Abstract
Vectors containing group B adenovirus (Ad) fibers are able to efficiently transduce gene therapy targets that are refractory to infection with standard Ad serotype 5 (Ad5) vectors, including malignant tumor cells, hematopoietic stem cells, and dendritic cells. Preliminary studies in mice indicate that, after intravenous injection, B-group fiber-containing Ads do not efficiently transduce most organs and cause less acute toxicity than Ad5 vectors. However, biodistribution and safety studies in mice are of limited value because the mouse analog of the B-group Ad receptor, CD46, is expressed only in the testis, whereas in humans, CD46 is expressed on all nucleated cells. Unlike mice, baboons have CD46 expression patterns and levels that closely mimic those in humans. We conducted a biodistribution and toxicity study of group B Ad fiber-containing vectors in baboons. Animals received phosphate-buffered saline, Ad5-bGal (a first-generation Ad5 vector), or B-group fiber-containing Ads (Ad5/35-bGal and Ad5/11-bGal) at a dose of 2 × 1012 VP/kg, and vector biodistribution and safety was analyzed over 3 days. The amount of Ad5/35-bGal and Ad5/11-bGal vector genomes was in most tissues one to three orders of magnitude below that of Ad5. Significant Ad5/35- and Ad5/11-mediated transgene (β-galactosidase) expression was seen only in the marginal zone of splenic follicles. Compared with the animal that received Ad5-bGal, all animals injected with B-group fiber-containing Ad vectors had lower elevations in serum proinflammatory cytokine levels. Gross and histopathology were normal in animals that received B-group Ad fiber-containing Ads, in contrast to the Ad5-infused animal, which showed widespread endothelial damage and inflammation. In a further study, a chimeric Ad5/35 vector carrying proapoptotic TRAIL and Ad E1A genes under tumor-specific regulation was well tolerated in a 30-day toxicity study. No major clinical, serologic, or pathologic abnormalities were noticed in this animal.
OVERVIEW SUMMARY
B-group Ad fiber-containing vectors are promising tools for gene therapy, for example, for the treatment of metastatic cancer or cardiovascular diseases, or for vaccination/immunotherapy. However, only a few studies of vectors containing B-group Ad fibers in mice have been conducted so far, and little is known about the mechanisms and effects of B-group Ad vector delivery in vivo. Before these vectors can be considered for clinical application, this knowledge gap must be filled. We performed biodistribution and safety studies after intravenous injection of chimeric Ad5 vectors containing Ad35 and Ad11 fibers into baboons. Our study suggests that Ad vectors possessing B-group Ad fibers have a better safety profile after intravenous injection than do conventional Ad5-based vectors.
INTRODUCTION
HUMAN ADENOVIRUSES (Ads) are classified into six groups (A through F). All Ad vectors used clinically so far have been based on serotype 5. The main disadvantages of Ad5-based vectors appear to be preexisting immunity in the majority of humans, low transduction of important gene therapy target cells (because of low expression of the primary Ad5 receptor, the coxsackie–adenovirus receptor [CAR]), and innate toxicity induced on intravascular application. Toxicity associated with Ad5 injection is characterized by complement activation, cytokine release, and consequent vascular damage leading to a systemic inflammatory response that can be fatal to the host (Schnell et al., 2001; Lozier et al., 2002; Morral et al., 2002; Raper et al., 2002; Gaggar et al., 2003). The innate response can be divided into two phases. The first phase of acute inflammation occurs within 24 hr of virus administration, and it depends entirely on virus capsid interactions with host cells (Liu and Muruve, 2003). This phase is therefore seen after administration of both first-generation and helper-dependent Ad vectors. The second phase, which is specific to first-generation Ad vectors, begins 3–4 days after Ad administration and requires expression of viral gene products. Ad5 uptake induces expression of a number of cytokines (including interleukin [IL]-6, IL-10, and IL-8, tumor necrosis factor α [TNF-α], and interferon γ[IFN-γ]) and chemokines (including macrophage inflammatory protein [MIP]-1α/β and MIP-2) (Lieber et al., 1997, 1998; Muruve et al., 1999), which, in turn, play a major causative role in inflammation, tissue damage, and the induction of an antiviral adaptive immune response. Induction of cytokine transcription is thought to be initiated by Ad interaction with cellular integrins and integrin-mediated signaling involving p38 MAPK (mitogen-activated protein kinase) and ERK1/2 (extracellular-signal regulated kinase 1/2), and subsequent activation of NF-κB (Borgland et al., 2000; Bowen et al., 2002; Liu and Muruve, 2003; Tamanini et al., 2003). Kupffer cells, endothelial cells, and leukocytes are considered to be the primary source of these cytokines and chemokines (Liu and Muruve, 2003).
Data show that vectors containing fibers from B-group Ads (including serotypes Ad35 and Ad11) efficiently transduce human cell types that are relatively refractory to Ad5 infection. These cell types include CD34+ cells (particularly subsets with potential stem cell activity) (Segerman et al., 2000; Shayakhmetov et al., 2000; Stecher et al., 2001; Yotnda et al., 2001; Mizuguchi and Hayakawa, 2002; Sakurai et al., 2003), immature human dendritic cells (Rea et al., 2001; Stone et al., 2002; Vogels et al., 2003; DiPaolo, 2004), primary tumor cells (Gaggar et al., 2003; Sova et al., 2004), bone marrow mesenchymal cells (Olmsted-Davis et al., 2002; Gugala et al., 2003), and myoblasts, amniocytes, and synoviocytes (Rea et al., 2001; Havenga et al., 2002). Furthermore, we found that intravenously injected vectors containing Ad35 or Ad11 fibers (which have short fiber shafts) only inefficiently transduce Kupffer cells (whose transduction requires long-shafted fibers) and therefore elicit significantly less innate toxicity compared with Ad5 vectors in mice (Shayakhmetov et al., 2004). Therefore, Ad vectors containing B-group fibers show promise for in vivo gene transfer, particularly for the treatment of metastatic cancer or for vaccination/immunotherapy. Unfortunately, only a limited number of murine studies on vectors containing B-group Ad fibers have been conducted so far (Koizumi et al., 2003; Seshidhar Reddy et al., 2003; Barouch et al., 2004), and little is known about mechanisms and effects of B-group Ad vector infection in vitro and in vivo.
We and others identified CD46 as a cellular receptor that is used by all B-group Ads and by B-group Ad fiber-containing vectors (Gaggar et al., 2003; Segerman et al., 2003; Sirena et al., 2004). CD46 is also a receptor for measles virus laboratory strains, for human herpes virus 6, and for certain pathogenic bacteria (for a review see Cattaneo, 2004). CD46 is a membrane protein that is expressed on all nucleated human cells. Initially, CD46 was identified as a regulator of complement activation that binds and cleaves C3b and C4b in concert with serine protease factor I and, consequently, protects cells from complement-mediated lysis. CD46 expression is greatly upregulated in malignant tumor cells (Hara et al., 1992; Thorsteinsson et al., 1998; Kinugasa et al., 1999; Murray et al., 2000) and hematopoietic stem cells (Cho et al., 1991; Manchester et al., 2002). CD46 is expressed at the blood–brain barrier and was suggested to promote passage of pathogens from blood to cerebrospinal fluid and particularly might be involved in the pathogenesis of meningococcal disease (Johansson et al., 2003). Specific expression of CD46 on the inner acrosomal membrane of spermatozoa and human placental trophoblasts, as well as restricted expression of rodent CD46 in testis, suggests that CD46 also plays a role in reproductive biology (Cervoni et al., 1992).
Whereas all human nucleated cells express CD46, expression of the murine CD46 homolog is restricted to the testis. In addition, there is no homology between mouse and human cytoplasmic domain sequences. This clearly limits the value of biodistribution and toxicity studies with B-group Ad fiber-containing vectors in mice. In contrast to mice, the CD46 expression profile in nonhuman primates is similar to that in humans (Hsu et al., 1997). Nonhuman primates have been extensively used for studies with measles virus (which also uses CD46 as a receptor) (Sakaguchi et al., 1986; McChesney et al., 1997; Premenko-Lanier et al., 2003).
In this study, we analyzed the biodistribution and safety of intravenously applied Ad5/35 and Ad5/11 vectors in a short-term study. We also conducted a 30-day toxicity study with an Ad5/35-based oncolytic adenovirus expressing E1A and TRAIL (TNF-related apoptosis-inducing ligand) in a tumor-specific manner.
MATERIALS AND METHODS
Adenovirus vectors
Ad5-bGal, Ad5/35-bGal, and Ad5/11-bGal are first-generation E1/E3-deleted adenovirus vectors expressing Escherichia coli β-galactosidase (β-Gal) under the control of the Rous sarcoma virus (RSV) promoter (Shayakhmetov and Lieber, 2000; Stecher et al., 2001; Bernt et al., 2002). Ad5/35.IR-E1A/TRAIL is a capsid-modified, conditionally replicating, oncolytic vector (Sova et al., 2004). Physical vector titers were determined spectrophotometrically and expressed as vector particles (VP) per milliliter. Infectious titers were measured as plaque-forming units or β-Gal-expressing units on 293 cells. The genome titers of all vectors were between 4 × 1012 and 8 × 1012 genomes (viral particles) per milliliter and the ratio of genomes to infectious titer was 20:1.
Virus was stored in 10 mM Tris (pH 7.5), 1 mM MgCl2, 10% glycerol at −80°C. Before infusion into animals, all viral preparations were dialyzed against 2000 volumes of phosphate-buffered saline (PBS) for 4 hr at 4°C. A preliminary study has shown that without this dialysis step, baboons develop nausea and hypotension during and immediately after intravenous infusion.
To measure contamination with E1+ replication-competent adenovirus, real-time polymerase chain reaction (PCR) analysis was performed with primers for E1A (GACCGTTTAC-GTGGAGACTC [F; forward primer] and CAGCCAGTAC-CTCTTCGATC [R; reverse primer]) and with primers for a sequence in the E4 region (TAAGCATAAGACGGACTACG [F] and GTAAGGCTGACTGTTAGGC [R]). Quantitative PCR (qPCR) was performed with the SYBR Green kit for the LightCycler (Roche Applied Science, Indianapolis, IN) and external standards for E1 and E4 (15 sec at 95°C, 5 sec at 57°C, and 17 sec 72°C). Only virus preparations that contained less than one E1+ (wild-type) viral genome in 1 × 108 genomes were used in these studies.
To measure contamination with bacterial endotoxin, the Limulus amebocyte lysate Pyrotell test kit was used, which detects as little as 0.03 endotoxin unit/ml by a gel-clot technique. Only preparations that tested negative for endotoxin were used in baboon studies.
Animals and procedures
All studies were performed at the Washington National Primate Research Center (Seattle, WA) in accordance with institutional guidelines of the University of Washington. The study was approved by the University of Washington Institutional Care and Use Committee. Six male baboons (Papio cynocephalus; age, 2.5 to 4.2 years) were used in this study. Animals were individually caged. Because of the age of the animals and contact with humans, serum samples from all animals were tested for neutralizing antibodies against Ad5-, Ad5/35-, and Ad5/11-bGal vectors, using an assay described elsewhere (Kay et al., 1997). No infection-blocking antibodies were detected at the lowest serum dilution (1:2). All viral vectors (in PBS) were infused through the femoral vein at a dose of 2 × 1012 VP/kg in a volume of 4 ml/kg of saline (5 × 1011 VP/ml) at a rate of 1 ml/min under sedation. Animals were sedated with ketamine (10 mg/kg). The animals were of the following ages and weights: mock injected: 4.2 years, 13.5 kg; Ad5-bGal injected: 2.5 years, 8.6 kg; Ad5/35-bGal injected: 5.0 years, 15.6 kg; Ad5/11-bGal injected: 2.7 years, 8.7 kg and 3.8 years, 12.1 kg; Ad5/35.IR-E1A/TRAIL injected: 3.2 years, 9.6 kg. Blood samples were collected from all animals before injection and 30 min, 60 min, 6 hr, 24 hr, and 72 hr postinjection. Mock-, Ad5-bGal-, Ad5/35-bGal-, and Ad5/11-bGal-injected animals were killed 72 hr postinjection. Tissue samples were collected as described elsewhere (Morral et al., 2002). The animal injected with Ad5/35.IR-E1A/TRAIL was monitored until day 30 postinjection and additional blood samples were drawn on days 3, 10, 14, 17, 21, 24, and 30 postinjection. During necropsy, animals were perfused with 3 liters of saline to remove blood from organs. Tissue samples were taken under conditions that avoid cross-contamination of material. Samples for DNA analysis were snap-frozen in liquid nitrogen. Isolation of mononuclear cells from peripheral blood and bone marrow was performed by Ficoll gradient centrifugation. Buffy coats of peripheral blood cells were collected by centrifugation at 1000 × g, collection of the leukocyte layer, and subsequent lysis of residual erythrocytes with ammonium chloride (PharmLyse; BD Biosciences Pharmingen, San Diego, CA).
Quantitative PCR for vector genomes
Isolation of cellular DNA from organs was performed as described elsewhere (Lieber et al., 1997). This method allows for efficient purification of the episomal 3–6 kb of Ad DNA together with cellular genomic DNA. Genomic DNA was isolated from 100 mg of tissue. Genomic DNA (500 ng) was used for real-time PCR, using vector-specific primers against the Ad5 L4 region and primers against the endogenous baboon β-globin gene as an internal standard (used to equalize vector genome numbers between different tissues). Samples were analyzed with a LightCycler and the SYBR Green kit, and the following primers were used: for viral DNA, F-L4 (5′-TGCAAGATACCCCTATCCTG-3′) and R-L4 (5′-CCTGTTGCAGAGCGTTTGC-3′); for baboon β-globin, F-β-globin (5′-CCTATCAGAAAGTGGTGGCTGG-3′) and R-β-globin (5′-TTGGACAGCAAGAAAGTGAGCTT-3′).
Blood analysis
Ca2+, Mg2+, amylase (Amy-Panc [pancreatic amylase] and Amy-Sal [salivary gland amylase]), alanine aminotransferase (ALT; glutamicpyruvic transaminase [GPT]), aspartate amino-transferase (AST; glutamic-oxaloacetic transaminase [GOT]), bilirubin (total and direct), lactate dehydrogenase (LDH), creatine kinase (CK), creatinine, white blood cell count (WBC), red blood cell count (RBC), hematocrit, hemoglobin, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC) were analyzed in the clinical laboratory of the University of Washington Medical Center (Seattle, WA) according to standard procedures (using assays developed for human blood biochemistry).
Cytokines
Serum cytokines were measured with OptEIA monkey enzyme-linked immunosorbent assay (ELISA) kits for monkey IL-6 and TNF-β (BD Biosciences Pharmingen).
RESULTS
Baboons were injected via the femoral vein with PBS (Mock) or viral vector (2 × 1012 VP/kg). All vectors had an Ad5-based genome deleted for E1 and E3 genes and expressed E. coli β-galactosidase (β-Gal) under the control of the RSV promoter. In the chimeric Ad5/35 and Ad5/11 vectors, the Ad5 fiber gene was substituted with a chimeric fiber that contains the Ad5 tail and the Ad35 or Ad11 shaft and knob domains.
In a short-term study, animals were injected with Ad5-bGal (n = 1), Ad5/35-bGal (n = 1), Ad5/11-bGal (n = 2), or PBS (n = 1) and monitored for 3 days. A long-term study (more than 30 days) was conducted with Ad5/35.IR-E1A/TRAIL (n = 1), which is an E1/E3-deleted Ad5 vector containing the Ad35 fiber and expressing Ad5 E1A and human TRAIL in a tumor-specific manner on homologous recombination between inverted repeats (IRs) (Sova et al., 2004). Transgene expression in Ad5/35.IR-E1A/TRAIL is under the control of the RSV promoter.
Short-term study
Biodistribution: vector genomes.
The level of vector genomes in major organs was determined by qPCR, using Ad-specific primers directed against the L4 region and total cellular DNA as a substrate. Differences in quality of DNA preparations were equalized by qPCR using primers directed against an endogenous baboon gene (β-globin). The genome concentration was expressed as the number of viral genomes per 104 cells (assuming a diploid cellular genome) (Fig. 1). For the mock-injected animal, no viral genomes were detected in all organs analyzed. One to three orders of magnitude more Ad5-bGal than Ad5/35-bGal or Ad5/11-bGal genomes were found in liver, brain, stomach, duodenum, testis, bone marrow mononuclear cells, and lymph nodes. The levels of Ad5-bGal, Ad5/35-bGal, and Ad5/11-bGal vector genomes were comparable in heart, pancreas, and esophagus. More Ad5/35-bGal and Ad5/11-bGal than Ad5-bGal genomes were found in the spleen. The differences between Ad5/11-bGal and Ad5/35-bGal genomes were not significant.
FIG. 1.
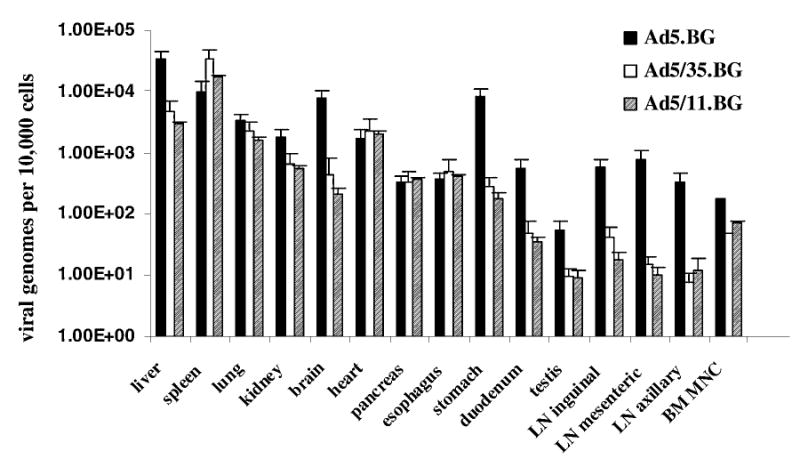
Quantitative comparison of viral genomes present in major organs 72 hr postinjection of Ad5-bGal, Ad5/35-bGal, and Ad5/11-bGal. Three independent tissue samples were analyzed and the SEM is shown. Genome concentration was expressed as the number of viral genomes per 104 cells (assuming that the mass of a diploid human genome is 6 pg). qPCR results for vector genomes were equalized on the basis of qPCR data for an endogenous (two copies per genome) monkey β-globin gene. Ad5-bGal (one animal), Ad5/35-bGal (one animal), Ad5/11 (two animals). BM-MNC, bone marrow mononuclear cells; LN, lymph node.
Biodistribution: β-Gal expression in organs.
Tissue sections were analyzed for β-Gal expression by 5-bromo-4-chloro-3-in-dolyl-β-d-galactopyranoside (X-Gal) staining (Table 1) and Ad5-mediated β-Gal expression was seen in most organs. More than 5% of hepatocytes were transduced (Fig. 2A). In the spleen, there were isolated β-Gal-positive cells in the red pulp that appeared to be endothelial cells lining the sinusoids (Fig. 2A). Areas with almost 100% transduction of arterial endothelium in lung were found (Fig. 2B). Furthermore, transduction of the arterial endothelium of the testis was observed with the Ad5-bGal vector (data not shown).
FIG. 2.
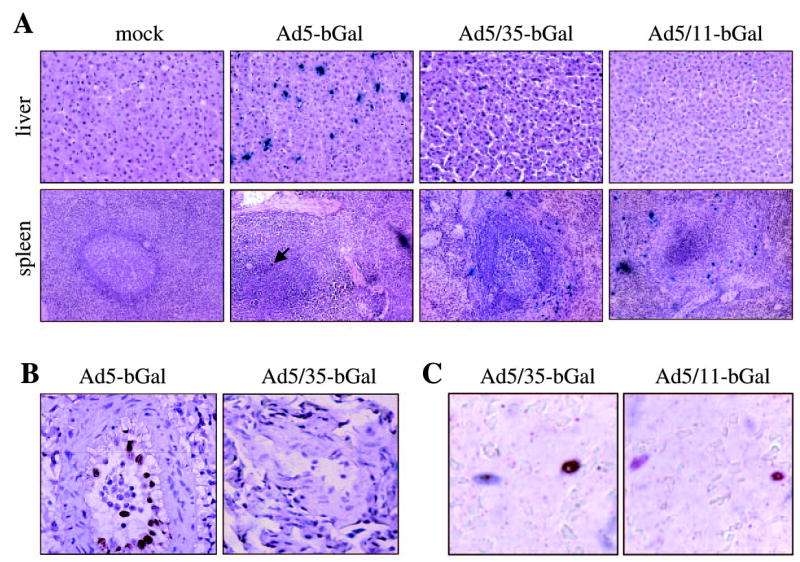
Analysis of β-galactosidase expression in selected tissues. (A) X-gal-stained sections of liver and spleen. (B) Immunohistochemistry for β-galactosidase in lung sections (positive staining appears brown). (C) Costaining of spleen section from the Ad5/35-bGal-injected animal with antibodies against β-Gal (blue) and S100 (red). Overlapping colors result in brown.
For Ad5/35-bGal- and Ad5/11-bGal-injected animals, β-galactosidase expression was detectable only in the marginal zone of splenic follicles (Fig. 2A). These β-Gal-expressing splenic cells also stained positive for S100, a marker for antigen-presenting cells (Fig. 2C). No Ad5/35-bGal- and Ad5/11-bGal-mediated β-Gal expression was seen in other organs.
Biodistribution: β-Gal expression in bone marrow and blood cells.
Mononuclear cells from bone marrow aspirates (BM-MNCs) were isolated by centrifugation in Ficoll gradients. Of 107 cells we found 40, 684, and 255 β-Gal-positive BM-MNCs, respectively, for Ad5-bGal-, Ad5/35-bGal-, and Ad5/11-bGal-injected animals (Fig. 3). The transduction level of peripheral leukocytes (buffy coats) was even lower, with 1 and 0.1 β-Gal-positive cells in 107 cells for Ad5/35-bGal- and Ad5/11-bGal-injected animals, respectively.
FIG. 3.
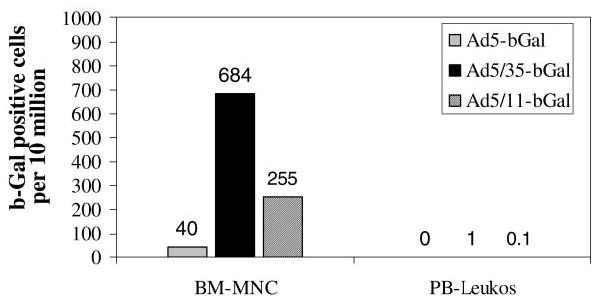
Transduction of peripheral blood and bone marrow cells after infusion of Ad vectors into baboons. A total of 107 bone marrow mononuclear cells (BM-MNCs) and peripheral blood cells (PB-leukos) from Ad-injected animals were analyzed for β-galactosidase expression by X-Gal staining (day 3 postinjection). Show is the total number of β-Gal-positive cells.
Taken together, biodistribution data from the three animals show that intravenous injection of Ad35 or Ad11 fiber-containing vectors resulted in low-level transduction of all major organs, including bone marrow and peripheral blood leukocytes. Significant transgene expression was found only in antigen-presenting cells of the spleekn. In agreement with earlier reports (Morral et al., 1999, 2002; Lozier et al., 2002; Brunetti-Pierri et al., 2004), our data from one animal injected with Ad5-bGal show transgene expression in most tissues, particularly in hepatocytes and vascular endothelial cells.
Toxicity: blood analysis.
We analyzed blood samples taken before Ad injection and 15 min, 30 min, 6 hr, 24 hr, and 72 hr postinfusion (Fig. 4). Serum levels of alanine aminotransferase (ALT; a marker of hepatocellular damage) were slightly increased for all Ad vectors at 30 and 60 min postinjection, particularly for Ad5-bGal (Fig. 4A). At 72 hr postinjection, ALT levels in the Ad5-bGal-injected animal increased further, whereas ALT levels in Ad5/35-bGal- and, to a lesser degree, Ad5/11-bGal-injected animals returned to normal. Serum as-partate aminotransferase (AST) levels were elevated only in the Ad5-bGal-injected animal at 72 hr. Markers of cholestasis, γ-glutamyltransferase (γ-GT) and bilirubin, were within normal ranges for all animals at all time points. Creatine kinase levels were elevated more than 10-fold in all Ad-injected animals at 30 and 60 min postinfusion. Kidney function as assessed by serum creatinine was normal for all animals at all time points.
FIG. 4.
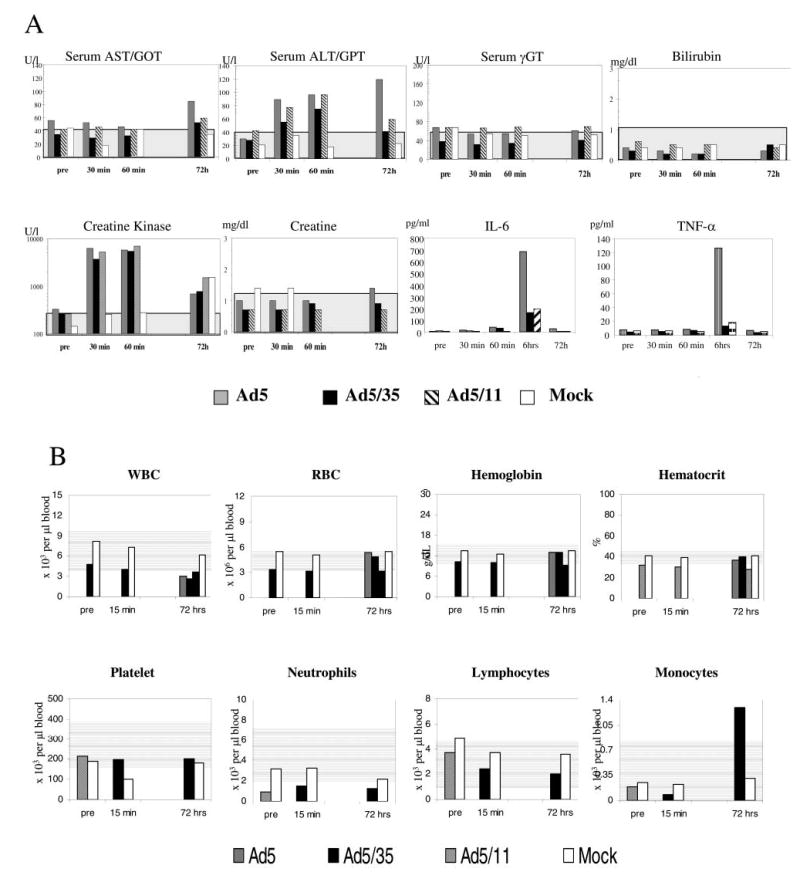
Blood analyses. (A) Analysis of serum markers of liver damage (ALT, γ-GT, and bilirubin), tissue damage (AST), muscle damage (creatine kinase), and kidney damage (creatinine) as well as proinflammatory cytokines (IL-6 and TNF-α) at various time points after Ad infusion. (B) Blood cell counts. Normal ranges are indicated by gray areas in the background of each panel.
We also analyzed serum IL-6 and TNF-α levels (markers of acute inflammation). Serum levels of these proinflammatory cytokines increased for all vectors and reached peak levels at 6 hr postinjection. For the Ad5-bGal vector, serum IL-6 increased from undetectable levels to 690 pg/ml at 6 hr postinfusion, whereas for the Ad5/35-bGal and Ad5/11-bGal-injected animals, IL-6 peak levels were 155 and 195 pg/ml, respectively. The peak TNF-α levels at 6 hr postinjection were 129, 11, and 14 pg/ml for Ad5-bGal-, Ad5/35-bGal-, and Ad5/11-bGal-injected animals, respectively. No clear signs of disseminated in-travascular coagulation (DIC), as determined on the basis of changes in prothrombin time and platelet counts, were observed in all tested animals. Overall, all blood cell counts were within normal ranges for all animals (Fig. 4B).
Histopathology.
Necropsy was performed 72 hr after injection. Over the 3-day observation period the animal injected with Ad5-bGal was inactive, with decreased food intake and an elevated body temperature. At necropsy, this animal had acute multifocal hemorrhage in the lung accompanied by fibrin accumulation (Fig. 5). The absence of inflammatory cells and obstruction of bronchi and bronchioles indicated that the hemorrhage was near terminal (within 24 hr) and that blood was not inhaled. The pulmonary hemorrhage appears to be an extreme manifestation of vascular leakage potentially via activation of innate inflammatory responses. The liver demonstrated centrilobular, mild hydropic changes. Ascites was noted grossly during necropsy. Spleen, lymph nodes, and tonsils displayed mild lymphoid hyperplasia. Heart tissue showed subacute epicarditis. Other histopathological changes included tracheitis and sialoadenitis. No renal, CNS, or muscle abnormalities were noted. The mock-, Ad5/35-bGal-, and Ad5/11-bGal-injected animals showed reduced activity on the first day, but exhibited normal behavior on the following 2 days. Gross pathology and histology were normal for these animals.
FIG. 5.
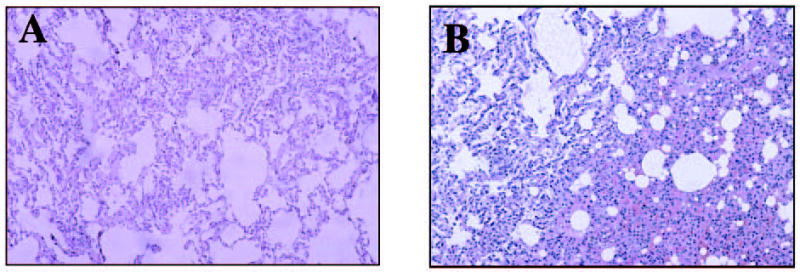
Lung histology at necropsy. (A) Mock injected; (B) Ad5-bGal injected. Note the diffuse hemorrhage. Lungs from Ad5/35-bGal- and Ad5/11-bGal-injected animals were normal.
The levels of serum markers indicated that mild tissue damage occurred rapidly after injection of all three vectors; however, liver damage was more pronounced for Ad5-bGal. A stronger acute inflammatory response after Ad5-bGal injection was reflected by 4- and 12-fold higher IL-6 and TNF-α levels, respectively, compared with the Ad5/35-bGal- and Ad5/11-bGal-injected animals. Severe gross and histopathological changes indicating vascular damage were observed at necropsy of the Ad5-bGal-injected animal.
Long-term study
One animal was intravenously injected with Ad5/35.IR-E1A/TRAIL at 2 × 1012 VP/kg (Sova et al., 2004). This vector has the same capsid and the same tropism as the Ad5/35-bGal vector used in the short-term study. Ad5/35.IR-E1A/TRAIL allows for expression of Ad E1A and TRAIL in a replication-dependent and tumor-restricted manner.
Histopathology
The animal was monitored for 30 days, until the planned time point for termination of the experiment. Over this period, the animal was healthy and active. Body temperature was measured twice per week and was normal. Blood samples were taken before injection and 1 min, 15 min, 6 hr, 24 hr, 72 hr, 8 days, 10 days, 14 days, 17 days, 21 days, 24 days, and 30 days after injection. Urine samples were taken on days 2 and 7 after injection. Tissue samples were harvested during necropsy as described above.
Biodistribution.
Distribution of Ad5/35.IR-E1A/TRAIL vector genomes was analyzed by qPCR in tissues on day 30 after vector infusion (Fig. 6A). Overall, the tissue distribution of vector genomes was comparable to that in the animal injected with Ad5/35-bGal (see Fig. 1); however, the total amount of vector genomes detected on day 30 postinfusion (compared with day 3 in the short-term study) was about 10-fold less in most tissues. E1A transgene expression was analyzed by immunohistochemistry of tissue sections and was absent in all tissues (liver, lung, spleen, kidney, lymph node, and heart). (As a positive control, we used tissue from mice injected with wild-type Ad5; Bernt et al., 2002.) Activation of transgene expression in normal tissue is not expected from Ad.IR vectors (Steinwaerder et al., 2001).
FIG. 6.

Quantitative analysis of Ad5/35.IR-E1A/TRAIL genomes. (A) Distribution of viral genomes in major tissues, 30 days after infusion. The genome concentration is expressed as the number of viral genomes per 104 cells (assuming that the mass of a diploid human genome is 6 pg). The average of three independent tissue samples ± SEM is shown. (B) Clearance of vector genomes from blood cells. Total blood cells were harvested at various time points. The average of three independent PCRs ± SEM is shown. The genome concentration is expressed as the number of viral genomes per 103 cells. qPCR results for vector genomes were equalized on the basis of qPCR data for the endogenous (two copies per genome) monkey β-globin gene.
Blood clearance.
We used qPCR for vector genomes to assess the kinetics of Ad clearance from the blood. Ad5/35.IR-E1A/TRAIL genomes in blood cells reached preinjection levels by 6 hr postinfusion. When we compared the amount of viral DNA found in blood cells with the total amount of injected Ad, we found that at 1 min postinfusion, ~2% of the virus input dose was associated with blood cells. The number of blood cell-associated vector genomes remained constant over a period of 72 hr and declined afterward. On day 8 postinjection, viral genomes associated with blood cells reached undetectable levels (Fig. 6B).
In contrast to humans, baboons express CD46 on erythrocytes (Hsu et al., 1997), which might affect vector distribution in blood cells. We therefore compared the number of genomes associated with white cells and total blood cells (white and red cells) on day 3 by qPCR. If Ad5/35.IR-E1A/TRAIL efficiently interacts with baboon erythrocytes, the number of Ad genomes found into total blood cell DNA should be ~3 orders of magnitude higher than the amount found in white cells as there are ~1000-fold more red cells than white cells (erythrocytes have no nuclei/nuclear DNA). We found, however, only 5-fold more vector genomes in total blood cells than in white cells (data not shown), indicating that Ad5/35 particles efficiently associated with white blood cells and not with erythrocytes.
No viral genomes were found at 48 hr in urine, analyzed at a 1:100 dilution.
Biochemical markers, blood cells, and cytokines.
After an initial slight increase, white blood cell counts decreased between 24 hr and day 8 postinfusion, after which they increased again (Fig. 7A). The number of monocytes and lymphocytes increased between day 8 and day 17. Importantly, all changes in blood cell numbers were within normal ranges (based on human hematological parameters).
FIG. 7.

(A) Analysis of blood cell numbers. (B–D) Analysis of serum markers for liver damage (ALT, γ-GT, and bilirubin), tissue damage (AST), and kidney damage (creatinine). (E and F) Analysis of serum proinflammatory cytokine levels (IL-6 and TNF-α) at various time points after Ad5/35.IR-E1A/TRAIL infusion.
Serum transaminase levels increased between 6 and 24 hr after infusion and returned to normal afterward (Fig. 7B). Bilirubin and γ-GT levels remained within normal limits (Fig. 7C and D). Creatinine levels also remained within the normal range (Fig. 7D). IL-6 levels increased at 6 hr postinfusion (as seen in the short-term study) and returned to normal by 24 hr (Fig. 7E). TNF-α levels were elevated for 14 days after infusion (Fig. 7F).
DISCUSSION
This is, to our knowledge, the first biodistribution and toxicity study of capsid-modified Ad vectors in nonhuman primates. In this study, Ad5-based chimeric vectors were used, in which the Ad5 fiber gene was replaced with either the Ad35 or Ad11 B-group virus sequence. We identified CD46 as the receptor used by B-group Ads for cell infection. In baboons, CD46 is expressed in a pattern and at a level comparable to humans (Hsu et al., 1997). Nonhuman primates have been used in studies with measles virus vectors that also utilize CD46 as a receptor (Combredet et al., 2003).
Biodistribution in this study was analyzed by two different methods: quantitative PCR and transgene expression. Quantitative PCR measures the number of viral genomes present in tissue DNA, but does not distinguish between virus that infects cells and virus that remains extracellular. Also, most tissues contain more than one cell type, and qPCR does not give information regarding the cell type targeted by the vector. Although we tried to minimize the presence of extracellular or intravascular viral DNA by aggressively flushing the vascular system with normal saline at necropsy, these potential confounding factors must be kept in mind when interpreting the results. Copy numbers measured by qPCR are therefore informative only in comparison. The total number must be taken with caution. This is exemplified by the results for viral DNA content in the liver where we found, on the basis of theoretical calculations, an average of five copies of Ad5-bGal per cell. In contrast, only approximately 5% of hepatocytes were found to express β-Gal. Transduced hepatocytes were found predominantly in the periportal area and probably represent cells that were exposed to the highest dose on intravenous vector injection. From published studies it is known that nonhuman primate hepatocyte transduction can be increased to ~50% by increasing the number of infused particles to 5.6 × 1012 VP (Morral et al., 2002). In murine studies, a large portion of intravenously infused viral particles is taken up by the reticuloendothelial system (RES), and a dose-dependent increase in hepatocyte transduction is observed only at doses that are higher than the RES saturating dose (Liu and Muruve, 2003). Much of the extracellular or RES-associated viral DNA would be expected to degrade by the time of analysis (72 hr). Nevertheless, it is possible that a substantial portion of the viral genomes detected by PCR were taken up by cells of the reticuloendothelial system rather than by hepatocytes.
Transgene expression, as analyzed by X-Gal staining, better reflects active transduction. We chose the RSV promoter for this study, one of the strongest viral promoters, as it is active in a wide range of tissues. In particular, the main target cell population of Ad5-based vectors, hepatocytes, supports transcription from this promoter. (Notably, cytomegalovirus [CMV] promoter-driven expression is downregulated in liver; Loser et al., 1998.)
Ad5-bGal transduction was found in all major organs, predominantly in liver and lung. Our data are in agreement with studies performed by others (Morral et al., 1999, 2002; Lozier et al., 2002; Brunetti-Pierri et al., 2004), and although we infused only one animal, we believe that these data are representative.
Overall, transduction of baboon tissue with B-group fiber-containing vectors (Ad5/35-bGal and Ad5/11-bGal, a total of three animals), as assessed by the number of vector genomes and β-Gal-positive cells, was less efficient than with the Ad5 vector. The following observations made in vitro might account for the low in vivo transduction efficiency of chimeric vectors, despite the fact that CD46 is expressed on all cells in baboons: (1) Ad5/35 transduction of CD46-expressing CHO cells (at a multiplicity of infection [MOI] of 100 plaque-forming units [PFU]/cell) directly correlates with CD46 density on the cell surface and is inefficient when fewer than 100 CD46 molecules are expressed per cell (Anderson et al., 2004). CD46 is expressed in normal cell only at low levels; and (2) the affinity of Ad35 and Ad11 fiber-containing Ads for CD46 is relatively low (Gaggar et al., 2003). Notably, two of the main criteria that determine the efficiency of viral infection (in addition to MOI) are the receptor density and the affinity for a given receptor.
The highest numbers of viral genomes and β-Gal-positive cells in animals infused with Ad5/35-bGal or Ad5/11-bGal were found in the spleen. Transduced cells were located in the marginal zone of splenic follicles. Notably, transduction of marginal zone cells has also been observed for certain measles virus strains, which also use CD46 as a cellular receptor (Sakaguchi et al., 1986; McChesney et al., 1997; Premenko-Lanier et al., 2003). The transduced cells were positive for S100, and negative for B cell markers. Further characterization of this cell population has been hindered by the limited availability of antibodies against baboon surface markers. Nevertheless, this limited analysis points toward an antigen-presenting cell as the main target for Ad5/35 and Ad5/11 transduction in the spleen. The immunological consequences of this effect are not clear. In theory, infection/transduction of splenic antigen-presenting cells can cause both an increased or decreased immune response against the viral vector and/or the transgene. A potential reduced immune response can be due to CD46 signaling that transiently affects the function of dendritic cells or T cells, in analogy to measles-induced immunosuppression (Marie et al., 2001, 2002). Importantly, no opportunistic infections or WBC abnormalities were observed in the 30-day observation of the Ad5/35.IR-E1A/TRAIL-injected animal. A more in-depth analysis of the clinical and immunological consequences of splenic antigen-presenting cell transduction is ongoing.
In summary, the biodistribution studies show, on the basis of the presence of viral genomes and transgene expression, an overall decrease in levels of viral uptake and transduction for vectors carrying group B fibers when compared with Ad5. This was most evident in the liver, the main target of Ad5 transduction. Uptake into bone marrow and peripheral blood cells was also low, despite theoretical concerns over in vivo transduction based on the in vitro infectibility of these cell populations by Ad5/35 and Ad/11 vectors (Shayakhmetov et al., 2000; Stecher et al., 2001). S100-positive cells in the marginal zone of splenic follicles represent an exception and are transduced at a fairly high level by vectors carrying group B fibers.
Differences in biodistribution between Ad5 and B-group fiber-containing vectors are likely the reason for the differences observed for the different vectors in short-term toxicity. Kupffer cells and other cells of the RES have been implicated as the main mediators of the cytokine “storm” triggered by the infusion of high doses of Ad5-based vectors (Liu et al., 2003; Muruve et al., 2004). Because Ad5/35 vectors possess short fiber shafts, uptake of Ad5/35 capsid-containing vectors into Kupffer cells (which is mediated by blood factors and requires long fiber shafts) is inefficient, as studies in mice have shown (Shayakhmetov et al., 2004). On the basis of our data, we hypothesize that not only in mice, but also in baboons, a direct consequence of this is greatly reduced expression of proinflammatory cytokines and tissue damage after intravenous Ad5/35 and Ad5/11 vector injection, when compared with Ad5. The changes seen in histopathology and blood biochemistry after infusion of Ad vectors correlate with the levels of proinflammatory serum cytokines. Ad5-bGal infusion resulted in greatly elevated IL-6 and TNF-α levels and generalized inflammatory responses characterized by histopathological and blood biochemistry abnormalities. In contrast, elevation of IL-6 and TNF-α levels on Ad5/35-bGal or Ad5/11-bGal infusion was markedly less and signs of acute inflammation were not detected.
Overall, the type and extent of clinical, serological, and pathological abnormalities in our Ad5-injected animal were consistent with other nonhuman primate studies (Morral et al., 1999, 2002; Lozier et al., 2002; Brunetti-Pierri et al., 2004). In a study by Morral et al. (2002), infusion of 1 × 1012 VP/kg into a baboon caused elevation of transaminases (AST, 84 U/liter; ALT, 107 U/liter) at 48 hr, similar to the range in our study (where 2 × 1012 VP/kg was injected). In contrast, a second animal receiving a dose of 1 × 1013 VP/kg showed a massive increase in transaminases (AST, 7440 U/ml), thrombocytopenia, and DIC. In the low-dose animal, WBC numbers were similar to our study, and no coagulation abnormalities were observed. Organ distribution of β-Gal expression was similar to our study, with the exception of endothelial transduction, which was observed only in the high-dose animal. IL-6 and TNF-α levels were, respectively, 709 and 325 pg/ml at 48 hr in the high-dose animal and normal in the low-dose, animal. However, a third animal (not otherwise discussed in this report) receiving 0.6 × 1012 VP/kg had an IL-6 level of 306 pg/ml at 24 hr, suggesting considerable variability and no strict dose dependency. We observed a peak of IL-6 and TNF-α at 6 hr, and it is possible that a small increase in these cytokines may have been missed in the low-dose animal studied by Morral et al. because no 6-hr sample was obtained. Interestingly, in that study, IL-6 levels continued to rise even after 24 hr in the animal that received the lethal dose. In another study, infusion of an Ad5-based, first-generation vector expressing human factor IX at doses of 0.34 × 1012, 1.7 × 1012, and 3.8 × 1012 VP/kg induced similar dose-dependent toxicity and inflammatory changes (Lozier et al., 2002). IL-6 levels peaked at 6 hr and were 18.2, 297, and 238 pg/ml, respectively. Elevation of transaminases was also observed. Partial thrombin time (PTT) abnormalities may have been exacerbated by transgene expression, particularly at the later time points. Interestingly, CK abnormalities were reported in this study, with elevation to 800–2000 U/liter.
In addition to the short-term toxicity study, we have conducted a 30-day clinical observation and subsequent histopathology analysis of a baboon injected with Ad5/35.IR-E1A/TRAIL. This vector is in consideration for a phase I clinical trial in cancer patients, and this study is part of the preclinical evaluation. Ad5/35.IR-E1A/TRAIL has the same capsid, and hence tropism, as Ad5/35-bGal. In addition, this vector employs a new concept for tumor-specific gene expression that is based on homologous recombination in Ad genomes (Ad.IR system) (Steinwaerder et al., 2001). Studies in primary normal human cells (fibroblasts, small airway epithelial cell, astrocytes, and mesenthelial cells) (Steinwaerder et al., 2001; Bernt et al., 2002; Sova et al., 2004), in mouse liver and baboon liver segments (Steinwaerder et al., 2001), demonstrated the absence of activation of gene expression from Ad.IR vectors in nontumor cells. It was therefore not expected that E1A and TRAIL would be expressed in baboon tissues that were successfully transduced with Ad5/35 IR-E1A/TRAIL. Toxicity after infusion of Ad5/35.IR-E1A/TRAIL, at a dose of 2 × 1012 VP/kg, was minimal with a small, transient increase in transaminases during the first 24 hr, a transient increase in IL-6 (4-fold less than with the Ad5-based vector), and almost no change in TNF-α levels. The animal showed no signs of illness or distress during the observation period. On necropsy, no gross or histopathologic abnormalities were found. No expression of adenoviral E1A was observed in any of the tissues analyzed, including the spleen. This finding suggests that the second level of specificity, the tumor-specific Ad.IR expression system, efficiently prevents toxicity in nontransformed cells that are susceptible to Ad5/35 infection.
Overall, this study suggests that Ad vectors possessing B-group Ad fibers have a better safety profile after intravenous injection than conventional Ad5-based vectors. This makes them potential candidates for gene therapy, for example, in the treatment of metastatic cancer or cardiovascular diseases.
Acknowledgments
We thank Mike Gough, Judy Johnson, and Denny Liggitt for help with the animal studies. We are grateful to Daniel Stone for help with the manuscript. This work was supported by funding from NIH grants HL-00-008 and P01 HL53750.
References
- ANDERSON BD, NAKAMURA T, RUSSELL SJ, PENG KW. High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer Res. 2004;64:4919–4926. doi: 10.1158/0008-5472.CAN-04-0884. [DOI] [PubMed] [Google Scholar]
- BAROUCH DH, PAU MG, CUSTERS JH, KOUDSTAAL W, KOSTENSE S, HAVENGA MJ, TRUITT DM, SUMIDA SM, KISHKO MG, ARTHUR JC, KORIOTH-SCHMITZ B, NEWBERG MH, GORGONE DA, LIFTON MA, PANICALI DL, NABEL GJ, LETVIN NL, GOUDSMIT J. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J Immunol. 2004;172:6290–6297. doi: 10.4049/jimmunol.172.10.6290. [DOI] [PubMed] [Google Scholar]
- BERNT K, LIANG M, YE X, NI S, LI ZY, YE SL, HU F, LIEBER A. A new type of adenovirus vector that utilizes homologous recombination to achieve tumor-specific replication. J Virol. 2002;76:10994–11002. doi: 10.1128/JVI.76.21.10994-11002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORGLAND SL, BOWEN GP, WONG NC, LIBERMANN TA, MURUVE DA. Adenovirus vector-induced expression of the C-X-C chemokine IP-10 is mediated through capsid-dependent activation of NF-κB. J Virol. 2000;74:3941–3947. doi: 10.1128/jvi.74.9.3941-3947.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOWEN GP, BORGLAND SL, LAM M, LIBERMANN TA, WONG NC, MURUVE DA. Adenovirus vector-induced inflammation: Capsid-dependent induction of the C-C chemokine RANTES requires NF-κB. Hum. Gene Ther. 2002;13:367–379. doi: 10.1089/10430340252792503. [DOI] [PubMed] [Google Scholar]
- BRUNETTI-PIERRI N, PALMER DJ, BEAUDET AL, CAREY KD, FINEGOLD M, NG P. Acute toxicity after high-dose systemic injection of helper-dependent adenoviral vectors into nonhuman primates. Hum Gene Ther. 2004;15:35–46. doi: 10.1089/10430340460732445. [DOI] [PubMed] [Google Scholar]
- CATTANEO R. Four viruses, two bacteria, and one receptor: Membrane cofactor protein (CD46) as pathogens’ magnet. J Virol. 2004;78:4385–4388. doi: 10.1128/JVI.78.9.4385-4388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CERVONI F, OGLESBY TJ, ADAMS EM, MILESIFLUET C, NICKELLS M, FENICHEL P, ATKINSON JP, HSI BL. Identification and characterization of membrane cofactor protein of human spermatozoa. J Immunol. 1992;148:1431–1437. [PubMed] [Google Scholar]
- CHO SW, OGLESBY TJ, HSI BL, ADAMS EM, ATKINSON JP. Characterization of three monoclonal antibodies to membrane co-factor protein (MCP) of the complement system and quantification of MCP by radioassay. Clin Exp Immunol. 1991;83:257–261. doi: 10.1111/j.1365-2249.1991.tb05624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COMBREDET C, LABROUSSE V, MOLLET L, LORIN C, DELEBECQUE F, HURTREL B, McCLURE H, FEINBERG MB, BRAHIC M, TANGY F. A molecularly cloned Schwarz strain of measles virus vaccine induces strong immune responses in macaques and transgenic mice. J Virol. 2003;77:11546–11554. doi: 10.1128/JVI.77.21.11546-11554.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAGGAR A, SHAYAKHMETOV D, LIEBER A. CD46 is a cellular receptor for group B adenoviruses. Nat Med. 2003;9:1408–1412. doi: 10.1038/nm952. [DOI] [PubMed] [Google Scholar]
- GUGALA Z, OLMSTED-DAVIS EA, GANNON FH, LINDSEY RW, DAVIS AR. Osteoinduction by ex vivo adenovirus-mediated BMP2 delivery is independent of cell type. Gene Ther. 2003;10:1289–1296. doi: 10.1038/sj.gt.3302006. [DOI] [PubMed] [Google Scholar]
- HARA T, KOJIMA A, FUKUDA H, MASAOKA T, FUKUMORI Y, MATSUMOTO M, SEYA T. Levels of complement regulatory proteins, CD35 (CR1), CD46 (MCP) and CD55 (DAF) in human haematological malignancies. Br J Haematol. 1992;82:368–373. doi: 10.1111/j.1365-2141.1992.tb06431.x. [DOI] [PubMed] [Google Scholar]
- HAVENGA MJ, LEMCKERT AA, OPHORST OJ, van MEIJER M, GERMERAAD WT, GRIMBERGEN J, van den DOEL MA, VOGELS R, van DEUTEKOM J, JANSON AA, DE BRUIJN JD, UYTDEHAAG F, QUAX PH, LOGTENBERG T, MEHTALI M, BOUT A. Exploiting the natural diversity in adenovirus tropism for therapy and prevention of disease. J Virol. 2002;76:4612–4620. doi: 10.1128/JVI.76.9.4612-4620.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HSU EC, DORIG RE, SARANGI F, MARCIL A, IORIO C, RICHARDSON CD. Artificial mutations and natural variations in the CD46 molecules from human and monkey cells define regions important for measles virus binding. J Virol. 1997;71:6144–6154. doi: 10.1128/jvi.71.8.6144-6154.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHANSSON L, RYTKONEN A, BERGMAN P, ALBIGER B, KALLSTROM H, HOKFELT T, AGERBERTH B, CATTANEO R, JONSSON AB. CD46 in meningococcal disease. Science. 2003;301:373–375. doi: 10.1126/science.1086476. [DOI] [PubMed] [Google Scholar]
- KAY MA, MEUSE L, GOWN AM, LINSLEY P, HOLLENBAUGH D, ARUFFO A, OCHS HD, WILSON CB. Transient immunomodulation with anti-CD40 ligand antibody and CTLA4Ig enhances persistence and secondary adenovirus-mediated gene transfer into mouse liver. Proc Natl Acad Sci USA. 1997;94:4686–4691. doi: 10.1073/pnas.94.9.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KINUGASA N, HIGASHI T, NOUSO K, NAKATSUKASA H, KOBAYASHI Y, ISHIZAKI M, TOSHIKUNI N, YOSHIDA K, UEMATSU S, TSUJI T. Expression of membrane cofactor protein (MCP, CD46) in human liver diseases. Br J Cancer. 1999;80:1820–1825. doi: 10.1038/sj.bjc.6690604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOIZUMI N, MIZUGUCHI H, SAKURAI F, YAMAGUCHI T, WATANABE Y, HAYAKAWA T. Reduction of natural adenovirus tropism to mouse liver by fiber–shaft exchange in combination with both CAR- and αv integrin-binding ablation. J Virol. 2003;77:13062–13072. doi: 10.1128/JVI.77.24.13062-13072.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIEBER A, HE CY, MEUSE L, SCHOWALTER D, KIRILLOVA I, WINTHER B, KAY MA. The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J Virol. 1997;71:8798–8807. doi: 10.1128/jvi.71.11.8798-8807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIEBER A, HE CY, MEUSE L, HIMEDA C, WILSON C, KAY MA. Inhibition of NF-κB activation in combination with bcl-2 expression allows for persistence of first-generation adenovirus vectors in the mouse liver. J Virol. 1998;72:9267–9277. doi: 10.1128/jvi.72.11.9267-9277.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU Q, MURUVE DA. Molecular basis of the inflammatory response to adenovirus vectors. Gene Ther. 2003;10:935–940. doi: 10.1038/sj.gt.3302036. [DOI] [PubMed] [Google Scholar]
- LIU Q, ZAISS AK, COLARUSSO P, PATEL K, HALJAN G, WICKHAM TJ, MURUVE DA. The role of capsid–endothelial interactions in the innate immune response to adenovirus vectors. Hum Gene Ther. 2003;14:627–643. doi: 10.1089/104303403321618146. [DOI] [PubMed] [Google Scholar]
- LOSER P, JENNINGS GS, STRAUSS M, SANDIG V. Reactivation of the previously silenced cytomegalovirus major immediate-early promoter in the mouse liver: Involvement of NFκB. J Virol. 1998;72:180–190. doi: 10.1128/jvi.72.1.180-190.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOZIER JN, CSAKO G, MONDORO TH, KRIZEK DM, METZGER ME, COSTELLO R, VOSTAL JG, RICK ME, DONAHUE RE, MORGAN RA. Toxicity of a first-generation adenoviral vector in rhesus macaques. Hum Gene Ther. 2002;13:113–124. doi: 10.1089/10430340152712665. [DOI] [PubMed] [Google Scholar]
- MANCHESTER M, SMITH KA, ETO DS, PERKIN HB, TORBETT BE. Targeting and hematopoietic suppression of human CD34+ cells by measles virus. J Virol. 2002;76:6636–6642. doi: 10.1128/JVI.76.13.6636-6642.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARIE JC, KEHREN J, TRESCOL-BIEMONT MC, EVLASHEV A, VALENTIN H, WALZER T, TEDONE R, LOVELAND B, NICOLAS JF, RABOURDIN-COMBE C, HORVAT B. Mechanism of measles virus-induced suppression of inflammatory immune responses. Immunity. 2001;14:69–79. doi: 10.1016/s1074-7613(01)00090-5. [DOI] [PubMed] [Google Scholar]
- MARIE JC, ASTIER AL, RIVAILLER P, RABOURDIN-COMBE C, WILD TF, HORVAT B. Linking innate and acquired immunity: divergent role of CD46 cytoplasmic domains in T cell induced inflammation. Nat Immunol. 2002;3:659–666. doi: 10.1038/ni810. [DOI] [PubMed] [Google Scholar]
- McCHESNEY MB, MILLER CJ, ROTA PA, ZHU YD, ANTIPA L, LERCHE NW, AHMED R, BELLINI WJ. Experimental measles. I Pathogenesis in the normal and the immunized host. Virology. 1997;233:74–84. doi: 10.1006/viro.1997.8576. [DOI] [PubMed] [Google Scholar]
- MIZUGUCHI H, HAYAKAWA T. Adenovirus vectors containing chimeric type 5 and type 35 fiber proteins exhibit altered and expanded tropism and increase the size limit of foreign genes. Gene. 2002;285:69–77. doi: 10.1016/s0378-1119(02)00410-9. [DOI] [PubMed] [Google Scholar]
- MORRAL N, O’NEAL W, RICE K, LELAND M, KAPLAN J, PIEDRA PA, ZHOU H, PARKS RJ, VELJI R, AGUILARCORDOVA E, WADSWORTH S, GRAHAM FL, KOCHANEK S, CAREY KD, BEAUDET AL. Administration of helper-dependent adenoviral vectors and sequential delivery of different vector serotype for long-term liver-directed gene transfer in baboons. Proc Natl Acad Sci USA. 1999;96:12816–12821. doi: 10.1073/pnas.96.22.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORRAL N, O’NEAL WK, RICE K, LELAND MM, PIEDRA PA, AGUILARCORDOVA E, CAREY KD, BEAUDET AL, LANGSTON C. Lethal toxicity, severe endothelial injury, and a threshold effect with high doses of an adenoviral vector in baboons. Hum Gene Ther. 2002;13:143–154. doi: 10.1089/10430340152712692. [DOI] [PubMed] [Google Scholar]
- MURRAY KP, MATHURE S, KAUL R, KHAN S, CARSON LF, TWIGGS LB, MARTENS MG, KAUL A. Expression of complement regulatory proteins—CD35, CD46, CD55, and CD59—in benign and malignant endometrial tissue. Gynecol Oncol. 2000;76:176–182. doi: 10.1006/gyno.1999.5614. [DOI] [PubMed] [Google Scholar]
- MURUVE DA, BARNES MJ, STILLMAN IE, LIBERMANN TA. Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum Gene Ther. 1999;10:965–976. doi: 10.1089/10430349950018364. [DOI] [PubMed] [Google Scholar]
- MURUVE DA, COTTER MJ, ZAISS AK, WHITE LR, LIU Q, CHAN T, CLARK SA, ROSS PJ, MEULENBROEK RA, MAELANDSMO GM, PARKS RJ. Helper-dependent adenovirus vectors elicit intact innate but attenuated adaptive host immune responses in vivo. J Virol. 2004;78:5966–5972. doi: 10.1128/JVI.78.11.5966-5972.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLMSTED-DAVIS EA, GUGALA Z, GANNON FH, YOTNDA P, McALHANY RE, LINDSEY RW, DAVIS AR. Use of a chimeric adenovirus vector enhances BMP2 production and bone formation. Hum Gene Ther. 2002;13:1337–1347. doi: 10.1089/104303402760128568. [DOI] [PubMed] [Google Scholar]
- PREMENKO-LANIER M, ROTA PA, RHODES G, VERHOEVEN D, BAROUCH DH, LERCHE NW, LETVIN NL, BELLINI WJ, McCHESNEY MB. DNA vaccination of infants in the presence of maternal antibody: A measles model in the primate. Virology. 2003;307:67–75. doi: 10.1016/s0042-6822(02)00036-3. [DOI] [PubMed] [Google Scholar]
- RAPER SE, YUDKOFF M, CHIRMULE N, GAO GP, NUNES F, HASKAL ZJ, FURTH EE, PROPERT KJ, ROBINSON MB, MAGOSIN S, SIMOES H, SPEICHER L, HUGHES J, TAZELAAR J, WIVEL NA, WILSON JM, BATSHAW ML. A pilot study of in vivo liver-directed gene transfer with an adenoviral vector in partial ornithine transcarbamylase deficiency. Hum Gene Ther. 2002;13:163–175. doi: 10.1089/10430340152712719. [DOI] [PubMed] [Google Scholar]
- REA D, HAVENGA MJ, van den ASSEM M, SUTMULLER RP, LEMCKERT A, HOEBEN RC, BOUT A, MELIEF CJ, OFFRINGA R. Highly efficient transduction of human monocyte-derived dendritic cells with subgroup B fiber-modified adenovirus vectors enhances transgene-encoded antigen presentation to cytotoxic T cells. J Immunol. 2001;166:5236–5244. doi: 10.4049/jimmunol.166.8.5236. [DOI] [PubMed] [Google Scholar]
- SAKAGUCHI M, YOSHIKAWA Y, YAMANOUCHI K, SATA T, NAGASHIMA K, TAKEDA K. Growth of measles virus in epithelial and lymphoid tissues of cynomolgus monkeys. Microbiol Immunol. 1986;30:1067–1073. doi: 10.1111/j.1348-0421.1986.tb03036.x. [DOI] [PubMed] [Google Scholar]
- SAKURAI F, MIZUGUCHI H, HAYAKAWA T. Efficient gene transfer into human CD34+ cells by an adenovirus type 35 vector. Gene Ther. 2003;10:1041–1048. doi: 10.1038/sj.gt.3301959. [DOI] [PubMed] [Google Scholar]
- SCHNELL MA, ZHANG Y, TAZELAAR J, GAO GP, YU QC, QIAN R, CHEN SJ, VARNAVSKI AN, LECLAIR C, RAPER SE, WILSON JM. Activation of innate immunity in nonhuman primates following intraportal administration of adenoviral vectors. Mol Ther. 2001;3:708–722. doi: 10.1006/mthe.2001.0330. [DOI] [PubMed] [Google Scholar]
- SEGERMAN A, MEI YF, WADELL G. Adenovirus types 11p and 35p show high binding efficiencies for committed hematopoietic cell lines and are infective to these cell lines. J Virol. 2000;74:1457–1467. doi: 10.1128/jvi.74.3.1457-1467.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEGERMAN A, ATKINSON JP, MARTTILA M, DENNERQUIST V, WADELL G, ARNBERG N. Adenovirus type 11 uses CD46 as a cellular receptor. J Virol. 2003;77:9183–9191. doi: 10.1128/JVI.77.17.9183-9191.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SESHIDHAR REDDY P, GANESH S, LIMBACH MP, BRANN T, PINKSTAFF A, KALOSS M, KALEKO M, CONNELLY S. Development of adenovirus serotype 35 as a gene transfer vector. Virology. 2003;311:384–393. doi: 10.1016/s0042-6822(03)00161-2. [DOI] [PubMed] [Google Scholar]
- SHAYAKHMETOV DM, LIEBER A. Dependence of adenovirus infectivity on length of the fiber shaft domain. J Virol. 2000;74:10274–10286. doi: 10.1128/jvi.74.22.10274-10286.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAYAKHMETOV DM, PAPAYANNOPOULOU T, STAMATOYANNOPOULOS G, LIEBER A. Efficient gene transfer into human CD34+ cells by a retargeted adenovirus vector. J Virol. 2000;74:2567–2583. doi: 10.1128/jvi.74.6.2567-2583.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAYAKHMETOV DM, LI ZY, NI S, LIEBER A. Analysis of adenovirus sequestration in the liver, transduction of hepatic cells, and innate toxicity after injection of fiber-modified vectors. J Virol. 2004;78:5368–5381. doi: 10.1128/JVI.78.10.5368-5381.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIRENA D, LILIENFELD B, EISENHUT M, KALIN S, BOUCKE K, BEERLI RR, VOGT L, RUEDL C, BACHMANN MF, GREBER UF, HEMMI S. The human membrane cofactor CD46 is a receptor for species B adenovirus serotype 3. J Virol. 2004;78:4454–4462. doi: 10.1128/JVI.78.9.4454-4462.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOVA P, REN XW, NI S, BERNT KM, MI J, KIVIAT N, LIEBER A. A tumor-targeted and conditionally replicating oncolytic adenovirus vector expressing TRAIL for treatment of liver metastases. Mol Ther. 2004;9:496–509. doi: 10.1016/j.ymthe.2003.12.008. [DOI] [PubMed] [Google Scholar]
- STECHER H, SHAYAKHMETOV DM, STAMATOY-ANNOPOULOS G, LIEBER A. A capsid-modified adenovirus vector devoid of all viral genes: Assessment of transduction and toxicity in human hematopoietic cells. Mol Ther. 2001;4:36–44. doi: 10.1006/mthe.2000.0410. [DOI] [PubMed] [Google Scholar]
- STEINWAERDER DS, CARLSON CA, OTTO DL, LI ZY, NI S, LIEBER A. Tumor-specific gene expression in hepatic metastases by a replication-activated adenovirus vector. Nat Med. 2001;7:240–243. doi: 10.1038/84696. [DOI] [PubMed] [Google Scholar]
- STONE D, STECHER H, CHEN SY, LIEBER A. Transduction of human CD34+ peripheral blood derived dendritic cells with a tumor antigen is more efficient with chimeric Ad5/11 and Ad5/35 than with Ad5. Mol Ther. 2002;5:S110. [Google Scholar]
- TAMANINI A, ROLFINI R, NICOLIS E, MELOTTI P, CABRINI G. MAP kinases and NF-κB collaborate to induce ICAM-1 gene expression in the early phase of adenovirus infection. Virology. 2003;307:228–242. doi: 10.1016/s0042-6822(02)00078-8. [DOI] [PubMed] [Google Scholar]
- THORSTEINSSON L, O’DOWD GM, HARRINGTON PM, JOHNSON PM. The complement regulatory proteins CD46 and CD59, but not CD55, are highly expressed by glandular epithelium of human breast and colorectal tumour tissues. APMIS. 1998;106:869–878. doi: 10.1111/j.1699-0463.1998.tb00233.x. [DOI] [PubMed] [Google Scholar]
- VOGELS R, ZUIJDGEEST D, van RIJNSOEVER R, HARTKOORN E, DAMEN I, DE BETHUNE MP, KOSTENSE S, PENDERS G, HELMUS N, KOUDSTAAL W, CECCHINI M, WETTERWALD A, SPRANGERS M, LEMCKERT A, OPHORST O, KOEL B, van MEERENDONK M, QUAX P, PANITTI L, GRIMBERGEN J, BOUT A, GOUDSMIT J, HAVENGA M. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: Efficient human cell infection and bypass of preexisting adenovirus immunity. J Virol. 2003;77:8263–8271. doi: 10.1128/JVI.77.15.8263-8271.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOTNDA P, ONISHI H, HESLOP HE, SHAYAKHMETOV D, LIEBER A, BRENNER M, DAVIS A. Efficient infection of primitive hematopoietic stem cells by modified adenovirus. Gene Ther. 2001;8:930–937. doi: 10.1038/sj.gt.3301488. [DOI] [PubMed] [Google Scholar]


