bone mineralization4. Similarly, alkaline phosphatase null mice are capable of developing a mineralized skeleton but develop postnatal osteopenia and fractures5.
The adherent bone marrow stromal cell is a mesenchymal stem cell that can be induced to form osteoblasts. This osteogenic capacity has been demonstrated in vitro in cells isolated from mice, rats, rabbits, and humans6,7. Curiously, the potency of individual inducers to induce alkaline phosphatase varies in a species-dependent manner. Several bone morphogenetic proteins (BMPs) are potent inducers of osteogenesis in both mouse and rat bone marrow stromal cells8–10. Glucocorticoids such as dexamethasone are potent inducers in human and rat stromal cells, but they have no effect on alkaline phosphatase activity in mouse stromal cells11–16. This species variation in inducer-dependent effects on mesenchymal stem cells is notable for two reasons. First, one must be cautious in assuming that stromal cells from other species may serve as models for inducible osteogenesis in human marrow stromal cells. Second, while cultured human stromal cells differentiate into osteoblasts in the presence of dexamethasone, this has questionable clinical relevance because glucocorticoids are potent inducers of osteoporosis in vivo.
In this study, we showed that BMP-2 alone does not induce osteogenesis in isolates of human bone marrow stromal cells as measured by stimulation of alkaline phosphatase expression. However, BMP-2 does induce other markers associated with differentiation of osteoblasts. Interestingly, we found that BMP-responsive alkaline phosphatase activity may be elicited by dexamethasone pretreatment of stromal cells from some patients, with the extent of this response apparently related to gender.
Materials and Methods
Isolation of Human Marrow Stromal Cells
Marrow samples were isolated from the proximal part of the medullary cavity of femora from a series of patients at the time of primary total hip replacement. The samples were placed on ice for transport. All steps in establishing primary marrow cultures were performed at room temperature unless otherwise noted. Individual marrow samples were washed twice by suspension in alpha-minimal essential medium and subsequent centrifugation to remove a fatty fraction. Following the second wash step, the marrow pellets were suspended in Hanks balanced salt solution and layered onto Ficoll-Paque minimal essential medium (Amersham Pharmacia Biotech, Piscataway, NJ). The gradient was centrifuged for thirty minutes at 1900 RCF (relative centrifugal force). This step concentrated the nucleated cells at the Hanks balanced salt solution/Ficoll-Paque interface. The nucleated cell fractions were collected and were washed in minimal essential medium. The cells were quantitated, and primary cultures were established at a plating density of 5 × 105 cells/cm2 in minimal essential medium/15% fetal calf serum and antibiotics. The medium was changed initially at day 4 and then every other day thereafter until the cultures reached confluence. Subsets of primary cultures from each isolate were treated with dexamethasone at a final concentration of 10−7M on day 1 and at subsequent changes of the medium.
Osteogenic studies were performed employing first-passage cultures plated at a density of 1 × 104 cells/cm2 (day 0). Four treatment groups were established on day 1 by the initial addition of osteogenic inducers: ascorbate-2-phosphate alone at 100 μg/mL; ascorbate-2-phosphate and BMP-2 (100 ng/mL); ascorbate-2-phosphate and dexamethasone (10−7M); or ascorbate-2-phosphate, BMP-2, and dexamethasone. Medium was changed every other day, and inducers were replenished at that time. Cells were harvested on day 6 for determination of alkaline phosphatase activity and RNA isolation.
Alkaline Phosphatase Assay
Alkaline phosphatase activity was determined kinetically by monitoring the conversion of p-nitrophenyl phosphate to p-nitrophenol as described previously8.
Isolation of Total RNA and Reverse Transcriptase-Polymerase Chain Reactions
Total RNA was isolated with use of TRI Reagent (Molecular Research Center, Cincinnati, OH) according to the manufacturer’s protocol. Phase separation was achieved by the addition of 1-bromo-3-chloropropane. The total RNA was precipitated from the aqueous phase with use of isopropanol, and the RNA pellet was washed with 75% ethanol. The RNA was quantitated spectrophotometrically at 260 and 280 nm, aliquoted, and stored at −70°C.
Reverse transcriptase-polymerase chain reaction was performed with use of a commercially available single tube system (Ready-To-Go PCR beads, Amersham Pharmacia Biotech) and used according to the manufacturer’s protocol with 2 μg total RNA for each reaction. Oligo dT was used as the first strand primer, and forward and polymerase chain reaction primers were added to a final concentration of 0.15μM each. Denaturation, annealing, and extension steps were carried out for one minute each at 95°C, 60°C, and 72°C, respectively.
Polymerase Chain Reaction Primers
The following forward and reverse primer pairs were used to amplify specific cDNAs:
Alkaline phosphatase: forward: 5′-ACCATTCCCACGT-CTTCACATTTG-3′; reverse: 5′-AGACATTCTCTCGTTCAC-CGCC-3′. BMP-2: forward: 5′-GAGTTGCGGCTGCTCAGC-ATGTT-3′; reverse: 5′-ACATGTCTCTTGGAGACACCT-3′. Noggin: forward: 5′-GGAGGAAGTTACAGATGTGGCTGT-3′; reverse 5′-CACTCGGAAATGATGGGGTACTG-3′. Osteopon-tin: forward: 5′-AGCCAGGACTCCATTGACTCGAAC-3′; reverse: 5′-GTTTCAGCACTCTGGTCATCCAGC-3′. Runx2 (Cbfa1): forward: 5′-AGATGATGACACTGCCACCTCTG-3′; reverse: 5′-GGGATGAAATGCTTGGGAACTGC-3′. Osteocal-cin: forward: 5′-ATGAGAGCCCTCACACTCCTC-3′; reverse: 5′-GCCGTAGAAGCGCCGATAGGC-3′. Bone sialoprotein: forward: 5′-AATGAAAACGAAGAAAGCGAAG-3′ reverse: 5′-ATCATAGCCATCGTAGCCTTGT-3′.
Real-Time Polymerase Chain Reactions
cDNA was prepared from 2-μg samples of total RNA isolated as described above with use of the SuperScript First Strand Synthesis System (Invitrogen, Carlsbad, CA). The cDNA samples were amplified in a real-time polymerase chain reaction cycling apparatus (Cepheid Smart Cycler, Sunnyvale, CA) with use of the LightCycler Fast-Start DNA Master SYBR Green I (Roche Molecular Biochemicals, Mannheim, Germany). The reactions were formulated according to the manufacturer’s protocol. After initial denaturations for ten minutes at 95°C, amplifications were carried out for forty-five cycles with use of three-step cycles of denaturation at 95°C for fifteen seconds, annealing for thirty seconds, and extension at 72°C for thirty seconds. The annealing temperature was individually calculated for each primer pair: alkaline phosphatase and bone sialoprotein at 57°C, noggin and osteocalcin at 65°C, BMP-2 at 60°C, and osteopontin at 68°C. At the termination of the polymerase chain reactions for each sample, a melting curve was generated over a 60°C to 96°C range at a rate of 0.2°C elevation/second.
Immunoblotting for Noggin Protein
Cultured cells were washed twice with ice-cold Hanks balanced salt solution and collected in 1mM Tris-HCl, pH 6.8; 20% glycerol; and 4% sodium dodecyl sulphate lysis buffer. Lysates were placed on ice denatured by heating at 100°C for six minutes in the presence of 5% β-mercaptoethanol. Equal amounts of protein were electrophoresed in a 10% Trisglycine/sodium dodecyl sulphate pre-cast gel (Invitrogen, Carlsbad, CA). Proteins were electro-blotted at 4°C onto a polyvinylidene difluoride membrane (Immuno-Blot PVDF, Bio-Rad Laboratories, Hercules, CA) at 100 V for one hour, and the membrane was then blocked in phosphate-buffered saline solution containing 3% nonfat, dehydrated milk for at least two hours at room temperature. Exposure to rat anti-human noggin primary antibody (provided by Regeneron, Tarrytown, NY) was performed overnight in the cold with use of 5 μg of primary antibody in a phosphate-buffered saline solution containing 1% nonfat, dehydrated milk. The membrane was then washed with five changes of phosphate-buffered saline solution. Peroxidase-linked sheep anti-rat secondary antibody (Santa Cruz) was added at a dilution of 1:1000 in phosphate-buffered saline solution with 1% milk, and incubation was performed for two hours at room temperature. The membrane was then washed with five changes of phosphate-buffered saline solution. The peroxidase-based signal was developed with use of a chemiluminescence reagent system according to the manufacturer’s instructions (Western Lightning, PerkinElmer Life Sciences, Boston, MA). The development of the blot was monitored on a Kodak Image Station 440 (Perkin Elmer Life Sciences, Norfolk, Connecticut).
Results
Subjects
The data presented here were gathered from bone marrow samples of twenty-four patients who underwent primary total hip arthroplasty. Samples were obtained from twelve female patients. Their ages ranged from thirty-five to seventy-five years. An additional twelve samples were obtained from male patients, ranging in age from twenty-three to seventy-eight years. No detailed medical history of the patients was sought.
Induction of Alkaline Phosphatase Activity in Cultured Human Marrow Stromal Cells
Since a high level of alkaline phosphatase activity is considered a hallmark of the osteogenic phenotype, we evaluated the effect of both BMP-2 and dexamethasone on alkaline phosphatase activity in first-passage human marrow stromal cell cultures cultured until day 6. As expected, dexamethasone significantly stimulated alkaline phosphatase activity in first-passage human marrow stromal cell cultures (Fig. 1, A). However, 100 μg/mL BMP-2 had no significant effect on alkaline phosphatase activity, even when the culture period was extended to fourteen days. Cultures of bone marrow derived from two additional patients, a fifty-six-year-old man and a forty-one-year-old woman, showed some BMP stimulation, with BMP-induced alkaline phosphatase activity that was at least 60% of that seen with dexamethasone treatment.
Fig. 1.
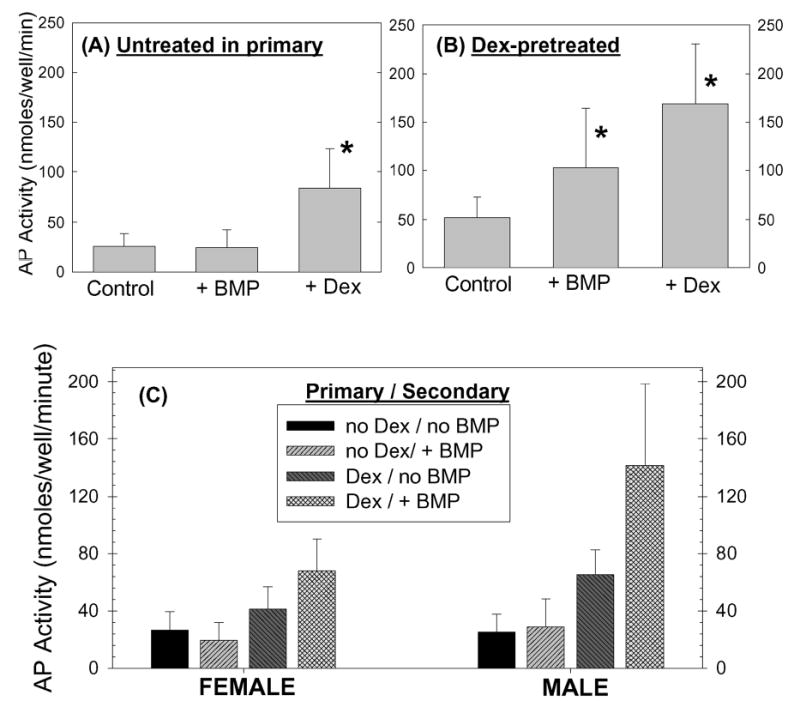
Alkaline phosphatase (AP) activity in day-6 first-passage human marrow-derived stromal cell cultures. First-passage human marrow-derived stromal cell cultures were treated and were harvested for analysis of alkaline phosphatase activity as described in Materials and Methods. A: First-passage cultures derived from untreated primaries did not show any response to BMP-2 treatment. Alkaline phosphatase activity was significantly (*) increased by dexamethasone (Dex). B: In first-passage cultures derived from dexamethasone-treated primaries, BMP-2 significantly increased alkaline phosphatase activity. Dexamethasone pretreatment also enhanced the dexamethasone stimulation of alkaline phosphatase seen in the first passage. C: The influence of dexamethasone pretreatment on BMP-2 induction of alkaline phosphatase in the first passage was greater in cultures of cells derived from male patients than it was in cultures of cells from female patients.
Dexamethasone Pretreatment and BMP-2 Induction of Alkaline Phosphatase Activity in Human Marrow Stromal Cells
Previously published data derived with use of rat marrow stromal cells demonstrated that dexamethasone treatment of primary marrow cell cultures had the capacity to maintain osteogenesis in subsequent passage8. Accordingly, we investigated the possibility that this same phenomenon would be elicited in cultured human marrow stromal cells. Primary cultures were treated with 10−7M dexamethasone at day 1 and at each subsequent medium change. At confluence, the dexamethasone-pretreated primary cells were passaged. First-passage cultures derived from dexamethasone-pretreated cells were divided among the four treatment groups described above and compared with cultures derived from nontreated cells.
First-passage cultures of cells pretreated with dexamethasone had higher baseline alkaline phosphatase activity than did first-passage cultures derived from untreated primaries (Fig. 1, A and B). Dexamethasone pretreatment also enhanced the dexamethasone responsiveness of first-passage cultures (Fig. 1, B). When results from all twenty-four patient samples were compiled, dexamethasone pretreatment was also seen to induce a BMP-2-dependent elevation in alkaline phosphatase activity (p = 0.01), which was absent in first-passage cultures derived from untreated primaries (Fig. 1, B). However, the patient samples appeared to divide into two groups: a male group in which dexamethasone pretreatment resulted in a marked response to BMP (p = 0.01) and a female group in which alkaline phosphatase levels in response to BMP were higher than control values but were significantly lower than those in the male group (Fig. 1, C). A summary of the data from these studies, sorted by gender, is presented in Table I.
TABLE I.
Alkaline Phosphatase Activity in Human Bone Marrow Cells with and without Dexamethasone Pretreatment*
| Primary Culture
|
First-Passage Culture (nmol product/min/well) |
||||
|---|---|---|---|---|---|
| With Dexa methasone? | With Dexa methasone? | No BMP | With BMP-2 | P Value for BMP vs. no BMP | |
| Males | No | No | 24.1 ± 11.9 | 24.2 ± 19.6 | >0.3 |
| Yes‡ | 78.7 ± 54.7 | 94.0 ± 57.5 | >0.3 | ||
| Yes† | No | 65.4 ± 17.2 | 142.0 ± 66.2 | 0.01 | |
| Yes‡ | 203.3 ± 64.0 | 323.6 ± 123.5 | 0.01 | ||
| Females | No | No | 24.4 ± 14.9 | 18.6 ± 11.9 | >0.3 |
| Yes‡ | 69.2 ± 40.0 | 78.3 ± 44.3 | >0.3 | ||
| Yes† | No | 41.0 ± 15.8 | 67.7 ± 24.3 | 0.02 | |
| Yes‡ | 137.9 ± 39.6 | 192.1 ± 60.4 | 0.03 | ||
Alkaline phosphatase activity was measured after individually culturing bone marrow cells from twelve men (average age, 45 ± 16 years) and twelve women (average age, 54 ± 11 years). Mononuclear cell samples from each patient were divided into two culture dishes for primary culture, with one of the dishes receiving 10−7M dexamethasone and the other not receiving dexamethasone. At confluence, the dexamethasone-treated cells and untreated cells were each replated (first passage), both with and without dexamethasone, and BMP was added as indicated. All results are the average of samples, assayed in triplicate, from all twelve men or all twelve women.
The values for male cells pretreated with dexamethasone were significantly higher than the values for female cells pretreated with dexamethasone; p < 0.01.
All values with dexamethasone in first passage were significantly higher than those without dexamethasone in first passage; p < 0.01.
BMP-2 Stimulation of BMP-Responsive Genes
The limited effect of BMP on alkaline phosphatase activity in first-passage cultures of human stromal cells led us to investigate whether this poor response extended to two genes reported to be regulated by BMP signaling: BMP-2 and the BMP-binding protein noggin. Reverse transcriptase-polymerase chain reaction analysis demonstrated that BMP-2 treatment of human marrow stromal cells produced increased expression of both BMP-2 mRNA and noggin. Addition of 10−7M dexamethasone to cultures caused decreased expression of BMP-2 mRNA and attenuation of the BMP-induced increase in BMP-2. In contrast, dexamethasone elevated noggin mRNA to levels comparable with those seen with BMP treatment (Fig. 2).
Fig. 2.
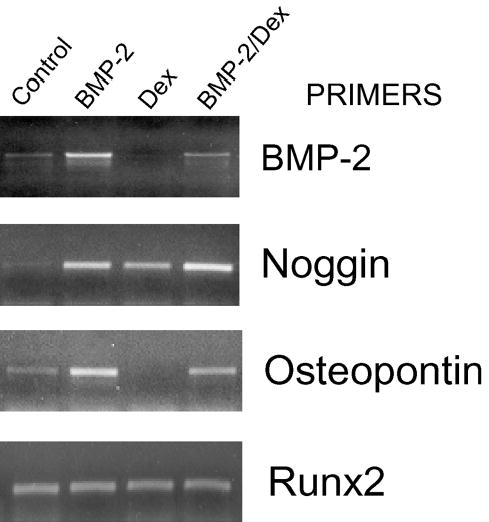
Reverse transcriptase-polymerase chain reactions for osteoblastic and BMP-inducible genes in human marrow-derived stromal cells. Conventional reverse transcriptase-polymerase chain reaction was performed on total RNA isolated from day-6 first-passage human marrow-derived stromal cells not pretreated with dexamethasone. BMP-2 had no effect on alkaline phosphatase, but it consistently increased BMP-2 and noggin mRNA. BMP also induced osteopontin in most samples. Dexamethasone also increased noggin mRNA and potentiated the effect of BMP. However, dexamethasone attenuated the BMP-2 upregulation of BMP-2 and osteopontin mRNA.
The effects on regulation of noggin and BMP-2 expression were confirmed by real-time polymerase chain reaction assays. Figure 3 displays results with a representative cDNA sample derived from human marrow stromal cells. BMP treatment increased both noggin and BMP-2 mRNA levels as demonstrated by the shift to the left, indicating detectable SYBR Green fluorescence at an earlier cycle number. These results indicate that BMP signaling is capable of inducing a transcriptional response in human marrow stromal cells. Analyses of RNA prepared from cultured cells obtained from several patients indicated that both noggin and BMP-2 mRNA levels were increased approximately fifteenfold by BMP treatment (Table II). Comparison of dexamethasone and BMP effects demonstrated both similarities and differences. Unlike the stimulatory effects seen with BMP, dexamethasone suppressed BMP-2 mRNA levels. However, dexamethasone increased noggin mRNA to levels comparable with those seen with BMP (Fig. 2, Table II), and the combination of dexamethasone and BMP resulted in significantly higher levels than did either compound alone. Since the ability of dexamethasone to regulate noggin levels has not been previously reported, we investigated whether noggin protein was similarly affected. Immunoblot analysis of whole cell extracts with use of a rat anti-human noggin antibody showed that both BMP-2 and dexamethasone increased noggin protein (Fig. 4). The increase in noggin protein seen when BMP and dexamethasone were both present was greater than that seen with each inducer alone.
Fig. 3.
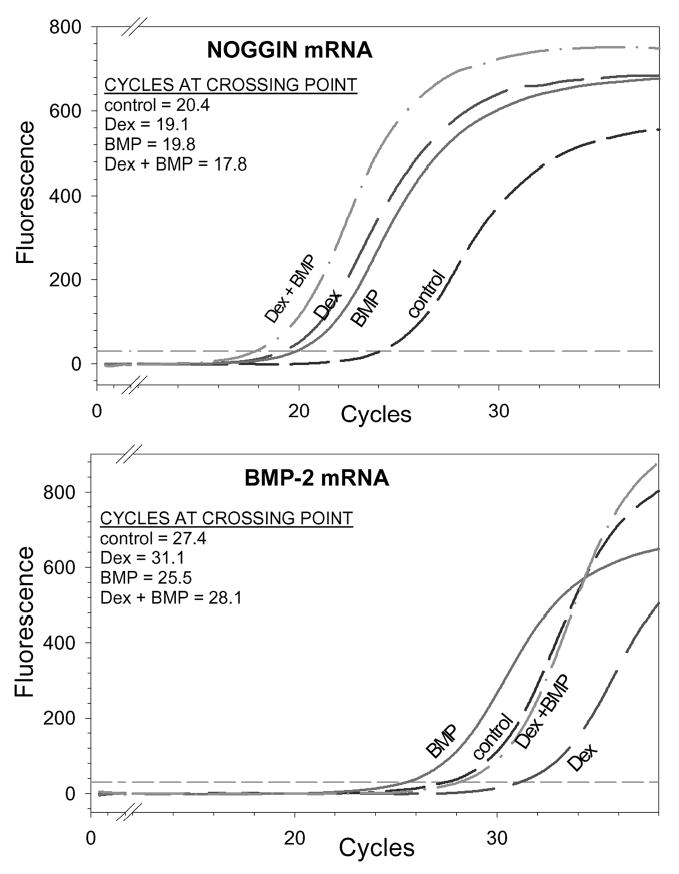
Expression of BMP-inducible genes in a representative sample of cultured first-passage human marrow-derived stromal cells. cDNA was prepared from mRNA of day-6 first-passage cultures and analyzed by real-time reverse transcriptase-polymerase chain reactions. SYBR Green fluorescence indicates formation of polymerase chain reaction product. The cycle number at which product fluorescence crosses the fluorescence threshold is the crossing point; larger amounts of initial mRNA result in detectable fluorescence at a lower crossing point. Both noggin and BMP-2 mRNA were increased in BMP-treated cultures. Dexamethasone (Dex) treatment increased noggin mRNA but decreased BMP-2 mRNA.
TABLE II.
Real-Time Polymerase Chain Reaction Analyses of mRNA from Human Marrow Stromal Cells Cultured with Dexamethasone or BMP
| Fold Change from Control* |
|||||
|---|---|---|---|---|---|
| mRNA | No. | 10−7M Dexamethasone | P Value† | 100 ng/mL BMP-2 | P Value† |
| Alkaline phosphatase | 14 | 7.3 ± 3.0 | <0.001 | –1.2 ± 3.2 | NS |
| Osteopontin | 5 | 0.4 ± 2.8 | NS | 8.1 ± 3.2 | 0.01 |
| Bone sialoprotein‡ | 3 | 12.7 ± 3.3 | 0.04 | 18.2 ± 4.0 | 0.03 |
| Noggin | 5 | 16.9 ± 1.9 | <0.001 | 16.7 ± 2.3 | <0.001 |
| BMP-2 | 3 | –7.0 ± 3.1 | 0.10 | 14.0 ± 4.1 | 0.05 |
Calculated by assuming that 3.3 cycles reflect a tenfold change in mRNA.
Probabilities calculated with use of a paired t test. NS = not significantly different from control.
Bone sialoprotein values were derived from cells cultured until day 10. All other assays were performed on RNA derived from day-6 cultures.
Fig. 4.
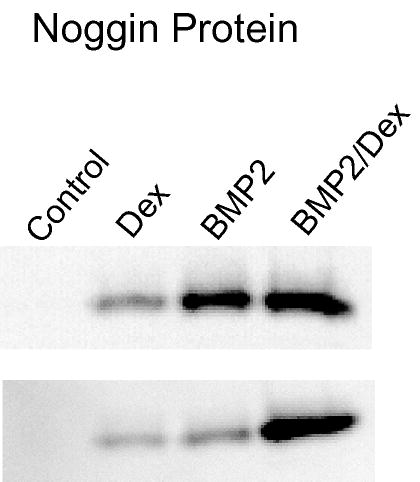
Immunoblot analysis for noggin protein in human marrow-derived stromal cells. Whole cell protein lysates from day-6 cultures were evaluated as described in Materials and Methods with use of a rat anti-human noggin antibody. Two representative samples from non-pretreated first-passage cultures are shown. The results mirror the reverse transcriptase-polymerase chain reaction results, demonstrating increased noggin protein associated with either BMP-2 or dexamethasone (Dex) treatment and the additive effect of combined treatment.
BMP Regulation of Genes Induced During Osteogenesis
Our analyses of noggin and BMP-2 levels suggested that although BMP had no effect on alkaline phosphatase activity in first-passage culture without dexamethasone pretreatment, the lack of effect could not be explained simply as defective BMP signaling in human marrow stromal cells. However, noggin and BMP-2 are genes that are BMP-responsive in a variety of cell types. We therefore examined the regulation of genes more specifically associated with osteoblast differentiation. Reverse transcriptase-polymerase chain reactions with use of primers specific for all isoforms of Runx2 (Cbfa1) showed high levels of this mRNA in human marrow stromal cells under all conditions. Quantitive real-time polymerase chain reaction analyses for bone sialoprotein and osteocalcin mRNA as well as alkaline phosphatase mRNA are shown in Figure 5, and the cumulative results from all analyses are presented in Table II.
Fig. 5.
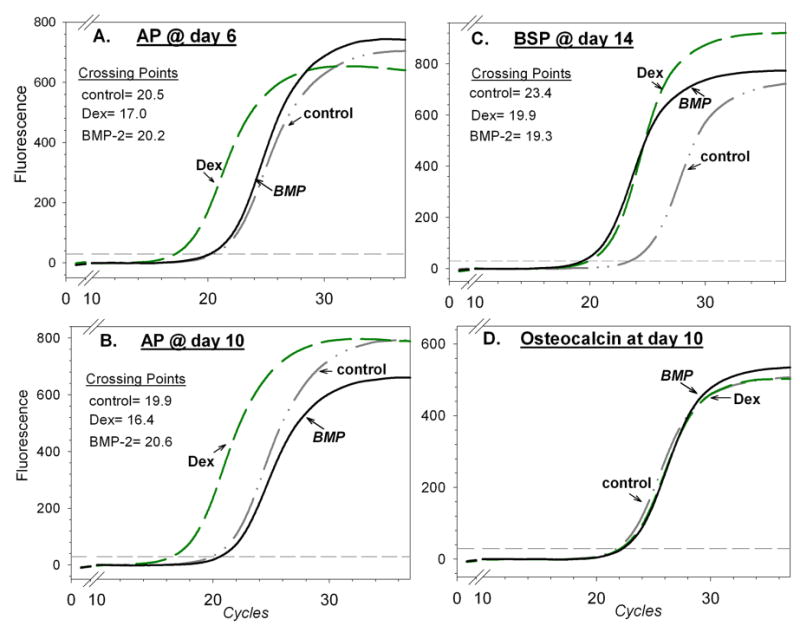
Expression of ostoblast genes in a representative sample of cultured first-passage human marrow-derived stromal cells. Results of real-time polymerase chain reactions were analyzed as described in the legend to Figure 3. While dexamethasone (Dex) treatment increased levels of alkaline phosphatase (AP) mRNA at both day 6 and day 10, BMP treatment did not. Bone sialoprotein (BSP) mRNA was increased by either dexamethasone or BMP, and osteocalcin mRNA was unaffected by either dexamethasone or BMP.
The changes in alkaline phosphatase mRNA levels at both six and ten days of culture correlated well with the changes seen in alkaline phosphatase activity (Fig. 5, A and B); BMP-treated cultures showed low alkaline phosphatase mRNA levels comparable with levels seen in untreated controls, while dexamethasone increased alkaline phosphatase mRNA levels five to tenfold. Even when human marrow stromal cells were cultured with BMP-2 for fourteen days, no increase in alkaline phosphatase mRNA was observed (data not shown). Culture with BMP-4 and BMP-7 yielded patterns similar to that with BMP-2, with no significant increase in alkaline phosphatase mRNA (data not shown). In contrast to the poor alkaline phosphatase response, mRNA for bone sialoprotein, a late marker of osteoblast differentiation, was elevated by BMP and reached levels comparable with those seen with dexamethasone treatment (Fig. 5, C). Osteocalcin, another late marker of osteoblast differentiation, was not regulated by either dexamethasone or BMP treatment (Fig. 5, D). Most samples also showed BMP stimulation of osteopontin mRNA (Table II), but cells from two patients showed little or no increase in osteopontin mRNA after culture with BMP.
Discussion
Bone marrow-derived stromal cells are inducible osteoprogenitors that play an important role in bone renewal processes such as remodeling and fracture callus formation. This osteogenic capacity is seen in stromal cells isolated from mice, rats, rabbits, and humans; however, cell behavior and efficacy of inducers varies in a species-dependent manner8–10,12,13,15,17,18. Using both a kinetic assay for alkaline phosphatase enzyme activity and real-time polymerase chain reactions to determine alkaline phosphatase mRNA, we showed that BMP-2 alone is a poor inducer of this osteoblast marker in human marrow-derived stromal cells. Similar results were obtained with BMP-4 and BMP-7. However, BMP treatment of human marrow-derived stromal cells does autoregulate BMP-2 expression and increase levels of noggin, osteopontin, and bone sialoprotein mRNA. Thus, among the genes tested, only the alkaline phosphatase gene seems resistant to BMP regulation in human marrow-derived stromal cells.
Our data show that the same marrow-derived stromal cell cultures that do not show BMP-induced alkaline phosphatase are responsive to dexamethasone induction of alkaline phosphatase, as has been reported previously12,14,19,20. Previous studies from our laboratory have shown that BMP induces alkaline phosphatase activity in rat marrow-derived stromal cells and that dexamethasone potentiates this BMP responsiveness8. In contrast, the results with human marrow-derived stromal cells reported in Table I indicate that combining dexamethasone and BMP has no effect on alkaline phosphatase expression beyond that seen with dexamethasone alone, unless the marrow-derived stromal cells had been previously cultured with dexamethasone. When pretreated with dexamethasone in primary culture and replated just prior to confluency, approximately half of the human marrow-derived stromal cells cultures responded to BMP-2 by an increase in alkaline phosphatase mRNA and enzyme activity. The potency of this effect was gender-related, being greater in samples derived from male patients. Our primary cultures of total mononuclear cells from bone marrow contained non-adherent hematopoietic cells for the first four days, and the ability of macrophage cell lines to produce osteoinductive signals has been demonstrated21. It is therefore plausible that the ability of dexamethasone to promote subsequent alkaline phosphatase expression in response to BMP is mediated, at least in part, by these non-adherent cells.
High levels of alkaline phosphatase are routinely used to confirm an osteoblast phenotype in cell culture, and cultured osteoblasts from alkaline phosphatase-null mice fail to mineralize their matrix22. However, despite the significant correlation with osteogenic potential, alkaline phosphatase is but one among an array of proteins that have an important role in regulating this phenotype. mRNA analyses indicated that BMP induction of both osteogenesis-related genes such as osteopontin and bone sialoprotein and the less tissue-specific genes noggin and BMP-2 operated efficiently in human marrow-derived stromal cells. These findings demonstrate that the lack of a BMP effect on alkaline phosphatase is not due to a general lack of the BMP signaling pathway in these cells.
There have been a limited number of reported studies in which human marrow-derived stromal cells have been cultured in the presence of BMPs, and, unfortunately, none of them directly compared BMP and dexamethasone effects. Fromigue et al., studying human marrow-derived stromal cells that had been pretreated with dexamethasone in primary culture, showed results similar to those reported here; BMP-2 elevated alkaline phosphatase activity two to threefold23. Similar results were obtained in studies with 2.5 ng/mL BMP-3, but the levels of alkaline phosphatase activity obtained were unusually low24. Using a conditionally immortalized human marrow-derived stromal cell line, Gori et al. found BMP stimulation of alkaline phosphatase activity if the culture medium contained dexamethasone, calcitriol, and 10% fetal bovine serum, but they found no BMP stimulation if the medium contained rabbit serum and lacked dexamethasone25. The same cell line has recently been used for gene array analysis with samples from BMP-treated and untreated cultures26. No change in alkaline phosphatase mRNA was observed in the presence of BMP-2 in gene array assays; however, the authors reported that BMP elevated alkaline phosphatase mRNA when measured by real-time polymerase chain reactions. Lecanda et al. reported that cultures of marrow-derived stromal cells derived from human ribs exhibit a marked increase in alkaline phosphatase activity after four-day exposure to BMP-227. Those authors also reported a BMP-induced increase in osteocalcin expression, which we did not observe. The discrepancy between our results and those of Lecanda et al. is not readily explained. It might be related to differences in the osteogenic potential of marrow-derived stromal cells from different bone sites; Lecanda et al. studied marrow-derived stromal cells from ribs, while we used marrow-derived stromal cells from femora. Another issue might be different culturing systems, as Lecanda et al. used heat-inactivated serum. Using selected isolates, we compared culture media containing different batches of serum, alpha-modified Eagle medium versus Dulbecco modified Eagle medium, and heat-inactivated serum; however, no significant differences in BMP responsiveness were observed. A final possibility might relate to the fact that our tests were done on individual isolates, while Lecanda et al. utilized pooled samples. In our studies, we observed a BMP response in two patient isolates, but the remaining twenty-four isolates, when cultured in the absence of dexamethasone, did not show elevated alkaline phosphatase activity in the presence of BMP.
The reason for the poor stimulation of alkaline phosphatase expression by BMP alone in human marrow-derived stromal cells is unknown, but it clearly differs from the effect of BMP on rodent marrow-derived stromal cells. An age-related effect on the source of marrow cells may be one possible explanation for the observed difference between rodent and human alkaline phosphatase regulation. Osteogenic studies of BMP-2 effects on rat marrow-derived stromal cells generally involve use of marrow obtained from very young animals8,28, whereas cells used in human studies tend to be from older individuals who require joint surgery. Studies of age-related effects on rat and mouse marrow-derived stromal cell osteogenesis support the hypothesis that cells from younger animals show greater osteogenic potential than do those from older animals, while studies examining the effects of aging on human marrow-derived stromal cell osteogenesis have shown conflicting results29–32.
Other explanations for the observed results relate to mechanisms that may be downstream of BMP signaling. Since the lack of BMP response seems limited to alkaline phosphatase expression, it cannot be attributed to a generalized lack of BMP signaling; e.g., the classic model of BMP signaling in which activated Smads function as transcriptional regulators. However, it is possible that activation of signaling pathways regulated by BMPs or acting parallel to Smad signaling may be important in regulation of alkaline phosphatase transcription. Many BMP-dependent osteogenic markers show modulation by MAP pathways, and evidence from several laboratories has suggested that activated Smads lead to downstream activation of ERK or p38 kinase29–33. These results raise the possibility that the problem with BMP-mediated alkaline phosphatase regulation in humans may result from a defect in a kinase pathway activated subsequent to Smad-mediated signaling. While this mechanism visualizes sequential actions involving Smad and MAP kinase signaling pathways, Nohe et al. recently suggested that alternative mechanisms of BMP receptor activation will result in alternative signaling pathways involving either Smad or p38 MAP kinase34. These authors hypothesized that, while many BMP-regulated genes are controlled by Smads activated by preformed heterodimeric BMP receptors, alkaline phosphatase is regulated by means of a Smad-independent mechanism involving initial BMP binding to a homodimeric type-I BMP receptor, leading to p38 activation. Yet another possibility arises from the observations that some BMP-responsive genes such as osteopontin and osteoprotegerin are regulated by Smad-mediated removal of a Hox-C8 repressive response element in their promoter regions35–37, leading to the possibility that an alkaline phosphatase-specific repressor behaves differently in humans and rodents. We are currently investigating the importance of these alternatives in BMP-treated human marrow-derived stromal cells.
Our conventional and real-time reverse transcriptase-polymerase chain reaction studies, while focusing on the role of BMP in human marrow-derived stromal cell osteogenesis, also revealed new information about dexamethasone action on cultured human marrow-derived stromal cells. Both dexamethasone and BMP increased bone sialoprotein mRNA, but only BMP increased expression of BMP-2 or osteopontin mRNA, and dexamethasone attenuated the BMP-2 induction of these two mRNAs. Surprisingly, dexamethasone, like BMP-2, induced noggin mRNA and potentiated the effect of BMP. The ability of BMPs to increase noggin expression is generally viewed as a negative feedback mechanism, operating to inhibit BMP signaling by binding to BMP and thereby preventing BMP receptor activation38,39. The observation that dexamethasone also increases noggin expression suggests a possible mechanism that may be operative in glucocorticoid-induced osteoporosis.
Finally, our in vitro data from human marrow-derived stromal cells appear to correlate with the evidence of BMP efficacy in human clinical trials. While laboratory animal studies have demonstrated a reliable capacity for BMPs to promote bone repair in vivo40,41, the success of BMP osteoinduction has been less impressive in human clinical trials42–45, with a large variation in response among individual patients46. The results presented here suggest that this may be due to a difference in the ability of therapeutic doses of BMPs to stimulate alkaline phosphatase expression and subsequent lack of control of osteoid mineralization.
Footnotes
Note: The authors thank Ms. Helen Dickson and Dr. Christopher Ferrante for their invaluable assistance in coordinating the procurement of marrow samples.
In support of their research or preparation of this manuscript, one or more of the authors received grant DE13962 from the National Institute of Dental and Craniofacial Research/National Institutes of Health. None of the authors received payments or other benefits or a commitment or agreement to provide such benefits from a commercial entity. No commercial entity paid or directed, or agreed to pay or direct, any benefits to any research fund, foundation, educational institution, or other charitable or nonprofit organization with which the authors are affiliated or associated. Genetics Institute (Wyeth Labs) supplied the human rBMPs, and Regeneron provided the rat anti-human noggin antibody.
References
- 1.Owen TA, Aronow M, Shalhoub V, Barone LM, Wilming L, Tassinari MS, Kennedy MB, Pockwinse S, Lian LB, Stein GS. Progressive development of the rat osteoblast phenotype in vitro: reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix. J Cell Physiol. 1990;143:420–30. doi: 10.1002/jcp.1041430304. [DOI] [PubMed] [Google Scholar]
- 2.Rodan GA. Introduction to bone biology. Bone. 1992;13 (Suppl 1):S3–6. doi: 10.1016/s8756-3282(09)80003-3. [DOI] [PubMed] [Google Scholar]
- 3.Hessle L, Johnson KA, Anderson HC, Narisawa S, Sali A, Goding JW, Terkeltaub R, Millan JL. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc Natl Acad Sci USA. 2002;99:9445–9. doi: 10.1073/pnas.142063399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mornet E, Stura E, Lia-Baldini AS, Stigbrand T, Menez A, Le Du MH. Structural evidence for a functional role of human tissue nonspecific alkaline phosphatase in bone mineralization. J Biol Chem. 2001;276:31171–8. doi: 10.1074/jbc.M102788200. [DOI] [PubMed] [Google Scholar]
- 5.Fedde KN, Blair L, Silverstein J, Coburn SP, Ryan LM, Weinstein RS, Waymire K, Narisawa S, Millan JL, MacGregor GR, Whyte MP. Alkaline phosphatase knock-out mice recapitulate the metabolic and skeletal defects of infantile hypophosphatasia. J Bone Miner Res. 1999;14:2015–26. doi: 10.1359/jbmr.1999.14.12.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Long MW. Osteogenesis and bone-marrow-derived cells. Blood Cells Mol Dis. 2001;27:677–90. doi: 10.1006/bcmd.2001.0431. [DOI] [PubMed] [Google Scholar]
- 7.Krebsbach PH, Kuznetsov SA, Bianco P, Robey PG. Bone marrow stromal cells: characterization and clinical application. Crit Rev Oral Biol Med. 1999;10:165–81. doi: 10.1177/10454411990100020401. [DOI] [PubMed] [Google Scholar]
- 8.Rickard DJ, Sullivan TA, Shenker BJ, Leboy PS, Kazhdan I. Induction of rapid osteoblast differentiation in rat bone marrow stromal cell cultures by dexamethasone and BMP-2. Dev Biol. 1994;161:218–28. doi: 10.1006/dbio.1994.1022. [DOI] [PubMed] [Google Scholar]
- 9.Balk ML, Bray J, Day C, Epperly M, Greenberger J, Evans CH, Niyibizi C. Effect of rhBMP-2 on the osteogenic potential of bone marrow stromal cells from an osteogenesis imperfecta mouse (oim) Bone. 1997;21:7–15. doi: 10.1016/s8756-3282(97)00075-6. [DOI] [PubMed] [Google Scholar]
- 10.Abe E, Yamamoto M, Taguchi Y, Lecka-Czernik B, O’Brien CA, Economides AN, Stahl N, Jilka RL, Manolagas SC. Essential requirement of BMPs-2/4 for both osteoblast and osteoclast formation in murine bone marrow cultures from adult mice: antagonism by noggin. J Bone Miner Res. 2000;15:663–73. doi: 10.1359/jbmr.2000.15.4.663. [DOI] [PubMed] [Google Scholar]
- 11.Leboy PS, Beresford JN, Devlin C, Owen ME. Dexamethasone induction of osteoblast mRNAs in rat marrow stromal cell cultures. J Cell Physiol. 1991;146:370–8. doi: 10.1002/jcp.1041460306. [DOI] [PubMed] [Google Scholar]
- 12.Rickard DJ, Kassem M, Hefferan TE, Sarkar G, Spelsberg TC, Riggs BL. Isolation and characterization of osteoblast precursor cells from human bone marrow. J Bone Miner Res. 1996;11:312–24. doi: 10.1002/jbmr.5650110305. [DOI] [PubMed] [Google Scholar]
- 13.Cheng SL, Yang JW, Rifas L, Zhang SF, Avioli LV. Differentiation of human bone marrow osteogenic stromal cells in vitro: induction of the osteoblast phenotype by dexamethasone. Endocrinology. 1994;134:277–86. doi: 10.1210/endo.134.1.8275945. [DOI] [PubMed] [Google Scholar]
- 14.Beresford JN, Joyner CJ, Devlin C, Triffitt JT. The effects of dexamethasone and 1,25-dihydroxyvitamin D3 on osteogenic differentiation of human marrow stromal cells in vitro. Arch Oral Biol. 1994;39:941–7. doi: 10.1016/0003-9969(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 15.Bellows CG, Ciaccia A, Heersche JN. Osteoprogenitor cells in cell populations derived from mouse and rat calvaria differ in their response to corticosterone, cortisol, and cortisone. Bone. 1998;23:119–25. doi: 10.1016/s8756-3282(98)00084-2. [DOI] [PubMed] [Google Scholar]
- 16.Lian JB, Shalhoub V, Aslam F, Frenkel B, Green J, Hamrah M, Stein GS, Stein JL. Species-specific glucocorticoid and 1,25-dihydroxyvitamin D responsiveness in mouse MC3T3-E1 osteoblasts: dexamethasone inhibits osteoblast differentiation and vitamin D down-regulates osteocalcin gene expression. Endocrinology. 1997;138:2117–27. doi: 10.1210/endo.138.5.5117. [DOI] [PubMed] [Google Scholar]
- 17.Krebsbach PH, Kuznetsov SA, Satomura K, Emmons RV, Rowe DW, Robey PG. Bone formation in vivo: comparison of osteogenesis by transplanted mouse and human marrow stromal fibroblasts. Transplantation. 1997;63:1059–69. doi: 10.1097/00007890-199704270-00003. [DOI] [PubMed] [Google Scholar]
- 18.Kuznetsov S, Gehron Robey P. Species differences in growth requirements for bone marrow stromal fibroblast colony formation in vitro. Calcif Tissue Int. 1996;59:265–70. doi: 10.1007/s002239900121. [DOI] [PubMed] [Google Scholar]
- 19.Ahdjoudj S, Lasmoles F, Oyajobi BO, Lomri A, Delannoy P, Marie PJ. Reciprocal control of osteoblast/chondroblast and osteoblast/adipocyte differentiation of multipotential clonal human marrow stromal F/STRO-1(+) cells. J Cell Biochem. 2001;81:23–38. doi: 10.1002/1097-4644(20010401)81:1<23::aid-jcb1021>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 20.Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64:295–312. [PubMed] [Google Scholar]
- 21.Champagne CM, Takebe J, Offenbacher S, Cooper LF. Macrophage cell lines produce osteoinductive signals that include BMP-2. Bone. 2002;30:26–31. doi: 10.1016/s8756-3282(01)00638-x. [DOI] [PubMed] [Google Scholar]
- 22.Wennberg C, Hessle L, Lundberg P, Mauro S, Narisawa S, Lerner UH, Millan JL. Functional characterization of osteoblasts and osteoclasts from alkaline phosphatase knockout mice. J Bone Miner Res. 2000;15:1879–88. doi: 10.1359/jbmr.2000.15.10.1879. [DOI] [PubMed] [Google Scholar]
- 23.Fromigue O, Marie PJ, Lomri A. Bone morphogenetic protein-2 and transforming growth factor-beta2 interact to modulate human bone marrow stromal cell proliferation and differentiation. J Cell Biochem. 1998;68:411–26. [PubMed] [Google Scholar]
- 24.Faucheux C, Ulysse F, Bareille R, Reddi AH, Amedee J. Opposing actions of BMP3 and TGF beta1 in human bone stromal cell growth and differentiation. Biochem Biophys Res Commun. 1997;241:787–93. doi: 10.1006/bbrc.1997.7792. [DOI] [PubMed] [Google Scholar]
- 25.Gori F, Thomas T, Hicok KC, Spelsberg TC, Riggs BL. Differentiation of human marrow stromal precursor cells: bone morphogenetic protein-2 increases OSF2/CBFA1, enhances osteoblast commitment, and inhibits late adipocyte maturation. J Bone Miner Res. 1999;14:1522–35. doi: 10.1359/jbmr.1999.14.9.1522. [DOI] [PubMed] [Google Scholar]
- 26.Locklin RM, Riggs BL, Hicok KC, Horton HF, Byrne MC, Khosla S. Assessment of gene regulation by bone morphogenetic protein 2 in human marrow stromal cells using gene array technology. J Bone Miner Res. 2001;16:2192–204. doi: 10.1359/jbmr.2001.16.12.2192. [DOI] [PubMed] [Google Scholar]
- 27.Lecanda F, Avioli LV, Cheng SL. Regulation of bone matrix protein expression and induction of differentiation of human osteoblasts and human bone marrow stromal cells by bone morphogenetic protein-2. J Cell Biochem. 1997;67:386–96. [PubMed] [Google Scholar]
- 28.Hanada K, Dennis JE, Caplan AI. Stimulatory effects of basic fibroblast growth factor and bone morphogenetic protein-2 on osteogenic differentiation of rat bone marrow-derived mesenchymal stem cells. J Bone Miner Res. 1997;12:1606–14. doi: 10.1359/jbmr.1997.12.10.1606. [DOI] [PubMed] [Google Scholar]
- 29.Justesen J, Stenderup K, Eriksen EF, Kassem M. Maintenance of osteoblastic and adipocytic differentiation potential with age and osteoporosis in human marrow stromal cell cultures. Calcif Tissue Int. 2002;71:36–44. doi: 10.1007/s00223-001-2059-x. [DOI] [PubMed] [Google Scholar]
- 30.Mueller SM, Glowacki J. Age-related decline in the osteogenic potential of human bone marrow cells cultured in three-dimensional collagen sponges. J Cell Biochem. 2001;82:583–90. doi: 10.1002/jcb.1174. [DOI] [PubMed] [Google Scholar]
- 31.Stenderup K, Justesen J, Eriksen EF, Rattan SI, Kassem M. Number and proliferative capacity of osteogenic stem cells are maintained during aging and in patients with osteoporosis. J Bone Miner Res. 2001;16:1120–9. doi: 10.1359/jbmr.2001.16.6.1120. [DOI] [PubMed] [Google Scholar]
- 32.Triffitt JT, Joyner CJ, Oreffo RO, Virdi AS. Osteogenesis: bone development from primitive progenitors. Biochem Soc Trans. 1998;26:21–7. doi: 10.1042/bst0260021. [DOI] [PubMed] [Google Scholar]
- 33.Lee KS, Hong SH, Bae SC. Both the Smad and p38 MAPK pathways play a crucial role in Runx2 expression following induction by transforming growth factor-beta and bone morphogenetic protein. Oncogene. 2002;21:7156–63. doi: 10.1038/sj.onc.1205937. [DOI] [PubMed] [Google Scholar]
- 34.Nohe A, Hassel S, Ehrlich M, Neubauer F, Sebald W, Henis YI, Knaus P. The mode of bone morphogenetic protein (BMP) receptor oligomerization determines different BMP-2 signaling pathways. J Biol Chem. 2002;277:5330–8. doi: 10.1074/jbc.M102750200. [DOI] [PubMed] [Google Scholar]
- 35.Shi X, Yang X, Chen D, Chang Z, Cao X. Smad1 interacts with homeobox DNA-binding proteins in bone morphogenetic protein signaling. J Biol Chem. 1999;274:13711–7. doi: 10.1074/jbc.274.19.13711. [DOI] [PubMed] [Google Scholar]
- 36.Wan M, Shi X, Feng X, Cao X. Transcriptional mechanisms of bone morphogenetic protein-induced osteoprotegrin gene expression. J Biol Chem. 2001;276:10119–25. doi: 10.1074/jbc.M006918200. [DOI] [PubMed] [Google Scholar]
- 37.Hullinger TG, Pan Q, Viswanathan HL, Somerman MJ. TGFbeta and BMP-2 activation of the OPN promoter: roles of smad- and hox-binding elements. Exp Cell Res. 2001;262:69–74. doi: 10.1006/excr.2000.5074. [DOI] [PubMed] [Google Scholar]
- 38.Nifuji A, Kellermann O, Noda M. Noggin expression in a mesodermal pluripotent cell line C1 and its regulation by BMP. J Cell Biochem. 1999;73:437–44. [PubMed] [Google Scholar]
- 39.Gazzerro E, Gangji V, Canalis E. Bone morphogenetic proteins induce the expression of noggin, which limits their activity in cultured rat osteoblasts. J Clin Invest. 1998;102:2106–14. doi: 10.1172/JCI3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li RH, Wozney JM. Delivering on the promise of bone morphogenetic proteins. Trends Biotechnol. 2001;19:255–65. doi: 10.1016/s0167-7799(01)01665-1. [DOI] [PubMed] [Google Scholar]
- 41.Yoon ST, Boden SD. Osteoinductive molecules in orthopaedics: basic science and preclinical studies. Clin Orthop. 2002;395:33–43. doi: 10.1097/00003086-200202000-00005. [DOI] [PubMed] [Google Scholar]
- 42.Service RF. Tissue engineers build new bone. Science. 2000;289:1498–500. doi: 10.1126/science.289.5484.1498. [DOI] [PubMed] [Google Scholar]
- 43.Chapman MW. Thoughts on clinical trials to evaluate the action and effectiveness of BMPs in bone healing. J Bone Joint Surg Am. 2001;83(Suppl 1Pt 2):S163–4. [PubMed] [Google Scholar]
- 44.Lane JM. BMPs: why are they not in everyday use? J Bone Joint Surg Am. 2001;83(Suppl 1Pt 2):S161–3. [PubMed] [Google Scholar]
- 45.Boden SD. Clinical application of the BMPs. J Bone Joint Surg Am. 2001;83(Suppl 1Pt 2):S161. [PubMed] [Google Scholar]
- 46.Groeneveld EH, Burger HE. Bone morphogenetic proteins in human bone regeneration. Eur J Endocrinol. 2000;142:9–21. doi: 10.1530/eje.0.1420009. [DOI] [PubMed] [Google Scholar]


