Abstract
Pathological expression of movement and muscle tone in human upper motor neuron disorders has been partly associated with impaired modulation of spinal inhibitory mechanisms, such as reciprocal or presynaptic inhibition. In addition, input from specific afferent systems contributes significantly to spinal reflex circuits coupled with posture or locomotion. Accordingly, the objectives of this study were to identify the involved afferents and their relative contribution to soleus H-reflex modulation induced by changes in hip position, and to relate these effects with activity of spinal interneuronal circuits. Specifically, we investigated the actions of group I synergistic and antagonistic muscle afferents (e.g. common peroneal nerve, CPN; medial gastrocnemius, MG) and tactile plantar cutaneous afferents on the soleus H-reflex during controlled hip angle variations in 11 motor incomplete spinal cord injured (SCI) subjects. It has been postulated in healthy subjects that CPN stimulation evokes an inhibition on the soleus H-reflex at a conditioning test (C-T) interval of 2–4 ms. This short latency reflex depression is caused mainly by activation of the reciprocal Ia inhibitory pathway. At longer C-T intervals (beyond 30 ms) the soleus H-reflex is again depressed, and is generally accepted to be caused by presynaptic inhibition of soleus Ia afferents. Similarly, MG nerve stimulation depresses soleus H-reflex excitability at the C-T interval of 6 ms, involving the pathway of non-reciprocal group I inhibition, while excitation of plantar cutaneous afferents affects the activity of spinal reflex pathways in the extensors. In this study, soleus H-reflexes recorded alone or during CPN stimulation at either short (2, 3, 4 ms) or long (80, 100, 120 ms) C-T intervals, and MG nerve stimulation delivered at 6 ms were elicited via conventional methods and similar to those adopted in studies conducted in healthy subjects. Plantar skin conditioning stimulation was delivered through two surface electrodes placed on the metatarsals at different C-T intervals ranging from 3 to 90 ms. CPN stimulation at either short or long C-T intervals and MG nerve stimulation resulted in a significant facilitation of the soleus H-reflex, regardless of the hip angle tested. Plantar skin stimulation delivered with hip extended at 10° induced a bimodal facilitation reflex pattern, while with hip flexed (10°, 30°) the reflex facilitation increased with increments in the C-T interval. This study provides evidence that in human chronic SCI, classically key inhibitory reflex actions are switched to facilitatory, and that spinal processing of plantar cutaneous sensory input and actions of synergistic/antagonistic muscle afferents interact with hip proprioceptive input to facilitate soleus H-reflex excitability. These actions might be associated with the pathological expression of neural control of movement in individuals with SCI, and potentially could be considered in rehabilitation programs geared to restore sensorimotor function in these patients.
Keywords: H-reflex, Hip, Cutaneous afferents, Postsynaptic/presynaptic inhibition, Rehabilitation
Introduction
It has been accepted for a long time that sensory afferent feedback integrated at several levels of the central nervous system plays a principal role in regulating human movements (Jankowska 1992). Sensory feedback might play even a more important role following a motor incomplete spinal cord injury (SCI) in humans.
Research performed mainly in animal preparations has identified specific afferent systems such as hip proprioceptors, group I muscle afferents, and plantar cutaneous afferents that control muscle activity during movements. Both hip and ankle muscle afferents responding to stretch and load respectively reset the locomotor rhythm, signifying their impact on the walking pattern (Hiebert et al. 1996; Kriellaars et al. 1994; Conway et al. 1987). Practically, the hip joint has to reach a certain extended angle for the swing phase to be initiated (Anderson and Grillner 1981), and the ankle extensor muscles to be unloaded (Duysens and Pearson 1980). In humans, evidence suggests that hip angle is critical for soleus H-reflex modulation (Chapman et al. 1991a; Knikou and Rymer 2002b; Brooke et al. 1993, 1995), and for switching reflex inhibitory actions to facilitatory (Knikou and Rymer 2002a).
Proprioceptive information from the foot sole contributes to the maintenance of erect posture in humans (Kavounoudias et al. 2001), probably by re-enforcing extensor muscle activity (Pierrot-Deseilligny et al. 1981), and by strengthening the ongoing motoneuronal activity (Kernell and Hultborn 1990). In the cat, excitation of low-threshold afferents from the foot delays or suppresses the initiation of swing, inhibits late flexion reflexes, and promotes stance (Duysens and Pearson 1976; Duysens 1977; Conway et al. 1995). Recent studies in the spinal cat further support the important role of the cutaneous afferents and show that these afferents contribute to the recovery of stepping following spinalization (Bouyer and Rossignol 2003).
Activity in specific neuronal pathways and associated mechanisms can be indirectly assessed in humans using specific electrophysiological techniques (reviewed in Hultborn 2003; Pierrot-Deseilligny and Mazevet 2000). Using these methods, it is now possible to describe some of the components that contribute to spinal pathophysiology of spasticity, when compared with similar findings in healthy subjects. For example, earlier studies in healthy subjects have described that the soleus H-reflex is depressed at conditioning test (C-T) intervals of 2–4 ms, when conditioned by electrical low-intensity strength stimulation of the ipsilateral common peroneal nerve (CPN). These effects are mediated via the muscle spindle of the flexor group Ia afferents, and the pathway involved is known as reciprocal Ia inhibition (Tanaka 1974; Crone et al. 1987; see review in Crone 1993). This reflex inhibition is reported to decrease considerably, and be eliminated during voluntary movement in individuals with established SCI (Boorman et al. 1996a; Morita et al. 2001; Crone et al. 2003), contributing to spasticity and to poor movement performance.
Further, presynaptic inhibition, which controls the transmission from Ia afferents gating selectively the sensory feedback from the periphery (Rudomin and Schmidt 1999), can also be indirectly assessed in humans. A conditioning volley to the CPN evokes a long lasting inhibition in the soleus H-reflex (Crone and Nielsen 1994; Zehr and Stein 1996). This reflex depression is attributed to presynaptic inhibition of soleus Ia afferents (reviewed in Katz 1999). In human SCI, modulation of Ia input is diminished under both static (Faist et al. 1994; Morita et al. 2001) and dynamic (Yang and Whelan 1993) conditions, suggesting that the timely regulation of presynaptic inhibition at the onset of stretch or movement in these patients might be lost.
Autogenic group I inhibition is evidenced on the soleus H-reflex by a preceded conditioning stimulus applied to the ipsilateral medial gastrocnemius (MG) nerve (Pierrot-Deseilligny et al. 1979). This reflex inhibition is reduced during voluntary activation (Pierrot-Deseilligny et al. 1982), and is replaced by group Ib excitation during walking (Stephens and Yang 1996) in healthy subjects. The reports on this reflex inhibition in human spasticity are conflicting. Delwaide and Oliver (1988) demonstrated that Ib inhibition is reduced in spasticity, while Downes et al. (1995) could not confirm the reduction in Ib inhibition, probably because of differences between cerebral and spinal spasticity. To summarize, changes in the organization of spinal neuronal pathways contribute to the pathological expression of movement and muscle tone after SCI. However, these spinal pathways have not been investigated under the influence of the afferent systems aforementioned.
Accordingly, in the present study we investigated whether a combination of signals registering hip position, excitation of synergistic and antagonistic muscle afferents and cutaneous afferents interact to influence the magnitude of the soleus H-reflex as a function of hip angle under static conditions in a group of subjects with motor incomplete SCI. Preliminary accounts of this work have been reported only in abstract form (Knikou and Rymer 2004).
Materials and methods
Subjects
All experiments were approved by the Institutional Review Board (IRB), Office for the Protection of Human Research Subjects at Northwestern University (Chicago IL, USA) and conducted according to the 1964 Declaration of Helsinki. Informed consent was obtained from all subjects or from their parents prior to testing. Eleven subjects with SCI ranging from C5 to T11 participated in the study. The impairment scale of the American Spinal Injury Association (ASIA) (Maynard et al. 1997) was employed to classify the completeness of the lesion. Clinical tests were performed to determine if cutaneous and joint proprioception were present. Joint and muscle proprioception was assessed by asking the subjects to identify the position and direction of movement in the joints of both lower extremities with eyes closed, performed passively by the experimenter. For assessing tactile sensation, subjects were asked to identify with eyes closed the site where a peace of cotton and/or needle touched them. Of the 11 subjects, one subject (S2) participated only in two tests before withdrawing from the study. None displayed signs of lower motoneuron disorder. Subjects’ characteristics are summarized in Table 1.
Table 1.
SCI subjects’ characteristics
| Subjects | Gender | Age (years) | Post-injury (months) | Ashworth score | ASIA scale | Lesion level | Sensation | Medication/day |
|---|---|---|---|---|---|---|---|---|
| S1 | M | 23 | 72 | 1 | C | C5 | Intact | – |
| S2 | M | 44 | 9 | 0 | B | C5 | Intact | Vallium 30 mg |
| S3 | M | 27 | 20 | 0 | C | C5 | Intact | – |
| S4 | M | 45 | 48 | 2 | C | C6 | Intact | Baclofen 60 mg |
| S5 | M | 20 | 15 | 2 | C | T11 | Intact | Baclofen 20 mg |
| S6 | M | 17 | 21 | 0 | D | C6 | Intact | Baclofen 20 mg |
| S7 | F | 59 | 20 | 0 | C | T9 | Intact | Baclofen 10 mg |
| S8 | M | 15 | 20 | 2 | C | T3 | No sensation of cold/warm | – |
| S9 | M | 42 | 32 | 0 | C | C8 | Intact | Plavix Lipotor 75 mg |
| S10 | M | 50 | 180 | 1 | C | C5 | No sensation of cold/warm | Baclofen 30 mg |
| S11 | M | 50 | 156 | 3 | C | C5 | No sensation of cold/warm | – |
Spasticity at the ankle was scaled according to the Ashworth scale (Ashworth 1964). The completeness of the lesion was classified according to the American Spinal Injury Association (ASIA) scale (Maynard et al. 1997), ASIA B motor complete but sensory incomplete, ASIA C sensory and motor incomplete, but with more than half of muscles below injury level having a muscle grade (estimated via manual muscle test) of less than 3 out of 5 ASIA D sensory and motor incomplete with at least half of the muscles below the level of the injury having a muscle grade of 3 or higher. F Female, M Male
Subject position
Experiments were conducted with subjects supine. The right lower limb was secured to a knee ankle foot orthosis (KAFO), previously used in similar studies in able-bodied and SCI subjects (a, Knikou and Rymer 2002b). For each subject, adjustments were made to fit shank and thigh lengths. The KAFO was connected to the motor head of an isokinetic dynamometer (Biodex Medical Systems, Shirley, NY, USA). In all subjects, ankle and knee joints were set at 20° of plantar flexion and 30° of flexion, respectively. With the KAFO secured to the lower limb, the center of the subjects’ hip joint was then aligned with the center of the Biodex motor head unit. The KAFO was moved in the sagittal plane by the experimenter. When the hip was placed in the tested angle, the lower limb was stabilized by the Biodex system. During the experiment, the head and arms were fully supported and subjects were instructed to relax.
Soleus H-reflex and M-wave (elicitation and recording protocol)
The soleus H-reflex was elicited by stimulating the right posterior tibial nerve in the popliteal fossa through a monopolar electrode using a 1-ms rectangular pulse generated by a constant current stimulator (DS7A; Digitimer Ltd., UK) and triggered once every 5 s. The indifferent electrode was placed just above the patella for selective stimulation of the nerve trunk (Hugon 1973).
Surface electromyograms (EMG) were recorded with single differential bipolar electrodes (DelSys Inc., Boston, MA, USA) placed over the soleus muscle. EMG activity of MG and tibialis anterior (TA) muscles was recorded in the experiments where the effects of MG and CPN stimulation on the soleus H-reflex were investigated during hip angle changes, respectively (for details on conditioning protocols see below). Light abrasion of the skin was performed before placement of surface electrodes, which were secured with surgical adhesive tape.
At the start of each test, a hand-held monopolar electrode was used as a probe to establish the correct site for stimulating the posterior tibial nerve. This was identified as the one during which the soleus H-reflex was present without the M-wave being present. Having established this site, the probe electrode was replaced by a permanent one (N-10-A, Ambu Inc., Denmark) under constant pressure, and the evoked responses were observed on the oscilloscope screen (Tektronics, USA). In case the surface electrode did not evoke the same response behavior the procedure was repeated. After this procedure was completed, the maximal M-wave was measured. Then, the stimulus strength was adjusted to give an H-reflex of 15–30% of the maximal M-wave, across subjects.
In this study, we used control H-reflexes of similar sizes (in percentages of maximal M-wave) across subjects to assess the effects of different types of sensorimotor conditioning stimulation (Crone et al. 1990) with hip positioned in different angles. This was based largely on that the susceptibility of the reflex to inhibition and to facilitation and the amount of reciprocal Ia inhibition depend on the size of the test reflex (Meinck 1980; Crone et al. 1985, 1990), while imposed hip angle changes influence substantially the magnitude of the soleus H-reflex in individuals with SCI (Knikou and Rymer 2002b). Based on these factors, at each hip angle tested (30° flexion, 10° extension) the stimulus strength was carefully adjusted to evoke a control H-reflex at each hip angle tested (Hohomonymous) (10° extension, 10° flexion, 30° flexion) that had the same amplitude as the control reflex recorded with hip flexed at 10°, since H-reflexes with these amplitudes have a minimal sensitivity to inhibition and facilitation (Crone et al. 1990). The average size (pool data from all tested subjects) of the Hohomonymous is presented as a percentage of the maximal M-wave in Fig. 1. Data indicated in this figure correspond to tests where the H-reflex was conditioned with plantar cutaneous afferents (Fig. 1a) and CPN (Fig. 1b) stimulation (see ‘sensorimotor conditioning’ below for specific protocols). Similar results were observed for MG nerve conditioning of the reflex. It is clear that control H-reflexes recorded with hip set in different angles and across conditioning protocols were of similar size. This allowed a valid comparison to be made on the amount of facilitation/inhibition due to the conditioning stimulation across hip angles tested and subjects.
Fig. 1.
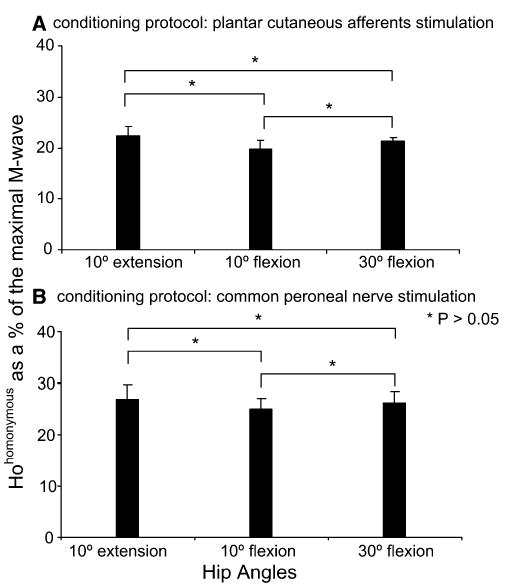
a, b Pool data showing the average size of the control H-reflex (Hohomonymous) recorded at each hip angle tested as a percentage of the maximal M-wave from two different experimental tests (H-reflex conditioning stimulation protocols). For all cases, no statistically significant differences between the Hohomonymous recorded across hip angles were encountered (P>0.05). Error bars denote the standard error of the mean(SEM)
For each reflex recorded in this study (control or conditioned), 20 repeated reflex responses evoked every 5 s were acquired and saved for further analysis. The recorded EMG signals were amplified 1,000 times and band-pass-filtered (10 Hz–1 kHz), and subjected to an analogue to digital conversion (PCI-MIO 16E, National Instruments Co., Austin, TX, USA). The digitized EMG signals were rectified and the size of the evoked M-waves and H-reflexes was measured as the area under the full-wave rectified waveforms (Matlab vs. 5.3., Mathworks).
The M-wave amplitude was used as a screening factor for accepting control H-reflexes across hip angles and conditioned reflexes at different C-T intervals at each hip angle tested. By keeping the M-wave amplitude stable, the number of group-Ia afferent fibers recruited by the stimulus can be well-controlled (Boorman et al. 1996b). This resulted in a constant Ia afferent test volley across hip angles tested. Control and conditioned soleus H-reflexes were recorded with the subjects supine and hip positioned randomly at 10°, 30° of flexion and extended to 10°.
Sensorimotor conditioning
Plantar cutaneous stimulation
Two Ag-AgCl surface electrodes (Ambu Inc., Ølstykke, Denmark) were placed transversely across the 1st and 3rd metatarsals. Using a constant current stimulator (DS7, Digitimer Ltd., UK), the perceptual threshold (PT) that corresponded to the stimulus intensity first perceived by the subject was established. All conditioning stimuli were equivalent to 3×PT. At this stimulation intensity no movement of the intrinsic muscles of the foot was elicited and no pain was reported, verifying that the conditioning afferent volley excited mainly cutaneous afferents of the foot sole. Tactile units remain largely intact after chronic SCI (Thomas and Westling 1995), thus transmission of plantar cutaneous sensation was not regarded as problematic in the current study.
The conditioning stimulus train consisted of five pulses with an inter-stimulus interval of 4.8 ms and pulse train duration of 24 ms, similar to that previously employed in healthy subjects to investigate the effects of plantar cutaneous afferents on the soleus H-reflex with the hip positioned in different angles (Knikou and Rymer 2002a). Conditioning stimuli were delivered every 5 s, and preceded the soleus H-reflex at variable C-T intervals. Each interval represents the time between the end of the conditioning stimulus train and the single pulse delivered to the posterior tibial nerve. Seven different C-T intervals were investigated when the soleus H-reflex was conditioned by plantar cutaneous afferent excitation with hip positioned in different angles. The intervals ranged from 3 to 90 ms (3, 6, 9, 15, 30, 60, 90 ms), so to observe early and late effects on the soleus H-reflex with hip positioned at different angles. These intervals were selected on the basis that non-nociceptive sural nerve (purely cutaneous) stimulation has early and late facilitatory effects on the soleus H-reflex in motor complete SCI and able-bodied subjects (Roby-Brami and Bussel 1990; Delwaide et al. 1981).
Antagonistic group I muscle afferent (CPN) stimulation
Methods for CPN stimulation used in the present study were similar to those described in several studies conducted in healthy subjects (Crone et al. 1987; Crone and Nielsen 1994). The stimulus to the CPN was a single shock, 1-ms in duration, generated by a constant current stimulator (DS7A, Digitimer Ltd., UK) and delivered with a bipolar electrode placed distal to the head of the fibula. The conditioning stimulus was delivered every 5 s at motor threshold (MT) level of the TA muscle, and was kept at this level during the experiment. Selective TA activation ensured on that activity in the peroneal muscles was not present, and with increases in stimulation intensity ankle dorsi flexion without eversion was evoked. Because the peroneal muscles are not antagonists to the soleus muscle, activation of their afferents might obscure the reciprocal inhibition. Thus, although TA and peroneus longus reflex arcs can be activated independently (Pérot and Mora 1993), it was essential that in all tests the motor threshold in the TA muscle was always lower than the one in the peroneal muscles. This was checked by palpating the tendon of the peroneal muscles. Given a possible spread of TA activity, only motor responses associated with a clear contraction in the peroneal muscles were taken as evidence of peroneal motor axons activation.
The MT in the TA muscle was determined by the appearance of EMG activity recorded by a differential bipolar surface electrode (DelSys Inc., MA, USA) placed over the TA muscle. During H-reflex conditioning, TA H-wave amplitude was monitored online to ensure stability of the conditioning afferent volley. In cases where the TA H-wave was absent constancy of the conditioning stimulation was ensured by very small in magnitude TA M-waves.
The ipsilateral CPN stimulation always preceded the soleus H-reflex at C-T intervals of short (2, 3, and 4 ms) and long (80, 100, and 120 ms) duration. These C-T intervals were selected on the basis that in quiescent healthy subjects the reciprocal Ia inhibitory pathway has the shortest latency with 2 to 3 ms between activation of the agonist and inhibition of the antagonist (Crone et al. 1987; Crone and Nielsen 1989), while the reflex inhibition produced by this weak stimulation at such long C-T intervals is predominantly presynaptic (Iles 1996; Zehr and Stein 1999).
Synergistic group I muscle afferents (MG nerve) stimulation
Methods for MG nerve stimulation used in the present study were similar to those described by Pierrot-Deseilligny et al. (1979). The stimulus to the MG nerve was a single shock, 1-ms in duration, generated by a constant current stimulator and delivered with a bipolar electrode placed at a site where a clear contraction of the MG muscle could be observed. This electrode was placed 6–10 cm below the cathode electrode to the posterior tibial nerve. The stimulus strength to the MG nerve was set at 0.95×MT to avoid recurrent inhibition (Rossi et al. 1994). The intensity of the MG stimulus was kept below the level that would generate an M-wave in the MG muscle. Surface electrodes (DelSys, USA) recording MG muscle activity were placed over the muscle site that showed contraction at minimal stimulation intensities.
The effects of MG nerve stimulation on the homologous muscle were observed online and were checked several times throughout the experiment to ensure that MG nerve stimulation did not change over the course of the experiment. MG nerve stimulation always preceded the soleus H-reflex at a C-T interval of 6 ms, delivered every 5 s. This interval was selected on the basis that in quiescent healthy subjects, MG nerve stimulation at 6 ms induces a stronger soleus H-reflex depression compared to other C-T intervals (Pierrot-Deseilligny et al. 1979).
Experimental procedures
With the KAFO secured to the right lower limb, the hip joint was set at 10° of flexion passively by the experimenter and was held stable in this position by the Biodex system. In this position, control and conditioned H-reflexes were recorded according to the methods previously described. The hip was then set to a new angle and reflexes (control and conditioned) were recorded again. At every hip angle tested, the control H-reflex (Hohomonymous) was recorded at least twice to establish reflex stability and was randomly alternated with the conditioned reflexes. This experimental protocol was adopted for conditioning the soleus H-reflex with CPN, MG, and plantar cutaneous afferents stimulation with the hip positioned in different angles (10°, 30° flexion, 10° extension) across subjects. For each subject, each conditioning protocol was completed in a different experimental session, and each test lasted 2 h.
Data analysis
For each subject, the 20 repeated responses of each of the conditioned H-reflex were expressed as a percentage of the mean size of the control reflex recorded at each hip angle tested. A one-way analysis of variance (ANOVA) along with post hoc Bonferroni tests were applied to the experimental data sets to determine if significant differences existed between the control and the conditioned H-reflexes at each hip angle, and to establish statistically significant differences between the conditioned H-reflexes across C-T intervals. This analysis was performed for every conditioning protocol separately.
The average size (n=20) of the conditioned H-reflex from each subject was then grouped by the hip angle and C-T interval tested. For each conditioning protocol, a two-way ANOVA with repeated measures was applied to the experimental data sets in order to establish changes in the magnitude of the conditioned H-reflex across hip angles and C-T intervals investigated.
For each trial, the sizes of the M-waves of the control and conditioned H-reflexes were expressed as a percentage of the maximal M-wave. A one-way ANOVA was used to test for differences between the M-waves of the reflexes recorded under control conditions and following sensorimotor conditioning. If significant differences were encountered the trial was rejected. Statistical analysis was also performed between the sizes of the M-waves in the Hohomonymous reflexes. When significant differences were identified the trial was rejected. Statistically significant differences between the control and the conditioned reflexes were established at 95% of confidence interval. Results are presented as mean values along with the standard error of the mean (SEM).
Results
Effects of plantar cutaneous afferents stimulation on the soleus H-reflex during hip angle changes
A summary of changes in the conditioned H-reflex following excitation of plantar cutaneous afferents for all C-T intervals and hip angles is illustrated in Fig. 2. Data in this figure indicate the average size of the conditioned H-reflex from all subjects tested. Excitation of plantar cutaneous afferents with the hip extended at 10° resulted in a significant facilitation of the H-reflex at the C-T interval of 3 ms. At this interval, the conditioned H-reflex reached an amplitude of 160 ± 36% of Hohomonymous (Fig. 2a). With increments in the C-T interval, the conditioned H-reflex reduced to control reflex values (9 and 15 ms), and then facilitation re-emerged during the longer C-T intervals investigated (e.g. 30–90 ms). It is thus apparent that excitation of low-threshold cutaneous afferent of the foot sole induced an early and a late soleus H-reflex facilitation with hip extended at 10°.
Fig. 2.
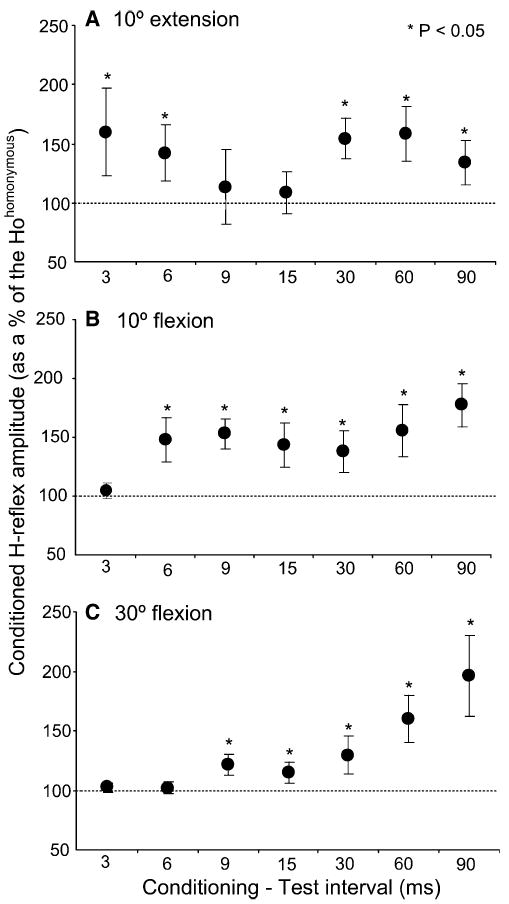
a–c Effects of plantar cutaneous afferent excitation on the soleus H− reflex with hip positioned at 10° of extension (a), 10° of flexion (b), and at 30° of flexion (c). For each conditioning test interval, the overall (from all subjects) average amplitude of the conditioned soleus H-reflex is presented as a percentage of the mean size of the Hohomonymous. Asterisks indicate statistically significant differences between the conditioned H-reflex size and the control H-reflex (Hohomonymous) (P<0.05). Error bars represent the SEM
In contrast, hip flexed at 10° and at 30° induced a reflex facilitation that became stronger with increments in the C-T interval. Specifically, with the hip flexed at 10° the conditioned soleus H-reflex reached an overall amplitude of 140 ± 19% and 180 ± 18% of Hohomonymous at the C-T intervals of 6 and 90 ms, respectively (Fig. 2b). Only at the C-T interval of 3 ms no significant changes in the conditioned soleus H-reflex were encountered (P>0.05). Similarly, the conditioned H-reflex following stimulation of plantar cutaneous afferents with hip flexed at 30° (Fig. 2c), at the C-T intervals of 9 and 90 ms reached an overall amplitude of 120 ± 9.18% and 195 ± 34% of Hohomonymous respectively. At the C-T intervals of 3 and 6 ms no significant changes in the size of the conditioned H-reflex were observed (P>0.05). The magnitude of the conditioned soleus H-reflex in response to plantar cutaneous afferents excitation was significantly different across C-T intervals and hip angles investigated (F(6, 35)=3.84, P<0.05).
Effects of CPN group I afferents excitation on the soleus H-reflex during hip angle changes
Figure 3 illustrates examples of EMG activity of TA and MG muscles recorded in one subject (S3) with the hip set in different angles of flexion and extension. EMG activity is shown when CPN stimulation was delivered alone and following conditioning of the soleus H-reflex with CPN stimulation at the C-T intervals of 3 and 100 ms. MG muscle activity was present at a latency of 33 ms, corresponding closely to that of the soleus H-reflex, indicating that a number of gastrocnemius group I afferents were excited following posterior tibial nerve stimulation, while no MG motor axons were excited.
Fig. 3.
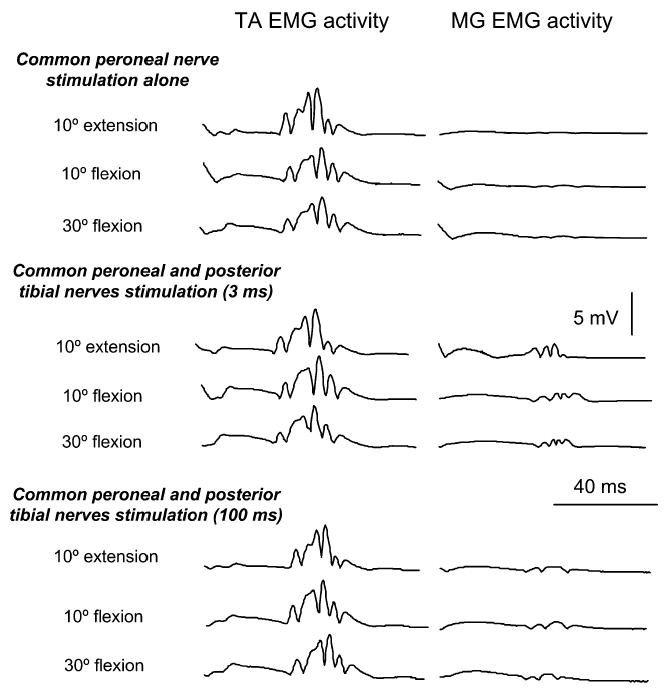
Full-wave rectified EMG activity in TA and MG muscles is shown for one subject (S3) under all conditions of testing. EMG activity of these muscles is shown with hip positioned at different angles of flexion and at 10° of extension during common peroneal nerve (CPN) stimulation alone and following conditioning of the soleus H-reflex (posterior tibial nerve stimulation) with CPN stimulation at 3 ms and at 100 ms of conditioning test intervals. Under all conditions motor axons were not excited while TA displayed a stable in amplitude H-reflex in all hip angles investigated
TA H-reflexes were present in 6 of the 11 subjects following CPN stimulation and following conditioning of the soleus H-reflex with CPN stimulation during imposed hip angle positioning. CPN stimulation alone induced an H-reflex in the TA muscle of similar amplitude at all hip angles tested (Fig. 3, left panel), demonstrating that excitation of antagonist group I afferents did not vary with changes in hip angle, and that conditioning stimulation occurred at low intensities exciting mainly flexor group Ia muscle afferents. The amplitude of the TA H-reflex recorded with the hip flexed at 10° was similar across subjects (P=0.51) when the soleus H-reflex was conditioned at 3 ms or at 100 ms. These TA H-reflexes were not significantly different from those recorded under control conditions (CPN stimulation alone). Similar findings were observed at the remaining hip angles.
CPN stimulation resulted in a significant facilitation of the soleus H-reflex with the hip set at different angles. Figure 4 shows the effects of CPN stimulation on the soleus H-reflex (dashed lines; average of 20 reflex responses) for the C-T intervals of 3 and 100 ms when the hip was positioned at 10° of flexion and extension (data are from subject 3). In all cases, reflex conditioning occurred without significant changes in the M-wave, verifying stable stimulation conditions. Moreover, the Hohomonymous (solid lines, average of 20 reflex responses) recorded with hip flexed at 30° and with hip extended at 10° were not significantly different from the control reflex recorded with the hip flexed at 10° (P>0.05), signifying further that the inhibitory and facilitatory effects of hip flexion and extension on the soleus H-reflex were counteracted (Knikou and Rymer 2002b), and thus the observed effects on the soleus H-reflex at these hip angles were due to the conditioning stimulation.
Fig. 4.
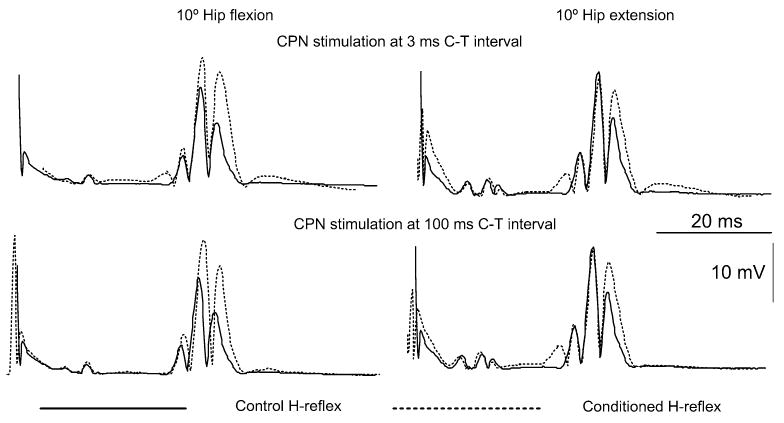
The average full-wave rectified H-reflex (n=20) recorded under control conditions (solid lines) and during conditioning of the soleus H-reflex with common peroneal nerve stimulation (dotted lines) at conditioning test intervals of 3 and 100 ms with hip flexed and extended at 10° are presented. Data shown are from subject S3. Note that the increment in the conditioned H-reflex size in all trials occurred without significant changes in the M-wave, signifying stable stimulation and recording conditions
A summary of changes in the conditioned H-reflex size following CPN stimulation is illustrated in Fig. 5. At the C-T interval of 2 ms, the conditioned H-reflexes reached overall amplitudes of 150 ± 18.3% and 120 ± 13% of Hohomonymous (P<0.05) with the hip flexed or extended at 10°, respectively. Facilitatory effects on the H-reflex were also observed when the conditioning stimulus was delivered with the hip flexed at 30° (126.4 ± 11% of Hohomonymous at 2 ms P<0.05). Likewise, CPN stimulation delivered at long C-T intervals (80, 100 and 120 ms) induced facilitatory effects on the soleus H-reflex with the hip set at different angles (Fig. 5b). The conditioned H-reflex reached an overall amplitude of 146 ± 12%, 150 ± 28% and 146 ± 23% of Hohomonymous (P<0.05) for C-T intervals of 80, 100, and 120 ms, respectively when the hip was extended at 10°. Facilitatory effects on the soleus H-reflex amplitude were also observed at the remaining hip angles. Two-way ANOVA applied to the pool data grouped according to the C-T interval and hip angle showed that the effects induced by CPN stimulation at either short (2 and 3 ms) or long (80, 100, 120 ms) C-T intervals did not show statistically significant differences across hip angles (P=0.266, P=0.112, respectively).
Fig. 5.
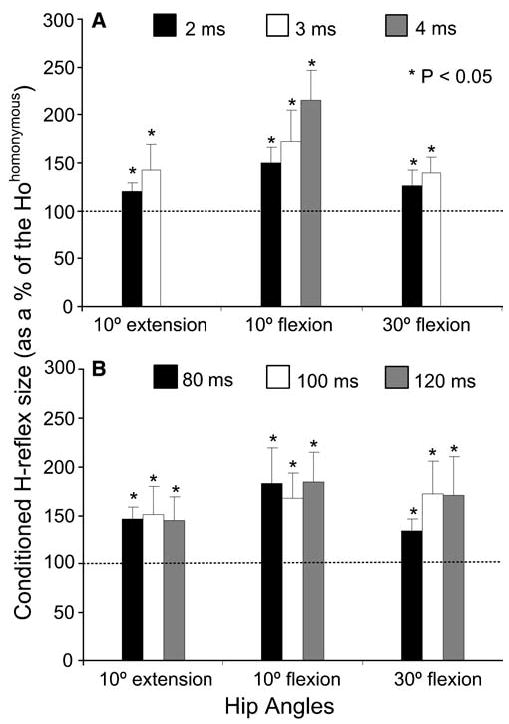
a, b Effects of variation on the conditioning action of common peroneal nerve stimulation on the soleus H-reflex during short (a) and long (b) conditioning test (C-T) intervals with the hip positioned at 10° , 30° of flexion and at 10° of extension. Data represent the overall mean size (pool data from all subjects tested) of the conditioned H-reflex expressed as a percentage of the Hohomonymous and grouped according to the C- T interval and hip angle. Asterisks indicate cases of statistically significant differences between the control and the conditioned reflex. In both graphs error bars designate the SEM
Effects of MG group I afferents excitation on the soleus H-reflex during hip angle changes
MG nerve stimulation delivered with subjects supine and hip set at various angles of flexion and extension resulted in significant changes in the size of the soleus H-reflex (Fig. 6). MG nerve stimulation with hip positioned at 30° of flexion resulted in facilitation of the reflex. The reflex reached an overall amplitude of 150 ± 17% of Hohomonymous at the C-T interval of 6 ms. Similarly, reflex facilitation was the only effect following MG nerve stimulation with the hip flexed or extended at 10°.
Fig. 6.
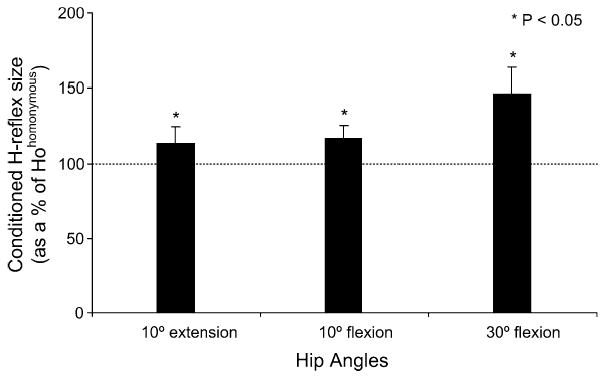
Histogram shows the effects of MG nerve stimulation on the magnitude of the soleus H-reflex at a conditioning test interval of 6 ms with the hip set at different angles. The overall (pool data) average amplitude of the H-reflex (expressed as a percentage of the mean size of the Hohomonymous) is plotted for each hip angle investigated. Asterisks indicate cases of statistically significant differences established at 95% of confidence level. Error bars represent the SEM
Discussion
Effects of plantar cutaneous afferent activation and hip angle changes on the soleus H-reflex
In all subjects of this study, hip-mediated sensory feedback influenced neuronal pathways conveying information from low-threshold plantar cutaneous afferents to soleus α motoneurons. The soleus H-reflex displayed a bimodal facilitation pattern with hip extended to 10°, while in the remaining two hip angles (10°, 30° flexion) the reflex facilitation was increased with increments in the C-T interval (Fig. 2). Our findings are in line with studies postulating changes in soleus H-reflex following activation of tactile afferents from the foot. Stimulation of purely cutaneous nerves (sural and saphenous) induces early and late soleus H-reflex facilitation in healthy subjects (Delwaide et al. 1981), and in intact and spinal cats (Schieppati and Crenna 1984; Crenna et al. 1982). Similar effects have also been reported for motor complete spinal-injured subjects (Roby-Brami and Bussel 1990). Nonetheless, no changes on the test H-reflex following excitation of plantar cutaneous afferents have also been reported (Pierrot-Deseilligny et al. 1981; Rossi and Mazzocchio 1988). The difference between these studies may be related to the protocol employed to excite cutaneous afferents, e.g. single pulse vs. pulse train.
Compared to what we have previously observed in healthy subjects (Knikou and Rymer 2002a), it appears that cutaneous afferent input during imposed hip angle changes influences spinal reflex excitability differently after SCI. So, it is possible that following SCI cutaneous afferent input augments spinal reflex excitability, triggered by signals registering hip position contributing in this way to exaggeration of muscle activity seen in these patients.
Plantar cutaneous afferents depress the actions of Ib reflex pathways to motoneurons supplying muscles at the knee but not at the ankle (Pierrot-Deseilligny et al. 1981), increase the reciprocal inhibition from TA onto soleus α motoneurons (Rossi and Mazzocchio 1988), and modulate transmission in presynaptic inhibitory pathways (Iles 1996). These findings suggest that tactile cutaneous afferents from the foot have spinal oligosynaptic and polysynaptic connections with the interneurons intercalated in the Ib, Ia reciprocal and presynaptic pathways. The contribution of cutaneous afferents in these neuronal pathways verifies that their signal integration at spinal level plays a significant role in the control of movement and posture in humans. This is clearly demonstrated in cases where such input is absent as in patients with sensory polyneuropathy, and postulated as large variations in their step cycle (van Wezel et al. 2000).
Potential mechanisms that contributed to the soleus H-reflex modulation following excitation of tactile plantar cutaneous afferents during imposed hip angle changes include decrement of presynaptic inhibition of Ia afferent terminals exerted by large cutaneous fibres (Iles 1996; Rudomin et al. 1974), interaction of cutaneous afferents with Ib inhibitory interneurons (Pierrot-Deseilligny et al. 1981), and cutaneous facilitation of α motoneurons via segmental interneuronal effects. At present however we have no evidence to support any of these mechanisms preferentially. To summarize, activation of tactile plantar cutaneous afferents increase soleus H-reflex excitability with the hip either flexed or extended, suggesting that sensory input from cutaneous mechanoreceptors of the foot sole interacts with hip-mediated sensory input to enhance H-reflex excitability in human SCI. The exaggerated H-reflex might be related to the enhanced muscle activity seen in these patients, observed clinically as extensor spasms.
Hip-induced modulation of spinal inhibitory mechanisms
CPN stimulation at either 2–4 ms or at 80–120 ms resulted in soleus H-reflex facilitation, regardless of the hip angle tested in all subjects (Fig. 5). In healthy subjects at rest, CPN stimulation induces soleus H-reflex depression at these C-T intervals. The reflex inhibition at the C-T intervals of 2 and 3 ms, involves mainly the reciprocal Ia inhibition, while the long latency reflex depression is primarily attributed to presynaptic inhibition of soleus Ia afferents (Crone et al. 1987; Zehr and Stein 1999). Our results indicate that both reciprocal and presynaptic inhibition were absent during hip angle changes, postulating a lack in key spinal inhibitory mechanisms in chronic human SCI when hip proprioceptive input is concurrently present.
The most prevalent possible mechanism to account for the manifestation of depression in reciprocal inhibition is the disruption of the descending pathways that excite spinal interneurons which in turn influence the reciprocal inhibition of α motoneurons (Crone and Nielsen 1994). However, an existing facilitatory pathway that becomes more potent following SCI should also be considered. This facilitatory pathway was evident with changes in hip position. The presence of a facilitatory pathway is supported by the differential modulation pattern of reciprocal and presynaptic inhibition during imposed static hip angle changes in neurologically intact subjects (Knikou and Rymer 2003), and the high incidence rate of the TA H-reflexes observed here. TA H-reflexes have previously reported in healthy subjects but at a much lower incidence rate (Crone et al. 1987; Pérot and Mora 1993). This suggests that TA motoneuronal excitability is increased in human SCI, and probably be mediated by a similar facilitatory pathway during which the actions of Ia reciprocal and presynaptic inhibitory interneurons were suppressed or absent during imposed hip angle changes.
Previous human studies have demonstrated diverse excitatory inputs onto Ia and presynaptic inhibitory interneurons in man, with their inhibitory actions to decrease or to increase depending on the direction of the movement (Petersen et al. 1999; Morita et al. 2001; Nielsen and Kagamihara 1993, Pierrot-Deseilligny 1997; Hultborn et al. 1987). These studies along with our findings suggest that the strength of reciprocal Ia and presynaptic inhibition is influenced by afferents of distal and proximal muscles and that some heteronymous connections postulated in healthy subjects (Meunier et al. 1990) are preserved following SCI.
However, it was not only the reciprocal Ia or presynaptic inhibition that was depressed during hip angle changes but also the non-reciprocal group I inhibition, suggesting of a generalized suppression of the spinal inhibitory mechanisms following SCI during hip angle changes. MG nerve stimulation is known to induce soleus H-reflex depression in healthy subjects at rest (Pierrot-Deseilligny et al. 1979; Jami 1992). This reflex depression is evoked mainly from Golgi tendon organ (Ib) afferents (Pierrot-Deseilligny et al. 1979); however, a contribution from MG group Ia afferents cannot be excluded (Gritti and Schieppati 1989). During walking, group Ib inhibition is replaced by a locomotor-related Ib excitation contributing to ankle stability during stance (Stephens and Yang 1996). In all subjects of this study, the disynaptic inhibitory input between MG and soleus α motoneurons was absent and was replaced by group I excitation independent of the hip angle, in contrast to that reported in able-bodied subjects (Knikou and Rymer 2002a). Group I excitation from the MG muscle during hip angle changes may resemble the reflex reversal observed during walking. However, one might consider that not only the conditions are different in several aspects, but also most importantly the absence of concurrent load input might modulate this neuronal pathway differently. Thus, there is a need for future studies on these reflex inhibitory actions during dynamic conditions in individuals with SCI.
Overall, reciprocal Ia, presynaptic and non-reciprocal group I inhibition were depressed with hip positioned at different angles. Practically, lack of these inhibitory mechanisms may be related to the manifestation of the extensor spasms observed in these patients. It is known that when patients with a SCI move from sit to supine, involving bilateral hip extension movement, extensor spasms are observed (Kuhn 1950). Given that the extensor spasms are a multi-joint response, some connections between distal and proximal afferents are preserved after SCI, and that imposed hip angle changes affect reflex pathways and actions of inhibitory interneurons coupled with human posture and locomotion, it is possible that the extensor spasms might be mediated by similar mechanisms that led to the depression of actions in these spinal inhibitory interneurons observed in this study.
Which afferents/receptors of the hip region were excited during imposed hip angle changes?
Given the current experimental protocol, it is difficult to identify the involved afferents that participated in the modulation of the actions of inhibitory interneurons and tactile cutaneous sensory input during imposed angle changes. Nonetheless, we should consider the most prevalent ones. Based largely on observations in analogous reduced animal preparations, the observed effects might be associated with the stretch of the muscles within the hip region.
Denervation of hip joint afferents does not affect the ability of the hip position to entrain the fictive locomotor rhythm in the spinal cat (Kriellaars et al. 1994), while selective activation of group II hip flexor muscle afferents (iliopsoas, sartorius) have the potential to prolong the swing phase of gait (Hiebert et al. 1996; Perreault et al. 1995). In a similar way, ankle muscle afferents responding to stretch modulate significantly soleus H-reflex excitability (Pinniger et al. 2001). It is worth noting that muscle receptors have been reported as receptors for detecting limb position, with their static discharges to depend on the amount of the muscle shortening or lengthening (Ribot-Ciscar et al. 2003). Further, in the absence of any background force, it is unlikely that group Ib afferents were activated during static hip angle changes. Thus, it is possible that muscle spindle afferents responding to stretch contributed to the observed effects.
Another question that arises at this point is under which mechanisms the hip muscle spindle afferents contributed to this reflex modulation. In this study we used the H-reflex as a test reflex to assess the effects of conditioning afferent volleys on a pool of soleus motoneurons. Reflex facilitation is likely to affect the motoneurons that were just recruited in the control reflex or that just failed to discharge (Pierrot-Deseilligny and Mazevet 2000). These motoneurons receive also oligosynaptic excitatory inputs (Burke et al. 1984). Given the uniform reflex facilitation observed in all hip angles following different types of conditioning stimulation, and that reflex modulation during hip angle changes coincides with shifts in reflex latency (Knikou and Rymer 2002b), there is a theoretical possibility that hip proprioceptive input increased the transmission of facilitatory interneurons and decreased the transmission of inhibitory interneurons affecting α motoneuronal excitability. However, future studies are necessary to address possible neuronal mechanisms using more detailed methods, such as the post-stimulus time histograms method, in which a single motoneuron is investigated.
Possible effects from inter-subject variability
Based on the consistent modulation pattern of the soleus H-reflex, following excitation of synergist/antagonist muscle afferents and tactile plantar cutaneous afferents with hip set at different angles of flexion and at 10° of extension, no conclusion could be drawn about differential effects due to the level or the nature of the injury. The spinal lesions were classified as ASIA C in 9 out of 11 subjects with their injury level to range from C5 to T9. Similarly, although 7 out of the 11 subjects were taking varying amounts of antispastic medications at the time of the study, across conditioning stimulations, we found no significant differences in the magnitude of the conditioned H-reflex when comparing data from subjects on spasticity medications to those without medications (P>0.05). However, the sample size for this comparison was small (see Table 1). It is worth noting at this point that the antispastic effect of baclofen, for example, in spastic multiple sclerosis patients has recently been demonstrated not to be due to changes on the transmitter release from Ia afferents or on disynaptic reciprocal Ia inhibition but from direct depression of motoneuronal excitability leading to decreased H-reflexes (Ørsnes et al. 2000). This strongly suggests that in patients who were under baclofen, their medication had minimal effects on the modulation pattern of reciprocal Ia and presynaptic inhibition observed here. Nevertheless, the effects of spasticity medications on the expression of these inhibitory/facilitatory reflex actions in response to imposed hip angle changes is an important topic for future studies since hip proprioceptive input influences walking in individuals with SCI (Dietz et al. 2002).
Conclusions
This study has demonstrated that hip afferent input interacts with post- and presynaptic inhibitory interneurons, and also with distal tactile cutaneous afferents such as those of the foot sole. The net effect on the soleus H-reflex expression following excitation of afferents from synergist/antagonist muscles and plantar cutaneous afferents was excitatory, verifying that key inhibitory reflex actions were switched to facilitatory, contributing to the pathological expression of muscle tone and movement in human SCI. Given the uniform reflex facilitation under all types of sensorimotor conditioning, we propose that transmission in facilitatory interneurons become more potent when input from hip proprioceptors is present, enhancing spinal reflex excitability. This multisegmental interneuronal excitatory drive may account for the manifestation of the extensor spasms seen in these patients. On the basis that combined input from cutaneous afferents and signals transmitting hip position interact and shape the walking pattern in individuals with established SCI (Dietz et al. 2002), there is a need for further investigation on these neuronal pathways in individuals with SCI during dynamic conditions.
Acknowledgments
The author would like to thank the patients for their patience and endurance on many experimental hours, and to acknowledge Dr. Elizabeth Kay for her help during the experiments. The suggestions and comments of Drs. GN Lewis, WZ Rymer and those of the anonymous reviewers are greatly appreciated. This project was supported by a research grant from the National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH), Grant No. R03 HD043951.
References
- Anderson O, Grillner S. Peripheral control of the cat’s step cycle I. Phase dependent effects of ramp-movements of the hip during ‘‘fictive locomotion’’. Acta Physiol Scand. 1981;113:89–101. doi: 10.1111/j.1748-1716.1981.tb06867.x. [DOI] [PubMed] [Google Scholar]
- Ashworth B. Preliminary trial of carisoprodol in multiple sclerosis. Practioner. 1964;192:540–542. [PubMed] [Google Scholar]
- Boorman GI, Lee RG, Becker WJ, Windhorst UR. Impaired ‘natural reciprocal inhibition’ in patients with spasticity due to incomplete spinal cord injury. Electroencephalogr Clin Neurophysiol. 1996a;101:84–92. doi: 10.1016/0924-980x(95)00262-j. [DOI] [PubMed] [Google Scholar]
- Boorman GI, Hoffer JA, Kallesoe K, Viberg D, Mah C. A measure of peripheral nerve stimulation efficacy applicable to H-reflex studies. Can J Neurol Sci. 1996b;23:264–270. doi: 10.1017/s0317167100038208. [DOI] [PubMed] [Google Scholar]
- Bouyer LJG, Rossignol S. Contribution of cutaneous inputs from the hindpaw to the control of locomotion. II. Spinal cats. J Neurophysiol. 2003;90:3640–3653. doi: 10.1152/jn.00497.2003. [DOI] [PubMed] [Google Scholar]
- Brooke JD, Misiaszek JE, Cheng J. Locomotor-like rotation of either hip or knee inhibits soleus H reflexes in humans. Somatosensory Motor Res. 1993;10:357–364. doi: 10.3109/08990229309028843. [DOI] [PubMed] [Google Scholar]
- Brooke JD, Cheng J, Misiaszek JE, Lafferty K. Amplitude modulation of the soleus H reflex in the human during active and passive stepping movements. J Neurophysiol. 1995;73:102–111. doi: 10.1152/jn.1995.73.1.102. [DOI] [PubMed] [Google Scholar]
- Burke D, Gandevia SC, McKeon B. Monosynaptic and oligosynaptic contributions to human ankle jerk and H-reflex. J Neurophysiol. 1984;52:435–448. doi: 10.1152/jn.1984.52.3.435. [DOI] [PubMed] [Google Scholar]
- Chapman CE, Sullivan SJ, Pompura J, Arsenault AB. Changes in hip position modulate soleus H-reflex excitability in man. Electromyogr Clin Neurophysiol. 1991;31:131–143. [PubMed] [Google Scholar]
- Conway BA, Hultborn H, Kiehn O. Proprioceptive input resets central locomotor rhythm in the spinal cat. Exp Brain Res. 1987;68:643–656. doi: 10.1007/BF00249807. [DOI] [PubMed] [Google Scholar]
- Conway BA, Scott DT, Riddell JS (1995) The effects of plantar nerve stimulation on long latency flexion reflexes in the acute spinal cat. In: Taylor A, Gladden MN, Durbada R (eds) Alpha and gamma motor systems. Plenum Press, New York, pp 593–595
- Crenna P, Schieppati M, DeCurtis M. Long-latency, nonreciprocal reflex responses of antagonistic hind limb muscles after cutaneous nerve stimulation in the cat. Exp Neurol. 1982;76:58–71. doi: 10.1016/0014-4886(82)90101-7. [DOI] [PubMed] [Google Scholar]
- Crone C. Reciprocal inhibition in man. Dan Med Bull. 1993;40:571–581. [PubMed] [Google Scholar]
- Crone C, Nielsen J. Spinal mechanisms in man contributing to reciprocal inhibition during voluntary dorsiflexion of the foot. J Physiol Lond. 1989;416:255–272. doi: 10.1113/jphysiol.1989.sp017759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C, Nielsen J. Central control of disynaptic reciprocal inhibition in humans. Acta Physiol Scand. 1994;152:351–363. doi: 10.1111/j.1748-1716.1994.tb09817.x. [DOI] [PubMed] [Google Scholar]
- Crone C, Hultborn H, Jespersen B. Reciprocal Ia inhibition from the peroneal nerve to soleus motoneurones with special reference to the size of the test reflex. Exp Brain Res. 1985;59:418–422. doi: 10.1007/BF00230924. [DOI] [PubMed] [Google Scholar]
- Crone C, Hultborn H, Jespersen B, Nielsen J. Reciprocal Ia inhibition between ankle flexors and extensors in man. J Physiol Lond. 1987;389:163–185. doi: 10.1113/jphysiol.1987.sp016652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C, Hultborn H, Mazieres L, Nielsen J, Pierrot-Deseilligny E. Sensitivity of monosynaptic test reflexes to facilitation and inhibition as a function of the test reflex size: a study in man and the cat. Exp Brain Res. 1990;81:35–45. doi: 10.1007/BF00230098. [DOI] [PubMed] [Google Scholar]
- Crone C, Johnsen LL, Biering-Sorensen F, Nielsen JB. Appearance of reciprocal facilitation of ankle extensors from ankle flexors in patients with stroke or spinal cord injury. Brain. 2003;126:495–507. doi: 10.1093/brain/awg036. [DOI] [PubMed] [Google Scholar]
- Delwaide PJ, Oliver E. Short-latency autogenic inhibition (IB inhibition) in human spasticity. J Neurol Neurosurg Psychiatry. 1988;51:1546–1550. doi: 10.1136/jnnp.51.12.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwaide PJ, Crenna P, Fleron MH. Cutaneous nerve stimulation and motoneuronal excitability. I: Soleus and tibialis anterior excitability after ipsilateral and contralateral sural nerve stimulation. J Neurol Neurosurg Psychiatry. 1981;44:699–707. doi: 10.1136/jnnp.44.8.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz V, Muller R, Colombo G. Locomotor activity in spinal man: significance of afferent input from joint and load receptors. Brain. 2002;125:2626–2634. doi: 10.1093/brain/awf273. [DOI] [PubMed] [Google Scholar]
- Downes L, Ashby P, Bugaresti J. Reflex effects from Golgi tendon organ (Ib) afferents are unchanged after spinal cord lesions in humans. Neurology. 1995;45:1720–1724. doi: 10.1212/wnl.45.9.1720. [DOI] [PubMed] [Google Scholar]
- Duysens J. Reflex control of locomotion as revealed by stimulation of cutaneous afferents in spontaneously walking premammillary cats. J Neurophysiol. 1977;40:737–751. doi: 10.1152/jn.1977.40.4.737. [DOI] [PubMed] [Google Scholar]
- Duysens J, Pearson KG. The role of cutaneous afferents from the distal hindlimb in the regulation of the step cycle of thalamic cats. Exp Brain Res. 1976;24:245–255. doi: 10.1007/BF00235013. [DOI] [PubMed] [Google Scholar]
- Duysens J, Pearson KG. Inhibition of flexor burst generation by loading ankle extensor muscles in walking cats. Brain Res. 1980;187:321–332. doi: 10.1016/0006-8993(80)90206-1. [DOI] [PubMed] [Google Scholar]
- Faist M, Mazevet D, Dietz V, Pierrot-Deseilligny E. A quantitative assessment of presynaptic inhibition of Ia afferents in spastics: differences in hemiplegics and paraplegics. Brain. 1994;117:1449–1455. doi: 10.1093/brain/117.6.1449. [DOI] [PubMed] [Google Scholar]
- Gritti I, Schieppati M. Short-latency inhibition of soleus motoneurones by impulses in Ia afferents from the gastrocnemius muscle in humans. J Physiol Lond. 1989;416:469–484. doi: 10.1113/jphysiol.1989.sp017772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiebert GW, Whelan PJ, Prochazka A, Pearson KG. Contribution of hind limb flexor muscle afferents to the timing of phase transitions in the cat step cycle. J Neurophysiol. 1996;75:1126–1137. doi: 10.1152/jn.1996.75.3.1126. [DOI] [PubMed] [Google Scholar]
- Hugon M (1973) Methodology of the Hoffmann reflex in man, proprioceptive reflexes and the H-reflexes. In: Desmedt JF (ed) New developments in electromyography and clinical neurophysiology. Karger, Basel, pp 277–293
- Hultborn H. Changes in neuronal properties and spinal reflexes during development of spasticity following spinal cord lesions and stroke: studies in animal models and patients. J Rehabil Med Suppl. 2003;41:46–55. doi: 10.1080/16501960310010142. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Meunier S, Pierrot-Deseilligny E, Shindo M. Changes in presynaptic inhibition of Ia fibres at the onset of voluntary contraction in man. J Physiol Lond. 1987;389:757–772. doi: 10.1113/jphysiol.1987.sp016681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iles JF. Evidence for cutaneous and corticospinal modulation of presynaptic inhibition of Ia afferents from the human lower limb. J Physiol Lond. 1996;491:197–207. doi: 10.1113/jphysiol.1996.sp021207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jami L. Golgi tendon organs in mammalian skeletal muscle: functional properties and central actions. Physiol Rev. 1992;72:623–666. doi: 10.1152/physrev.1992.72.3.623. [DOI] [PubMed] [Google Scholar]
- Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Progress Neurobiol. 1992;38:335–378. doi: 10.1016/0301-0082(92)90024-9. [DOI] [PubMed] [Google Scholar]
- Katz R. Presynaptic inhibition in humans: A comparison between normal and spastic patients. J Physiol Paris. 1999;93:379–385. doi: 10.1016/s0928-4257(00)80065-0. [DOI] [PubMed] [Google Scholar]
- Kavounoudias A, Roll R, Roll J-P. Foot sole and ankle muscle inputs contribute jointly to human erect posture regulation. J Physiol Lond. 2001;532:869–878. doi: 10.1111/j.1469-7793.2001.0869e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernell D, Hultborn H. Synaptic effects on recruitment gain: a mechanism of importance for the input-output relations of motoneurone pools? Brain Res. 1990;507:176–179. doi: 10.1016/0006-8993(90)90542-j. [DOI] [PubMed] [Google Scholar]
- Knikou M, Rymer WZ. Effects of changes in hip joint angle on H-reflex excitability in humans. [Erratum in Exp Brain Res (2002) 144:558] Exp Brain Res. 2002a;143:149–159. doi: 10.1007/s00221-001-0978-4. [DOI] [PubMed] [Google Scholar]
- Knikou M, Rymer WZ. Hip angle induced modulation of H reflex amplitude, latency and duration in spinal cord injured humans. Clin Neurophysiol. 2002b;113:1698–1708. doi: 10.1016/s1388-2457(02)00285-7. [DOI] [PubMed] [Google Scholar]
- Knikou M, Rymer WZ (2003) Possible mechanisms associated with hip-motion induced H-reflex modulation in humans. Program No. 186.1 Abstract Viewer/Itinerary Planner. Society for Neuroscience, Washington, DC
- Knikou M, Rymer WZ (2004) Changes in intersegmental spinal integration examined using imposed hip angle changes in human SCI. Program No. 311.5 Abstract Viewer/Itinerary Planner. Society for Neuroscience, Washington, DC
- Kriellaars DJ, Brownstone RM, Noga BR, Jordan LM. Mechanical entrainment of fictive locomotion in the decerebrate cat. J Neurophysiol. 1994;71:2074–2086. doi: 10.1152/jn.1994.71.6.2074. [DOI] [PubMed] [Google Scholar]
- Kuhn R. Functional capacity of the isolated human spinal cord. Brain. 1950;73:1–51. doi: 10.1093/brain/73.1.1. [DOI] [PubMed] [Google Scholar]
- Maynard FM, Bracken MB, Creasey G, Ditunno JF, Donovan WH, Ducker TB, Garber SL, Marini RJ, Stover SL, Tator CH, Waters RL, Wilberger JP, Young W. International standards for neurological and functional classification of spinal cord injury. Spinal Cord. 1997;5:266–274. doi: 10.1038/sj.sc.3100432. [DOI] [PubMed] [Google Scholar]
- Meinck HM. Facilitation and inhibition of the human H reflex as a function of the amplitude of the control reflex. Electroencephalogr Clin Neurophysiol. 1980;48:203–211. doi: 10.1016/0013-4694(80)90305-3. [DOI] [PubMed] [Google Scholar]
- Meunier S, Penicaud A, Pierrot-Deseilligny E, Rossi A. Monosynaptic Ia excitation and recurrent inhibition from quadriceps to ankle flexors and extensors in man. J Physiol Lond. 1990;423:661–675. doi: 10.1113/jphysiol.1990.sp018046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita H, Crone C, Christenhuis D, Petersen NT, Nielsen JB. Modulation of presynaptic inhibition and disynaptic reciprocal Ia inhibition during voluntary movement in spasticity. Brain. 2001;124:826–837. doi: 10.1093/brain/124.4.826. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Kagamihara Y. The regulation of presynaptic inhibition during co-contraction of antagonistic muscles in man. J Physiol Lond. 1993;464:575–593. doi: 10.1113/jphysiol.1993.sp019652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ørsnes G, Crone C, Krarup C, Petersen N, Nielsen J. The effect of baclofen on the transmission in spinal pathways in spastic multiple sclerosis patients. Clin Neurophysiol. 2000;111:1372–1379. doi: 10.1016/s1388-2457(00)00352-7. [DOI] [PubMed] [Google Scholar]
- Pérot C, Mora I. H reflexes in close muscles: cross-talk or genuine responses? Electroencephalogr Clin Neurophysiol. 1993;89:104–107. doi: 10.1016/0168-5597(93)90091-3. [DOI] [PubMed] [Google Scholar]
- Perreault MC, Angel MJ, Guertin P, McCrea DA. Effects of stimulation of hindlimb flexor group II afferents during fictive locomotion in the cat. J Physiol Lond. 1995;487:211–220. doi: 10.1113/jphysiol.1995.sp020872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen N, Morita H, Nielsen J. Modulation of reciprocal inhibition between ankle extensors and flexors during walking in man. J Physiol Lond. 1999;520:605–619. doi: 10.1111/j.1469-7793.1999.00605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E. Assessing changes in presynaptic inhibition of Ia afferents during movement in humans. J Neurosci Methods. 1997;74:189–199. doi: 10.1016/s0165-0270(97)02249-8. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Mazevet D. The monosynaptic reflex: a tool to investigate motor control in humans. Interests and limits. Neurophysiol Clin. 2000;30:67–80. doi: 10.1016/s0987-7053(00)00062-9. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Katz R, Morin C. Evidence for Ib inhibition in human subjects. Brain Res. 1979;166:176–179. doi: 10.1016/0006-8993(79)90660-7. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Bergego C, Katz R, Morin C. Cutaneous depression of Ib reflex pathways to motoneurons in man. Exp Brain Res. 1981;42:351–361. doi: 10.1007/BF00237500. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Bergego C, Katz R. Reversal in cutaneous control of Ib pathways during human voluntary contraction. Brain Res. 1982;233:400–403. doi: 10.1016/0006-8993(82)91213-6. [DOI] [PubMed] [Google Scholar]
- Pinniger GJ, Nordlund MM, Steele JR, Cresswell AG. H-reflex modulation during passive lengthening and shortening of the human triceps surae. J Physiol Lond. 2001;534:913–923. doi: 10.1111/j.1469-7793.2001.00913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribot-Ciscar E, Bergenheim M, Albert F, Roll J-P. Proprioceptive population coding of limb position in humans. Exp Brain Res. 2003;149:512–519. doi: 10.1007/s00221-003-1384-x. [DOI] [PubMed] [Google Scholar]
- Roby-Brami A, Bussel B. Effects of FRA stimulation on the soleus H-reflex in patients with a complete spinal cord lesion: Evidence for presynaptic inhibition of Ia transmission. Exp Brain Res. 1990;81:593–601. doi: 10.1007/BF02423509. [DOI] [PubMed] [Google Scholar]
- Rossi A, Mazzocchio R. Cutaneous control of group I pathways from ankle flexors to extensors in man. Exp Brain Res. 1988;73:8–14. doi: 10.1007/BF00279655. [DOI] [PubMed] [Google Scholar]
- Rossi A, Zalaffi A, Decchi B. Heteronymous recurrent inhibition from gastrocnemius muscle to soleus motoneurons in humans. Neurosci Lett. 1994;169:141–144. doi: 10.1016/0304-3940(94)90376-x. [DOI] [PubMed] [Google Scholar]
- Rudomin P, Schmidt RF. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp Brain Res. 1999;129:1–37. doi: 10.1007/s002210050933. [DOI] [PubMed] [Google Scholar]
- Rudomin P, Nunez R, Madrid J, Burke RE. Primary afferent hyperpolarization and presynaptic facilitation of Ia afferent terminals induced by large cutaneous fibres. J Neurophysiol. 1974;37:413–429. doi: 10.1152/jn.1974.37.3.413. [DOI] [PubMed] [Google Scholar]
- Schieppati M, Crenna P. Natural cutaneous stimulation induces late and long-lasting facilitation of extensor motoneurons in the cat. Brain Res. 1984;293:259–267. doi: 10.1016/0006-8993(84)91233-2. [DOI] [PubMed] [Google Scholar]
- Stephens MJ, Yang JF. Short latency, non-reciprocal group I inhibition is reduced during the stance phase of walking in humans. Brain Res. 1996;743:24–31. doi: 10.1016/s0006-8993(96)00977-8. [DOI] [PubMed] [Google Scholar]
- Tanaka R. Reciprocal Ia inhibition during voluntary movements in man. Exp Brain Res. 1974;21:529–540. doi: 10.1007/BF00237171. [DOI] [PubMed] [Google Scholar]
- Thomas CK, Westling G. Tactile unit properties after human cervical spinal cord injury. Brain. 1995;118:1547–1556. doi: 10.1093/brain/118.6.1547. [DOI] [PubMed] [Google Scholar]
- van Wezel BMH, van Engelen BMG, Gabreëls FJM, Gabreëls-Festen AAWM, Duysens J. Aβ fibers mediate cutaneous reflexes during human walking. J Neurophysiol. 2000;83:2980–2986. doi: 10.1152/jn.2000.83.5.2980. [DOI] [PubMed] [Google Scholar]
- Zehr PE, Stein RB. Interaction of the Jendrassik maneuver with segmental presynaptic inhibition. Exp Brain Res. 1999;124:474–480. doi: 10.1007/s002210050643. [DOI] [PubMed] [Google Scholar]


