Abstract
Purpose
To sensitize NSCLC to radiotherapy by tumor-specific delivery of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) gene.
Experimental Design
The TRAIL gene was delivered to human NSCLC cell lines and normal human bronchial epithelial cells by the replication-defective adenoviral vector Ad/TRAIL-F/RGD using a tumor specific human telomerase reverse transcriptase promoter. Cancer growth was studied using XTT and clonogenic assays. Activation of the apoptosis signal transduction pathway was analyzed in a Western blot, and sub-G1 DNA accumulation was evaluated by a flow cytometry assay. A xenograft mouse model was established, intratumoral injections of Ad/TRAIL-F/RGD were given, and the tumors were locally irradiated at a dose of 5 Gy per tumor; the other groups received one of these treatments alone or a control agent. Apoptosis and TRAIL expression in tumors were analyzed using TUNEL assay and immunohistochemistry respectively.
Results
Ad/TRAIL-F/RGD specifically targets human NSCLC cells without significant effect in NHBE. The combination of TRAIL gene therapy and radiotherapy significantly improved cell-killing effect in all NSCLC cancer cell lines tested (P < 0.05) but not in NHBE. Expression of TRAIL showed a dose-dependent relationship with Ad/TRAIL-F/RGD, and radiation appeared to increase TRAIL expression. Activation of the apoptosis by TRAIL and radiation was demonstrated by activation of caspase-9, caspase-8, caspase-3, and poly-ADP-ribose polymerase and increased DNA sub-G1 accumulation. The combination of TRAIL and radiotherapy significantly inhibited tumor growth and prolonged mean survival in mice bearing human NSCLC to 43.7 days, compared with 23.7 days with TRAIL only, 16.5 days for radiotherapy only (P < 0.05). Combination of Ad/TRAIL-F/RGD and radiation significantly increased apoptosis in animal tumor samples (29.8%, P <0.001) compared with radiation alone (9.7%), Ad/TRAIL-F/RGD alone (13.5%), radiotherapy plus Ad/CMV-GFP (10.3%).
Conclusions
The combination of tumor-specific TRAIL gene therapy and radiotherapy significantly improved therapeutic efficacy in suppressing NSCLC tumor growth and prolonging survival in mice bearing human NSCLC. Ad/TRAIL-F/RGD may improve the therapeutic ratio of radiotherapy in NSCLC.
Keywords: Tumor-specific targeting, TRAIL gene therapy, radiosensitivity, NSCLC, apoptosis
INTRODUCTION
Lung cancer remains the leading cause of cancer death in both men and women in the United States, with non-small cell lung cancer (NSCLC) accounting for 80% of all cases (1). Approximately 50% of patients with NSCLC have locally advanced (stage III) disease at presentation and require multimodality management. However, the median survival times for patients receiving such therapy is only 15 to 17 month (2). Radiation resistance is one of main reasons for treatment failure. Although radiation dose escalation may improve local control, considerable toxicities are associated with higher-dose radiotherapy, particularly with concurrent chemotherapy. Given the dismal outcomes of the current treatment and the considerable treatment-related toxicities in lung cancer, a novel approach with a different toxicity profile is urgently needed to sensitize lung cancer to radiotherapy.
Advances in our understanding of the molecular and cellular biology of cancer offer a broad range of possible radiosensitizing approaches. Gene therapy, for example, may be an alternative approach for treating patients with radiation-resistant lung cancer (3–5). One agent that might be very useful for such gene therapy is the tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) gene, which is able to specifically kill malignant cells but mostly spare normal cells (6–8). We and others have shown that adenoviral vector mediated introduction of the TRAIL gene into cancer cells induces apoptosis and suppresses tumor growth in vitro and in vivo (9–11). Moreover, nontransfected neighboring cancer cells can be killed by a TRAIL-mediated bystander effect (10, 12). We also reported that TRAIL-related hepatocyte toxicity could be prevented by using the human telomerase reverse transcriptase (hTERT) promoter, which is highly active in more than 85% of human cancer cells but inactive in most somatic cells (11, 13).
Preliminary data obtained in a breast cancer model showed that the combination of TRAIL and radiotherapy has a synergistic effect on an established human breast cancer xenograft model (14). In another study, most NSCLC tissue samples and all NSCLC cells tested were positive for telomerase expression (15). It is also known that hTERT dosage correlates with telomerase activity in human lung cancer cells (16). In addition, death receptor 4 (DR4), DR5, and TRAIL were expressed in 99%, 82%, 91%, respectively, of tumor biopsy samples from patients with stage III NSCLC (17). These data suggest that NSCLC is a good candidate for TRAIL gene targeting, particularly with the tumor-specific hTERT promoter.
However, we also found that repeated application of apoptosis-inducing adenovectors can result in the selection and expansion of resistant cells. One mechanism involved in this acquired resistance is resistance to adenovector infections (18) presumably because of low expression of the initial binding receptor, the coxsackie-adenovirus receptor (CAR; 19, 20). Reduced expression of CAR also has been reported in primary tumors, suggesting overcoming resistance to adenovirus in cancer cells is critical for future success of adenovirus-mediated cancer gene therapy (21, 22). Accumulating evidence has shown that adenoviral capsid proteins can be modified to retarget adenovectors to CAR-independent binding molecules. It has been reported that incorporation of an integrin-binding motif Arg-Gly-Asp (RGD)-containing peptide in the HI loop of the adenoviral vector fiber protein provided the ability of the adenovirus to utilize an alternative receptor during the cell entry process, that results in efficient transduction in cells resistant to conventional adenoviral vector particularly primary tumor cells (23, 24).
To develop a more efficient gene delivery system for clinical use, we recently modified our previous adenoviral vector and constructed a new adenoviral vector system, Ad/TRAIL-F/RGD, that contains an integrin-binding motif argine-glycine-aspartate (RGD) sequence in the HI loop of the adenoviral fiber and expresses the TRAIL gene from the hTERT promoter via GAL4 gene-regulatory components that can augment transgene expression from the tumor-specific promoter without losing target specificity (25–28). Our data showed that Ad/TRAIL-F/RGD could effectively suppress the growth of orthotopic pancreatic tumors (26).
Moreover, various preclinical and clinical studies have demonstrated that the combination of gene therapy and some type of conventional anticancer therapy can improve therapeutic benefit (27). Therefore, we hypothesized that combination of Ad/TRAIL-F/RGD and radiotherapy would lead to enhanced cell killing of NSCLC cells and prolong survival in mice bearing human NSCLC. In this study, we evaluated the efficacy of Ad/TRAIL-F/RGD combined with radiotherapy in vitro in three NSCLC cell lines and in vivo in a NSCLC tumor model. Our results showed that combination of Ad/TRAIL-F/RGD and radiotherapy dramatically inhibited the growth of human NSCLC cells in vitro and in vivo, which provides the experimental basis for the potential clinical use of this combination.
MATERIALS AND METHODS
Cell Lines and Cell Culture
Human NSCLC cell lines A549, H1299, H358 were obtained from the American Type Culture Collection (Manassas, VA). The A549 line, which contains the wild-type p53 gene, was maintained in Ham’s F12 medium supplemented with 10% fetal calf serum. The H1299 and H358 lines, which have an internal homozygous deletion of the p53 gene and a mutated p53 gene, respectively, were maintained in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 1% glutamine, and 1% antibiotics; these lines were cultured at 37°C in a humidified incubator containing 5% CO2. Normal human bronchial epithelial cells (NHBE) purchased from Clonetics (San Diego, CA), were cultured in medium recommended by the manufacture.
Adenovectors
Both Ad/CMV-GFP, an adenoviral vector with a green fluorescent protein (GFP) under the control of the CMV promoter, and Ad/g-TRAIL, an adenoviral vector with a GFP marker and TRAIL gene under the control of the hTERT promoter, have been described previously (10–12). To develop a more efficient gene delivery system for clinical use, we recently modified the Ad/g-TRAIL vector and constructed a new adenoviral vector system, namely Ad/TRAIL-F/RGD. In this construct, the gene marker GFP was removed because it was not needed for therapeutic purposes. To overcome the lack of adenoviral vectors receptor for some cancer cells, we inserted an RGD sequence containing integrin-binding motif argine-glycine-aspartate (RGD) in the HI loop of adenoviral fiber. In addition, to produce a large amount of TRAIL gene expression, we used the coexpression of a GAL4VP16 fusion protein, a very strong transcription factor for GT promoter (the GAL4 DNA binding site plus TATA box). GT is a very strong promoter that can only be activated by GAL4VP16 (28). In this new construct, hTERT controlled tumor-specific expression of GAL4VP16, and GAL4VP16 activated the GT promoter that initiates TRAIL expression.
The expansion, purification, titration, and quality analyses of all of these vectors were performed at the vector core facility of The University of Texas M. D. Anderson Cancer Center, as described previously (10–12). All viral preparations were free of the E1+ adenovirus, according to the results of a polymerase chain reaction assay, and free of endotoxin, according to the results of an endotoxin detection assay (LAL detection kit; BioWhittaker, Inc., Walkersville, ME). The titer used in this study was determined by the absorbency of the dissociated virus at A260 nm (one A260 nm unit = 1012 viral particles [VPs]/ml], and the titers determined with a plaque assay were used to determine additive information. Unless otherwise specified, Ad/CMV-GFP was used as the vector control, and phosphate-buffered saline (PBS) was used as a mock control.
Growth Inhibition and XTT Assay
The inhibition of tumor cell growth caused by Ad/TRAIL-F/RGD or Ad/g-TRAIL or radiation or combination treatment with Ad/TRAIL-F/RGD and radiation or Ad/CMV-GFP and radiation was analyzed by quantitatively determining cell viability using an improved XTT assay (Cell Proliferation Kit II; Roche Molecular Biochemicals, Indianapolis, IN). Briefly, cells were plated in 96-well microtiter plates at 1 × 103 cells/well in 100 μl of medium. One day after the cells were plated, the medium was removed from each well and replaced with a 100-μl aliquot of medium containing an Ad/TRAIL-F/RGD or an Ad/g-TRAIL or an Ad/CMV-GFP vector at various MOIs. After a 24-h incubation with the adenoviral vectors, cells in the 96-well plates were irradiated with various doses of γ-radiation in a 137Cs unit (Model E-0103; U. S. Nuclear Corp., Burbank, CA) at room temperature. Cells were then incubated at 37°C in a humidified atmosphere containing 5% CO2. Three days after incubation, cell growth and viability were quantified by an XTT assay. Briefly, the culture medium was removed, and 50 μl of an XTT reaction mixture was added to each well with fresh medium, to a final concentration of 0.3 mg/ml per well. Cells were then incubated for 2 h at 37°C. The absorbance was measured at a wavelength of 450 nm against a reference wavelength of 630 nm in a microplate reader (Model MRX; Dynatech Laboratories, Chantilly, VA). The percentage of viable cells was calculated in terms of the absorbency of treated cells compared with the absorbency of untreated control cells. Each experiment was performed in quadruplicate and repeated at least twice.
Cell Clonogenic Survival
Cells were cultured overnight in 10-cm dishes in normal culture medium and then treated with Ad/TRAIL-F/RGD or Ad/CMV-GFP at various MOIs (MOI for H1299 cells, 300 VPs; for H358 cells, 100 VPs; for A549 cells, 3,000 VPs; and for NHBE cells 1000 VPs). Cells treated with PBS alone were used as a control. After a 24-h exposure to Ad/TRAIL-F/RGD or Ad/CMV-GFP, cells in the 10-cm dishes were irradiated with various doses of radiation in a 137Cs unit at room temperature. The irradiated cells were then seeded in triplicate onto 10-cm dishes at a density of 1,000 cells per dish to yield 50 to 200 colonies per dish. The cells were then cultured in an incubator containing 5% CO2 at 37°C for 14 days. Individual colonies (> 50 cells/colony) were fixed and stained with a solution containing 0.25% crystal violet and 10% ethanol for 10 min. The colonies were counted with an imaging system (FluorChem 8800 Imaging System; Alpha Innotech, San Leandro, CA) using a visible light source. Each experiment was performed in triplicate and repeated at least twice.
Flow Cytometry Assay
Cells (2 × 105) were plated into 6-well plates 1 day before treatment. These cells were then treated with Ad/TRAIL-F/RGD or Ad/g-TRAIL or Ad/CMV-GFP at various MOI. Cells treated with PBS alone were used as a control. After 24 h of exposure to Ad/TRAIL-F/RGD or Ad/CMV-GFP, the cells were irradiated or not with 5 Gy of radiation in a 137Cs unit at room temperature. Three days after treatment, floating and attached cells were harvested and washed with PBS. One part was used for the analysis of GFP expression, which involved determining the percentage of GFP-positive cells using a FACS system (Becton-Dickinson, Mansfield, MA, USA). The second part of the sample, which was fixed by 70% ethanol overnight and stained with propidium iodide before analysis, was used to quantify the apoptotic cells. This was done using flow cytometry, which measured the sub G0/G1 cellular DNA content using Cell Quest software (Becton-Dickinson, San Jose, CA, USA).
Western Blot Analysis
Cells were cultured overnight in 10-cm dishes in normal culture medium and then treated with Ad/TRAIL-F/RGD or Ad/g-TRAIL or Ad/CMV-GFP at an MOI of 300 VPs. After a 24-h exposure to Ad/TRAIL-F/RGD or Ad/CMV-GFP, the cells were irradiated or not with 5 Gy of radiation in a 137Cs unit at room temperature. Three days after the treatment, cells were washed with cold PBS and underwent lysis in Laemmli’s lysis buffer. Equal amounts of protein and lysate were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and then transferred to enhance chemiluminescence membranes (Hybond; Amersham Corp., Arlington Heights, IL). These membranes were then blocked with a buffer containing 5% fat-free milk and PBS with 0.05% Tween 20 for 1 h or overnight at 4°C, washed three times with PBS with 0.05% Tween 20, and incubated with primary antibodies for at least 1 h at room temperature. Rabbit anti-human caspase-3 and caspase-9 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit anti-human caspase-8, mouse anti-human poly (ADP-ribose) polymerase (PARP), and mouse anti-human TRAIL were obtained from BD Pharmingen (San Diego, CA). After a second washing with PBS and 0.05% Tween 20, membranes were incubated with peroxidase-conjugated secondary antibodies and developed with a chemiluminescence detection kit (ECL kit; Amersham Bioscience, Buckinghamshire, United Kingdom). β-Actin was used as a loading control.
Animal Studies
All animals in this study were maintained and animal experiments were performed in accordance with National Institutes of Health requirements and institutional guidelines established for the animal core facility at M. D. Anderson. Female nu/nu mice (4–6 weeks of age) were purchased from Charles River Laboratories (Wilmington, MA). Prior to tumor cell inoculation, mice were subjected to 3.5 Gy of total body irradiation from a 137Cs radiation unit (ModelE-0103; U.S. Nuclear Corp.). H1299 NSCLC cells were used to establish subcutaneous tumors in mice. Briefly, 3 × 106 cells were injected into the two hind legs of each mouse. When the average size of tumors reached 80 to 100 mm3, mice were randomly divided into six treatment groups with eight mice per group; groups were treated with PBS alone, Ad/CMV-GFP alone, Ad/TRAIL-F/RGD alone, radiation alone, Ad/CMV-GFP plus radiation, or Ad/TRAIL-F/RGD plus radiation. On day 1, each mouse was intratumorally injected with either Ad/TRAIL-F/RGD at a dose of 5 × 1010 VP/tumor, Ad/CMV-GFP at a dose of 5 × 1010 VP/tumor, or PBS at a volume of 0.1 ml; on day 2, tumors in mice that were assigned to receive radiation were locally irradiated with a vertical 60Co-γ-radiation beam (Theratron 780C cobalt unit; Theratronics International Ltd., Kanata, ON, Canada) at a dose of 5 Gy/tumor. Each mouse was given anesthesia, restrained in a custom-designed jig, and positioned in the radiation field so that only the tumor xenografts implanted on the hind legs were exposed to the radiation beam; the rest of the mouse’s body was shielded by a lead block. On day 3, each mouse that had been injected with an adenoviral vector on day 1 was reinjected with the same vector. Mice were ear-tagged so that data obtained from individual animals could be traced. Tumor dimensions were measured three times per week using a digital caliper. Tumor volume was calculated using the equation V (mm3) = a × b2/2, in which a is the largest diameter and b is the smallest diameter.
The mice were killed at the animal core facility in accordance with our institution’s policy when the tumor size and the mice’s general condition suggested that the mice would die within 1 week. The survival times of mice were estimated based on the date of killing.
Immunohistochemistry
4 μm paraformaldehyde- fixed tumor sections were rehydrated and then incubated with a 1:200 of rabbit polyclonal antibody to TRAIL (Santa Cruz Biotech, Santa Cruz, CA, USA) for 60 min. The slides were stained with DakoCytomation Envision + System-HRP(DAB) by using Dakoautostainer (DakoCytomation CO.Carpinteria, CA) to detect the expression of TRAIL. Counterstaining was performed with haematoxylin, and the slides were covered with Kaiser’s glycerin-gelatin. Brown color indicates TRAIL expression. Percentage of TRAIL positive cells was counted under a light microscope (×400 magnification) in randomly chosen fields and calculated as a percentage of at least 1000 scored cells.
TUNEL Assay
To detect apoptotic cells in tumor, we used the in situ Cell Death Detection kit, POD (Roche Applied Science, Indianapolis, IN, USA). The staining was performed according to the manufacturer’s procedures. Counterstained with hamatoxylin, and viewed under a light microscope. Brown color indicates apoptotic nuclei as visualized using DAB substrate. Apoptotic cells were counted under a light microscope (×400 magnification) in randomly chosen fields, and the apoptosis index was calculated as a percentage of at least 1000 scored cells.
Statistical Analysis
Differences among the treatment groups were analyzed by analysis of variance using statistical software (Statistica; StatSoft, Tulsa, OK). A difference was considered statistically significant when the P value was 0.05 or less. Differences in tumor growth in vivo among the treatment groups were assessed by analysis of variance with a repeated measurement module. Analysis of variance was performed to determine statistical significance between each treatment group by using the SAS procedure mixed with SAS version 6.12 software; Survival was assessed by using the Kaplan-Meier method.
RESULTS
Ad/TRAIL-F/RGD Is More Effective than Ad/gTRAIL in Human NSCLC Cell Lines
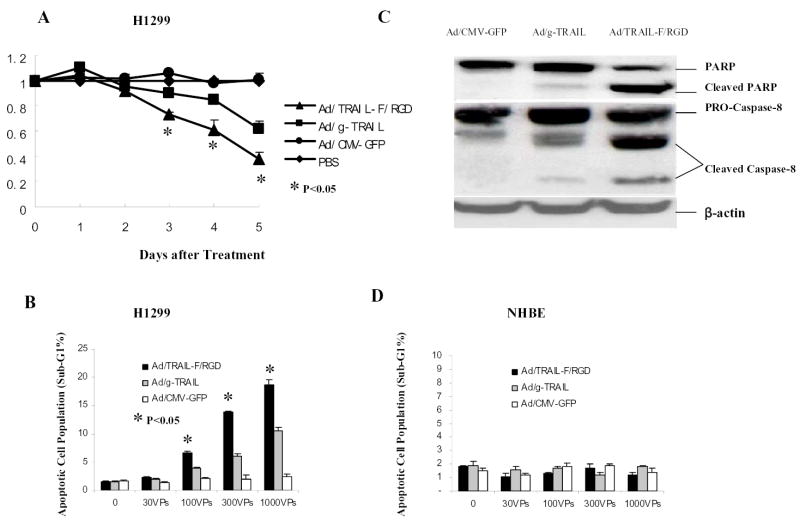
To test the antitumor activity of Ad/TRAIL-F/RGD, we treated H1299 cells with Ad/CMV-GFP, Ad/gTRAIL, and Ad/TRAIL-F/RGD. The cell-killing effect of Ad/TRAIL-F/RGD was analyzed by measuring cell viability via XTT assay (Fig. 1A) and by quantifying apoptotic cells via flow cytometry (Fig. 1B). Treatment of H1299 cells with Ad/TRAIL-F/RGD at 300 VPs dramatically reduced cell viability compared with Ad/gTRAIL or Ad/CMV-GFP with same MOIs at day 3 to day 5 (P < 0.05) (Fig. 1A). The result was supported by flow cytometry study showing treatment of H1299 cells with Ad/TRAIL-F/RGD at MOIs of 100 VPs to 1000 VPs significantly increased the number of apoptotic cells compared with Ad/gTRAIL or Ad/CMV-GFP at the same MOIs (P <0.05) (Fig. 1B). The induction of apoptosis by Ad/TRAIL-F/RGD was confirmed by Western blot analysis. (Fig. 1C). Caspase-8 and poly ADP-ribose polymerase (PARP ) cleavage were observed in cells treated with Ad/gTRAIL or Ad/TRAIL-F/RGD. However, in comparison with Ad/gTRAIL, treatment with Ad/TRAIL-F/RGD notably increased the cleavage of Caspase-8 and PARP. Taken together, our data demonstrated that Ad/TRAIL-F/RGD is more effective than Ad/g-TRAIL in cell killing and apoptosis induction in human NSCLC cells.
Fig. 1. Ad/TRAIL-F/RGD is more effective than Ad/g-TRAIL in human NSCLC cell lines.
A, Cell viability determined by the XTT assay in H1299 cells for 5 days after various treatment at 300 MOIs. Cells treated with PBS were used as a control, with their viability set at 1. Each experiment was performed in quadruplicate and repeated at least twice. Values are means of quadruplicate assay results. Bars, standard deviation. B, Flow cytometric analysis of apoptotic (sub-G1) cells in H1299 at third day after various vector treatment at the indicated MOIs. C, Activation of the apoptotic signal transduction pathway in H1299 at third day after various vector treatment at 300MOIs using Western blot analysis. D, Flow cytometric analysis of apoptotic (sub-G1) cells in NHBE cells at third day after various vector treatment at the indicated MOIs.
Dose Dependent Cell-Killing Effect of Ad/TRAIL-F/RGD alone in Human NSCLC Cell Lines but not in NHBE
To test the combined effect of TRAIL gene therapy and radiotherapy, we first evaluated the dose effect of Ad/TRAIL-F/RGD alone. Treatment with Ad/TRAIL-F/RGD resulted in a dose-dependent cell-killing effect at MOIs of 0 to 3,000 VPs in all cancer cell lines tested but not in NHBE cells (Fig. 2A). H358 cells were the most sensitive, H1299 cells were the next sensitive, A549 cells were the least sensitive, and NHBE cells were not sensitive. This result is consistent with the levels of transgene expression seen in the tumors treated with Ad/g-TRAIL and Ad/CMV-GFP at MOIs of 0 to 3,000 VPs (Fig. 2B). Treatment with Ad/gTRAIL or Ad/CMV-GFP resulted in similar levels of GPF-positive cells in all NSCLC cell lines but not in NHBE. There was a significant difference levels of GPF-positive cells between treatment with Ad/g-TRAIL versus Ad/CMV-GFP in NHBE cells (P <0.001). More than 80% of NHBE cells treated with Ad/CMV-GFP at 3000 VPs were positive for GFP, whereas only 5% of NHBE cells treated with Ad/g-TRAIL were positive for GFP (Fig. 2B), indicating tumor specific transgene expression under control of hTERT promotor in Ad/g-TRAIL. The level of transgene TRAIL expression in NSCLC cell was also found by Western blot analysis to depend on the vector dose (Fig. 2C).
Fig. 2. Dose dependent cell-killing effect of Ad/TRAIL-F/RGD alone in human NSCLC cell lines but not in NHBE.
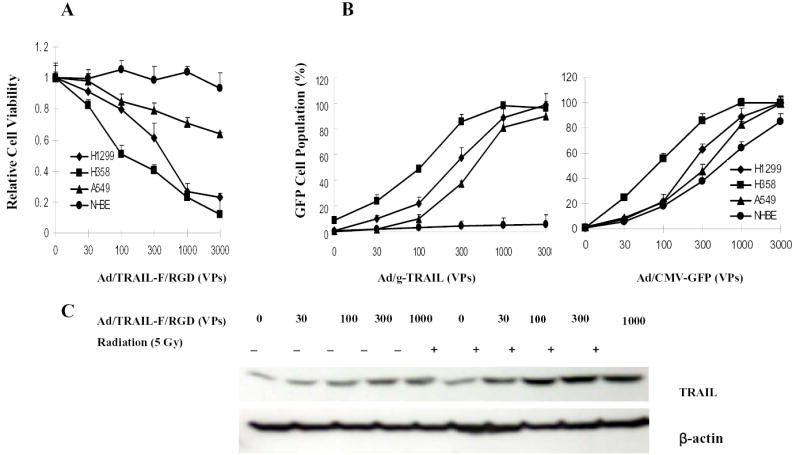
A, Cell viability determined by the XTT assay in different cell lines at the fourth day after treated by Ad/TRAIL-F/RGD at MOIs range from 0 to3000VPs. B, Results of a flow cytometry analysis of transgene expression in a GFP cell population. Four cell lines were treated with Ad/g-TRAIL or Ad/CMV-GFP and harvested 2 days after treatment. Levels of GFP in the cells were determined by using fluorescence-activated cell sorting. C, Level of TRAIL expression in H1299 cells after treatment with different doses of Ad/TRAIL-F/RGD plus/minus radiation (5 Gy), as determined by Western blot analysis. β-actin was used as a loading control.
To confirm no toxic effect in normal NHBE with Ad/TRAIL-F/RDG, we also evaluated apoptosis induction of Ad/TRAIL-F/RDG in NHBE by quantifying the sub-G1 population after treatment with Ad/TRAIL-F/RDG, Ad/g-TRAIL and Ad/CMV-GFP using fluorescence-activated cell sorting analysis. As showed in Fig. 1D, only background levels of apoptosis were observed in NHBE cells after treatment with Ad/CMV-GFP, Ad/g-TRAIL, or Ad/TRAIL-F/RDG at various MOIs.
Improved Cell-Killing Effects of Combined Ad/TRAIL-F/RGD and Radiotherapy in NSCLC Cells but not in NHBE
Treatment with ionizing radiation (0–20 Gy) alone led to a dose-dependent inhibition of cell growth in the three NSCLC cell lines and NHBE cells according to results of the XTT assay (Fig. 3). We then studied whether the combined treatment of ionizing radiation and Ad/TRAIL-F/RGD could inhibit profoundly the growth of these cells. Cells treated with PBS and Ad/CMV-GFP were used as mock and vector controls, respectively. Improved inhibitory effect on cell growth was observed in three NSCLC cell lines treated with various doses of Ad/TRAIL-F/RGD and ionizing radiation. In contrast, there was no difference observed among treatment groups with various doses of radiation combined with Ad/TRAIL-F/RGD, Ad/CMV-GFP, PBS in NHBE cell, indicating no radiation sensitization in NHBE with Ad/TRAIL-F/RGD. Fig. 3 shows representative data. In H1299 cells, a single treatment with 5 Gy of ionizing radiation alone caused 30.9% growth inhibition, and treatment with Ad/TRAIL-F/RGD alone (at an MOI of 300 VPs) caused 38.7% growth inhibition; however, the combined treatment caused 73.8% growth inhibition, according to the results of the XTT assay. A similar growth inhibition was observed with the other two cancer cell lines but not in NHBE (Fig. 3). The data thus showed that combined Ad/TRAIL-F/RGD and radiation improved cell-killing effect on NSCLC cell lines but not in NHBE.
Fig. 3. Improved cell-killing effects of combined Ad/TRAIL-F/RGD and radiotherapy in NSCLC cells but not in NHBE by XTT assay.
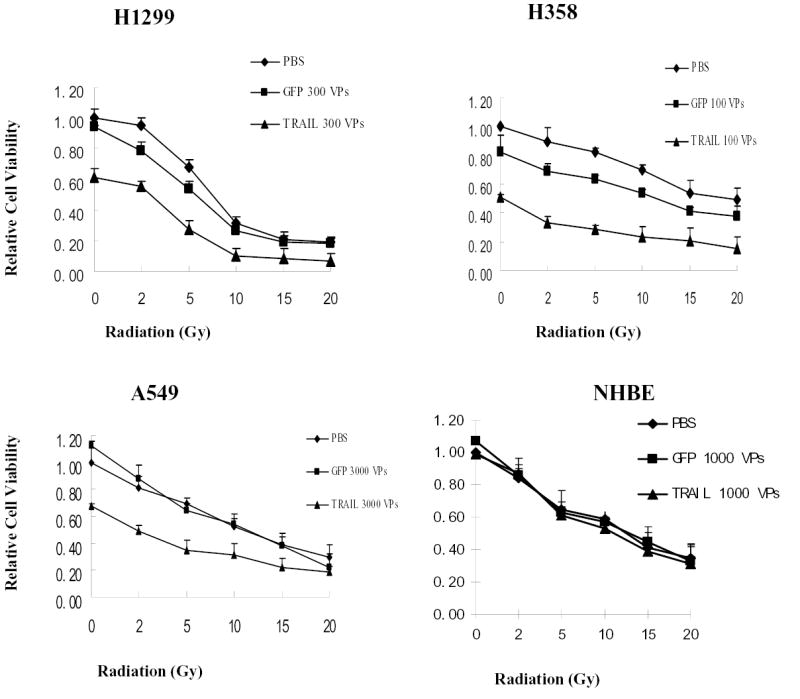
Four cell lines were treated with Ad/TRAIL-F/RGD at the indicated MOIs for 4 days and at the indicated doses of radiation for 3 days. Cell viability was then determined by the XTT assay. Cells treated with PBS were used as a control, with their viability set at 1. Each experiment was performed in quadruplicate and repeated at least twice. Values are means of quadruplicate assay results. Bars, standard deviation. GFP: Ad/CMV-GFP (vector control).
To confirm the combination effect of TRAIL gene therapy and radiotherapy, we conducted a clonogenic formation assay in the three NSCLC cell lines and NHBE cell lines after treatment with radiotherapy, Ad/TRAIL-F/RGD, or both. PBS and Ad/CMV-GFP were again used as mock and vector controls, respectively. Compared with the control group or the groups receiving single-agent treatment, the group receiving a combination of Ad/TRAIL-F/RGD and radiotherapy showed significantly reduced clonogenic formation (Fig. 4AB, P < 0.05) in the three NSCLC cell lines. Nevertheless, combination of Ad/TRAIL-F/RGD and radiotherapy resulted in almost similar colony formation compared with radiotherapy plus Ad/CMV-GFP group and radiotherapy plus PBS group in NHBE cell lines.
Fig. 4. Enhanced cell-killing effects of combined Ad/TRAIL-F/RGD (TRAIL) and radiotherapy in NSCLC cells but not in NHBE by the colony-forming assay.
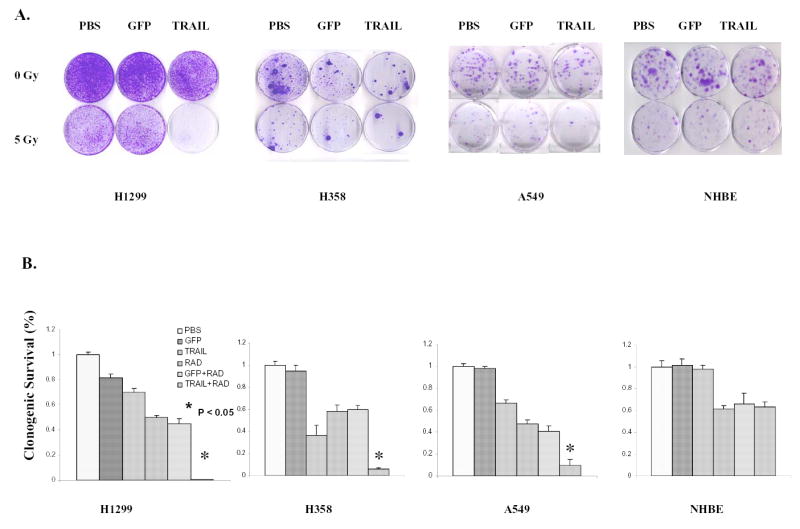
The influence of Ad/TRAIL-F/RGD on radiosensitivity was examined on the basis of the clonogenic survival of cell lines after exposure to PBS, Ad/CMV-GFP (GFP), Ad/TRAIL-F/RGD (TRAIL), Radiation (RAD), Ad/CMV-GFP and Radiation (GFP+RAD), Ad/TRAIL-F/RGD and Radiation (TRAIL+RAD). The MOIs for the cells were as follows: H1299 cells, 300 VPs; H358 cells, 100 VPs; A549 cells, 3000 VPs; and NHBE cells 1000 VPs. A, Representative dish by colony-forming assay of four cell lines at 14 days after treatment. Top of panels, gene treatment; left of panels, radiation dose; bottom of panels, name of each cell line. B, Relative clonogenic survival of four cell lines. Bottom of panels, name of each cell line. Each experiment was performed in triplicate and repeated at least twice. Values are means of the results of triplicate assays. Bars, standard deviation.
In H1299 cells, 5 Gy of radiation inhibited 50.2% of colony formation, Ad/TRAIL-F/RGD treatment at an MOI of 300 VPs suppressed 30.1% of colony formation. However, the combination of radiation and Ad/TRAIL-F/RGD treatment inhibited 99.5% of colony formation. A similar colony formation inhibition was observed in the other two cancer cell lines (Fig. 4B). The different percentages of inhibition shown by the XTT and clonogenic assays were due to the different sensitivities of these two assays with the different treatments. These results suggested that TRAIL gene therapy significantly sensitize NSCLC cells to radiotherapy. Interestingly, radiotherapy increased TRAIL gene expression at different concentrations of the vectors (Fig. 2C).
Enhanced Apoptosis Induction and Activation of the Apoptotic Signal Transduction Pathway by the Combination of TRAIL Gene Therapy and Radiotherapy
To test whether the enhanced cell killing produced by combination treatment is due to apoptosis, we quantified the sub-G1 population of H1299 cells after treatments with TRAIL gene therapy and radiotherapy in a fluorescence-activated cell sorting analysis. As showed in Fig. 5A, Ad/TRAIL-F/RGD alone or radiation alone induced a less than 5% sub-G1 accumulation. However, combined treatment with Ad/TRAIL-F/RGD and radiation resulted in a substantially increased sub-G1 accumulation (10.2%). These data suggested that apoptosis is significantly enhanced by the combination of TRAIL gene therapy and radiotherapy.
Fig. 5. Enhanced apoptosis induction and activation of the apoptotic signal transduction pathway by the combination of TRAIL gene therapy and radiotherapy (RAD).

A, Fluorescence-activated cell sorting was used to quantify apoptotic cells 4 days after one of six treatments. The percentage of sub-G1 cells appears in each panel. Data are shown from one of two experiments yielding similar results. B, Western blot analysis of cleavage of PARP, caspase-8, caspase-9, and caspase-3 in H1299 cells 4 days after Ad/TRAIL-F/RGD (TRAIL) treatment combined with radiotherapy. Cells were treated with PBS (Lane 1), radiation (5Gy) (Lane 2), Ad/CMV-GFP (GFP; MOI of 300 VPs) (Lane 3), Ad/CMV-GFP (MOI of 300 VPs) plus radiation (5 Gy) (Lane 4), Ad/TRAIL-F/RGD (MOI of 300 VPs) (Lane 5), and Ad/TRAIL-F/RGD (MOI of 300 VPs) plus radiation (5 Gy) (Lane 6). β-Actin was used as a loading control.
To further elucidate the apoptotic signal transduction pathway used by TRAIL gene therapy and radiotherapy, we performed a Western blot study to evaluate the activation of caspase-8, caspase-3, and caspase-9 and the cleavage of PARP after treatment with PBS, Ad/CMV-GFP alone, Ad/TRAIL-F/RDG alone, radiotherapy alone, and radiotherapy plus Ad/CMV-GFP or Ad/TRAIL-F/RDG. H1299 cells were harvested 4 days after treatment with Ad/TRAIL-F/RGD or Ad/CMV-GFP and 3 days after radiotherapy, and the activation of caspase-8, caspase-3, and caspase-9 and the cleavage of PARP were analyzed. Treatment with Ad/TRAIL-F/RGD alone resulted in the cleavage of caspase-8, caspase-3, caspase-9, and PARP, as expected (Fig. 5B). The combined treatment with Ad/TRAIL-F/RGD and radiation resulted in the cleavage of caspase-8, caspase-3, caspase-9, and PARP that was slightly increased over that produced by treatment with Ad/TRAIL-F/RGD alone (Fig. 5B). This result indicated that apoptosis was responsible for the cell death produced by the combination of TRAIL gene therapy and radiotherapy and also suggested that the combination treatment enhanced apoptosis.
Inhibition of Tumor Growth in Vivo and Prolongation of Mouse Survival by Combined Ad/TRAIL-F/RGD Treatment and Radiotherapy
We then tested the therapeutic efficacy of the combination of Ad/TRAIL-F/RDG treatment and radiotherapy in vivo in a human NSCLC xenograft mouse model. As shown in Fig. 6A, radiotherapy had a minimal inhibitory effect and Ad/TRAIL-F/RDG alone had a moderate inhibitory effect on tumor growth at the tested dose regimens; however, the combination of Ad/TRAIL-F/RGD and radiation significantly inhibited tumor growth compared with treatment with PBS alone, radiotherapy alone, Ad/TRAIL-F/RDG alone, Ad/CMV-GFP alone, or Ad/CMV-GFP plus radiotherapy (Day 14, P = 0.00907).
Fig. 6. Inhibition of tumor growth in vivo and prolongation of mouse survival by combined Ad/TRAIL-F/RGD (TRAIL) treatment and radiotherapy.
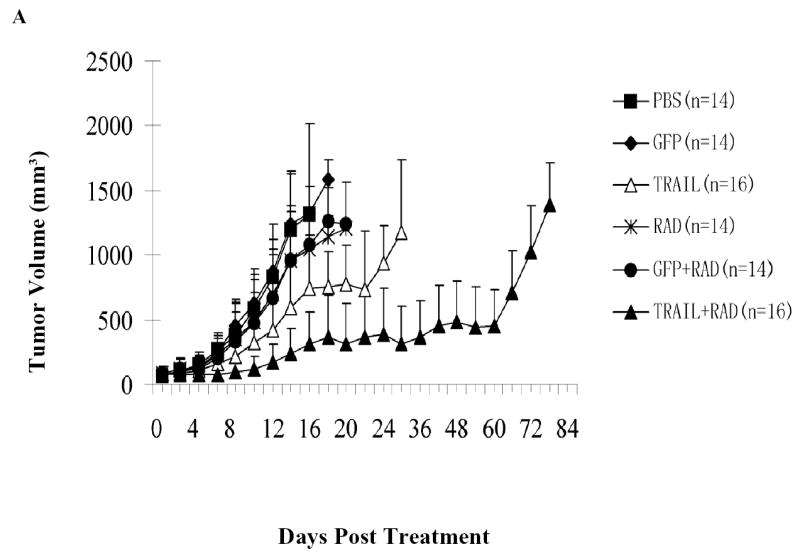
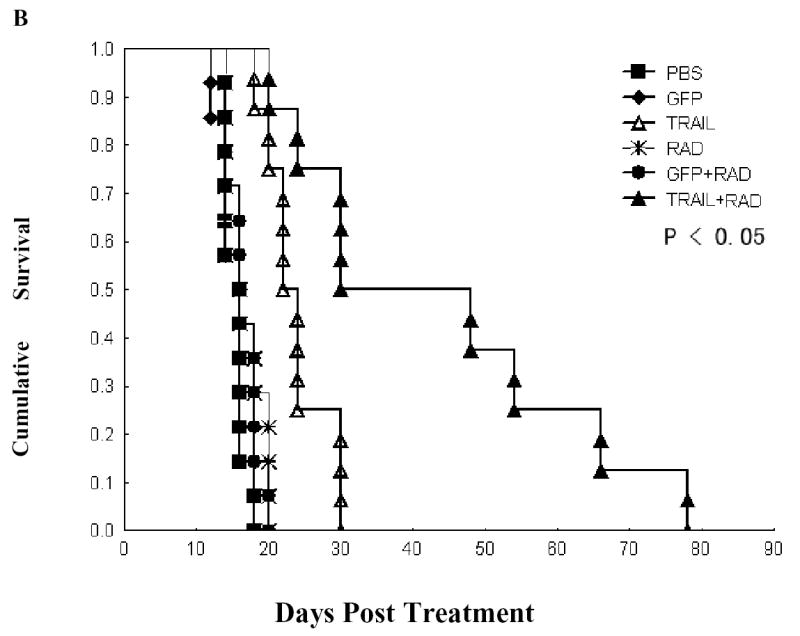
H1299 cells were grown as xenograft tumors in nude mice, and mice bearing tumors of 80 to 100 mm3 were treated with PBS, Ad/CMV-GFP alone (GFP), Ad/TRAIL-F/RGD alone, radiation (RAD) alone, Ad/CMV-GFP plus radiation, or Ad/TRAIL-F/RGD plus radiation. Tumor volumes were then measured. A, Tumor growth delay curves. Data are presented as the mean ± standard error. Analysis of variance was performed to determine statistical significance between each treatment group by using the SAS procedure mixed with SAS version 6.12 software; B, Animals alive as a function of time after treatment. We used the Kaplan-Meier method for our survival analysis.
The mouse survival data also showed a significant prolongation of survival time for the combination of Ad/TRAIL-F/RDG and radiotherapy, with a mean survival of 43.7 days, compared with 23.7 days for Ad/TRAIL-F/RGD alone, 16.5 days for radiotherapy plus Ad/CMV-GFP, 16.5 days for radiation alone, 15.4 days for Ad/CMV-GFP alone, and 15.7 days for PBS alone (P =0.00002) (Fig. 6B). Treatment with Ad/TRAIL-F/RDG or Ad/TRAIL-F/RDG plus radiotherapy significantly lengthened survival time compared with treatment with PBS only, the control vector only, radiation only, or the control vector plus radiation (P =0.00186). Furthermore, combined treatment with Ad/TRAIL-F/RGD and radiation prolonged survival time significantly compared with treatment with Ad/TRAIL-F/RGD alone (P =0.03354). Half of the mice treated with combined Ad/TRAIL-F/RGD and radiation remained alive for more than 48 days, compared with the mice treated with Ad/TRAIL-F/RGD alone, all of which died within 30 days, and the mice treated with radiotherapy alone all of which died within 20 days. We observed no marked treatment-related toxicities affecting the weight of mice or their general behavior.
Combination of Ad/TRAIL-F/RGD and Radiation Enhanced Apoptosis Induction in Vivo.
To assess apoptosis induction in vivo, we performed terminal deoxynucleotidyl transferase-mediated dUTP labeling (TUNEL) staining on tumor sections 3 days after radiation in all six treated groups (Fig. 7A). Brown color indicates apoptotic nuclei as visualized using DAB substrate. Apoptotic cells were counted under a light microscope in randomly chosen fields, and the apoptosis index was calculated as a percentage of at least 1000 scored cells. As showed in Fig. 7B, combination of Ad/TRAIL-F/RGD and radiation resulted in a significantly higher apoptotic index (29.8%) compared with Ad/TRAIL-F/RGD alone (13.5%), radiation alone (9.7%), and radiotherapy plus Ad/CMV-GFP (10.3%) after subtracting the background level of 2.2% (P =0.00016). We also determined TRAIL protein expression by immunohistochemical analysis of tumor specimens from same six treated groups. We observed strong expression of TRAIL protein in tumor tissues obtained from animals that were treated with Ad/TRAIL-F/RGD combined radiation or treated with Ad/TRAIL-F/RGD alone (Fig. 7. A and C). We did not observe TRAIL expression in tumors that were treated with PBS or radiation alone, or GFP alone or GFP plus radiation. The percentage of positive cells in tumor tissues treated with Ad/TRAIL-F/RGD plus radiation was obviously higher (30.1%) than that treated with Ad/TRAIL-F/RGD alone (16.8%, P =0.00306). This result also is consistent with our results in vitro (Fig. 2C) that radiotherapy increased TRAIL gene expression.
Fig. 7. Ad/TRAIL-F/RGD (TRAIL) combined with radiotherapy enhances apoptosis in vivo.
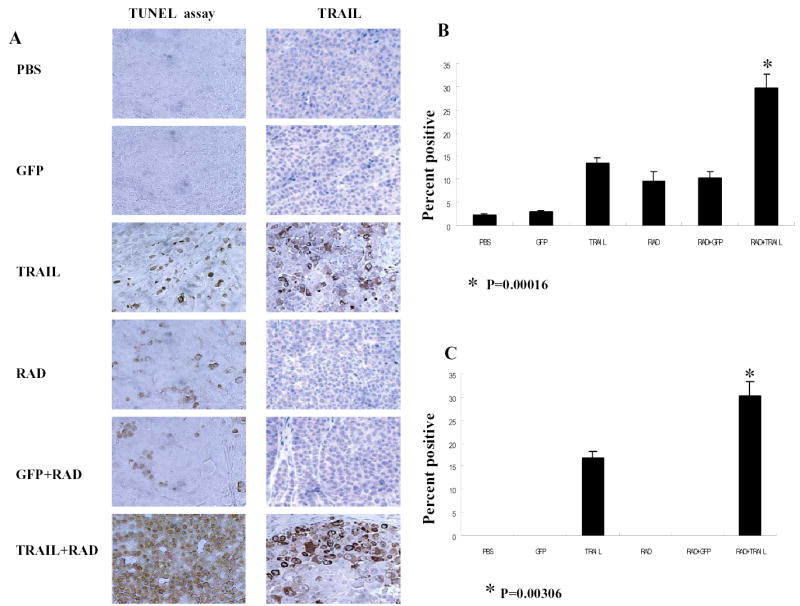
A, Representative fields of TRAIL expression by immunohistochemistry (right panel) and tumor cell apoptosis by TUNEL assay (left panel) in H1299 tumors receiving various treatments were harvested 3 days after radiation. Left of panels, treatment; top of panels, staining. B, Apoptotic index in the various treatment groups as determined by counting at least 1000 cells per sample under a light microscope. The apoptosis index was calculated as a percentage of total number of cells scored. Bars, SE. C. The percentage of TRAIL positive tumor cells as counted under a light microscope.
DISCUSSION
In this study, the tumor-specific TRAIL gene targeting achieved by using Ad/TRAIL-F/RDG was found to sensitize lung cancer cells to radiotherapy, with significantly increased tumor growth inhibition and survival of mice bearing a human NSCLC xenograft. For the radiotherapy (5 Gy) only group, the tumor growth inhibition effect was minimal, and there was no survival benefit; the mean survival was 16.5 days in mice receiving radiotherapy only, compared with 15.7 days in PBS-treated mice. These data suggest that the NSCLC cell line H1299 is resistant to this dose of radiotherapy. Ad/TRAIL-F/RDG alone moderately improved the tumor inhibition effect and prolonged the survival time of mice. However, combination of radiotherapy and Ad/TRAIL-F/RDG achieved a dramatic improvement with more than doubled mean survival (43.7 days). Of further importance, there were no observable toxicities noted in the combination treatment group compared with controls based on mice weight and general behavior. The combination of Ad/TRAIL-F/RDG and radiotherapy may serve as a novel approach to increasing the therapeutic ratio of radiotherapy in NSCLC that is radiation resistant.
In terms of toxicity, radiotherapy commonly causes acute adverse effects, including esophagitis, pneumonitis, and chronic conditions such as lung fibrosis, esophageal stricture, and fistula; these complications are the major dose-limiting toxicities of radiotherapy. Gene therapy, in contrast, causes different toxicity profiles, with common systemic reactions such as low-grade fever (5), which do not overlap with radiotherapy-induced adverse effects. Therefore, combined gene therapy and radiotherapy may improve therapeutic efficacy on cancer cells, with tolerable toxicities.
Among the family of death receptors ligands, tumor necrosis factor and Fas have been extensively investigated, and a phase I clinical trial has been completed that assessed the effect of intratumoral injections of tumor necrosis factor delivered by an adenoviral vector (29). However, potential ischemic and hemorrhagic reactions in normal tissues and liver toxicity were of major concern (30, 31). Treatment with TRAIL is a more potent cancer therapy that causes little toxicity in normal tissues (8, 9, 32); the only reported toxicity is the apoptosis of human primary hepatocytes and human brain cells in vitro (33–35). However, more detailed studies particularly in vivo are needed to further clarify the anti-cancer activity of TRAIL delivered by different biochemical preparations of TRAIL proteins the possible toxicities in humans (35, 36).
To minimize such potential toxicities, we developed a tumor-specific adenoviral vector–mediated TRAIL gene delivery system by expressing the TRAIL gene from the hTERT promoter via GAL4 gene regulatory components that can augment transgene expression from the tumor-specific promoter without losing target specificity (11, 25, 28). As showed in our data, TRAIL under control of tumor specific hTERT induced apoptosis, inhibited growth, sensitized radiotherapy in lung cancer cells but spared normal cell such as NHBE cells (Fig. 1D, Fig. 2AB, Fig. 3, and Fig. 4). The heterogeneity of human cancer cells is an additional problem because some cells do not have an adenoviral receptor in their cell membrane, thus hindering adenoviral vector delivery. To overcome this obstacle, we inserted a gene sequence containing the integrin-binding RGD motif sequence into the HI loop of the adenoviral fiber. Compared with previous vector Ad/g-TRAIL without RGD, Ad/TRAIL-F/RDG significantly improved cancer inhibition effect and apoptosis induction without losing its tumor specificity (Fig. 1).
The hTERT promoter has the ability to specifically target cancer cells. However, its promoter strength may not be strong enough to express the large amount of TRAIL required to kill cancer cells in patients with lung cancer. To increase promoter strength, a GAL4VP16 fusion protein, a very strong transcription factor for GT, was coexpressed in this construct. In this way, expression of GAL4VP16 under control of tumor specific hTERT promoter activates the GT promoter, which initiates TRAIL expression. Our preliminary data from the use of this new construct showed both the increased efficacy of gene delivery without a loss of tumor specificity and the minimally detectable gene expression in normal tissues, even bone marrow stem cells that is considered to have the highest hTERT promoter activity among all the normal tissues (26).
An enhanced anticancer effect of the combination of TRAIL protein therapy with radiotherapy has been seen in various other cancer cells, including breast (14), colon (37), leukemia, and lymphoma (38) cells, in several studies published over the past 4 years. For example, Chinnaiyan et al. (14) examined TRAIL protein therapy in combination with radiation therapy in a breast cancer model. An enhanced apoptotic response was tested for in several human breast cancer cell lines and in an established breast cancer xenograft mouse model. The results showed an enhanced tumor growth delay in response to combination treatment compared with either treatment alone. Enhanced apoptosis was also observed in histological sections taken from these tumors after the combination treatment. No significant toxicity was observed in the mice that received systemic treatment with TRAIL protein (14). This synergistic effect was found to be p53-dependent and may be the result of radiation-induced up-regulation of the TRAIL-receptor DR5. In our study, all the three NSCLC cell lines tested no matter what are their p53 status (A549 line, which contains the wild-type p53 gene, H1299 and H358 lines, which have an internal homozygous deletion of the p53 gene and a mutated p53 gene respectively) showed radiation sensitization by Ad/TRAIL-F/RDG, indicating p53 independent pathway.
Ravi and Bedi (37) showed that the TRAIL protein sensitizes human colon adenocarcinoma cell lines to radiation. TRAIL sensitized HCT116 cells to radiation independently of their p53 status. However, the Bax-deficient line was insensitive to TRAIL alone, radiation alone, and their combination. Gong and Almasan (38) tested the efficacy of the combination of TRAIL protein therapy and radiation in human leukemia and lymphoma cell line treated in vitro. The combination of radiation and the recombinant TRAIL protein had a synergistic effect on the loss of clonogenic survival in both cell lines. Interestingly, data from a study of leukemic cell systems showed that the TRAIL protein sensitized malignant but not normal erythroblastic cells to radiotherapy (39).
Although TRAIL-mediated gene therapy has shown promise, certain cancer cells, especially those that make up highly malignant tumors, are TRAIL resistant. The mechanisms of the resistance to TRAIL-induced apoptosis include dysfunctions of DR4 and DR5, which are essential for TRAIL binding to cells; a defect of either the adaptor protein Fas-associated death domain or caspase 8, which are essential for the TRAIL-induced apoptotic signal transduction pathway, overexpression of Bcl-2; or a lack of Bax or Bak (40). As shown by the results of our clonogenic assay, H549 cells are resistant to Ad/TRAIL-F/RDG therapy, with an MOI of 3,000 VPs required inhibit only 33.6% the cancer cells; in contrast, 63.3% inhibition of H358 cells can be achieved with Ad/TRAIL-F/RDG at an MOI of only 100 VPs. However, the combination of Ad/TRAIL-F/RDG and radiotherapy (5 Gy) inhibited 90.5% of colony formation in H549 cells. These data suggest that radiotherapy is also useful as a tool to overcome TRAIL resistance in gene therapy.
Several mechanisms explain the observed enhanced therapeutic effects between the TRAIL gene and radiotherapy. First, the TRAIL gene and radiation activate distinct apoptotic pathways, which, when jointly triggered, result in an amplified response (41, 42). TRAIL binds with its cell surface receptors and then mediates the recruitment of the FADD adapter molecule to the receptor complex. This complex directly activates caspase 8 (43) and subsequently activates caspase 3 resulting in activation of cascade of caspases (44). In addition, caspase 8 can also activate Bid that triggers cytochrome c release, with subsequent activation of caspase-9 and -3, thereby strongly amplifying the initial apoptotic signal (45). In contrary receptor induced apoptosis, radiation directly damages DNA and triggers p53-mediated transcriptional activation of Bax, Bak, Noxa and Puma, resulting in the mitochondrial damage by breakdown of the mitochondrial membrane potential and release of cytochrome c (46–48). Cytoplasmic cytochrome c forms a complex with Apaf-1 and dATP, leading to activation of caspase-9 (49). Activated caspase-9 subsequently activates caspase-3 and then again activates the cascade of caspases. Alternatively, radiation may directly damage cellular membrane resulting in the release of ceramide (50). Ceramide, once released into the cytoplasm, can directly damage mitochondrial membrane and then stimulate the initiation of apoptosis by releasing cytochrome c (51). This mitochondrial pathway could be p53-independent. Triggering both pathways simultaneously explain the effective therapeutic response seen in vitro and in vivo, as our data showed.
Another source of the enhanced antitumor effects of combined radiotherapy and gene therapy may be that ionizing radiation improves the transfection/transduction efficiency and transgene integration, as shown by our and other studies (52–54). Moreover, gene therapy and radiotherapy target at different phases of the cell cycle, with the S phase of the cell cycle being most responsive to gene therapy and the M and G2 phases being most radiosensitive (55). In addition, it has been reported that radiation and chemotherapy can cause up-regulation of expression of the DR4 or DR5 proteins, or of both (14, 56, 57). By expressing more of the TRAIL receptor, cells may become more sensitive to TRAIL therapy. Further experimentation is needed to confirm this mechanism in radiotherapy.
In summary, our data showed that tumor-specific TRAIL gene targeting can sensitize NSCLC cells to radiotherapy and thus improve survival while spare the normal cells. Ad/TRAIL-F/RGD may improve the therapeutic ratio of radiotherapy, which is crucial for radiation-resistant NSCLC. In addition, radiotherapy may also overcome the TRAIL resistance in highly malignant cancer cells.
Our results provide important preclinical evidence for the future design of multimodality clinical trials that use combined TRAIL gene therapy and radiotherapy. The combination of Ad-TRAIL gene therapy and radiotherapy may be a feasible and effective method for the treatment of NSCLC, particularly for radiation-resistant cancers.
Acknowledgments
We would like to thank Dr. Raymond E Meyn for his advice and review of the manuscript. We also want to thank Dr. James D. Cox and Dr. Jack A. Roth for their support of this project. We thank the Department of Scientific Publications and Barbara E. Lewis for their assistance in the preparation of this manuscript.
Footnotes
Grant support: This research was supported by a Radiology Society of North America (RSNA) Research Scholar Award (to J.Y.C.), Career Development Award from The University of Texas Lung Cancer SPORE grant from National Cancer Institute (to J. Y. C.), National Cancer Institute grants RO1 CA 092487-01A1 and RO1 CA 098582-01A1 (to B. F.), and a grant from the institutional research startup fund (to R. M. C).
References
- 1.Jemal A, Tiwari RC, Murray T, et al. Cancer statistics, 2004 CA Cancer. J Clin. 2004:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Curran WJ, Scott CB, Langer CJ, et al. Long-term benefit is observed in a phase III comparison of sequential vs concurrent chemo-radiation for patients with unresected stage III NSCLC: RTOG 9410 [abstract] Proceedings of the American Society of Clinical Oncology. 2003;22:621. [Google Scholar]
- 3.Nishikawa T, Ramesh R, Munshi A, Chada S, Meyn RE. Adenovirus-mediated mda-7 (IL24) gene therapy suppresses angiogenesis and sensitizes NSCLC xenograft tumors to radiation. Mol Ther. 2004;9:818–28. doi: 10.1016/j.ymthe.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 4.Nishizaki M, Meyn RE, Levy LB, et al. Synergistic inhibition of human lung cancer cell growth by adenovirus-mediated wild-type p53 gene transfer in combination with docetaxel and radiation therapeutics in vitro and in vivo. Clin Cancer Res. 2001;7:2887–97. [PubMed] [Google Scholar]
- 5.Swisher SG, Roth JA, Komaki R, et al. Induction of p53-regulated genes and tumor regression in lung cancer patients after intratumoral delivery of adenoviral p53 (INGN 201) and radiation therapy. Clin Cancer Res. 2003;9:93–101. [PubMed] [Google Scholar]
- 6.Ashkenazi A, Pai RC, Fong S, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155–62. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walczak H, Miller RE, Ariail K, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157–63. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 8.Wickelgren I. Mining the genome for drugs. Science. 1999;285:998–1001. doi: 10.1126/science.285.5430.998. [DOI] [PubMed] [Google Scholar]
- 9.Griffith TS, Anderson RD, Davidson BL, Williams RD, Ratliff TL. Adenoviral-mediated transfer of the TNF-related apoptosis-inducing ligand/Apo-2 ligand gene induces tumor cell apoptosis. J Immunol. 2000;165:2886–94. doi: 10.4049/jimmunol.165.5.2886. [DOI] [PubMed] [Google Scholar]
- 10.Kagawa S, He C, Gu J, Koch P, et al. Antitumor activity and bystander effects of the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) gene. Cancer Res. 2001;61:3330–8. [PubMed] [Google Scholar]
- 11.Lin T, Gu J, Zhang L, et al. Targeted expression of green fluorescent protein/tumor necrosis factor-related apoptosis-inducing ligand fusion protein from human telomerase reverse transcriptase promoter elicits antitumor activity without toxic effects on primary human hepatocytes. Cancer Res. 2002;62:3620–5. [PubMed] [Google Scholar]
- 12.Huang X, Lin T, Gu J, et al. Cell to cell contact required for bystander effect of the TNF-related apoptosis-inducing ligand (TRAIL) gene. Int J Oncol. 2003;22:1241–1245. [PubMed] [Google Scholar]
- 13.Chang JY. Telomerase: a potential molecular marker and therapeutic target for cancer. J Surg Oncol. 2004;87:1–3. doi: 10.1002/jso.20076. [DOI] [PubMed] [Google Scholar]
- 14.Chinnaiyan AM, Prasad U, Shankar S, et al. Combined effect of tumor necrosis factor-related apoptosis-inducing ligand and ionizing radiation in breast cancer therapy. Proc Natl Acad Sci U S A. 2000;97:1754–9. doi: 10.1073/pnas.030545097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee JC, Jong HS, Yoo CG, Han SK, Shim YS, Kim YW. Telomerase activity in lung cancer cell lines and tissues. Lung Cancer. 1998;21:99–103. doi: 10.1016/s0169-5002(98)00034-8. [DOI] [PubMed] [Google Scholar]
- 16.Saretzki G, Petersen S, Petersen I, Kolble K, von Zglinicki T. hTERT gene dosage correlates with telomerase activity in human lung cancer cell lines. Cancer Lett. 2002;176:81–91. doi: 10.1016/s0304-3835(01)00644-9. [DOI] [PubMed] [Google Scholar]
- 17.Spierings DC, de Vries EG, Timens W, Groen HJ, Boezen HM, de Jong S. Expression of TRAIL and TRAIL death receptors in stage III non-small cell lung cancer tumors. Clin Cancer Res. 2003;9:3397–405. [PubMed] [Google Scholar]
- 18.Zhang L, Gu J, Lin T, Huang X, Roth JA, Fang B. Mechanisms involved in development of resistance to adenovirus-mediated proapoptotic gene therapy in DLD1 human colon cancer cell line. Gene Ther. 2002;9:1262–70. doi: 10.1038/sj.gt.3301797. [DOI] [PubMed] [Google Scholar]
- 19.Bergelson JM, Cunningham JA, Droguett G, et al. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–3. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 20.Miller CR, Buchsbaum DJ, Reynolds PN, et al. Differential susceptibility of primary and established human glioma cells to adenovirus infection: targeting via the epidermal growth factor receptor achieves fiber receptor-independent gene transfer. Cancer Res. 1998;58:5738–48. [PubMed] [Google Scholar]
- 21.Jee YS, Lee SG, Lee JC, et al. Reduced expression of coxsackievirus and adenovirus receptor (CAR) in tumor tissue compared to normal epithelium in head and neck squamous cell carcinoma patients. Anticancer Res. 2002;22:2629–34. [PubMed] [Google Scholar]
- 22.Kim M, Su merel LA, Belousova N, et al. The coxsackievirus and adenovirus receptor acts as a tumour suppressor in malignant glioma cells. Br J Cancer. 2003;88:1411–6. doi: 10.1038/sj.bjc.6600932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dmitriev I, Krasnykh V, Miller CR, et al. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J Virol. 1998;72:9706–13. doi: 10.1128/jvi.72.12.9706-9713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wickham TJ, Tzeng E, Shears LL, et al. Increased in vitro and in vivo gene transfer by adenovirus vectors containing chimeric fiber proteins. J Virol. 1997;71:8221–9. doi: 10.1128/jvi.71.11.8221-8229.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koch PE, Guo ZS, Kagawa S, Gu J, Roth JA, Fang B. Augmenting transgene expression from carcinoembryonic antigen (CEA) promoter via a GAL4 gene regulatory system. Mol Ther. 2001;3:278–83. doi: 10.1006/mthe.2001.0273. [DOI] [PubMed] [Google Scholar]
- 26.Jacob D, Davis J, Zhu H, et al. Suppressing orthotopic pancreatic tumor growth a fiber-modified adenovector expressing the TRAIL gene from the human telomerase reverse transcriptase promoter. Clin Cancer Res. 2004;10:3535–41. doi: 10.1158/1078-0432.CCR-03-0512. [DOI] [PubMed] [Google Scholar]
- 27.Fang B, Roth JA. The role of gene therapy in combined modality treatment strategies for cancer. Curr Opin Mol Ther. 2003;5:475–82. [PubMed] [Google Scholar]
- 28.Fang B, Ji L, Bouvet M, Roth JA. Evaluation of GAL4/TATA in vivo. Induction of transgene expression by adenovirally mediated gene codelivery. J Biol Chem. 1998;273:4972–5. doi: 10.1074/jbc.273.9.4972. [DOI] [PubMed] [Google Scholar]
- 29.Senzer N, Mani S, Rosemurgy A, et al. TNFerade biologic, an adenovector with a radiation-inducible promoter, carrying the human tumor necrosis factor alpha gene: a phase I study in patients with solid tumors. J Clin Oncol. 2004;22:592–601. doi: 10.1200/JCO.2004.01.227. [DOI] [PubMed] [Google Scholar]
- 30.Debs RJ, Fuchs HJ, Philip R, Brunette EN, Duzgunes N, Shellito JE, Liggitt D, Patton JR. Immunomodulatory and toxic effects of free and liposome-encapsulated tumor necrosis factor alpha in rats. Cancer Res. 1990;50:375–80. [PubMed] [Google Scholar]
- 31.Tracey KJ, Cerami A. Metabolic response to cachectin/TNF. A brief review. Ann N Y Acad Sci. 1990;587:325–31. doi: 10.1111/j.1749-6632.1990.tb00173.x. [DOI] [PubMed] [Google Scholar]
- 32.Zhang XD, Nguyen T, Thomas WD, Sanders JE, Hersey P. Mechanisms of resistance of normal cells to TRAIL induced apoptosis vary between different cell types. FEBS Lett. 2000;482:193–9. doi: 10.1016/s0014-5793(00)02042-1. [DOI] [PubMed] [Google Scholar]
- 33.Jo M, Kim TH, Seol DW, et al. Apoptosis induced in normal human hepatocytes by tumor necrosis factor-related apoptosis-inducing ligand. Nat Med. 2000;6:564–7. doi: 10.1038/75045. [DOI] [PubMed] [Google Scholar]
- 34.Nitsch R, Bechmann I, Deisz RA, et al. Human brain-cell death induced by tumour-necrosis-factor-related apoptosis-inducing ligand (TRAIL) Lancet. 2000;356:827–8. doi: 10.1016/S0140-6736(00)02659-3. [DOI] [PubMed] [Google Scholar]
- 35.Lawrence D, Shahrokh Z, Marsters S, et al. Differential hepatocyte toxicity of recombinant Apo2L/TRAIL versions. Nat Med. 2001;7:383–5. doi: 10.1038/86397. [DOI] [PubMed] [Google Scholar]
- 36.Nicholson DW. From bench to clinic with apoptosis-based therapeutic agents. Nature. 2000;407:810–6. doi: 10.1038/35037747. [DOI] [PubMed] [Google Scholar]
- 37.Ravi R, Bedi A. Requirement of BAX for TRAIL/Apo2L-induced apoptosis of colorectal cancers: synergism with sulindac-mediated inhibition of Bcl-x(L) Cancer Res. 2002;62:1583–7. [PubMed] [Google Scholar]
- 38.Gong B, Almasan A. Apo2 ligand/TNF-related apoptosis-inducing ligand and death receptor 5 mediate the apoptotic signaling induced by ionizing radiation in leukemic cells. Cancer Res. 2000;60:5754–60. [PubMed] [Google Scholar]
- 39.Di Pietro R, Secchiero P, Rana R, et al. Ionizing radiation sensitizes erythroleukemic cells but not normal erythroblasts to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)–mediated cytotoxicity by selective up-regulation of TRAIL-R1. Blood. 2001;97:2596–603. doi: 10.1182/blood.v97.9.2596. [DOI] [PubMed] [Google Scholar]
- 40.Zhang L, Fang B. Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther 2004 Nov 19; [Epub ahead of print]. [DOI] [PubMed]
- 41.Ferreira CG, Epping M, Kruyt FA, Giaccone G. Apoptosis: target of cancer therapy. Clin Cancer Res. 2002;8:2024–34. [PubMed] [Google Scholar]
- 42.Belka C, Jendrosek V, Pruschy M, Vink S, Verheij M, Budach W. Apoptosis-modulating agents in combination with radiotherapy—current status and outlook. Int J Radiat Oncol Biol Phys. 2004;58:542–54. doi: 10.1016/j.ijrobp.2003.09.067. [DOI] [PubMed] [Google Scholar]
- 43.Muzio M, Chinnaiyan AM, Kischkel FC, et al. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 44.Stennicke HR, Jurgensmeier JM, Shin H, et al. Pro-caspase-3 is a major physiologic target of caspase-8. J Biol Chem. 1998;273:27084–27090. doi: 10.1074/jbc.273.42.27084. [DOI] [PubMed] [Google Scholar]
- 45.Luo X, Budihardjo I, Zou H, et al. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 46.Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7:683–694. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 47.Oda E, Ohki R, Murasawa H, et al. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 2000;288:1053–1058. doi: 10.1126/science.288.5468.1053. [DOI] [PubMed] [Google Scholar]
- 48.Wei MC, Zong WX, Cheng EH, et al. Proapoptotic BAX and BAK: A requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li P, Nijhawan D, Budihardjo I, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91 :479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 50.Haimovitz-Friedman A, Kan CC, Ehleiter D, et al. Ionizing radiation acts on cellular membranes to generate ceramide and initiate apoptosis. J Exp Med. 1994;180:525–535. doi: 10.1084/jem.180.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhivotovsky B, Joseph B, Orrenius S. Tumor radiosensitivity and apoptosis. Exp Cell Res. 1999;248:10–17. doi: 10.1006/excr.1999.4452. [DOI] [PubMed] [Google Scholar]
- 52.Jain PT, Gewirtz DA. Sustained enhancement of liposome-mediated gene delivery and gene expression in human breast tumour cells by ionizing radiation. Int J Radiat Biol. 1999;75:217–23. doi: 10.1080/095530099140672. [DOI] [PubMed] [Google Scholar]
- 53.Stevens CW, Zeng M, Cerniglia GJ. Ionizing radiation greatly improves gene transfer efficiency in mammalian cells. Hum Gene Ther. 1996;7:1727–34. doi: 10.1089/hum.1996.7.14-1727. [DOI] [PubMed] [Google Scholar]
- 54.Zeng M, Cerniglia GJ, Eck SL, Stevens CW. High-efficiency stable gene transfer of adenovirus into mammalian cells using ionizing radiation. Hum Gene Ther. 1997;8:1025–32. doi: 10.1089/hum.1997.8.9-1025. [DOI] [PubMed] [Google Scholar]
- 55.Simons JW, Marshall FF. The future of gene therapy in the treatment of urologic malignancies. Urol Clin North Am. 1998;25:23–38. doi: 10.1016/s0094-0143(05)70430-4. [DOI] [PubMed] [Google Scholar]
- 56.Gibson SB, Oyer R, Spalding AC, Anderson SM, Johnson GL. Increased expression of death receptors 4 and 5 synergizes the apoptosis response to combined treatment with etoposide and TRAIL. Mol Cell Biol. 2000;20:205–12. doi: 10.1128/mcb.20.1.205-212.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nagane M, Pan G, Weddle JJ, Dixit VM, Cavenee WK, Huang HJ. Increased death receptor 5 expression by chemotherapeutic agents in human gliomas causes synergistic cytotoxicity with tumor necrosis factor-related apoptosis-inducing ligand in vitro and in vivo. Cancer Res. 2000;60:847–53. [PubMed] [Google Scholar]